Abstract
Purpose
The purpose of this review is to describe the evolution of the use of dental adhesives to form a tight seal of freshly prepared dentin to protect the pulp from bacterial products, during the time between crown preparation and final cementum of full crowns. The evolution of these “immediate dentin sealants” follows the evolution of dental adhesives, in general. That is, they began with multiple-step, etch-and-rinse adhesives, and then switched to the use of simplified adhesives.
Methods
Literature was reviewed for evidence that bacteria or bacterial products diffusing across dentin can irritate pulpal tissues before and after smear layer removal. Smear layers can be solubilized by plaque organisms within 7–10 days if they are directly exposed to oral fluids. It is likely that smear layers covered by temporary restorations may last more than one month. As long as smear layers remain in place, they can partially seal dentin. Thus, many in vitro studies evaluating the sealing ability of adhesive resins use smear layer-covered dentin as a reference condition. Surprisingly, many adhesives do not seal dentin as well as do smear layers.
Results
Both in vitro and in vivo studies show that resin-covered dentin allows dentinal fluid to cross polymerized resins. The use of simplified single bottle adhesives to seal dentin was a step backwards. Currently, most authorities use either 3-step adhesives such as Scotchbond Multi-Purposea or OptiBond FLb or two-step self-etching primer adhesives, such as Clearfil SEc, Unifil Bondd or AdheSEe, respectfully.
Introduction
When indirect restorations are used to restore function, dentists must seal the exposed dentin with temporary materials during the interval required to fabricate and cement the final restoration. Full crown preparations expose up to 1 cm2 of dentin that contains more than 3 million tubules/cm2.1 Such tubules represent millions of microscopic “pathways to the pulp” because they all terminate in the tooth pulp. Both enamel and cementum are impermeable and nerve-free. These peripheral seals have very low permeabilities. However, once these surface sealing hard tissues are removed from dentin surfaces, the exposed dentin becomes highly permeable and very sensitive to hydrodynamic stimuli2. Because dentinal tubules contain collagen fibrils/fibers, constrictions etc. their functional diameter (0.1 µm) is far smaller than their 1.0 µm anatomical diameter3. This allows dentin to function like a 0.1 µm Millipore filter to prevent bacteria from invading the pulp via dentinal tubules. However, soluble bacterial products can permeate through dentin to the pulp where they provoke immunological reactions and pulpal inflammation that threaten pulpal health4–6.
Prevention of pulpal inflammation
Current prosthodontists recommend conservative tooth preparations using copious air-water spray and intermittent cutting. This is followed by temporizing the preparations using a variety of temporary filling materials and crown formers as long as there are no pre-existing signs of pulp pathology. The rationale is that a healthy pulpodentin complex reacts to tooth preparation by the deposition of tertiary dentin under those tubules that were cut during cavity preparation that should wall off the prepared dentin and prevent bacterial invasion7.
On the other hand, old teeth have smaller pulps with fewer mesenchymal cells, and a poorer blood supply8. Tertiary dentin requires more than 30 days to begin to form and that dentin will not form if the pulp under the cut tubules produces an inflammatory response7. Old pulps contain pulp stones that interfere with endodontic treatment. If a temporary crown is lost and the cut dentin is exposed, bacterial products will begin to diffuse down the tubules toward the pulp. Pulpal cells will react to these bacterial antigens as if actual bacteria were invading the pulp. This will trigger neurogenic inflammation in the pulp leading to pulpal symptoms9,10.
Those that advocate immediate dentin sealing (IDS) of freshly prepared dentin seek to protect the pulp from bacteria and bacterial products, using adhesive resins11–24. These resins should prevent dentinal fluid from permeating from inside dentin, through polymerized resin to the surface. The resins should also prevent the inward diffusion of bacterial products through the polymerized resin. This involves acid-etching dentin with 37% phosphoric acid for 15 sec when using etch-and-rinse adhesives. When using self-etching adhesives, the two-bottle primer adhesives are preferred over single-bottle systems because the acidic primer is covered with a solvent-free adhesive, rich in dimethacrylates that create stronger resin films than monomethacrylates. Thicker resin seals are better than thinner coatings, because they are less likely to be lacerated by multiple setting of crowns during their final adjustments.
Combinations of resin adhesives covered by flowable composites provide tougher IDS than resin films alone. Optibond FLb is an example of a 3-step, etch-and-rinse adhesive that uses an adhesive that contains 48% fumed SiO2 and barium aluminoborosilicate Na2SiF625. Scotchbond Multi-Purposea adhesive contains few fillers and is often covered with a flowable composite to make the IDS stronger. The final adhesive material is covered with glycerin gel before polymerization to prevent the formation of an oxygen-inhibited layer. Such a layer of unpolymerized comonomers interferes with polymerization of impression material26.
Advantages of IDS include eliminating post-cementation sensitivity27, eliminating the potential risk of bacterial leakage and pulpal irritation. Local anesthesia is often not required for try-ins and occlusal adjustments. IDS should be considered for any patients at increased risk of pulpitis due to immunosuppression, aged pulps, previous history of tooth sensitivity, patients taking bisphosphonates, etc. who are at risk of developing bisphosphonate-induced osteonecrosis of the jaw28. If such patients require root canal therapy and the roots are perforated during RCT, such extraction sites fail to heal properly. Abutment teeth in such patients should receive IDS as a precautionary measure.
The influence of the thickness of IDS materials has also been reported29. That group found that thicker adhesives increased fracture resistance of IDS Empress 2 ceramic crowns.
The purpose of this review was to follow the evolution of the use of dental adhesives to create an immediate dentin seal to protect the pulpodentin complex from inflammatory insults, in vitro. This review will begin with etch-and-rinse adhesives, followed by self-etching primer/adhesives and finally single-bottle, simplified self-etching adhesives.
Historical review
The work of Bergenholtz4,5 showed that bacterial products could diffuse across freshly prepared dentin to induce pulpal inflammation. This lead Pashley et al. in 1992 to propose sealing freshly prepared dentin with adhesive resins11. This was endorsed by Davidson’s group in 199612, Paul and Schaerer 199713, and Özturk et al.14. Prof. Tagami advocated “resin coating” of freshly cut dentin to prevent pulpal irritation15–17, and to increase adhesion to dentin. Others have also stressed the importance of resin sealing18–24. In their 1992 paper, Pashley et al.,11, the authors mounted the crowns of extracted human third molars on plexiglass blocks and prepared full crown preparations (Fig. 1). They measured dentin permeability as a hydraulic conductance before and after sealing the preparations with Prisma Univeral Bond 2f, Scotchbond 2a, Superbond C&Bg, Amalgambondh, Gluma Bondi or Clearfil Photobondc. All adhesives reduced dentin permeability in vitro by at least 50%, with Prisma Universal Bond 2f sealing the best followed by Superbond C&Bg, Amalgambondh and Scotchbond 2a. The worst seals were produced by Gluma Bondi. Bonds made with Scotchbond 2a and Clearfil Photobondc gave excellent initial seals, but began to leak after thermocycling the bonded dentin.
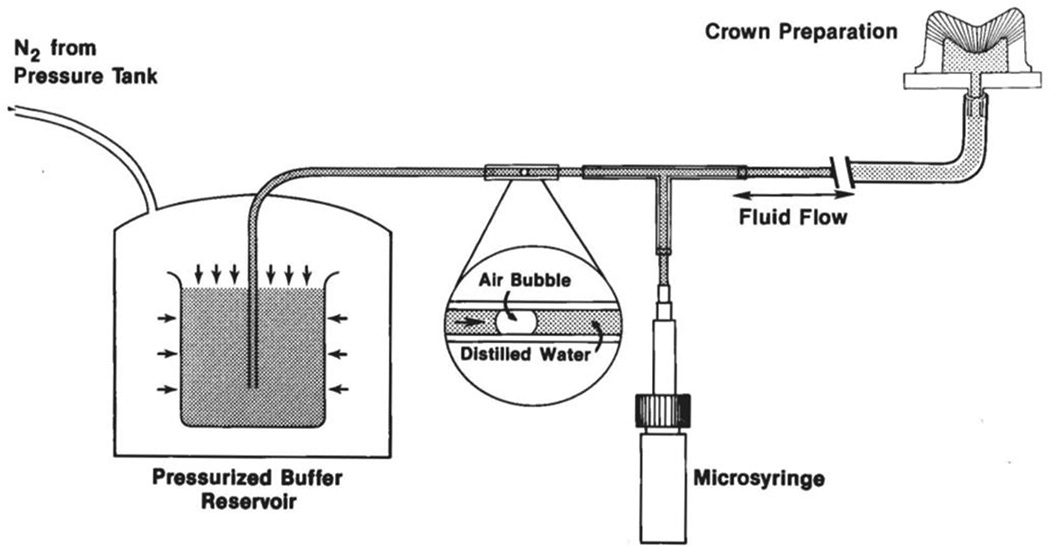
Fig. 1.
Schematic of the apparatus used to measure dentin permeability. N2 gas passed into a pressure vessel containing a beaker of water. The water passes through the system at whatever pressure is used. The rate of movement of a tiny air bubble in a 25 µL micropipette quantitates how much water permeates across exposed dentin. The microsyringe adjusts the position of the air bubble for the next trial (from Pashley et al.11, with permission).
Bouillaguet et al.20 used extracted human third molar crowns flattened on the occlusal surface and glued to a plexiglass base penetrated by 18 ga. stainless steel tubing to permit measurement of dentin permeability. They measured the hydraulic conductance of acid-etched dentin before and after bonding with Scotchbond Multi-Purposea, Prime & Bond 2.0f or All-Bond 2j (Table 1). All of these etch-and-rinse adhesives reduced dentin permeability by 83%, 90% or 96.6%, respectively. Unfortunately, the authors only measured the initial reduction in permeability. When Gregorie et al.30 evaluated the sealing ability of Optibond Solo Plusb, Single Bonda, Excitee and Prime & Bond NTf, all these etch-and-rinse adhesives only reduced dentin permeability to a residual value of 40%, while the self-etching adhesives Clearfil SE Bondc, and Prompt-L Popa only reduced dentin permeability down to a residual value of 36 and 16%, respectively (Table 2). They also only measured initial permeability. Better initial permeability results were reported when Vaysman et al.31 using Clearfil SE Bondc and Optibond Solo Plusf to seal acid-etched dentin. Both adhesives sealed dentin 80–95%. All of the above authors remarked that no dental adhesive reduced dentin permeability 100%.
Table 1.
Effects of bonding on the permeability of human dentin.
| Hydraulic conductance of dentin (µL min−1 cm−2 cm H2O−1) | |||
|---|---|---|---|
| Adhesive Systems |
Smeared dentin | Etched dentin | Bonded dentin |
| SBMP Plusa | b1.1 ± 0.7×10−2 (24.4%) | a4.5 ± 3.0×10−2 (100%) | b0.78 ± 0.6×10−2 (17%) |
| P&B 2.0f | b1.0 ± 0.6×10−2 (20%) | a5.0 ± 3.2×10−2 (100%) | c0.5 ± 0.4×10−2 (10%) |
| All-Bond 2j | b1.2 ± 1.0×10−2 (23%) | a5.2 ± 3.9×10−2 (100%) | c0.24 ± 0.1×10−2 (3.4%) |
Values are mean hydraulic conductances ± SD (n=12). Values in parentheses are the hydraulic conductance expressed as a percent of the control, acid-etched values that were defined as 100%. Groups identified by the same superscript are not significantly different (from Bouillaguet et al.20, with permission).
Table 2.
Ability of various adhesives to seal dentin.
| Bonding agent | Acid-etch permeability | Residual permeability after bonding |
|---|---|---|
| Excitee | 150.13 ± 56.26a | 40.21 ± 23.09a |
| Optibond Solo Plusb | 175.77 ± 30.19a | 38.53 ± 21.87a |
| Single Bonda | 138.30 ± 27.08a | 42.79 ± 13.61a |
| Prime & Bond NTf | 137.82 ± 36.32a | 41.87 ± 14.926a |
| Clearfil SE Bondc | -- | 36.42 ± 20.30ab |
| Prompt L-Popa | -- | 16.24 ± 15.66b |
Values expressed in percentage decrease (−) or increase (+) with respect to baseline value (n=6). Mean ± SD, Duncan’s test (different superscript letters, a, b, or ab, indicate different groups (from Grégorie et al.30, with permission).
Elgalaid et al.32 were the third group to attempt to seal full crown preparations with Prime & Bond NTf or leave them covered with a smear layer as controls. They were surprised to discover that smear layers sealed dentin as well as the adhesive, but that either approach only reduced dentin permeability by 55–64% for up to 3 weeks (the longest time studied). Unfortunately, smear layers are very acid-labile and can solubilize in as little as one week when exposed33. Carrilho et al.34 repeated Elgalaid’s32 work in 2007, and obtained similar in vitro results. That is, smear layer/smear plugs seal dentin better than adhesive resins/resin tags.
Water movement across resins, in vivo
Unfortunately, few clinical studies of “immediate dentin sealing” have been reported. Hu and Zhu18 reported that the incidence of post-cementation hypersensitivity was significantly lower in teeth that received immediate dentin sealing with Prime & Bond NTf compared to abutment teeth that were prepared and temporized without immediate dentin sealing. Tay35 and others have prepared human teeth for full crowns and then taken polyvinyl siloxane impressions of smear layer-covered dentin (controls) or adhesive-coated dentin.
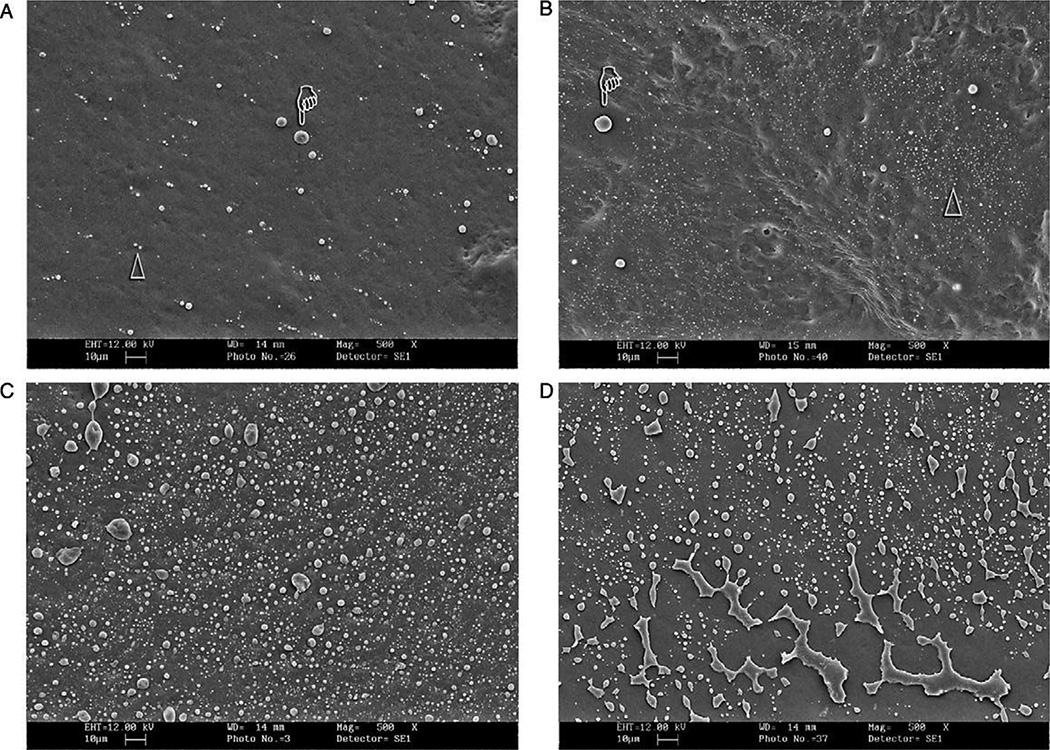
These impressions were poured up in epoxy resin for SEM examination of in vivo fluid transudation across dentin “sealed” with smear layers or adhesives, as evidenced by the presence of tiny bubbles of dentinal fluid on smear layer-covered or “adhesive-sealed” dentin (Fig. 2). All two-step, self-priming, total-etch adhesives tested (Single Bonda, Prime & Bond NTf, One-Stepj, Excite DSCe) were covered with fluid droplets indicating that dentinal fluid had transudated across the polymerized adhesives in the 3–4 min required for self-polymerization of the polyvinyl siloxane impression material. Less fluid droplets came across smear-layer covered dentin than across polymerized resins.
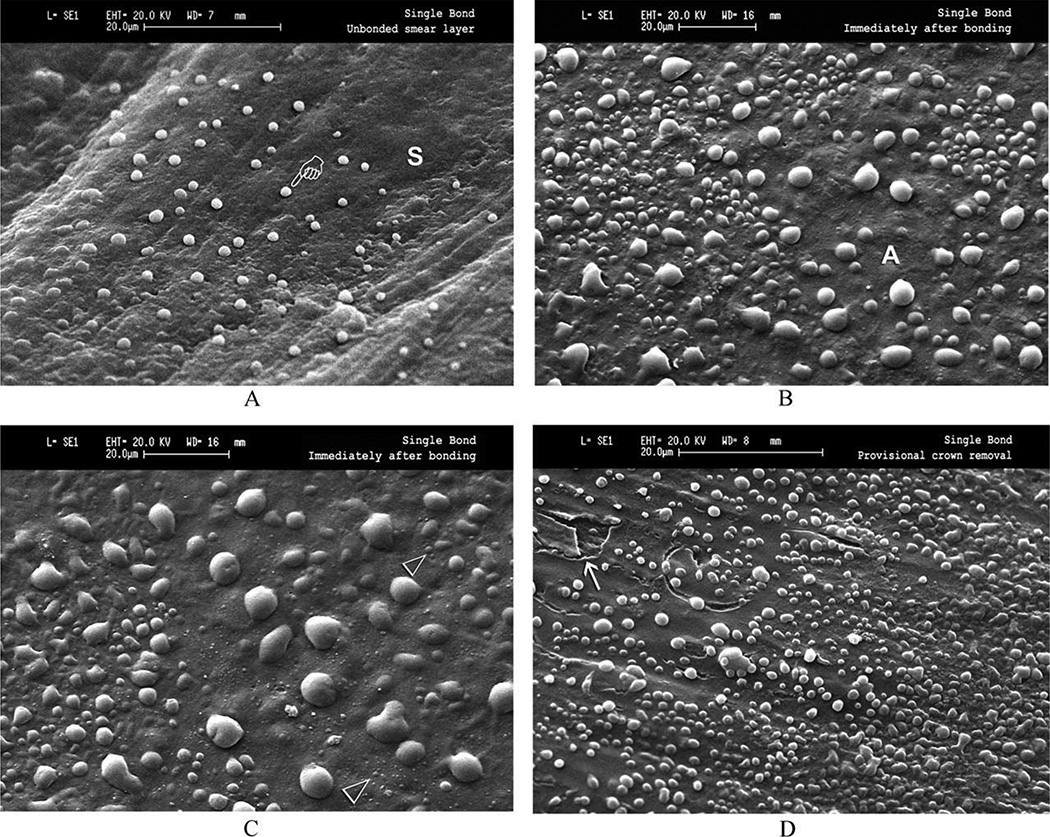
Fig. 2.
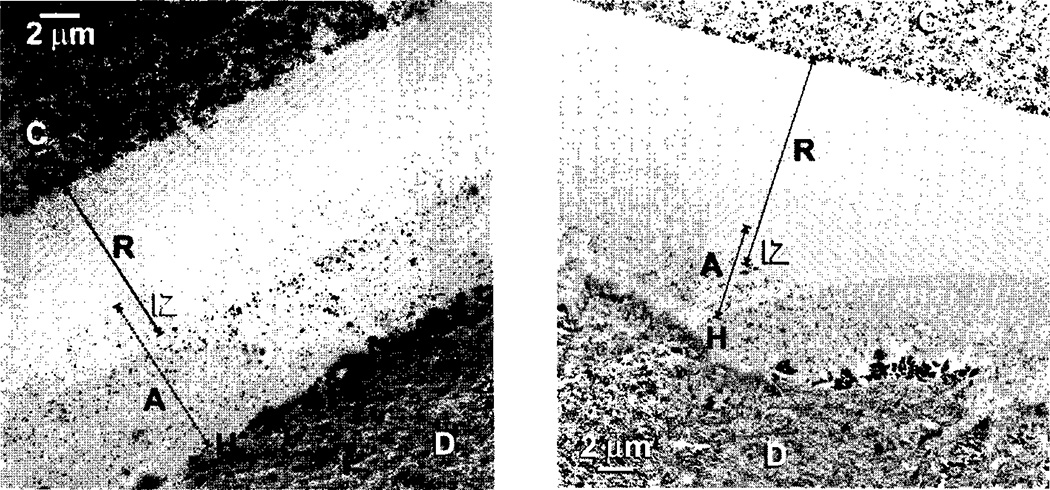
SEM micrographs of epoxy resin replicas of crown preparations taken in vivo. (A) Smear layer(s) covered dentin showing how microdrops of dentinal fluid transudated across the smear layer and were trapped by the impression material in the 2–3 min required for the setting time. (B) Resin-covered crown preparation sealed with Single Bonda, a 2-step one-bottle adhesive. Note the presence of extensive fluid mirodrops. (C) Higher magnification of (B) showing the presence of nanodroplets of dentinal fluid. (D) Resin-coated dentin after gentle clearing with pumice slurry. This produced cracks and partial removal of adhesive (arrow) exposing underlying dentin (from Tay et al.35, with permission).
Chersoni et al.36 extended the in vivo work of Tay et al.35 by making replicas of resin-covered dentin in vivo and also conducting fluid filtration studies across crown segments in vitro (Fig. 3). When self-etch adhesives Adper Prompta, Xeno IIIf, iBondi and One-Up Bond Fk were used to seal dentin, Adper Prompta and Xeno IIIf could not seal dentin as well as did smear layer-covered dentin (Table 3). One-Up Bond Fk sealed dentin as well as did smear layers. When the two-step self-etching system Unifil Bondd was used to seal dentin, it reduced dentin permeability to 2.1% of smear layer covered dentin values (Table 3)! This was due to the fact that the acidic primer layer was covered by a solvent-free hydrophobic layer (Fig. 3). Unifil Bondd produced the best seal of dentin, in part, because it was a self-etching system, and because it was covered with a neat hydrophobic resin that absorbed little water30.
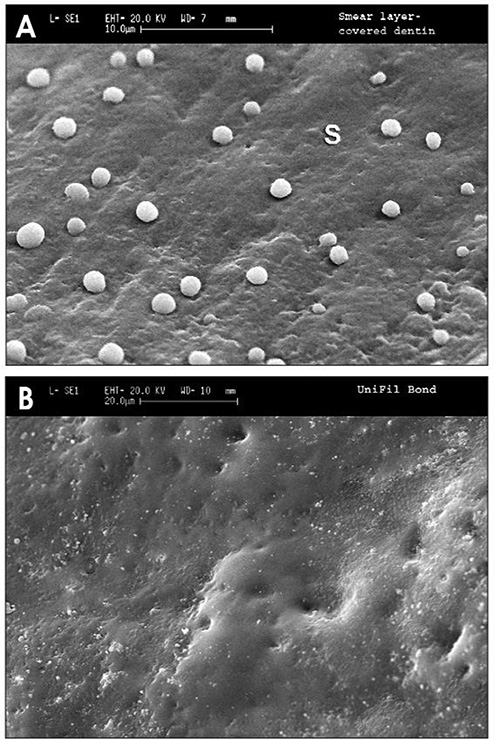
Fig. 3.
SEMs of resin replicas of crown preparations of vital teeth covered with (A) smear layer. Note presence of a few microdroplets of transudated dentinal fluid trapped by the impression material. (B) Unifil Bondd bonded surface after removal of O2-inhibited resin. No fluid droplets were seen when using this two bottle, self-etching primer adhesive (from Chersoni et al.36, with permission).
Table 3.
Fluid conductance across dentin before and after bonding.
| % Fluid flow compared to acid-etched controls | ||
|---|---|---|
| Adhesives self-etch | Smear layer covered | Bonded dentin |
| Adper Prompta | 17.3 ± 4.5A | 28.3 ± 4.4A |
| Xeno IIIf | 12.4 ± 6.2A | 24.2 ± 2.9A,B |
| iBondi | 15.1 ± 5.6A | 18.7 ± 3.3B |
| One-Up Bond Fk | 14.0 ± 3.1A | 14.9 ± 5.0B |
| Two-step | ||
| Unifil Bondd | 18.2 ± 5.0A | 2.1 ± 2.1C |
Groups identified by different superscript letters are significantly different (p<0.05) (from Chersoni et al.36, with permission).
Clearly, not all polymerized resins should be used for immediate dentin sealing, for if dentinal fluid can transudate across the “resin-sealed dentin,” those water droplets might interfere with polymerization of impression materials. Ghiggi et al.37 sealed dentin with Clearfil SE Bondc (CSE) alone or CSE covered with a glycerin jelly to exclude oxygen and polymerized through jelly. Other specimens were treated with CSE that was polymerized in air, but the oxygen-inhibited layer was removed with alcohol, or the CSE was covered with Protect Liner Fc (PLF), a flowable composite alone or the flowable was covered with a glycerin jelly or the polymerized PLF was scrubbed with alcohol before taking impressions with Express XTa or Impreguma. Their results showed that small amounts of impression material remained attached to dentin sealed with CSE alone or PLF, but were not found on CSE covered with jelly or scrubbed with alcohol. Magne and Nielsen24 reported that only CSE used with Extrudeb generated ideal impressions. They did not recommend Impreguma for taking impressions of immediate dentin seals.
Immediate dentin seals, if treated properly, can serve as a foundation for indirect composite inlays. Duarte et al.38 found that immediate dentin sealing with Adper Single Bonda gave microtensile bond strengths of 51.1 MPa, while Adper Prompt L-Popa only gave 1.7 MPa bond strength. If dentin is temporized without immediate dentin sealing, the subsequent use of Optibond FLb or CSEc for indirect restorations falls 11.6 MPa or 1.81 MPa, respectively39. When those same adhesives were used for immediate dentin sealing and the final restoration delayed 1–12 weeks, the microtensile bond strengths exceeded 45 MPa for both adhesives39. Dillenburg et al.40 reported that immediate dentin seals should be cleaned of temporary cement with aluminum oxide powder and 37% phosphoric acid treatment, followed by a second layer of the same adhesive. These microtensile bond strengths were similar to the controls that were never temporized.
Water movement across polymerized resins, in vitro
Özok et al.41 prepared class II cavities (Fig. 4) in crown segments in vitro, and measured the permeability of smear layer-covered dentin. Then the cavities were bonded with Scotchbond 1a or Prompt L-Popa following the manufacturer’s instructions, under 15 cm H2O of pulpal pressure or 0 cm H2O pulpal pressure. Scotchbond 1a reduced dentin permeability 48% when bonded under 15 cm H2O pressure, but 88% when no pressure was applied (Table 4). In contrast, Prompt L-Popa reduced dentin permeability 88% when 15 cm H2O was applied during bonding, but 95% when no pulpal pressure was applied.
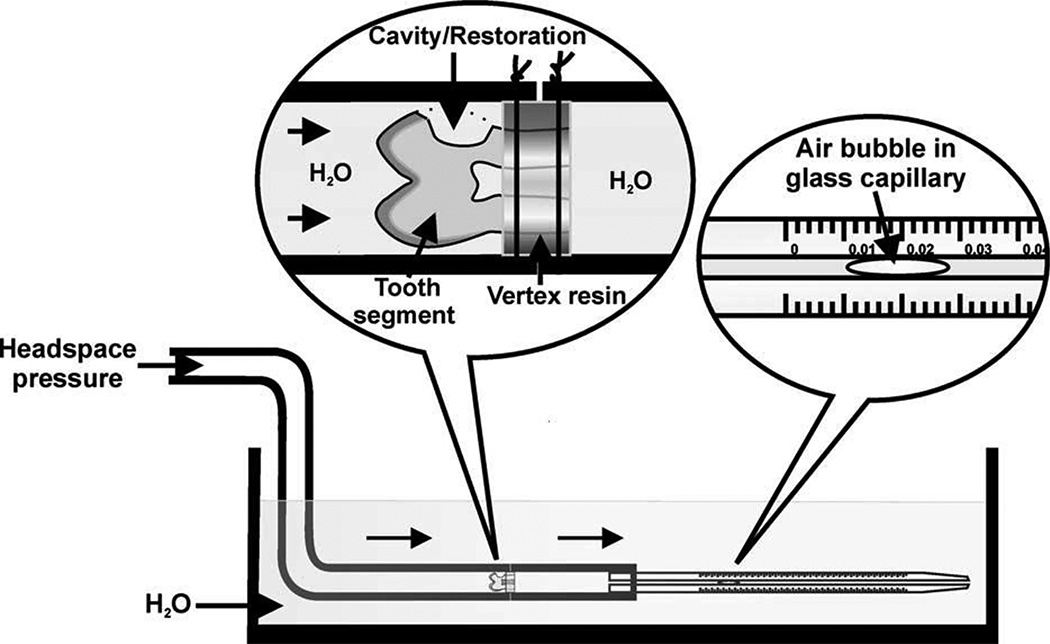
Fig. 4.
Schematic of the apparatus used for measuring the dentin permeability of class V cavities before and after restoration with bonded composites (from Özok et al.41, with permission).
Table 4.
Percent reduction in dentin permeability in class V composite restorations bonded with Scotchbond or Prompt L-Pop with or without pulpal pressure (15 cm H2O).
| Percent reduction in permeability | ||
|---|---|---|
| Adhesive | with pulp pressure | without pulpal pressure |
| Scotchbond-1a | 48.2 ± 49.2a | 88.3 ± 19.1b |
| Prompt L-Popa | 88.4 ± 17.3b | 94.6 ± 7.8b |
Groups identified by different superscript letters were significantly different (p<0.05) (from Özok et al.41, with permission).
Itthagarun et al.42 created exposed, flat dentin surfaces in human dentin and then bonded them with Prompt-L-Popa, Etch & Prime 3.0m, One-Up Bond Fk, Reactmer Bondl or Unifil Bondd. Unifil Bondd lowered dentin permeability 98% to a residual post-bonded permeability of only 2% of smear layer values (similar to the results of Chersoni et al.36 above), while Prompt L-Popa and Etch & Prime 3.0m didn’t seal dentin any better than did the smear layer (Table 5). One-Up Bond Fk and Reactmer Bondl were better than Prompt L-Popa and Etch & Primem but not as good as Unifil Bondd (Table 5).
Table 5.
Fluid flow across bonded dentin as a percent of maximum acid-etched values.
| Fluid flow (% of maximum) measured | ||
|---|---|---|
| Adhesives | across smear layers | across bonded dentin |
| Single-step | ||
| Prompt L-Popa | 18.9 ± 5.3A | 19.2 ± 3.6A |
| Etch & Prime 3.0m | 18.9 ± 4.4A | 17.4 ± 5.8A,B |
| One-Up Bond Fk | 14.8 ± 4.4A | 7.7 ± 4.9C |
| Reactmer Bondl | 13.1 ± 3.9A | 8.9 ± 5.7B,C |
| Two-step | ||
| Unifil Bondd | 18.2 ± 5.0A | 2.1 ± 2.1D |
Groups identified by different superscript letters are significantly different (p<0.05) (from Itthagarun et al.42, with permission).
Magne23 recommended the etch-and-rinse adhesive, OptiBond FLb, to seal dentin. After acid-etching and priming of dentin, the final adhesive layer is solvent-free and relatively hydrophobic, like the adhesive of Unifil Bondd and creates an excellent seal. Indeed, Scotchbond Multi-Purpose’sa adhesive produces good dentin sealing because it is also solvent-free. Clearfil SE Bondc adhesive is another example of a solvent-free, relatively hydrophobic resin blend that seals dentin well.
Grégorie et al.43 bonded smear layer-covered dentin in vitro with ten self-etching adhesives. Smear layers reduced dentin permeability 40–50% compared to acid-etched values. However, when they bonded the 4000 grit SiC smear layers with Xeno IIIf, AdhSEe, Adper Prompt L-Popa, Etch & Primem or One-Up Bond Fk, the post-adhesion permeability fell 50–60% compared to the initial acid-etched value (Table 6). Optibond Solo Plusb, Prime & Bond NTf-treated dentin reduced dentin permeability 45.6 to 42.3%. Prime & Bondf non-rinse conditioner and 1 layer of Prompt L-Popa only reduced dentin permeability 16% (Table 6).
Table 6.
Dentin permeability of specimens after bonding with self-etching adhesives, compared to acid-etched controls.
| Adhesives | Reductions in permeability after bonding |
|---|---|
| Xeno IIIf | −68.9 ± 15.7%a |
| AdhSEe | −58.2 ± 26.8%a,b |
| Adper Prompt L-Popa | −51.0 ± 33.2%a,b |
| Etch & Prime 3.0m | −56.8 ± 26.3%a,b |
| One-Up Bondk | −60.5 ± 27.3%a,b |
| Optibond Solo Plus SEb | −45.6 ± 34.4%a,b |
| Prime & Bond NT controlf | −42.3 ± 5.1%b |
| Clearfil SE Bondc | −35.5 ± 14.7%b,c |
| Prime & Bond NRC NTf | −16.4 ± 33.3%c |
| Prompt L-Popa | −16.3 ± 13.3%c |
Values are mean ± SE, N=10. Groups identified by different superscript letters are significantly different (p<0.05). Smear layer was created using 4000 grit SiC paper. Only 1 layer of adhesive was used with Prompt L-Pop, while multiple layers were used with Adper Prompt L-Pop. Prime & Bond NRC NT is a no rinse conditioner that does not require water rinsing, used with Prime & Bond NT adhesive (from Gregorié et al.43, with permission).
Use of potassium oxalate to seal tubules with crystals
Yiu et al.44 reported that the in vitro dentin permeability of smear layer-covered dentin varied from 9–11% of acid-etched values. When acid-etched dentin was treated with BisBlockj (2.8% oxalic acid), the permeability fell to between 9.4- 11.9% of acid-etched values, much like the smear layer covered dentin (Table 7). When acid-etched dentin was bonded with One-Stepj, Single Bonda, OptiBond Solo Plusb or Prime & Bond NTf, the permeability only fell to 31–34% of acid-etched values that were all significantly higher than the smear layer or BisBlockj values (Table 7). However, when BisBlockj was used to pretreat dentin prior to bonding with One-Stepj or Single Bonda, the dentin permeability fell to values that were only 2 or 6%, respectively, far lower permeability values than were produced by smear layers or by BisBlockj alone, indicating that a double seal by calcium oxalate crystals and resin tags lowers dentin permeability. One-Stepj and Single Bonda reduced dentin permeability almost to zero. In contrast, when BisBlockj-treated dentin was bonded with OptiBond Solo Plusb or Prime & Bond NTf, the permeability of dentin only fell to 27–28%, about like the use of these adhesives without BisBlockj (Table 7). Further studies were done to try to determine why OptiBond Solo Plusb and Prime & Bondf were not as effective as One Stepj and Single Bonda. Table 7 shows that the pH of OptiBond Solo Plusb and Prime & Bond NTf were 2.8 and 2.7, respectively. Clearly, OptiBond Solo Plusb and Prime and Bond NTf were more acidic than the other two adhesives. When the adhesives were analyzed for ionic fluoride, One-Stepj and Single Bonda contained 70 and 130 ppm F-, while OptiBond Solo Plusb contained 4527 ppm F- and Prime & Bond NTf contained 3641 ppm F- (Table 7). Further, TEM studies of ammoniacal silver nitrate immersed specimens revealed the presence of globular deposits into dentinal tubules that may be CaF2-phosphate complexes. These were not found in One-Stepj or Single Bonda bonded specimens.
Table 7.
Effects of adhesives on fluid conductance of dentin.
| Percent of fluid flow at 20 cm H2O pressure | ||||||
|---|---|---|---|---|---|---|
| Adhesive | Smear layer covered |
Oxalate Tx only |
Oxalate Tx plus adhesive |
Adhesive only |
pH | F− ppm |
| One Stepj | 11.1 ± 2.0b | 9.4 ± 1.9b | 2.0 ± 0.9a | 33.6 ± 8.9c | 4.6 | 70 |
| Single Bonda | 10.8 ± 1.8b | 9.9 ± 2.5b | 6.0 ± 1.7a,b | 34.2 ± 11.3c | 3.6 | 130 |
| OptiBond Solo Plusb | 10.7 ± 5.9b | 11.9 ± 5.6b | 28.3 ± 12.2c | 31.4 ± 4.7c | 2.8 | 4527 |
| Prime & Bond NTf | 8.7 ± 1.7b | 10.9 ± 4.7b | 27.5 ± 10.8c | 31.1 ± 6.3c | 2.7 | 3641 |
Fluid flow from acid-etched dentin was assigned a value of 100%. Each tooth served as its own control. Groups identified by different superscript letters are significantly different (p<0.05) (from Yiu et al.44, with permission).
When low viscosity polyvinyl siloxane impressions were made of the BisBlockj pretreated, resin-bonded surfaces, epoxy resin replicas were made for SEM examination. Figure 5 shows only a few droplets of water on the surface of One-Stepj or Single Bonda bonded dentin, but extensive amounts of water had filtered across the polymerized resin of Prime & Bond NTf or Optibond Solo Plusb in the 3–4 min required for setting of the impression material.
Fig. 5.
SEM micrograph of epoxy resin replicas taken from acid-etched dentin treated with 2.7% potassium oxalate (pH 2.7) prior to bonding with adhesives. (A) One Stepj bonded dentin with only a few microdrops of fluid transudation. (B) Single Bonda bonded dentin showed few microdrops (pointer) and nanodrops (triangle). (C) Prime & Bond NTf bonded dentin showed massive numbers of microdrops that had transudated across the polymerized adhesives. (D) Optibond Solo Plusb bonded dentin contained so many microdroplets that they coalesced into pools of fluid (from Yiu et al.44, with permission).
Clearly, low pH and high fluoride concentrations somehow interfere with the sealing properties of OptiBond Solo Plusb and Prime & Bond NTf, but not with One Stepj or Single Bonda. When less acidic, low fluoride adhesives are combined with pretreatment by oxalic acid, one obtains double sealing of dentinal tubules45.
Fluid movement across polymerized resins
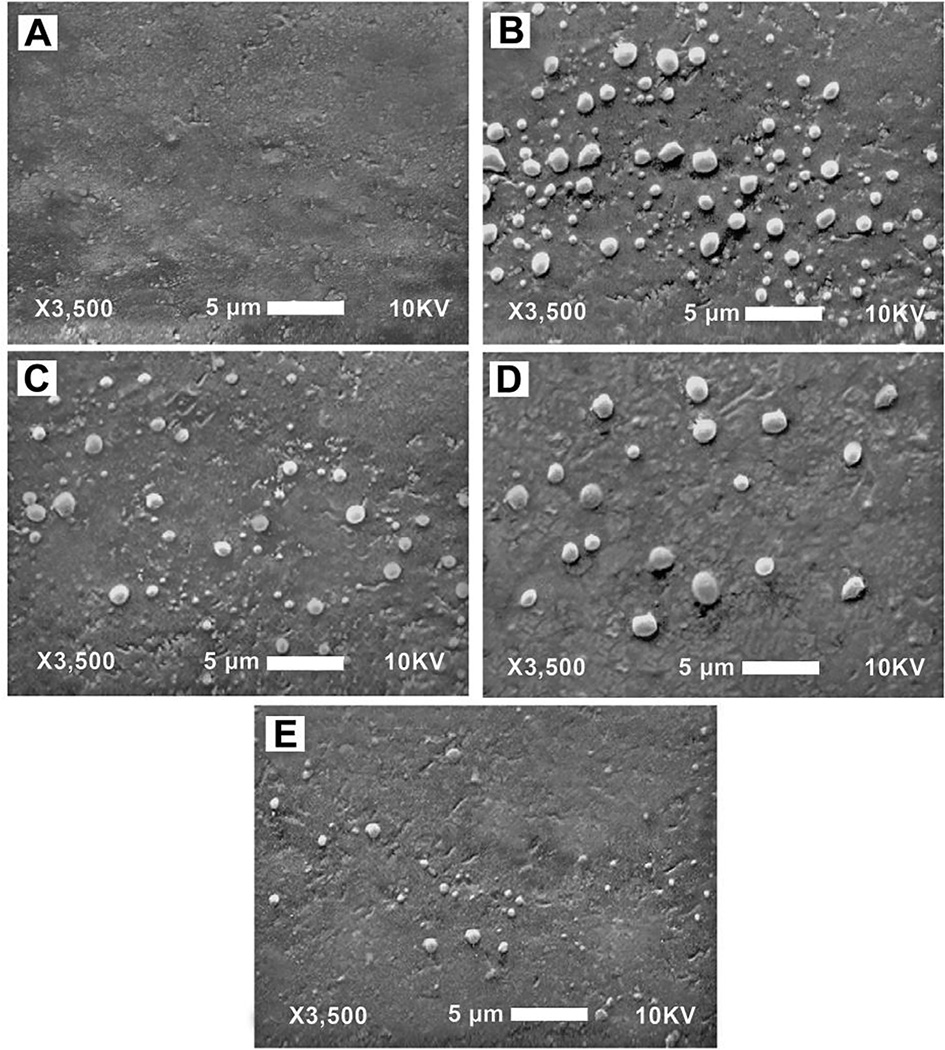
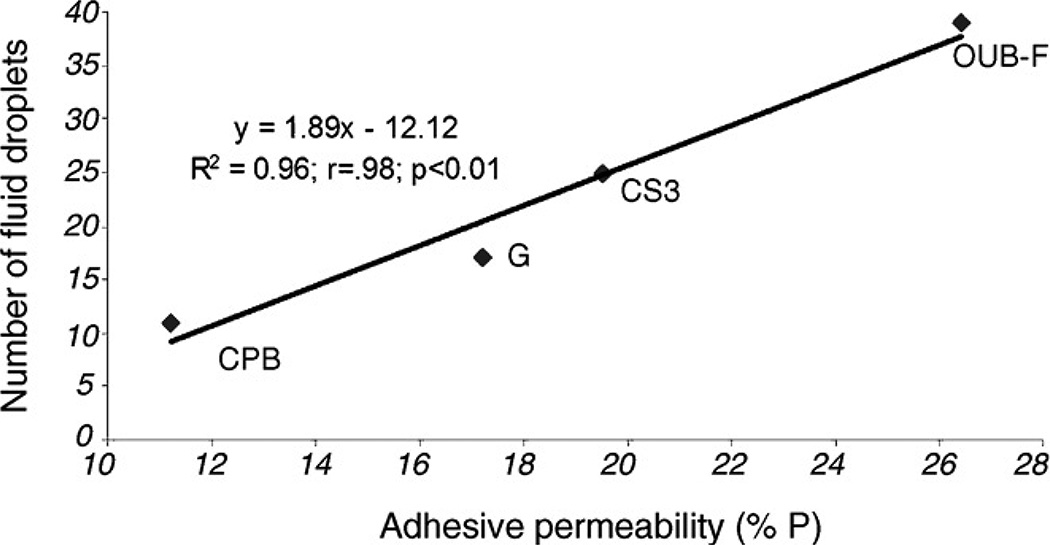
Sauro et al.46 extended the work of Tay et al.35 and Chersoni et al.36 by quantitating the number of water droplets on various adhesive resins bonded to dentin (Fig. 6), and to then correlate it with actual fluid flow transudation across the bonded dentin using the hydraulic conductance technique (Fig. 7). They published a highly significant positive linear regression equation showing an R2 = 0.96 of the relationship between the number of fluid droplets on resin surfaces and the permeability of the adhesives (Fig. 7).
Fig. 6.
SEM microscopy impression replicas of bonded dentin before and 3 min after application of a physiologic pulpal pressure (20 cm H2O). (A) Dentin bonded with One Up Bond F Plusk with no pulpal pressure. (B) Same specimen after application of pulpal pressure. (C) Dentin specimens bonded with Clearfil S3c with pressure. (D) Specimen bonded with G-Bondd with pressure. (E) Dentin specimens bonded with Clearfil Protect Bondc, a two-bottle self-etching adhesive where the primed dentin is covered by a solvent-free adhesive (from Sauro et al.46, with permission). Abbreviations: CPB = Clearfil Protect Bondc; G = G-Bonda; CS3 = Clearfil S3-Bondc, OUB-F = One-Up Bond F Plusk.
Fig. 7.
Regression analysis between adhesive permeability (abscissa) and number of microdroplets of fluid on the bonded surface (ordinate) after application of 20 cm H2O pressure. A significant (p<0.001) positive correlation (R2 = 0.96) was seen between the two variables (from Sauro et al.46, with permission).
Two years later, Sauro et al.47 measured droplet formation by tandem confocal scanning microscopy (TSM) dentin sealed with G-Bondd, DC-Bondc, Single Bonda, OptiBond FLb or Filtek Siloranea. The best sealing was obtained using G-Bondd. This was the exact opposite of their results obtained in 2007. The authors explain that the polymerized G-Bondd films looked frothy, but did not allow much fluid transudation. OptiBond FLb sealed dentin gave the next to the best seal. DC Bondc and Single Bonda gave the worst seals.
Grégorie et al.48 tested the moisture-sensitivity of Prompt L-Popa versus Scotchbond 1XTa by measuring the ability of these resins to seal dentin compared to their acid-etched values. Scotchbond 1XTa was more sensitive to overly dry dentin than was Prompt L-Popa. Scotchbond 1XTa reduced dentin permeability by 55%, while Prompt L-Popa reduced it by 62%, although these values were not significantly different. These relatively poor seals suggest that the ability of adhesive resins to seal dentin had not improved much over the 2000–2009 period.
Use of resin-modified glass ionomers
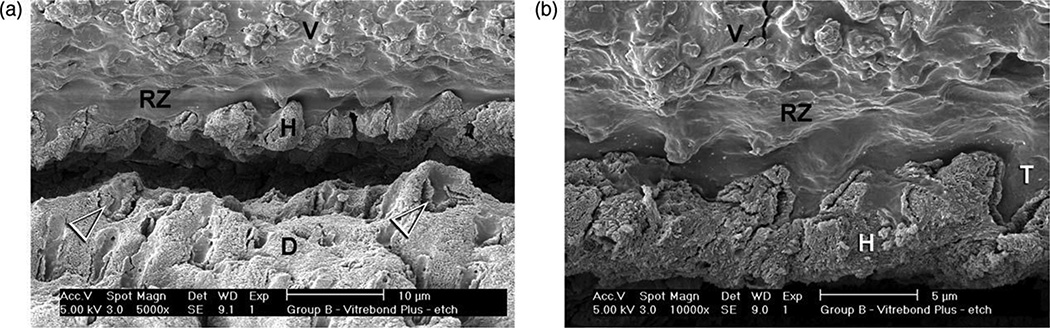
Rusin et al.49 evaluated the resin-modified glass ionomer (Vitrebond Plusa) for its ability to seal dentin, when applied to acid-etched or smear layer-covered dentin. When applied to smear layers, Vitrebond Plusa reduced dentin permeability 98.9 ± 1.1%, because it was a second layer on top of a smear layer that contained smear plugs in the tubules. This is the best seal that we have seen published in the literature. When Vitrebond Plusa was placed on acid-etched dentin it created a hybrid layer complete with resin tags (Fig. 8). The Vitremer Plusa matrix separated from the filler particles creating Vitremer Plus matrix resin tags about 2 µm long. Vitrebond Plusa reduced the permeability of acid-etched dentin by 87.7 ± 18.6%. This means that some specimens produced perfect seals, while others only sealed dentin 69%.
Fig. 8.
SEM of Vitrebond Plusa light-cured glass ionomer liner/base applied to acid-etched dentin. Resin tags of the Vitrebonda matrix separated from the GIC fillers, extended 2 µm into open tubules (from Rusin et al.49, with permission).
Use of 2-bottle self-etching primer adhesives
Clearly, most resins do not seal dentin as well as do enamel or cementum. Two step, self-etching adhesives tend to seal better than do etch-and-rinse adhesives because the mildly (pH 2–2.4) acidic versions barely etch through smear layers (Fig. 9). As many smear plugs are more than 2 µm long and are covered by 1 µm thick smear layers, the mild etching of self-etching adhesives fails to remove the smear plugs, preventing dentinal fluid from contaminating dentin surfaces44. Self-etching adhesives only contain 25–35% water, while etching dentin with phosphoric acid and rinsing with water, leaves demineralized dentin floating in 70 vol% water41. Thus, it is easier to displace 25–35% water from thin hybrid layers than trying to displace 70 vol% water using etch-and-rinse adhesives.
Fig. 9.
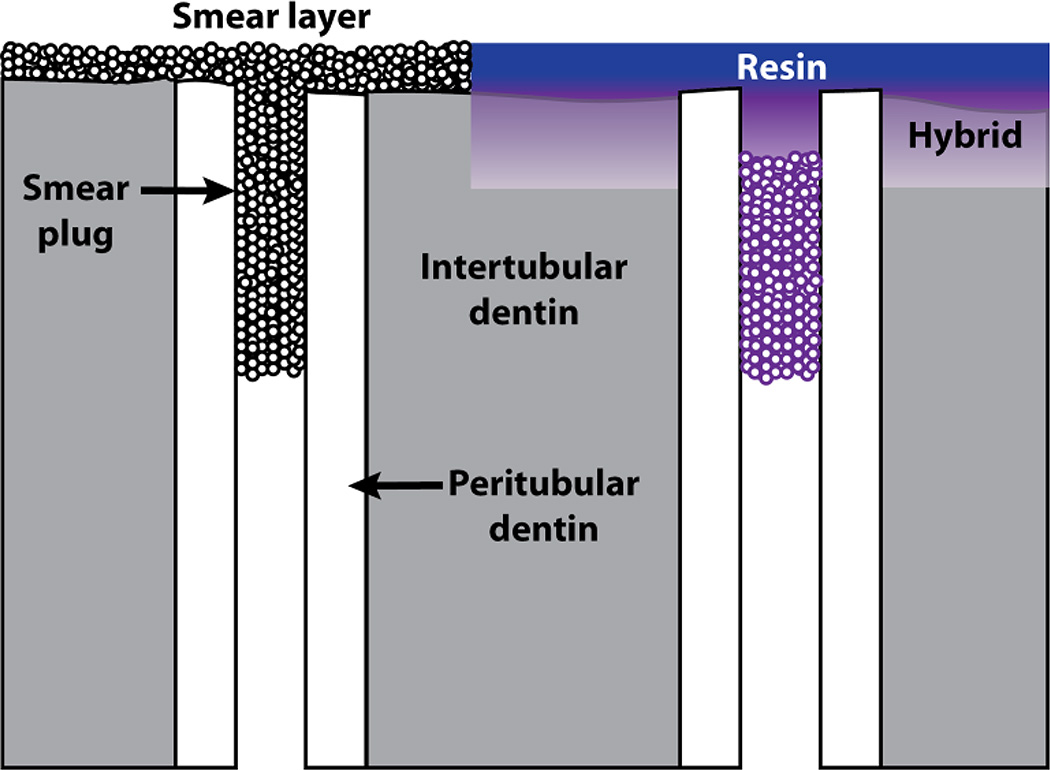
Schematic illustrating the smear layer/smear plug complex on the left. When a weakly acidic primer is applied, it loosens up the smear layer and the smear plug and infiltrates them with resin (Violet). The presence of residual smear plugs prevents outward seepage of dentinal fluid during bonding. After application of the water-free, neutral adhesive (purple), the bond is well-sealed (from Pashley59, with permission).
For some etch-and-rinse adhesives (One-Stepj and Single Bonda), surface water can be controlled by applying oxalic acid to acid-etched dentin prior to bonding. Oxalates do not work with more acidic, high fluoride-containing products like OptiBond Solob or Prime & Bond NTf44.
The two-step self-etching primer/adhesives like Unifil Bondd, Clearfil SE Bondc or Clearfil Protect SEc or AdheSEe, confine their water and acidic monomers to their primers and cover the primed dentin with solvent-free, relatively hydrophobic resins that seal dentin much better than do simplified, single bottle adhesives (e.g. G-Bondi, iBondj, Xeno IIIf, etc.). If we assume that higher resin-dentin bond strengths represent improved dentin sealing, then there are several papers that show that multiple resin coats improve dentin bonding.
Attempts to seal dentin using multiple coatings of adhesives has resulted in mixed success. Pashley et al.50 compared applications of one versus two coats of Prompt L-Popa. Using one layer of adhesive, as recommended, produced microtensile bond strength of only 14.2 ± 7.2 MPa (n=24). Application of two layers produced a µTBS of 29.7 ± 5.7 (n=23, p<0.001). Transmission electron microscopy of the bonded specimens revealed a thin layer of polymerized resin on top of the hybrid layer. There was about 10–15 µm of oxygen-inhibited adhesive on top of the 5–7 µm polymerized resin. This layer was responsible for the low bond strength of single layers of adhesives.
Use of multiple layers of resin
In 2004, Hashimoto et al.51 tested the effects of multiple applications of OptiBond Solo Plusb or Single Bonda on both microtensile bond strength and silver nanoleakage. The results showed a progressive increase in bond strength and decrease in nanoleakage with each additional layer, up to 4 coats. Solvent evaporation after each coating increased the thickness of the adhesive layer. This result was confirmed by Ito et al.52 using the single bottle, self-etch adhesives Xeno IIIf or iBondi. The results are summarized in Table 8.
Table 8.
Effects of multiple layers of two self-etch adhesives on microtensile bond strength.
| Layers | ||||||
|---|---|---|---|---|---|---|
| Groups | 1 | 2 | 3 | 4 | 5 | 1 + SBMP |
| Xeno IIIf (evaporation) |
7.2 ± 6.3a | 22.6 ± 9.2a,b | 30.0 ± 9.9b | 43.5 ± 7.7c | 41.4 ± 8.0c | 27.8 ± 5.9b |
| Xeno IIIf (evaporation + 1c) |
7.2 ± 6.3a | 47.3 ± 19.2b | 65.4 ± 20.4d | 81.8 ± 20.8d | 68.9 ± 22.6d | 64.7 ± 21.2d |
| iBondi (evaporation) |
12.2 ± 7.5A | 18.5 ± 6.2A | 30.6 ± 7.0B | 34.2 ± 6.0B,C | 51.6 ± 14.8B,C | 62.3 ± 23.0C |
| iBondi (evaporation + 1c) |
12.2 ± 7.1A | 39.6 ± 15.2B | 42.7 ± 12.3B,C | 36.6 ± 7.7B | 35.8 ± 13.4B | 58.0 ± 23.5C |
Values are mean ± SD in MPa, N=16. Groups identified horizontally by different superscript letters are significantly different (p<0.05) (from Ito et al.52, with permission). +lc = with light-curing of each layer.
The results revealed that the bond strengths with Xeno IIIf increased with the number of coatings, just as in the Hashimoto et al.51, but the bond strengths were significantly higher after each coating, if the layer was light-cured (Table 8). The reverse was true with iBondi. Using iBondi, simply evaporating the solvent of each layer increased bond strengths faster than if each layer was evaporated and light-cured. While the results were very interesting, the technique requiring multiple solvent evaporations and multiple light-curing is so laborious that most clinicians would not adopt it.
There was one final group in the Ito et al.52 study, where after application of the first layer of self-etching adhesive, the “primed” dentin surface was covered with a layer of solvent-free Scotchbond Multi-Purposea (SBMP) adhesive, which was then light-cured. In the group 1 specimens, where only the solvent in the first layer was evaporated, but not light-cured, application of one layer of SBMPa and its light-curing gave a bond strength of 27.8 ± 5.9 MPa. In group 2, where the single layer of Xeno IIIf was both evaporated and light-cured prior to application of SBMPa, the bond strength increased 232% to 64.7 ± 21.2 MPa (Table 8, 1 + SBMP column). Using iBondi in groups 3 and 4, the bond strengths were above 55 MPs regardless of whether the single layer of iBondi was only evaporated or was evaporated and light-cured prior to application of SBMPa adhesive. Silver nanoleakage studies showed that self-etching single-bottle adhesives exhibit much more nanoleakage than does SBMPa adhesive because self-etch adhesives contain residual water, while SBMPa adhesives are water-free (Figs. 10A and 10B).
Fig. 10.
TEM micrographs of (A) dentin bonded with a coating of iBondi, followed by solvent evaporation and light-curing. The bonded surface was then coated with Scotchbond Multi-Purposea adhesive that was light-cured. After soaking in 50% ammoniacal silver nitrate overnight, the specimens were processed for transmission electron microscopy (TEM). Note the moderate silver nanoleakage in the bottom layer of iBondi adhesive (A) and the near absence of nanoleakage in the SBMPa adhesive (R). (B) Dentin bonded with one layer of Xeno IIIf, then the solvent was evaporated and the surface light-cured. The bonded surface was covered with one coat of water-free SBMPa adhesive (R). Note there is much less silver nanoleakage in (R) than in (A) (from Ito et al.52, with permission).
The results of the Ito et al.52 study clearly show the benefits of covering self-etching adhesives with solvent-free, dimethacrylate-rich adhesives. This concept was reinforced by the paper by Brackett et al.53. In that paper, the microtensile bond strengths of three single-bottle self-etching adhesives Prompt L-Popa, iBondi and Xeno IIIf were compared when they were used according to the manufacturer’s instructions and light-cured, or when the cured adhesive was covered with an additional layer of a solvent-free, neutral, dimethacrylyte-rich adhesive. The results are shown in Table 9, confirming that the use of solvent-free adhesives over simplified self-etch adhesives improves bond strengths.
Table 9.
Microtensile bond strengths of dentin specimens bonded with simplified, all-in-one adhesives alone, or plus an additional layer of hydrophobic resin from the same manufacturer.
| Adhesive | Microtensile bond strength (MPa) |
|---|---|
| iBondi | 41.0 ± 17.7a |
| iBond + Gluma Solid Bond Si | 53.5 ± 19.0b |
| Prompt L-Popa | 16.4 ± 9.9c |
| Prompt L-Pop + Scotchbond Multi-Purpose adhesivea | 56.4 ± 21.8b |
| Xeno IIIf | 10.3 ± 8.3c |
| Xeno III + experimental adhesivef | 25.8 ± 8.0c |
Values are mean ± SD, N=5. Groups identified by different superscript letters are significantly different (p<0.05) (from Brackett et al.53, with permission).
The question of whether resin-dentin bond strengths can be increased using multiple layers of resin, seems to have been answered in the affirmative. However, Reis et al.54 asked whether the durability of one-step self-etching adhesives would be improved by double application or by an extra layer of hydrophobic dentin. Both the double layer and the hydrophobic layer either improved the immediate bond strength, or did not differ from the results obtained with the manufacturer’s directions (Table 10). Resin-dentin bond strengths after 6 months of water storage were higher when the additional layer of hydrophobic resin was used compared to the results obtained with the manufacturer’s directions. These results should be repeated but measuring dentin permeability instead of bond strength, and extended to 12 months of water storage. Reis et al.55 repeated their 2008 study54 in a clinical trial of retention of resin composites bonded with the Clearfil S3c or iBondi using either the manufacturer’s directions or an additional hydrophobic layer (in this study, the solvent-free adhesive of Scotchbond Multi-Purposea Adhesive system) bonded to noncarious cervical lesions for 18 months. The results showed higher 18-month retention rates for both adhesives if they were covered with an extra layer of hydrophobic resin, especially iBondi (p<0.05)55.
Table 10.
Microtensile bond strength of self-etching adhesives to dentin measured immediately or after 6 months, using manufacturer’s instructions or double application or an extra layer of hydrophobic resin.*
| Microtensile bond strength to dentin | ||||
|---|---|---|---|---|
| Adhesive | Time | Manufacturer Directions |
Double application |
Hydrophobic layer |
| Prompt L-Popa | Immediately | 24.3 ± 3.1b | 45.3 ± 6.4a | 29.5 ± 4.9b |
| 6 month | 16.9 ± 4.1c | 17.2 ± 4.6b | 29.1 ± 3.6b | |
| Xeno IIIf | Immediately | 30.0 ± 1.2a | 33.8 ± 3.4b | 49.8 ± 3.7a |
| 6 month | 25.2 ± 3.9b | 30.3 ± 4.2b | 43.2 ± 4.8a | |
| iBondi | Immediately | 19.1 ± 2.4b | 29.2 ± 2.2a | 37.6 ± 3.4a,b |
| 6 month | 18.1 ± 4.8b | 26.4 ± 3.9b | 28.2 ± 3.1b | |
Values are means ± SD, in MPa.
Hydrophobic layer was the adhesive from Clearfil SE Bondc. Groups identified by different superscript letters are significantly different at p<0.05 (from Reis et al.54, with permission).
Evaluation of sealing using silver nitrate
Another approach to evaluating the sealing ability of various adhesives to dentin is to measure silver nanoleakage56. This is done by immersing resin-bonded dentin into 50 wt% ammoniacal silver nitrate for 24 hrs, followed by rinsing off excess AgNO3 and exposing the specimens to photodeveloper and/or bright light to reduce the silver ions to metallic silver grains that cannot diffuse after reduction.
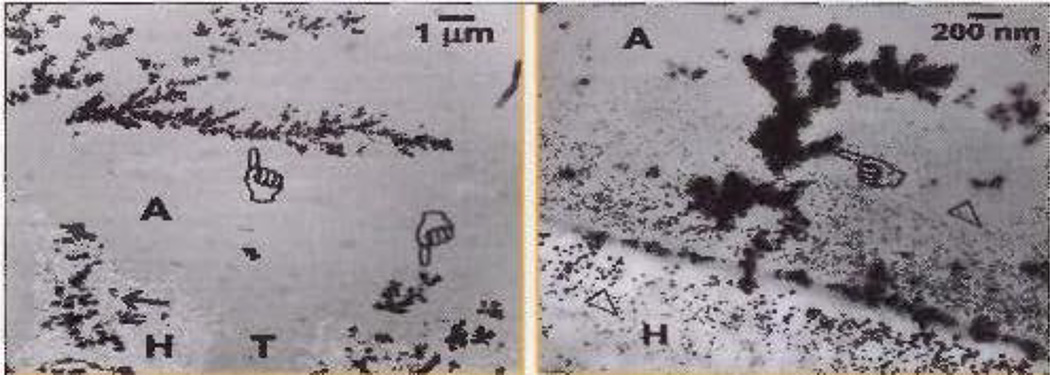
Silver nitrate uptake discloses the presence and distribution of water-filled channels within the hybrid layer and overlying adhesive layer. Figure 11 shows two transmission electron micrographs of resin-dentin bonds made with single-bottle, simplified self-etching adhesive. These adhesives contain 25–35% water to ionize their acidic monomers. Although clinicians attempt to evaporate the residual water in the adhesives prior to light-curing, 5–15 sec is insufficient to remove all water57. Some of that residual water resides in the small lateral branches of dentinal tubules (Fig. 11, pointer) that are sometimes referred to as “water-trees”58. Water-trees sometimes seem to originate from hybrid layers (often from one or two dentinal tubules), that extend up into the overlying adhesive layer (Fig. 11). Water-trees are seldom seen in dentin bonds made with 2-step self-etching adhesives like Clearfil SE Bondc because the water in the acidic primer is sealed by the water-free hydrophobic adhesive like Clearfil SE Bondc adhesive or Scotchbond Multi-Purposea adhesive50.
Fig. 11.
Transmission electron micrographs of dentin bonded with an all-in-one adhesive, incubated in 37°C water for 24 h, followed by immersion in 50% ammoniacal silver nitrate overnight to show the presence and distribution of residual water in hybrid layers (H) and adhesive layer (A) by silver grains. Silver filled branching channels are called “water-trees” (pointers). Spot-like silver deposits are simply called nanoleakage (from Pashley59, with permission).
Role of matrix metalloproteinase enzymes in bond degradation
When using etch-and-rinse adhesives, the etchant is 37% phosphoric acid. This acid is strong enough to solubilize and extract all extrafibrillar and intrafibrillar apatite crystallites from the top 8–10 µm of acid-etched dentin. Since the mineral content of dentin is about 70 vol%, the removal of all mineral from the dentin matrix is replaced by 70% water60. It is very difficult to replace 70% water with adhesive monomers in the 30–60 sec most clinicians use to infiltrate adhesives into dentin. The result of incomplete resin-infiltration is incomplete water removal. Silver ions saturate any residual water-filled spaces56,60,61.
During acid-etching, not only is the dentin matrix exposed, but the matrix metalloproteinases (MMPs) bound to the matrix is are uncovered and activated by acid-etching62. MMPs slowly degrade collagen by adding water across specific peptide bonds in collagen62. The more residual water in resin-dentin bonds, the greater the rate of hydrolysis. Collagen degradation not only lowers resin-dentin bond strength, but slowly increases dentin permeability by loosening resin tags and destroying the hybrid layer60. Thus, for many reasons, it is important to minimize residual water in resin-dentin bonds.
One approach to minimizing residual water at bonded interfaces is to treat the 8–10 µm demineralized layer with 10% NaOCl gel63. This treatment dissolved some of the collagen fibrils in the hybrid layer and lowered the bond strength of Single Bonda by 69% and Prime & Bond NTf by 69%63. Others have shown that a solution of 5% NaOCl is more effective than gels. The use of 5% NaOCl as an endodontic irrigant to remove exposed collagen is well known64. It is used, in part, to disinfect root canals of contaminating bacteria as well as remove exposed collagen so that endodontic seals are placed on stiff mineralized dentin rather than soft, demineralized dentin. Sodium hypochlorite has a pH of 12. It is a strong oxidizing agent. Its action lowers the bond strength of free-radical polymerizing resins. This oxidized state can be reversed within 2–5 min by treating NaOCl-treated dentin with 10% sodium ascorbate65,66. The only disadvantage is that a 3 GPa adhesive layer comes in direct contact with 18 GPa underlying mineralized dentin, since most of the 8–10 µm thick demineralized layer is removed by NaOCl.
Are extra steps of pretreatment able to improve resin sealing of dentin as was seen in the Yiu et al.44 study with oxalate? Oxalates can be used with some etch-and-rinse adhesives but not with others.
Multi-step approach to sealing dentin
Abu-Nawareg67 compared the dentin sealing ability of three adhesive systems: Adper Single Bond Plusa, AdheSEe, and G-Bondd representing an etch-and-rinse adhesive, a two-step self-etching primer adhesive, and a single bottle, simplified self-etching adhesive.
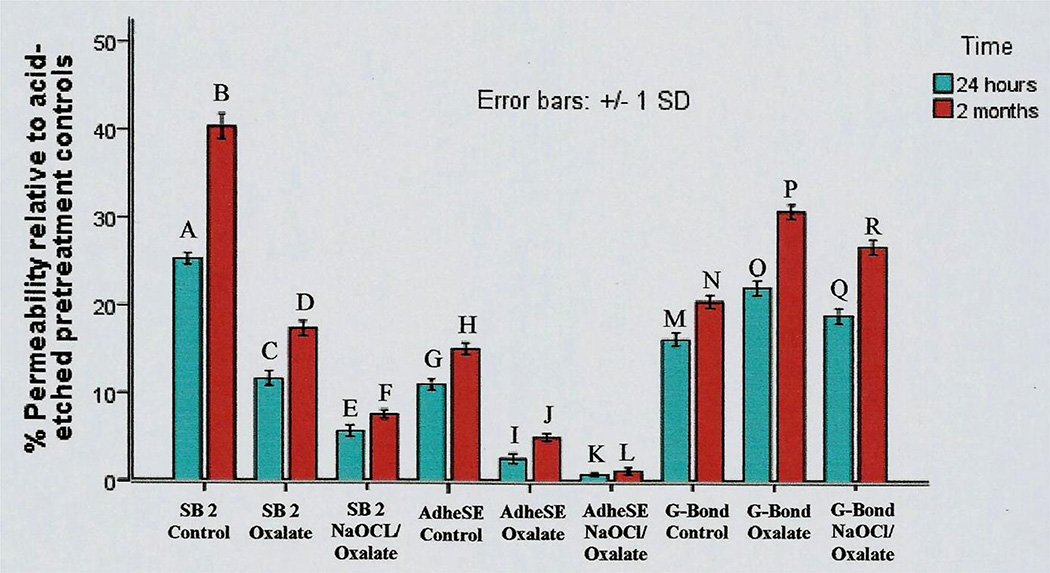
Single Bond Plusa controls (no pretreatments) showed the highest residual permeabilities, 25.3 ± 0.7% at day 1 and 40.4 ± 1.4% (p<0.05) after 2 months in a simulated body fluid at 37°C. When Single Bond Plusa specimens were pretreated with oxalate (BisBlockj) just before bonding, their 1 day permeabilities were only 11.7 ± 0.8% and their 2 month values were 17.4 ± 0.8% (p<0.05). Sequential pretreatment of etched dentin with both NaOCl and oxalate produced a significant (p<0.05) further reduction in residual permeability to 5.7 ± 0.6 and 7.6 ± 0.5% after 1 day or 2 months, respectively (Fig. 12).
Fig. 12.
Permeability of human dentin after bonding with Single Bonda, AdhSEe or G-Bondd using the manufacturer’s instructions, or after pretreatment with 2.7% potassium oxalate (pH 2.7), or after sequential pretreatment with 5.25% NaOCl followed by oxalate, after 1 day or 2 months (from Abu-Nawareg67, Adhesive sealing of dentin, Cairo University, PhD Thesis, 2011, with permission).
Control specimens bonded with AdheSEe showed the greatest sealing of dentin by exhibiting residual post-bonded permeabilities of only 11.0 ± 0.6 and 15.1 ± 0.7% after 1 day or 2 months, respectively (Fig. 12). When AdheSEe bonded specimens were pretreated with oxalate (BisBlockj), their dentin permeability fell (p<0.05) to 2.6 ± 0.6 and 5.0 ± 0.4% after 1 day or 2 months storage, respectively. Sequential pretreatment of dentin with NaOCl, plus oxalate produced the lowest permeability recorded in that study, 0.80 ± 0.2 and 1.2 ± 0.4%, respectively, after 1 day or 2 months.
Control specimens bonded with G-Bondd produced intermediate residual permeabilities of 16.2 ± 0.8 and 20.5 ± 0.7% after 1 day or 2 months, respectively. Oxalate pretreated specimens bonded with G-Bondd showed significantly (p<0.05) higher permeabilities 22.1 ± 0.9 (day 1) versus 30.8 ± 0.8% (60 days), respectively (Fig. 12). When G-Bondedd specimens were subsequently pretreated with NaOCl and oxalate, their permeabilities were significantly higher from the oxalate alone values (Fig. 12).
The superior sealing ability of AdheSEe, a two-bottle, self-etching primer adhesive, mimics the excellent results obtained by others36,37 using Unifil Bondd. In such two bottle systems, the water-containing acidic primer is scrubbed on the dentin for 20 sec, dried, and then covered with a water-free, dimethacrylate-containing adhesive that provides an excellent seal. Other two-bottle self-etching adhesives such as Clearfil SE Bondc or Clearfil Protect SEc or Optibond Solob should work equally well.
The use of Adper Prompt L-Popa for dentin sealing of crown preparations is not recommended because it etches far more deeply and removes smear plugs as well as smear layers, allowing dentinal fluid access to the bonded interface. As the depth of etch is nearly as deep as that produced by 37% phosphoric acid, there is concern that adhesive monomers may not infiltrate to the full depth of the etch58, allowing dentin proteases to degrade the resin-dentin bond62.
Recent expanded applications of resin sealing of dentin
In the U.S., Pashley’s group began sealing the coronal top of root canal fillings with unfilled adhesives so that the pink gutta percha could be visualized through an adhesive intracoronal seal68 in case the root canal filling needed to be removed sometime later. Others used Vitrebonda, but it is not transparent70. Intracanal seals protect root canal fillings during the time interval between completion of root canal treatment and permanent restoration of the tooth.
In Japan, Tagami’s group were also advocating intracoronal seals to prevent microleakage in endodontically-treated teeth69,71. Imazato et al.72 recommended the use of antibacterial monomers. Nikaido et al.73 reported that the bond strength of resin cements to resin-coated dentin could be improved by covering the adhesive with a flowable composite. Magne’s latest recommendations for “immediate dentin sealing” also recommend covering resin seals with flowable composites74. To treat exposed root surfaces in elderly patients with cervical caries, Daneshmehr et al.75 and Tajima et al.71 demonstrated that coating root surfaces with adhesive resins decreased biofilm adherence compared to untreated root surfaces. Others have incorporated antimicrobial quaternary ammonium methacrylates like 12-methacryloyloxydodecyl pyridinium bromide (MDPB) into adhesives72. The use of MDPB is indicated when bonding to dentin containing bacteria72. More recently, MDPB has been shown to be a potent inhibitor of dentin MMPs and cathepsin K77. Thus, resins can be bioengineered to have therapeutic effects76. By increasing the concentration of acidic monomers in adhesive blends, Brambilla et al.78 were able to inhibit S. mutans colonization.
Summary
The use of adhesive resins for immediate sealing of dentin (ISD) began in 1992, using early generation dentin bonding agents alone. As bonding agents improved, in vitro studies demonstrated that Scotchbond Multi-Purposea (SBMP) adhesive or Optibond FLb sealed dentin better than most other adhesives and so they were widely employed in ISD. When self-etching adhesives were developed, the best dentin seals were produced by Unifil Bondd36 or Clearfil SE Bondc24,37,39 (CSE). These adhesive systems seal the acid-etched dentin with a solvent-free adhesive that is rich in dimethacrylates that have been shown to minimize water sorption into the bonded assembly75,76. Others empirically found that covering SBMPa or CSEc with a flowable composite such as Protect Liner Fc provides additional sealing benefits24,37,38, while various pretreatments can improve resin sealing of dentin67, though they require extra bonding steps. More clinical studies need to be done to determine if IDS prevents later adverse pulpal responses to indirect restorative procedures.
Magne’s latest recommendations74 include the use of flowable composite over unfilled adhesives as an alternative to the use of filled adhesives, and to cover the resin sealed preparation with glycerine gel to prevent forming a layer of oxygen inhibited resin at the surface that interferes with polymerization of impression material. More controlled clinical trials need to be done to statistically test whether post-restorative pulpal health is significantly better in teeth protected by IDS, compared to the use of conventional temporization.
CLINICAL SIGNIFICANCE.
Although newly developed adhesive resins have attempted to improve dentin sealing, many such attempts failed. The use of two-step self-etching primer adhesives that combine the use of an acidic primer with a solvent-free adhesive layer give excellent sealing in vitro. Alternatively, the use of three-step, etch-and-rinse adhesive systems like Scotchbond Multi-Purposea or OptiBond FLb that also use solvent-free adhesives seal dentin very well, in vitro.
Acknowledgments
This work was supported, in part, by grant R01 DE015306 from the NIDCR, a GRU/GT Seed Grant to DP (PI) and by the Deanship of Scientific Research (DSR), King Abdulaziz University, under grant No. (1 - 165 - 35 - HiCi). The authors, therefore, acknowledge technical and financial support of KAU. The authors are grateful to Mrs. Michelle Barnes for her outstanding secretarial support.
This study was not supported by any manufacturer.
Dr. Abu-Nawareg is an Associate Professor of Restorative Dentistry and Biomaterials, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia and Faculty of Oral and Dental Medicine, Cairo University, Egypt; Dr. Ahmed Zidan is an Assistant Professor of Restorative Dentistry and Biomaterials, Faculty of Dentistry, Umm Al-Qura University, Mekkah, Saudi Arabia and Faculty of Dentistry, University of Modern Sciences and Arts, Egypt; Dr. Jianfeng Zhou is a member of the Faculty of Dentistry, Peking University School and Hospital of Stomatology, Beijing, P.R. of China; Dr. Akaya Chiba is a graduate student of Department of Cariology and Operative Dentistry, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan; Dr. Junji Tagami is the Chairman of the Department of Cariology and Operative Dentistry, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan; Dr. David Pashley is an Emeritus Regents Professor of Oral Biology, College of Dental Medicine, Georgia Regents University, Augusta, GA, USA.
Footnotes
3M ESPE, St. Paul, MN, USA
Kerr, Orange, CA, USA
Kuraray America Inc., New York, NY, USA
dG-C Corp., Tokyo, Japan
Ivoclar Vivadent, Schaan, Liechtenstein
Dentsply, York, PA, USA
Sun Medical Co. Ltd., Shiga, Japan
Parkell, Edgewood, NY, USA
Heraeus Kulzer, Hanau, Germany
Bisco, Inc., Schaumberg, IL, USA
Tokuyama Corp., Tokyo, Japan
Shofu Inc., Kyoto, Japan
Degussa AG, Hanau, Germany
Disclosure Statement: Drs. Abu Nawareg, Zidan, Zhou, Chiba, Tagami and Pashley have no commercial interest in any of the products used in this study.
None of the authors claim any conflict of interest.
References
- 1.Pashley DH. Dynamics of the pulpo-dentin complex. Crit Rev Oral Biol Med. 1996;7:104–133. doi: 10.1177/10454411960070020101. [DOI] [PubMed] [Google Scholar]
- 2.Pashley DH, Tay FR, Haywood VB, Collins MA, Drisko CL. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. Compendium. 2008;29(Spec Iss):1–35. [Google Scholar]
- 3.Michelich V, Pashley DH, Whitford GM. Dentin permeability: A comparison of functional vs. anatomical tubular radii. J Dent Res. 1978;57:1019–1024. doi: 10.1177/00220345780570110301. [DOI] [PubMed] [Google Scholar]
- 4.Bergenholtz G. Pathogenic mechanisms in pulpal disease. J Endod. 1990;16:98–101. doi: 10.1016/S0099-2399(06)81571-2. [DOI] [PubMed] [Google Scholar]
- 5.Bergenholtz G. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Crit Rev Oral Biol Med. 2000;11:467–480. doi: 10.1177/10454411000110040501. [DOI] [PubMed] [Google Scholar]
- 6.Pashley DH. How can sensitive dentine become hypersensitive and can it be reversed. J Dent. 2013;41S4:S49–S55. doi: 10.1016/S0300-5712(13)70006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith AJ. Formation and repair of dentin in the adult. Chapter 2. In: Hargreaves KM, Goodis HE, Tay FR, editors. Seltzer and Bender’s Dental Pulp. Chicago: Quintessence Publishing Co., Inc.; 2012. p. 27046. [Google Scholar]
- 8.Goodis HE, Kahn A, Simon S. Aging and the pulp. Chapter 18. In: Hargreaves KM, Goodis HE, Tay FR, editors. Seltzer and Bender’s Dental Pulp. Chicago: Quintessence Publishing Co., Inc.; 2012. pp. 429–445. [Google Scholar]
- 9.Gay NJ, Gangloff M, Weber AN. Toll-like receptors activate T-cells and dendritic cells. Nat Rev Immunol. 2006;6:693–698. doi: 10.1038/nri1916. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino K, Takeuchi O, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira S. Cutting edge: Tole-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 11.Pashley EL, Comer RW, Simpson MD, Horner JA, Pashley DH, Caughman WF. Dentin permeability: sealing the dentin crown of preparation. Oper Dent. 1992;17:13–30. [PubMed] [Google Scholar]
- 12.Cagidaco MC, Ferrari M, Garberoglio R, Davidson CL. Dentin contamination protection after mechanical preparation for veneering. Am J Dent. 1996;9:57–60. [PubMed] [Google Scholar]
- 13.Paul SJ, Schaerer P. The dual bonding technique: A modified method to improve adhesive luting procedures. Int J Periodont Restor Dent. 1997;17:536–545. [PubMed] [Google Scholar]
- 14.Özturk N, Aykent F. Dentin bond strengths of two ceramic inlay systems after cementation with three different techniques and one bonding system. J Prosthet Dent. 2003;89:275–281. doi: 10.1067/mpr.2003.37. [DOI] [PubMed] [Google Scholar]
- 15.Jayasooriya PR, Pereira PN, Nikaido T, Burrow MF, Tagami J. The effect of a “resin coating” on the interfacial adaptation of composite inlays. Oper Dent. 2003;28:28–35. [PubMed] [Google Scholar]
- 16.Okuda M, Nikaido T, Maruoka R, Foxton RM, Tagami J. Microtensile bond strengths to cavity floor dentin in indirect composite restorations using resin coating. J Esthet Dent. 2005;17:144–155. doi: 10.1111/j.1708-8240.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 17.Islam MR, Takada T, Weerasinghe DS, Uzzaman MA, Foxton RM, Nikaido T, Tagami J. Effect of resin coating on adhesion of composite crown restoration. Dent Mater J. 2006;25:272–279. doi: 10.4012/dmj.25.272. [DOI] [PubMed] [Google Scholar]
- 18.Hu J, Zhu Q. Effect of immediate sealing on preventive treatment for post-cementation hypersensitivity. Int J Prosthodont. 2010;23:49–52. [PubMed] [Google Scholar]
- 19.Johnson GH, Hazelton LR, Bales DJ, Lepe X. The effect of a resin-based sealer on crown retention for three types of cement. J Prosthet Dent. 2004;91:428–435. doi: 10.1016/S0022391304000770. [DOI] [PubMed] [Google Scholar]
- 20.Bouillaguet S, Duroux B, Ciucchi B, Sano H. Ability of adhesive systems to seal dentin surfaces: An in vitro study. J Adhes Dent. 2000;2:201–208. [PubMed] [Google Scholar]
- 21.Magne P, Douglas WH. Porcelain veneers: dentin bonding optimization and biomimetic recovery of the crown. Int J Prosthodont. 1999;12:111–121. [PubMed] [Google Scholar]
- 22.Del Nero MO, De La Macorra JC. Sealing and dentin bond strengths of adhesive systems. Oper Dent. 1999;24:194–202. [PubMed] [Google Scholar]
- 23.Magne P. Immediate dentin sealing: a fundamental procedure for indirect bonded restorations. J Esthet Dent. 2005;17:144–155. doi: 10.1111/j.1708-8240.2005.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 24.Magne P, Nielsen B. Interactions between impression materials and immediate dentin sealing. J Prosthet Dent. 2009;102:298–305. doi: 10.1016/S0022-3913(09)60178-5. [DOI] [PubMed] [Google Scholar]
- 25.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–3785. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 26.Ghiggi PC, Steiger AK, Mareondes ML, Mota EG, Junior BLH, Spohr AM. Does immediate dentin sealing influence the polymerization of impression materials. Eur J Dent. 2014;8:366–372. doi: 10.4103/1305-7456.137650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosaka S, Kajihara H, Kurashige H, Tanaka T. Effect of resin coating as a means of preventing marginal leakage beneath full cast crowns. Dent Mater J. 2006;25:272–279. doi: 10.4012/dmj.24.117. [DOI] [PubMed] [Google Scholar]
- 28.Mawardi H, Giro G, Kajiya M, Ohta K, Almazrooa SB, Nishimura I, Kowai T. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res. 2011;90:1339–1345. doi: 10.1177/0022034511420430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spohr AM, Borges GA, Platt JA. Thickness of immediate dentin sealing materials and its effect on the fracture load of a reinforced all-ceramic crown. Eur J Dent. 2013;7:474–483. doi: 10.4103/1305-7456.120682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grégorie G, Joniot S, Guignes P, Millas A. Dentin permeability: self-etching and one-bottle dentin bonding systems. J Prosthet Dent. 2003;90:42–49. doi: 10.1016/s0022-3913(03)00258-0. [DOI] [PubMed] [Google Scholar]
- 31.Vaysman T, Rajan N, Thompson VP. Effect of bur cutting patterns and dentin bonding agents on dentin permeability in a fluid flow model. Oper Dent. 2003;28(5):522–528. [PubMed] [Google Scholar]
- 32.Elgalaid TO, Youngson CC, Mc Hugh S, Hall AF, Creanor SL, Foye RH. In-vitro dentin permeability: The relative effect of a dentin bonding agent on crown preparations. J Dent. 2004;32:413–421. doi: 10.1016/j.jdent.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Kerns DC, Scheidt MJ, Pashley DH, Strong SL, Van Dyke TE. Dentinal tubule occlusion and root hypersensitivity. J Periodontol. 1991;62:421–428. doi: 10.1902/jop.1991.62.7.421. [DOI] [PubMed] [Google Scholar]
- 34.Carrilho MR, Tay FR, Sword J, Donnelly AM, Agee KA, Nishitani Y, Sadek FT, Carvalho RM, Pashley DH. Dentin sealing provided by smear layer/ smear plugs vs adhesive resin/resin tags. Eur J Oral Sci. 2007;115:321–329. doi: 10.1111/j.1600-0722.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 35.Tay FR, Frankenberger R, Krejci I, Bouillaguet S, Pashley DH, Carvalho RM, et al. Single-bottle adhesives behave as permeable membranes after polymerization. I. In-vivo evidence. J Dent. 2004;32:611–621. doi: 10.1016/j.jdent.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Chersoni S, Suppa P, Grandini S, Goracci C, Monticelli F, Yiu C, et al. In-vivo and in-vitro permeability of one-step self-etch adhesives. J Dent Res. 2004;83(6):459–464. doi: 10.1177/154405910408300605. [DOI] [PubMed] [Google Scholar]
- 37.Ghiggi PC, Steiger AK, Marcondes ML, Mota EG, Burnett LH, Jr, Spohr AM. Does immediate dentin sealing influence the polymerization of impression materials? Eur J Dent. 2014;8:366–372. doi: 10.4103/1305-7456.137650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duarte S, Jr, de Freitas CR, Saad JR, Sadan A. The effect of immediate dentin sealing on the marginal adaptation of bond strength of total-etch and self-etch adhesives. J Prosthet Dent. 2009;102:1–9. doi: 10.1016/S0022-3913(09)00073-0. [DOI] [PubMed] [Google Scholar]
- 39.Magne P, Sows, Cascione D. Immediate dentin sealing supports delayed restoration placement. J Prosthet Dent. 2007;98:166–174. doi: 10.1016/S0022-3913(07)60052-3. [DOI] [PubMed] [Google Scholar]
- 40.Dillenburg AL, Soares CG, Paranhos MP, Spohr AM, Loguercio AD, Burnett LH., Jr Microtensile bond strength of prehybridized dentin: storage time and surface treatments. J Adhes Dent. 2009;11:231–237. [PubMed] [Google Scholar]
- 41.Özok AR, Wu MK, De Gee AJ, Wesselink PR. Effect of dentin perfusion on the sealing ability and microtensile bond strengths of a total-etch versus an all-in-one adhesive. Dent Mater. 2004;20:479–486. doi: 10.1016/j.dental.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Itthagarun A, Tay FR, Pashley DH, Wefel JS, Garcia-Godoy F, Wei SHY. Single-step, self-etch adhesives behave as permeable membranes after polymerization. Part III. Evidence from fluid conductance and artificial caries inhibition. Am J Dent. 2004;17:394–400. [PubMed] [Google Scholar]
- 43.Grégorie G, Guignes P, Millas A. Effect of self-etching adhesives on dentin permeability in a fluid flow model. J Prosth Dent. 2005;93:56–63. doi: 10.1016/j.prosdent.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Yiu CKY, Hiraishi N, Chersoni S, Breschi L, Ferrari M, Prati C, King NNM, Pashley DH, Tay FR. Single bottle adhesives behave as permeable membranes after polymerization. II. Differential permeability reduction with an oxalate desensitiser. J Dent. 2006;34:106–116. doi: 10.1016/j.jdent.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Pashley DH, Carvalho RM, Pereira JC, Villanueva R, Tay FR. The use of oxalate to reduce dentin permeability under adhesive restorations. Am J Dent. 2001;14:89–94. [PubMed] [Google Scholar]
- 46.Sauro S, Pashley DH, Montanari M, Chersoni S, Carvalho RM, Toledano M, et al. Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent Mater. 2007;23:705–713. doi: 10.1016/j.dental.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Sauro S, Mannocci F, Toledano M, Osorio R, Thompson I, Watson TF. Influence of the hydrostatic pulpal pressure on droplets formation in current etch-and-rinse and self-etch adhesives: a video rate/TSM microscopy and fluid filtration study. Dent Mater. 2009;25:1392–1402. doi: 10.1016/j.dental.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Grégorie G, Guignes P, Nasr K. Effects of dentine moisture on the permeability of total-etch and one-step self-etch adhesives. J Dent. 2009;37:691–699. doi: 10.1016/j.jdent.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Rusin RP, Agee KA, Suchko M, Pashley DH. Effect of a new linear/base on human dentin permeability. J Dent. 2010;38:245–252. doi: 10.1016/j.jdent.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Pashley EL, Agee KA, Tay FR, Pashley DH. Effects of one versus two applications of an unfilled all-in-one adhesive on dentin bonding. J Dent. 2002;30:83–90. doi: 10.1016/s0300-5712(02)00002-7. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto M, Sano H, Yoshida E, Hori M, Kaga M, Oguchi H, Pashley DH. Effects of multiple adhesives coatings on dentin bonding. Oper Dent. 2004;29:416–423. [PubMed] [Google Scholar]
- 52.Ito S, Tay FR, Hashimoto M, Yoshiyama M, Saito T, Brackett WW, Waller JL, Pashley DH. Effects of multiple coatings of two all-in-one adhesives on dentin bonds. J Adhes Dent. 2005;7:133–141. [PubMed] [Google Scholar]
- 53.Brackett WW, Ito S, Tay FR, Haisch LD, Pashley DH. Microtensile bond strength of self-etching resins: Effect of a hydrophobic layer. Oper Dent. 2005;20:733–738. [PubMed] [Google Scholar]
- 54.Reis A, Albuquerque M, Pegoraro M, Mattei G, Bauer JRO, Grande RHM, Klein-Junior CA, Baumhardt-Neto R, Loguercio AD. Can the durability of one-step self-etch adhesives be improved by double application or by an extra layer of hydrophobic resin? J Dent. 2008;36:309–315. doi: 10.1016/j.jdent.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Reis A, Leite TM, Matte K, Michels R, Amaral RC, Geraldeli S, Loguercio AD. Improving clinical retention of one-step, self-etching adhesive systems with an additional hydrophobic adhesive layer. JADA. 2009;140:877–885. doi: 10.14219/jada.archive.2009.0281. [DOI] [PubMed] [Google Scholar]
- 56.Sano H, Yoshiyama M, Ebisu S, Burrow MF, Takatsu T, Ciucchi B, Carvalho R, Pashley DH. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995;20:160–167. [PubMed] [Google Scholar]
- 57.Yiu CKY, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26:6863–6872. doi: 10.1016/j.biomaterials.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CKY. Water treeing in simplified dentin adhesives – Déjá Vu? Buonocore Memorial Lecture. Oper Dent. 2005;30:561–579. [PubMed] [Google Scholar]
- 59.Pashley DH. The evolution of dentin bonding. Dentistry Today. 2003;22:1–6. [PubMed] [Google Scholar]
- 60.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brackett MG, Brackett WW, Sword RJ, Qi YP, Niu LN, Pucci CR, Dib A, Pashley DH, Tay FR. The critical barrier to progress in dentin bonding with etch-and-rinse technique. J Dent. 2011;39:238–248. doi: 10.1016/j.jdent.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol LS, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR, Pashley DH. Review: Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinase and cysteine cathepsins. Dent Mater. 2013;29:116–135. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perdigão J, Lopes M, Geraldeli S, Lopes GC, Garcia-Godoy F. Effect of a sodium hypochlorite gel on dentin bonding. Dent Mater. 2000;16:311–323. doi: 10.1016/s0109-5641(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 64.Morris MD, Lee K-W, Agee KA, Bouillaguet S, Pashley DH. Effects of sodium hypochlorite and RC-Prep on bond strengths of resin cement to endodontic surfaces. J Endod. 2001;27:753–757. doi: 10.1097/00004770-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Lai SCN, Mak YF, Cheung GSP, Osorio R, Toledano M, Carvalho RM, Pashley DH. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001;80:1919–1924. doi: 10.1177/00220345010800101101. [DOI] [PubMed] [Google Scholar]
- 66.Vongphan N, Senawongse P, Somsiri W, Harnirattisai C. Effects of sodium ascorbate on microtensile bond strength of total-etching adhesives to NaOCl treated dentin. J Dent. 2005;33:689–695. doi: 10.1016/j.jdent.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 67.Abu-Nawareg MM. PhD Thesis, Faculty of Oral and Dental Medicine. Cairo, Egypt: Cairo University; 2007. The effect of different surface treatments on dentin and adhesive permeability. [Google Scholar]
- 68.Galvan RR, West LA, Liewehr FR, Pashley DH. Coronal microleakage of five materials used to create an intracoronal seal in endodontically treated teeth. J Endod. 2002;28:59–61. doi: 10.1097/00004770-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Makuroka R, Nikaido T, Ikeda M, Ishizuka T, Foxton RM, Tagami J. Coronal leakage inhibition in endodontically-treated teeth using resin coating technique. Dent Mater J. 2006;25:97–103. doi: 10.4012/dmj.25.97. [DOI] [PubMed] [Google Scholar]
- 70.Rajakumar V, Indira R. Effect of glass-ionomer cement as an intra-canal barrier in post space prepared teeth: An in vitro study. J Conserv Dent. 2009;12:65–68. doi: 10.4103/0972-0707.55620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tajima K, Nikaido T, Inoue G, Ikeda M, Tagami J. Effect of coating root dentin surfaces with adhesive materials. Dent Mater J. 2009;28:578–586. doi: 10.4012/dmj.28.578. [DOI] [PubMed] [Google Scholar]
- 72.Imazato S, Torii Y, Takatsuka T, Inoue K, Ebi N, Ebisu S. Bactericidal effect of dentin primer containing antibacterial monomer methacryloyloxydodecyl pyridinium bromide (MDPB) against bacteria in human carious dentine. J Oral Rehabil. 2001;28:314–319. doi: 10.1046/j.1365-2842.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- 73.Nikaido T, Cho E, Nakajima M, Tashiro H, Toba S, Burrow MF, Tagami J. Tensile bond strengths of resin cements to bovine dentin using resin coating. Am J Dent. 2003;16:A41–A46. [PubMed] [Google Scholar]
- 74.Magne P. IDS:Immediate dentin sealing for tooth preparations. J Adhes Dent. 2014;16:594. doi: 10.3290/j.jad.a33324. [DOI] [PubMed] [Google Scholar]
- 75.Daneshmehr L, Matin K, Nikaido T, Tagami J. Effects of root dentin surface coating with all-in-one adhesives materials on biofilm adherence. J Dent. 2008;36:33–41. doi: 10.1016/j.jdent.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Imazato S, Ma S, Chen J-h, Xu HHK. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent Mater. 2014;30:97–104. doi: 10.1016/j.dental.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tezvergil-Mutluay A, Agee KA, Mazzoni A, Carvalho RM, Carrilho M, Tersariol IL, Nascimento FD, Imazato S, Tjäderhane L, Breschi L, Tay FR, Pashley DH. Can quaternary ammonium methacrylates inhibit matrix MMPs and cathepsins? Dent Mater. 2015;31:e25–e32. doi: 10.1016/j.dental.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brambilla E, Ionescu A, Mazzoni A, Cadenaro M, Gagliani M, Ferraroni M, Tay FR, Pashley DH, Breschi L. Hydrophilicity of dentin bonding systems influences in vitro Streptococcus mutans biofilm formation. Dent Mater. 2014;30:926–935. doi: 10.1016/j.dental.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]