Abstract
Rationale
Nitric oxide (NO) bioavailability is reduced in the setting of heart failure. Nitrite (NO2) is a critically important NO intermediate that is metabolized to NO during pathological states. We have previously demonstrated that sodium nitrite ameliorates acute myocardial ischemia/reperfusion (MI/R) injury.
Objective
No evidence exists as to whether increasing NO bioavailability via nitrite therapy attenuates heart failure severity following pressure overload-induced hypertrophy.
Methods and Results
Serum from heart failure patients exhibited significantly decreased nitrosothiol and cGMP levels. TAC was performed in mice at 10–12 weeks of age. Sodium nitrite (50 mg/L) or saline vehicle (VEH) was administered daily in the drinking water post-operative from day 1 for 9 weeks. Echocardiography was performed at baseline and at 1, 3, 6, and 9 weeks post TAC to assess left ventricular dimensions and ejection fraction (LVEF). We observed increased cardiac nitrite, RXNO, and cGMP levels in mice treated with nitrite. Sodium nitrite preserved LVEF and improved LV dimensions) at 9 weeks (p < 0.001 vs. VEH). In addition, circulating and cardiac brain natriuretic peptide (BNP) levels were attenuated in mice receiving nitrite (p < 0.05 vs. VEH). Western blot analyses revealed upregulation of Akt-eNOS-NO-cGMP-GS3Kβ signaling early in the progression of hypertrophy and heart failure.
Conclusions
These results support the emerging concept that nitrite therapy may be a viable clinical option for increasing NO levels and may have a practical clinical use in the treatment of heart failure.
Keywords: Hypertrophic cardiomyopathy, nitric oxide, left ventricular function, cyclic-GMP, heart failure, Akt, nitric oxide synthase
INTRODUCTION
Left ventricular hypertrophy is a maladaptive response to chronic pressure overload and an important risk factor for atrial fibrillation, diastolic heart failure, systolic heart failure and sudden death in patients.1
The integrity of the cardiovascular system is dependent on the continuous generation of nitric oxide (NO). As such, reduction in the bioavailability of NO is central to the development of cardiovascular disorders, including heart failure.2 For instance, class III heart failure patients have blunted endothelium-dependent flow-mediated dilatation in response to acetylcholine infusion indicative of diminished NO release. NO bioavailability is affected by the activity of the three nitric oxide synthases (NOS) and the presence of reactive oxygen species (ROS). In heart failure, the activity of endothelial NOS (eNOS) is reduced and there is an increase in ROS levels due to decreased antioxidant defenses. 3 Further evidence that eNOS/NO provide protection against heart failure comes from animal studies in which genetic overexpression of eNOS protects against4 whereas the genetic deficiency of eNOS enhances the development of heart failure.5 Pharmacological approaches to upregulate eNOS function have been shown to decrease the severity of heart failure.6 Therefore, therapeutic agents that enhance NO bioavailability, improve NO signaling, or provide a more favorable shift in redox balance may improve the outcome of heart failure.7
Nitrite is a promising therapeutic agent for the treatment of cardiovascular disease.8 Nitrite is a stable oxidative metabolic product of NO that is readily reduced back to NO in hypoxic and acidic environments.9 Previously it has been demonstrated that administration of nitrite attenuates the severity of ischemia/reperfusion injury (I/R) in a number of organs.10, 11 We have shown that nitrite reduces myocardial I/R injury.10, 12 However, no evidence exists as to whether increasing NO bioavailability through nitrite therapy attenuates chronic heart failure. The purpose of the current study was to investigate the effects of sustained sodium nitrite therapy following transverse aortic constriction.
METHODS
Experimental animals
Male C57BL/6J mice and CS-eNOS Tg mice at 10–12 weeks of age (Jackson Labs, Bar Harbor, ME) were used. All animals were housed in a temperature-controlled animal facility with a 12-hour light/dark cycle, with water and rodent chow provided ad libitum. All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the NIH (Publication No. 85-23, Revised 1996).
Serum samples from heart failure patients- Tacc study
Serum samples were obtained from patients enrolled in the Atlanta Cardiomyopathy Consortium (TACC). This prospective cohort study enrolls patients from the Emory University-affiliated teaching hospitals. All patients undergo detailed medical history surveys, electrocardiogram, standardized questionnaires, and blood and urine sample collection at baseline. All patients provide written informed consent prior to enrollment. The Emory University Institutional Review Board has approved this study.
Transverse aortic constriction (TAC) protocol
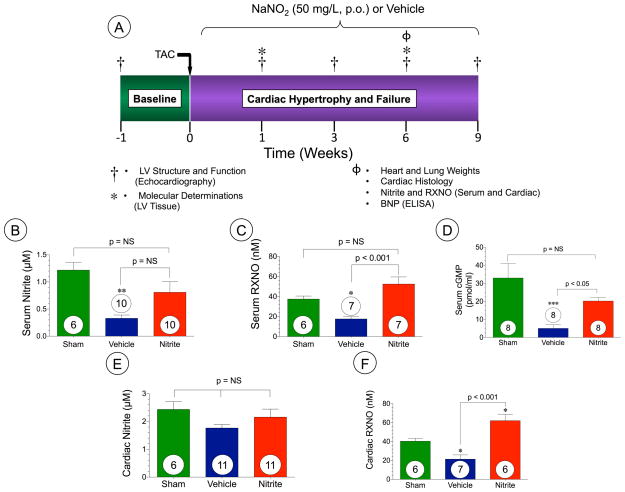
To establish myocardial pressure overload and heart failure transverse aortic constriction surgery was performed as described previously13. The complete experimental protocol for these studies is depicted in Figure 2A.
Figure 2. Oral sodium nitrite therapy augments NO metabolites in mice at 6 weeks following TAC.
(A) Experimental protocol for transverse aortic constriction (TAC) model in mice. (B) serum nitrite (μM), (C) serum RXNO (nM), (D) Serum cGMP (pmol/ml), (E) cardiac nitrite (μM) and (F) Cardiac RXNO (nM). Results are expressed as mean ± SEM.
Echocardiography and hemodynamic assessment
One week prior to TAC procedure, baseline transthoracic echocardiogram was performed using 30-MHz probe on a Vevo 2100 (Visualsonics) under anesthesia with isoflurane (0.25 to 0.50%) supplemented with 100% O2. Following TAC procedure, echocardiography was also performed in the same manner for up to 9 weeks at fixed intervals and pulsed-wave (PW) Doppler mode was used to measure hemodynamic assessment of aortic peak flow velocity at 1week.
Western blot analysis
Western blot analysis was performed as described previously.14
Myocardial measurement of NO metabolites
Nitrite (NO2−) nitroso products (RXNO) analysis of cardiac tissue and blood plasma were performed as previously described.15
Measurements of serum and myocardial brain natriuretic peptide (BNP)
Serum levels of brain natriuretic peptide (BNP EIA kit, Phoenix Pharmaceuticals, Inc.) were determined by ELISA at 6 weeks following TAC. Myocardial RNA levels of BNP were determined using PCR methods as described previously using. 16
cGMP radioimmunoassay (RIA)
The RIA was modified from17 Briefly, cGMP standards (Sigma) and samples were acetylated by adding 8 μl of 5 NKOH and 2 μl of acetic anhydride in a volume of 200 μl, and incubated at room temperature for 30 min. Then tubes were placed on ice to stop the reaction. All determinations were performed in duplicate. Each tube contained 50 μl acetylated standards or samples, 50 μl iodinated cGMP (14000 to 16000 cpm), and 50 μl cGMP antibody (Sigma). The reaction mixture was incubated overnight at 4°C. Then cGMP-antibody complex were precipitated by 12% polyethylene glycol 8000.
Histology and immunochemistry
Hearts were collected at the specific time points and fixed in 10% buffered formalin, embedded in paraffin stained with Masson’s trichome and Picrosirius Red to detect fibrosis Immunohistochemistry was performed to visualize vascular density with a commercially available kit (Blood Vessel staining kit, Millipore). In additional studies, mice were subjected to TAC for 6 weeks at which time lungs were harvested, fixed in 10% buffered formalin, embedded in paraffin, sectioned and stained with Hematoxylin and Eosin stain to determine the extent of pulmonary inflammation and edema.
Statistical analysis
All data are expressed as mean ± SEM. Student’s t test or a one-way ANOVA with Tukey’s or Dunnett’s post-hoc analysis was performed using Prism 5 (GraphPad Software). Values greater than two Standard deviations outside the mean were considered as outliers. A value of p < 0.05 was considered as statistically significant.
RESULTS
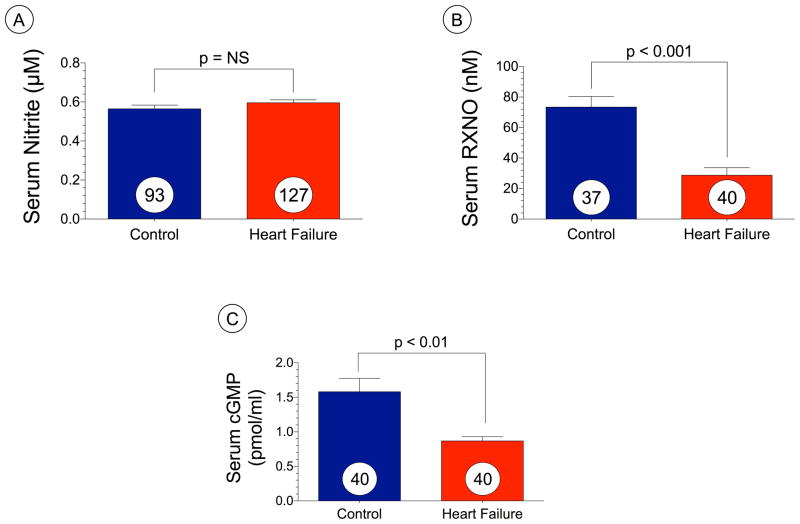
Serum levels of NO metabolites and cGMP are decreased in heart failure
We evaluated circulating levels of nitric oxide intermediates in heart failure patients (n=127) as compared to age-matched control subjects (n=93) and these data are depicted in Figure 1A–C. We failed to observe any differences in serum nitrite levels (μM) in heart failure compared to age-matched controls (Figure 1A). However, total serum RXNO (nM) were significantly (p < 0.001) reduced in heart failure patients (Figure 1B). Serum cGMP levels (pmol/ml) were also reduced in the heart failure (p < 0.01 vs. control) as compared to the controls (Figure 1C).
Figure 1. Serum levels of NO metabolites and cGMP are reduced in humans with heart failure.
(A) serum nitrite (μM), (B) serum RXNO (nM), and (c) serum cGMP. Results are expressed as mean ± SEM.
Effects of nitrite therapy on NO metabolites and cGMP levels in mice
To investigate the role of nitrite therapy in pressure overload induced hypertrophy and heart failure we performed TAC surgery in mice (Figure 2). Oral nitrite therapy (50 mg/L) was initiated postoperative day 1 following TAC surgery and was provided in drinking water continuously for 9 weeks. This oral nitrite resulted in the delivery of approximately 9–12 mg/kg/day to the mice (Supplemental Figure I). In order to evaluate the effects of nitrite therapy on NO bioavailability and signaling we examined serum and cardiac nitrite, RXNO, and cGMP levels in mice subjected to TAC (Figure 2). Similar to our clinical data TAC-induced heart failure resulted in decreased levels of serum nitrite (p<0.05 vs. Sham), serum RXNO, serum cGMP, and cardiac RXNO. Nitrite therapy following TAC restores serum RXNO (p < 0.001), serum cGMP (p < 0.05), and cardiac RXNO (p < 0.001) levels as compared to sham levels of NO bioavailability, however there was no significant difference in serum nitrite (Figure 2B–F).
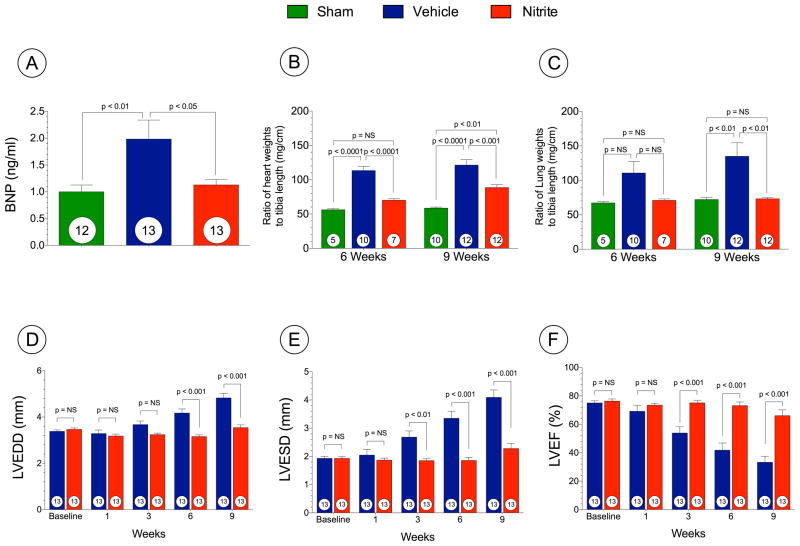
Adverse cardiac remodeling and pulmonary edema are attenuated with preserved LV function
Representative photomicrographs of sham mouse hearts and TAC + VEH or TAC + Nitrite are shown in Supplemental Figure 2. We evaluated circulating BNP levels (ng/ml), heart to tibia length (mg/cm), and lung to tibia weights (mg/cm) and these data are presented in Figures 3A–C. Cardiac structure and function were evaluated using echocardiography (Figure 3D–F). Circulating brain natriuretic peptide (BNP) levels serve as a reliable biomarker of heart failure severity in both humans and animal models. BNP levels increased significantly in TAC-VEH mice at 6 weeks compared to sham mice, and nitrite treatment significantly (p < 0.05) inhibited BNP levels as compared to VEH following TAC (Figure 3A). Myocardial tissue BNP mRNA expression was also determined using quantitative PCR methods (Supplemental Figure II).
Figure 3. Nitrite therapy prevents cardiac dilatation and dysfunction following TAC at 9 weeks.
(A) Circulating BNP levels (ng/ml). (B) The ratio of heart weight to tibia lengths. (C) The ratio of lung weight/tibia lengths. (D) Left ventricular end-diastolic diameter (LVEDD) (E) Left ventricular end-systolic diameter LVESD (F) Left ventricular ejection fraction (LVEF %) from 1 week to 9 weeks following TAC. Results are expressed as mean ± SEM.
Cardiac enlargement was confirmed by the heart weight/tibia length ratios demonstrating that the hearts of VEH mice were significantly increased compared to sham mice at 6 and 9 weeks following TAC (p < 0.001). Nitrite treated mice exhibited significantly increased heart weight to tibia length compared to sham mice at 9 weeks following TAC (p < 0.01) but these increases were less compared to VEH mice (Figure 3B). Nitrite treated mice displayed significantly less pulmonary edema when compared to VEH mice at 9 weeks (Figure 3C).
Nitrite treatment significantly prevented cardiac dilatation (LVEDD at 9 weeks; 4.8 ± 0.2 mm in VEH vs. 3.5 ± 0.1 mm in nitrite and LVESD at 9 weeks; 4.1 ± 0.3 mm in VEH vs. 2.3 ± 0.2 mm in nitrite (Figure 3D and E). Nitrite also attenuated LV dysfunction (Figure 3F) as assessed by left ventricular ejection fraction (LVEF) (33.3 ± 4.3% in VEH vs. 66.1 ± 4.1% in nitrite, p < 0.001 at 9 weeks). Despite robust cardiac protection we failed to observe any changes in survival with nitrite following TAC (Supplemental Figure III). Survival of mice receiving VEH was 59% and in mice receiving nitrite was 65% (p = NS).
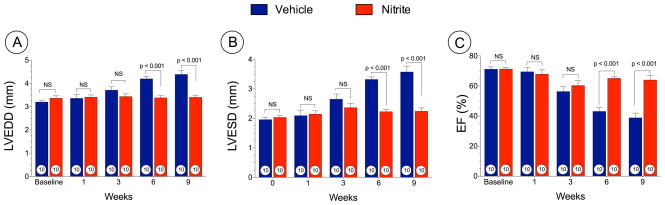
We also investigated the effects of oral nitrite started at 3 weeks following TAC (i.e., delayed nitrite therapy) and evaluated cardiac function at baseline, 1, 3, 6, and 9 weeks following TAC (Figure 4). Nitrite treatment significantly prevented cardiac dilatation (LVEDD at 9 weeks; 4.38 ± 0.1 mm in VEH vs. 3.4 ± 0.1 mm in nitrite and LVESD at 9 weeks; 3.5 ± 0.2 mm in VEH vs. 2.2 ± 0.1 mm in nitrite (Figure 4A and 4B). Nitrite also attenuated LV dysfunction (Figure 4C) as assessed by left ventricular ejection fraction (LVEF) (38.8 ± 3.1% in VEH vs. 63.9 ± 3.0% in nitrite, p < 0.001 at 9 weeks).
Figure 4. Delayed nitrite therapy prevents cardiac dilatation and dysfunction following TAC at 9 weeks.
Mice were subjected to TAC surgery and nitrite therapy (50 mg/L) was initiated at 3 weeks following TAC. (A) Left ventricular end-diastolic diameter (LVEDD) (B) Left ventricular end-systolic diameter LVESD (C) Left ventricular ejection fraction (LVEF %) from 1 week to 9 weeks following TAC. Results are expressed as mean ± SEM.
Hemodynamic assessment of aorta after TAC (PVb)
We measured aortic peak velocity after aortic banding in both the groups at 1 week following TAC. PVb was elevated significantly in both the groups after TAC as compare to sham mice without TAC at each time (data not shown). There was no significant difference between the groups (3055.4 ± 42 mm/s in VEH vs. 3265.3 ± 67.1 mm/s in nitrite) at 1 week thus indicating a similar severity of TAC in both study groups.
Overexpression of eNOS attenuates cardiac dysfunction following TAC
Cardiac-restricted overexpression of eNOS significantly increases nitrite and other NO metabolites in the heart and circulation18. We examined whether overexpression of eNOS within the cardiac myocyte attenuates cardiac hypertrophy and/or dysfunction following TAC using CS-eNOS Tg mice (Supplemental Figure V). There was no difference in the mortality between both groups (data not shown). CS-eNOS Tg mice exhibited significantly less cardiac enlargement and pulmonary edema, as assessed by the ratio of heart and lung weights to tibia length (mg/cm) when compared to wild-type controls (Supplemental Figure VA and VB). CS-eNOS Tg mice exhibited less cardiac dilatation (p < 0.001 vs. wild-type) and dysfunction (p < 0.001 vs. wild-type) from 3 weeks to 9 weeks following TAC (Supplemental Figure VC–E). Furthermore, while CS-eNOS Tg mice exhibited a significant difference in IVSd thickness compared to wild-type mice at 1 week following TAC (p < 0.01) this difference diminished after 3 weeks to 9 weeks (Supplemental Figure VF).
Nitrite attenuates cardiac fibrosis and augments vascular density
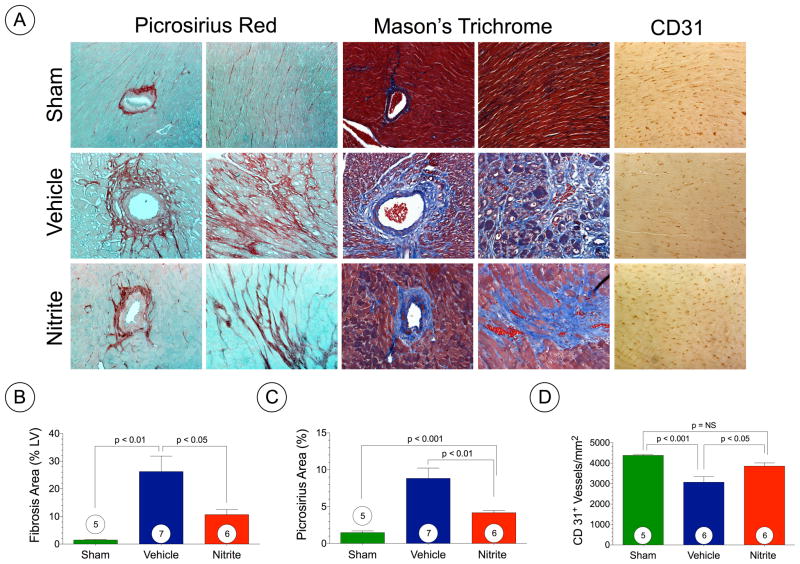
Masson’s Trichome and Picrosirius Red staining at 6 weeks following TAC (Figure 5A–C) revealed extensive areas of intermuscular and perivascular fibrosis in hearts of VEH TAC mice (p < 0.05 vs. sham) with significantly less fibrosis following nitrite therapy (Figure 5B–C). We also evaluated myocardial vascular density at 6 weeks following TAC surgery in VEH and nitrite treated hearts (Figure 5A and 5D). These data indicate significant vascular dropout following TAC that is partially restored with nitrite treatment (p < 0.05 vs. VEH).
Figure 5. Nitrite attenuates myocardial fibrosis following TAC.
(A) Representative photomicrographs of Masson’s Trichrome, Picrosirius Red, and CD31 stained heart sections from sham, TAC + vehicle, and TAC + nitrite mice at 6 weeks of TAC. (B) Summary of fibrosis area as % of the LV as calculated from Masson’s Trichrome sections. (C) Summary of fibrosis area as % of the LV calculated from the Picrosirius Red sections. (D) Summary of CD31+ vessels per area (mm2). Results are expressed as mean ± SEM.
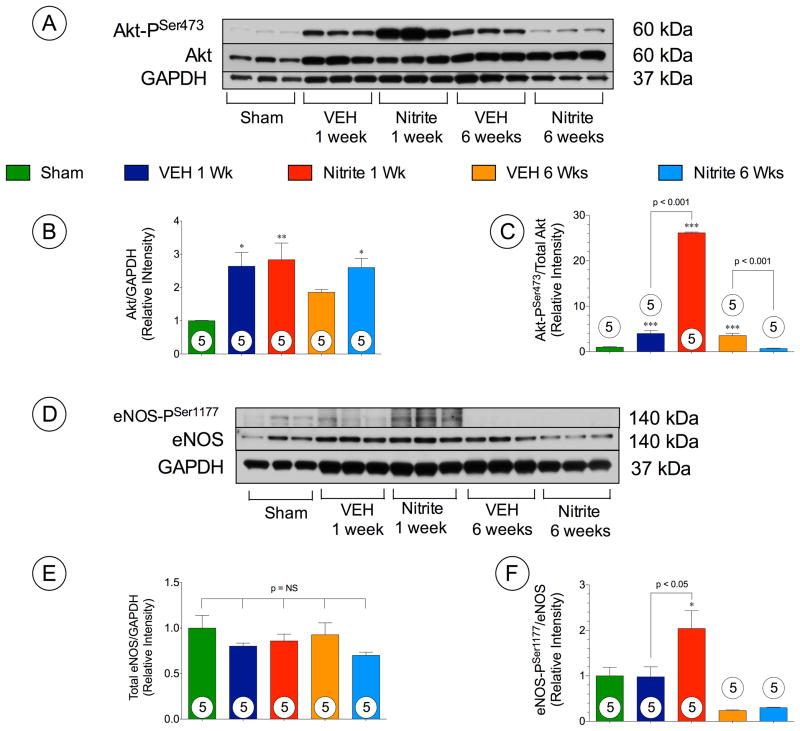
Nitrite augments Akt-eNOS signaling acutely following TAC
The serine/threonine kinase Akt regulates cardiac growth, angiogenesis, and survival19. We investigated whether nitrite altered Akt phosphorylation in the heart following TAC (Figure 6A–C). Representative Western blots for Akt phosphorylation status in the heart at 1 week and 6 weeks following TAC are shown in Figure 6A. Total Akt levels in the both VEH and nitrite treated mice significantly increased at 1 week following TAC, and in nitrite treated mice at 6 weeks. Nitrite treatment significantly increased the phosphorylated Akt at threonine residue 308 (Akt-PThr308) (p < 0.001) and serine residue 473 (Akt-PSer473) (p < 0.001) when compared to VEH mice at 1 week following TAC (Figure 6B and C). Interestingly, both Akt-PThr308 (p < 0.05) and Akt-PSer473 (p < 0.001) were significantly attenuated by nitrite treatment compared to VEH mice at 6 weeks following TAC (Figure 6B and C).
Figure 6. Nitrite upregulates Akt and endothelial nitric oxide (eNOS).
(A) Representative immunoblots of Akt phosphorylation. (B–C) Total Akt and Akt-PSer473 (D) Representative immunoblots of eNOS phosphorylation in TAC + vehicle, and TAC + nitrite treated mice following TAC at 1 week and 6 weeks. Results are expressed as mean ± SEM.
Upregulation of Akt results in eNOS activation to generate NO that modulates vascular angiogenesis and promote vascular and myocardial cytoprotection.20 To investigate the involvement of eNOS function following TAC, the expression and the phosphorylation status of eNOS at serine residue 1177 (eNOS-PSer1177) were assessed by Western blot analysis (Figure 6D–F). There were no differences in total eNOS expression in the heart among all groups (Figure 6E). However, the eNOS activation site (eNOS-PSer1177) exhibited significantly greater phosphorylation (Figure 6F) in the nitrite group compared to either sham (p < 0.01) or VEH mice (p < 0.01) at 1 week. Similar to the activation of Akt, the increase in eNOS phoshorylation at Ser1177 was transient and not observed at 6 weeks following TAC.
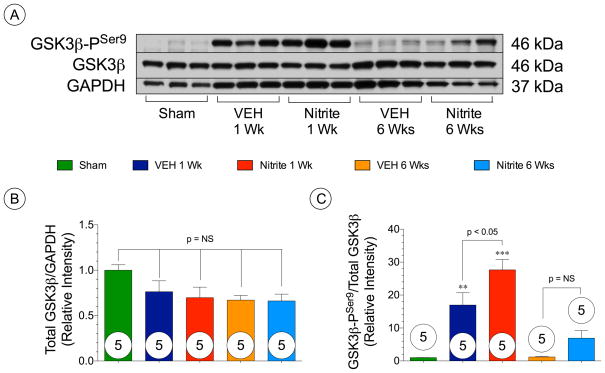
Nitrite augments GSK3β signaling inhibition in acute phase following TAC
Phosphorylation of protein kinase B/Akt (PKB/Akt) has been linked with the inhibition of GSK3β through phosphorylation at serine residue 9.21 Further, We investigated whether nitrite treatment altered GSK3β phosphorylation in the heart following TAC (Figure 7). Representative Western blots for GSK3β phosphorylation status in the heart at 1 week and 6 weeks following TAC are shown in Figure 7A. Total GSK3β levels in the both VEH and nitrite treated mice remained the same at 1 week and 6 weeks following TAC (Figure 7B). Nitrite treatment significantly increased the expression of phosphorylated-GSK3β at ser9 when compared to VEH mice (p < 0.05) at 1 week (Figure 7C). There were no differences in GSK3β phosphorylation between the nitrite and VEH groups at 6 weeks post-TAC as shown in Figure 7C.
Figure 7. Nitrite upregulates GSK3β activity.
(A) Representative immunoblots of (B) total GSK3β and (C) GSK3β-PSer9 in Sham, TAC + vehicle, and TAC + nitrite mice at 1 week and 6 weeks following TAC. Results are expressed as mean ± SEM.
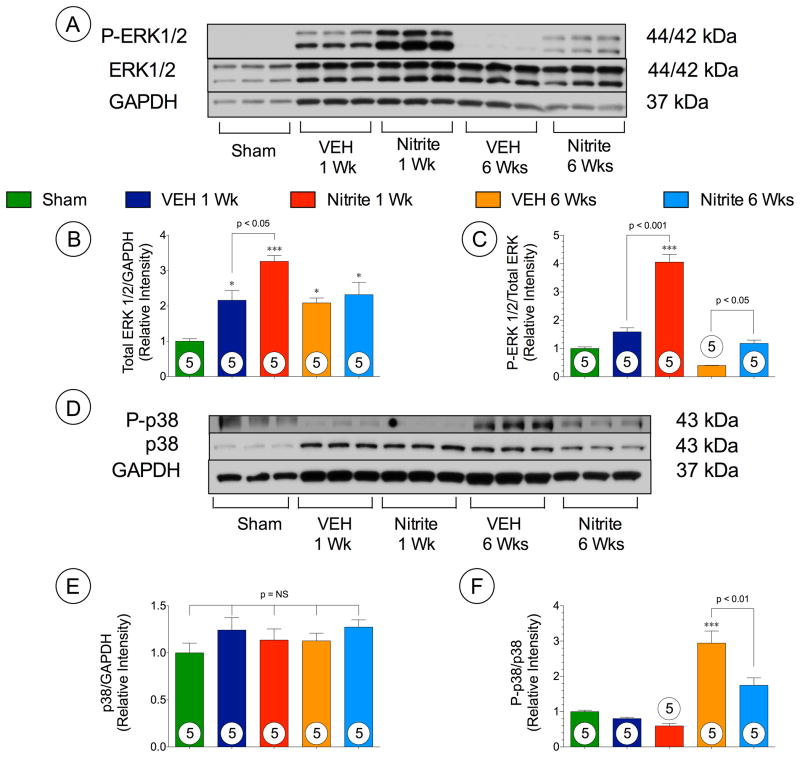
Nitrite augments ERK1/2 signaling following TAC
ERK1/2 is a known regulator of cardiac hypertrophy.22 We investigated whether nitrite treatment altered ERK phosphorylation in the heart following TAC (Figure 8A–C). Representative Western blots for ERK phosphorylation status in the heart at 1 week and 6 weeks following TAC are shown in Figure 8A. Total ERK levels (Figure 8B) in the both VEH and nitrite treated mice significantly increased at 1 week and 6 weeks following TAC. Nitrite treatment significantly increased the expression of phosphorylated- ERK1/2 when compared to VEH mice (p < 0.001) at 1 week and 6 weeks (p < 0.05) following TAC (Figure 8C).
Figure 8. Nitrite upregulates ERK1/2 and attenuates p38 phosphorylation.
(A) Representative immunoblots of (B) total ERK1/2 and (C) P-ERK1/2. (D) Representative immunoblots of (D) total p38 and P-p38 in Sham, TAC + vehicle, and TAC + nitrite treated mice following TAC at 1 week and 6 weeks. Results are expressed as mean ± SEM.
Nitrite attenuates p38 signaling following TAC
Previous studies have shown that activation of p38 leads to cardiac dysfunction by reducing LV function and increasing cardiac fibrosis.23, 24 We investigated the phosphorylation status of p38 at 1 and 6 weeks following TAC (Figure 8D–F). Representative Western blots for P38 phosphorylation status in the heart at 1 week and 6 weeks following TAC are shown in Figure 8D. We found that nitrite treatment significantly attenuated p38 activation at 6 weeks compared to the vehicle group, but failed to show any change at 1 week following TAC (Figure 8F).
Nitrite attenuates lung inflammation and edema
We also investigated the effect of oral nitrite on lung tissue after transverse aortic constriction in both the control and nitrite treated mice at 6 weeks. We found that in the nitrite treatment group, there is less inflammation and edema as well less thicker alveolar walls as compared to the vehicle group at 6 weeks. Representative pictures were taken at 5x and 20x magnification for both the groups (Supplemental Figure IV).
DISCUSSION
Alterations in NO bioavailability play a prominent role in the development of heart failure.7, 25 Studies have shown that enhancing NO levels through genetic manipulation leads to improved survival 4, decreased remodeling 26 and improved cardiac function following ischemia-induced heart failure. Furthermore, eNOS-derived NO exerts antihypertrophic effects in the heart as evidenced by the findings that eNOS deficient animals exhibit hypertension and cardiac hypertrophy27,28 Moreover, cardiac-specific overexpression of eNOS attenuates isoproterenol induced cardiac hypertrophy.29 The current study provides several lines of evidence to support these previous findings. Our study clearly demonstrates reductions in circulating levels of nitrosothiols and cGMP in patients with heart failure. We also demonstrate significant reductions in circulating and cardiac NO metabolites and cGMP in the setting of TAC-induced heart failure in mice. We also demonstrate that chronic oral nitrite therapy provides protection against the adverse remodeling and LV dysfunction associated with heart failure. Our findings suggest that increasing NO bioavailability in the myocardium protects against the development of pressure-induced heart failure.
The role of NO in the pathophysiology and treatment of chronic heart failure is not without controversy. Studies report that NO contributes to detrimental cardiac function in heart failure.30 Additionally, Takimoto et al. suggested that pressure overload results in eNOS-uncoupling resulting in increased myocardial oxidant production and exacerbated cardiac dysfunction.31 In sharp contrast to these findings, we found that the genetic overexpression of eNOS in the heart provided protection against the adverse remodeling and impaired LV function associated with TAC, and not exacerbated cardiac pathology. Additionally, the effect of NO on the mitochondria is recognized as one of its cardioprotective actions, as this leads to a decrease of myocardial injury by extending the zone of adequate tissue cellular oxygenation away from vessels.32 Furthermore, physicians have been successfully using drugs which are able to activate eNOS, (i.e. ACE-I and ARB) or increase nitric oxide bioavailability (i.e., ISDN-hydralazine-Bidil®) in the treatment of heart failure.33 Likewise, increasing the NO second messenger, cGMP, following sildenafil treatment has been shown to improve exercise capacity in patients with heart failure.34 Finally, statins augment eNOS-NO signaling, have been shown to prolong survival in a murine model of heart failure.35 Therefore, evidence is accumulating that under the appropriate conditions, NO in general, and eNOS-derived NO in particular, impart cardioprotective effects in the failing heart36
In an effort to provide insights into the mechanisms regulating the progression of heart failure, Haq et al investigated the activity of several signaling cascades in heart samples taken from patients with compensated cardiac hypertrophy and advanced heart failure. Although they found a clear pro-hypertrophic activity profile in both patient populations, the signaling cascades activated in both were distinct. The results of the current study suggest that nitrite therapy prevents the development of heart failure in response to TAC. In an effort to understand the molecular mechanisms associated with this prevention, we evaluated the effects of nitrite therapy on several signal transduction pathways activated in response to heart failure. We focused our analysis on two time points representing an acute onset of hypertrophy (1 week) and heart failure (6 weeks).
The serine/threonine protein kinase Akt regulates cardiac growth, contractile function and cell death and also is an important factor for VEGF-mediated angiogenesis37. Our data suggest that nitrite therapy rapidly induces Akt activation resulting in eNOS phosphorylation and inhibition of GSK3β at one week following the induction of aortic constriction. At 6 weeks of TAC, Akt phosphorylation remained elevated in the vehicle group, but returned to baseline levels in the nitrite group. Previous studies suggest that short term activation of Akt in cardiac muscle protects from contractile dysfunction in the failing heart38, whereas long-term activation switches the heart from an adaptive, compensated state with preserved function to a pathological state with cardiac dysfunction39. Our findings support the notion that Akt can exerts a dual role in the development of heart failure following TAC. The activation of Akt in the failing heart is due to a number of stress-related stimuli 40. As such, the persistent activation of Akt observed in the vehicle-treated heart at 6 weeks following TAC could be indicative of still present stimuli. Likewise, the decrease in Akt activation observed in the nitrite-treated hearts could be due to a reduction in these stimuli at the 6-week time point. Akt signaling is well known to occur in cardiac hypertrophy and perhaps some of the mixed results could reflect a combination of survival signaling and cell growth stimulation. Further studies are required to determine the exact mechanism by which nitrite therapy modulates Akt activation during the development of heart failure.
GSK3β regulates a wide variety of cellular functions and serves as a master regulator of cell growth and death in cardiac myocytes in response to hypertrophic stimuli. 41 The activity of GSK3β is negatively regulated by Akt and inhibiting GSK3β is critical to the antiapoptotic effects of Akt and to the hypertrophic response of cardiomyocytes.40, 42 Our findings suggests that nitrite therapy prevents the transition from compensated to decompensated heart failure via the inhibition GSK3β.
We also demonstrate that nitrite therapy increases the activation of MEK1-ERK1/2 signaling and attenuates p38 activation. Specifically, we found that nitrite therapy rapidly induces ERK1/2 activation at one week following the induction of aortic constriction. At 6 weeks of TAC, ERK1/2 phosphorylation levels remained higher in the nitrite-treated group compared to the vehicle-treated group. However, the level of phosphorylation in the nitrite-treated group was similar to those found in Sham animals. In regards to p38 activation, we found that nitrite therapy reduced the level of p38 phosphorylation at 6 weeks of TAC. It has been demonstrated that activation of MEK1-ERK1/2 signaling regulates hypertrophic response in vivo22 and promotes compensated cardiac hypertrophy and preservation of cardiac function43. In addition to the reported hypertrophic effects of ERK1/2 signaling, the enhanced activation of this pathway has been shown to provide cardioprotection via robust anti-apoptotic actions 44. Previous studies have indicated that p38 activation has a detrimental effect on cardiac function that leads to pathological hypertrophy rather than physiological compensation23, 24. The enhanced activation of a MEK1-ERK1/2 signaling pathway and inhibition of p38 in the nitrite-treated hearts is also responsible for preventing the transition from compensated to decompensated heart failure.
We also investigated its effect in an ischemic induced heart failure model. Previous studies indicate that nitrite is both a storage form of NO in tissues and also a source of NO in ischemic conditions45. Nitrite is cardioprotective during I/R injury via increasing nitrosothiol levels.11,46 Our results demonstrate that chronic nitrite therapy leads to significantly higher plasma nitrosothiol levels and higher myocardial nitrite levels.
Although the current study demonstrates that chronic nitrite therapy improves left ventricular function following heart failure, there are some limitations that need to be noted. Because a mouse model was used, these data may not accurately predict human disease. Future studies need to be conducted in large animal models that are more clinically relevant. Another limitation is used mice in this study that are without any other co-morbid conditions like hypertension, hypercholesterolemia and diabetes. We also started nitrite therapy at 24 hours following transverse aortic constriction that attenuated the progression of heart failure. Future studies need to evaluate nitrite therapy at a time when heart failure is fully developed.
In summary, we have demonstrated a cardioprotective action for sodium nitrite therapy on the severity of heart failure following TAC. Specifically, the current study provides evidence that chronic oral administration of sodium nitrite increases NO bioavailability and signaling following TAC-induced cardiac hypertrophy and failure. These results support the emerging concept that nitrite therapy may be a viable clinical option for increasing NO levels and may have a practical clinical use in the treatment of heart failure.
Supplementary Material
Novelty and Significance.
What Is Known?
In preclinical studies nitrite therapy (i.e., sodium nitrite) has been found to reduce ischemia/reperfusion injury in various organs by increasing the bioavailability of nitric oxide (NO) and augmenting NO signaling.
In a recent clinical trial, chronic nitrite therapy has been shown to be safe and well tolerated in humans with peripheral artery disease (i.e., SONIC Trial). Currently, there are 37 ongoing clinical trials (clinicaltrials.gov) to investigate the cytoprotective actions of sodium nitrite.
Recent evidence suggests that sodium nitrite is not effective in reducing myocardium infarct size following myocardial ischemia and reperfusion. There was no reported reduction in myocardial infarct size in the NIH CAESAR Preclinical Cardioprotection Consortium (mice, rabbit, and pig) and in the NIAMI (Nitrites in Acute Myocardial Infarction) human clinical trial.
What New Information Does This Article Contribute?
Nitric oxide (NO) bioavailability is reduced in the myocardium and in circulation during heart failure in both mice and humans.
In mice, chronic oral sodium nitrite therapy increases NO bioavailability and attenuates the severity of heart failure induced by chronic pressure overload..
Sodium nitrite increased myocardial vascular growth while decreasing cardiac fibrosis in chronic heart failure.
In the present study, we demonstrate a cardioprotective action for sodium nitrite therapy on the severity of heart failure following transverse aortic constriction. The study provides evidence that chronic oral administration of sodium nitrite increases NO bioavailability and signaling following transverse aortic constriction-induced heart failure. These results support the emerging concept that nitrite therapy may be a viable therapeutic option for increasing NO levels and potentially, for treating heart failure patients.
Acknowledgments
We thank Bradley Schwartz, Nabil Sabbak and Benjamin Hayes for their entire expert technical help during the course of these experimental studies.
SOURCES OF FUNDING
This work was supported by grants from the National Heart, Lung, and Blood Institute (National Institutes of Health; 5R01HL092141, 5R01HL093579, 1U24HL 094373, and 1P20HL113452 to Dr. Lefer and 5R01HL098481 to Dr. Calvert).
This research was also supported by a research grant from the American Diabetes Association, ADA grant 1-12-BS212, to Dr. Ya-xiong Tao, Ph.D.
Non-standard Abbreviations and Acronyms
- NO
Nitric oxide
- NaNO2
Sodium nitrite
- MI/R
Myocardial ischemia and reperfusion
- TAC
Transverse aortic constriction
- LVEF
Left ventricular ejection fraction
- LVEDD
Left ventricular end diastolic diameter
- LVESD
Left ventricular end systolic diameter
- VEH
Vehicle
- eNOS
Endothelial nitric oxide synthase
- NOS
Nitric oxide synthases
- RXNO
Nitrosothiol
- BNP
Brain natriuretic peptide
Footnotes
DISCLOSURES
D.J.L. has served on the Scientific Advisory Board of Theravasc, Inc. Theravasc is currently developing novel nitrite formulations for the treatment of peripheral arterial disease (PAD) and cardiovascular diseases.
References
- 1.Katholi RE, Couri DM. Left ventricular hypertrophy: Major risk factor in patients with hypertension: Update and practical clinical applications. International journal of hypertension. 2011;2011:495349. doi: 10.4061/2011/495349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2002;53:503–514. [PubMed] [Google Scholar]
- 3.Smith CJ, Sun D, Hoegler C, Roth BS, Zhang X, Zhao G, Xu XB, Kobari Y, Pritchard K, Jr, Sessa WC, Hintze TH. Reduced gene expression of vascular endothelial no synthase and cyclooxygenase-1 in heart failure. Circulation research. 1996;78:58–64. doi: 10.1161/01.res.78.1.58. [DOI] [PubMed] [Google Scholar]
- 4.Jones SP, Greer JJ, van Haperen R, Duncker DJ, de Crom R, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104:1286–1291. doi: 10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- 6.Fraccarollo D, Widder JD, Galuppo P, Thum T, Tsikas D, Hoffmann M, Ruetten H, Ertl G, Bauersachs J. Improvement in left ventricular remodeling by the endothelial nitric oxide synthase enhancer ave9488 after experimental myocardial infarction. Circulation. 2008;118:818–827. doi: 10.1161/CIRCULATIONAHA.107.717702. [DOI] [PubMed] [Google Scholar]
- 7.Taylor AL. Nitric oxide modulation as a therapeutic strategy in heart failure. Heart failure clinics. 2012;8:255–272. doi: 10.1016/j.hfc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Calvert JW, Lefer DJ. Clinical translation of nitrite therapy for cardiovascular diseases. Nitric oxide: biology and chemistry/official journal of the Nitric Oxide Society. 2010;22:91–97. doi: 10.1016/j.niox.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejam A, Hunter CJ, Schechter AN, Gladwin MT. Emerging role of nitrite in human biology. Blood cells, molecules & diseases. 2004;32:423–429. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. The Journal of clinical investigation. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Sr, Gojon G, Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2s protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhushan S, Kondo K, Predmore BL, Zlatopolsky M, King AL, Pearce C, Huang H, Tao YX, Condit ME, Lefer DJ. Selective beta2-adrenoreceptor stimulation attenuates myocardial cell death and preserves cardiac function after ischemia-reperfusion injury. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1865–1874. doi: 10.1161/ATVBAHA.112.251769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aragon JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ. Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. Journal of the American College of Cardiology. 2011;58:2683–2691. doi: 10.1016/j.jacc.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng R, Calvert JW, Sibmooh N, Piknova B, Suzuki N, Sun J, Martinez K, Yamamoto M, Schechter AN, Lefer DJ, Noguchi CT. Acute erythropoietin cardioprotection is mediated by endothelial response. Basic research in cardiology. 2011;106:343–354. doi: 10.1007/s00395-011-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiner AL, Parker CW, Kipnis DM. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972;247:1106–1113. [PubMed] [Google Scholar]
- 18.Elrod JW, Greer JJ, Bryan NS, Langston W, Szot JF, Gebregzlabher H, Janssens S, Feelisch M, Lefer DJ. Cardiomyocyte-specific overexpression of no synthase-3 protects against myocardial ischemia-reperfusion injury. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1517–1523. doi: 10.1161/01.ATV.0000224324.52466.e6. [DOI] [PubMed] [Google Scholar]
- 19.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circulation research. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 20.Calvert JW, Lefer DJ. Clinical translation of nitrite therapy for cardiovascular diseases. Nitric oxide: biology and chemistry/official journal of the Nitric Oxide Society. 2010;22:91–97. doi: 10.1016/j.niox.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, Clerk A, Sugden PH. Glycogen synthase kinases 3alpha and 3beta in cardiac myocytes: Regulation and consequences of their inhibition. Cellular signalling. 2008;20:206–218. doi: 10.1016/j.cellsig.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circulation research. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 23.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki J, Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, Hori M, Otsu K. P38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Molecular and cellular biology. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiological reviews. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz SD, Khan T, Zeballos GA, Mathew L, Potharlanka P, Knecht M, Whelan J. Decreased activity of the l-arginine-nitric oxide metabolic pathway in patients with congestive heart failure. Circulation. 1999;99:2113–2117. doi: 10.1161/01.cir.99.16.2113. [DOI] [PubMed] [Google Scholar]
- 26.Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, Jans P, Scherrer-Crosbie M, Picard MH, Szelid Z, Gillijns H, Van de Werf F, Collen D, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation research. 2004;94:1256–1262. doi: 10.1161/01.RES.0000126497.38281.23. [DOI] [PubMed] [Google Scholar]
- 27.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 28.Ichinose F, Bloch KD, Wu JC, Hataishi R, Aretz HT, Picard MH, Scherrer-Crosbie M. Pressure overload-induced lv hypertrophy and dysfunction in mice are exacerbated by congenital nos3 deficiency. American journal of physiology. Heart and circulatory physiology. 2004;286:H1070–1075. doi: 10.1152/ajpheart.00940.2003. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki M, Kawashima S, Yamashita T, Hirase T, Ohashi Y, Inoue N, Hirata K, Yokoyama M. Overexpression of endothelial nitric oxide synthase attenuates cardiac hypertrophy induced by chronic isoproterenol infusion. Circulation journal: official journal of the Japanese Circulation Society. 2002;66:851–856. doi: 10.1253/circj.66.851. [DOI] [PubMed] [Google Scholar]
- 30.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: Ten years after, and continuing. Circulation research. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 31.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. The Journal of clinical investigation. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: Implications for the perivascular dynamics of no and o2. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark AL. Almanac 2011: Heart failure. The national society journals present selected research that has driven recent advances in clinical cardiology. Heart. 2011;97:1643–1649. doi: 10.1136/heartjnl-2011-300897. [DOI] [PubMed] [Google Scholar]
- 34.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 35.Greer JJ, Kakkar AK, Elrod JW, Watson LJ, Jones SP, Lefer DJ. Low-dose simvastatin improves survival and ventricular function via enos in congestive heart failure. American journal of physiology. Heart and circulatory physiology. 2006;291:H2743–2751. doi: 10.1152/ajpheart.00347.2006. [DOI] [PubMed] [Google Scholar]
- 36.Prabhu SD. Nitric oxide protects against pathological ventricular remodeling: Reconsideration of the role of no in the failing heart. Circulation research. 2004;94:1155–1157. doi: 10.1161/01.RES.0000129569.07667.89. [DOI] [PubMed] [Google Scholar]
- 37.Chaanine AH, Hajjar RJ. Akt signalling in the failing heart. European journal of heart failure. 2011;13:825–829. doi: 10.1093/eurjhf/hfr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiojima I, Schiekofer S, Schneider JG, Belisle K, Sato K, Andrassy M, Galasso G, Walsh K. Short-term akt activation in cardiac muscle cells improves contractile function in failing hearts. The American journal of pathology. 2012;181:1969–1976. doi: 10.1016/j.ajpath.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated akt in the heart. The Journal of biological chemistry. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 40.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 41.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: A novel regulator of cardiac hypertrophy and development. Circulation research. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 43.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The mek1-erk1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. The EMBO journal. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouyssegur J, Pages G, De Windt LJ, Doevendans PA, Molkentin JD. Mek1-erk2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 45.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. American journal of physiology. Heart and circulatory physiology. 2006;291:H2026–2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, 3rd, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.