Abstract
BACKGROUND
Adverse event reports from North America have raised concerns that medications for attention deficit-hyperactivity disorder (ADHD) increase risk of serious cardiovascular events.
METHODS
We conducted a retrospective cohort study with automated data from four health plans (Tennessee Medicaid, Kaiser Permanente California, OptumInsight Epidemiology, Washington State Medicaid), with 1,200,438 children and youth aged 2–24 years and 2,579,104 person-years of follow-up, including 373,667 person-years of current ADHD medication use. We identified serious cardiovascular events (sudden cardiac death, acute myocardial infarction, and stroke) from health plan data and vital records, with endpoints validated by medical record review. We estimated the relative risk for endpoints in current users compared to nonusers with hazard ratios from Cox regression models.
RESULTS
Cohort members had 81 serious cardiovascular events (3.1/100,000 person-years). Current ADHD medication users had no increased risk for serious cardiovascular events (adjusted hazard ratio 0.75; 95% confidence interval [CI] 0.31 to 1.85). Risk was not increased for any of the individual endpoints, or for current users compared to former users (adjusted hazard ratio 0.70; 95% CI 0.29 to 1.72). Alternative analyses addressing several study assumptions also found no significant association between ADHD medication use and the risk of study endpoints.
CONCLUSIONS
Although there was no evidence of increased risk of serious cardiovascular events for current users of ADHD medications, the upper bound of the 95% confidence interval indicates that up to a two-fold increased risk cannot be ruled out. However, the absolute magnitude of such an increased risk would be low.
Medications used to treat attention deficit-hyperactivity disorder (ADHD) are prescribed for more than 2.7 million children in the U.S. each year1 and have been considered to be relatively safe.2–5 However, adverse event reports from Canada and the U.S. that included cases of sudden death, myocardial infarction, and stroke in conjunction with their use have raised concerns about the safety of these drugs.6, 7 Although case reports from adverse event reporting systems can be an important source for identifying medication safety signals, they cannot reliably quantify risk. Thus, there is a compelling need to obtain better safety data for these medications. We used data from four large, geographically and demographically diverse U.S. health plans to conduct a retrospective cohort study, with medical record review to validate study endpoints, of the use of ADHD medications and the risk of serious cardiovascular events in children and youth.
Methods
Data Sources
We obtained study data from computerized health records of four health plans which together annually covered 22.4 million persons during the study period [Tennessee Medicaid, Washington State Medicaid, Kaiser Permanente California (Northern and Southern regions), and OptumInsight Epidemiology (national private insurance health plan data)]. Health plan data were augmented with linkage to state death certificates and the National Death Index. Health plan data included enrollment records, outpatient and inpatient claims, and records of filled prescriptions (including the dispensing date, drug name, dose, quantity, and days supply), which have been shown to be good measures of medication use.8–11 The beginning of the study differed by site based on the earliest availability of the site’s computerized data (ranging from 1986 to 2002); follow-up concluded for all sites at the end of 2005. Each site prepared standardized files from their health plan data and used computer programs from the lead site (Vanderbilt) to define study variables and create anonymized files sent to the lead site for analyses.
Study Population
To assemble the cohort, we identified ADHD medication users who met the following criteria: 1) use of an ADHD medication (methylphenidate, dexmethylphenidate, dextroamphetamines, amphetamine salts, atomoxetine, and pemoline) during the study period; 2) age of 2 to 24 years on the first day of qualifying use, defined as t0; 3) continuous enrollment with drug benefits for 365 days preceding t0 (allowing for short administrative gaps in enrollment); and, 4) absence of possibly life-threatening serious (Appendix 1). Because children and youth with congenital heart disease may be vulnerable to ADHD medication effects, they were included in the study. Cohort members could not have a hospital discharge in the preceding 365 days with a primary diagnosis of acute myocardial infarction or stroke. The last day of study follow up (t1) was the last day of the study or when the person no longer met study criteria. A given child or youth was allowed to re-enter the cohort, as long as all of the cohort eligibility requirements were met.
For each ADHD medication user, we randomly selected up to two nonuser controls from the same site’s health plan members who were enrolled on t0 and who also met study inclusion criteria 2–4 above. Nonusers were matched for calendar year, age, and gender and were allowed to have prior non-qualifying use of ADHD medications before, but not on t0. Follow-up for nonusers began on t0 for the matched ADHD medication user and ended on the nonuser’s last day of study follow up, t1 (Appendix 2). Follow-up time did not include time during hospitalization and the 30 days after discharge because in-hospital deaths were not considered study end points and health plan files do not include drugs dispensed in the hospital.
Study medications
Every person-day during study follow-up was classified according to use of ADHD medications (Appendix 2). Current use was defined as the period between the prescription start date and the end of the days of supply (including up to a 7-day carryover from previous prescriptions). Former use included person-time that occurred following current use through the end of study follow-up. Nonuse included person-time with no prescribed use of ADHD medications on the day being classified or any preceding days. Former users and nonusers could become current users of ADHD medications during follow-up, and when this occurred their user person-time was classified as described above.
Study endpoints
The primary study endpoint was a serious cardiovascular event, defined as sudden cardiac death, myocardial infarction, or stroke. Sudden cardiac death was defined as a sudden, pulseless condition or collapse consistent with a ventricular tachyarrhythmia occurring in a community setting, and included both fatal and resuscitated cardiac arrest.12–16 Diagnosis of acute myocardial infarction required hospitalization and met the international diagnostic criteria for myocardial infarction.17–19 Stroke was defined as an acute neurologic deficit of sudden onset that persisted more than 24 hours, corresponded to a vascular territory, and was not explained by other causes (e.g. trauma, infection, vasculitis, or profound systemic hypotension).17, 20, 21
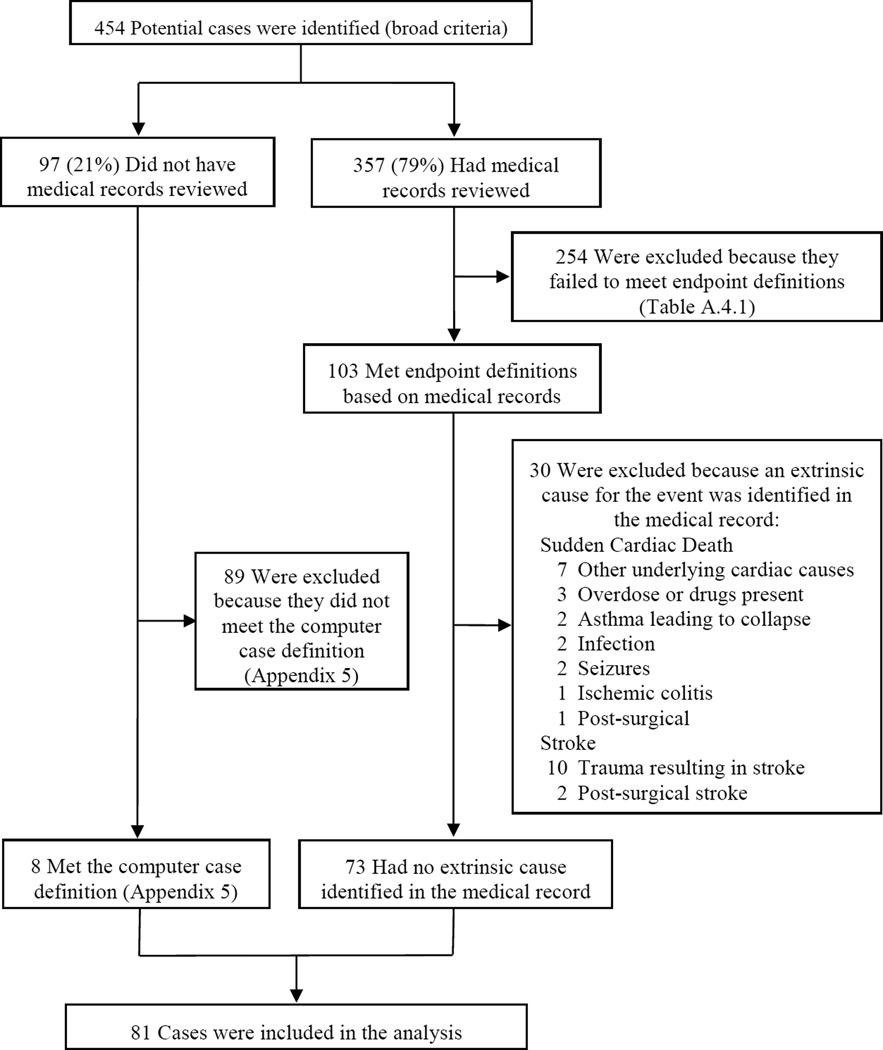
Potential endpoints were identified from claims and vital records and adjudicated through review of all pertinent medical records, including hospitalizations, emergency medical services reports, autopsies, and death certificates (Appendix 3). Criteria for potential cases were intentionally broad to increase sensitivity because we anticipated that study endpoints would be rare and planned to review medical records for all potential cases. All events were adjudicated by two cardiologists (sudden cardiac death and acute myocardial infarction) or two neurologists (stroke), who reviewed cases from all sites and were unaware of exposure status (Appendix 4). Disagreements (<5% of cases) were resolved by consensus among adjudicators and the study principal investigator. Cases were excluded if the documentation suggested a non-cardiovascular cause as the etiology (e.g. motor vehicle accident, drug overdose) or for sudden cardiac death, if clinically severe heart disease was present and sudden cardiac death was not unexpected (e.g. end-stage congestive heart failure). Congenital heart defects undiagnosed until autopsy were noted, but did not result in exclusion of the potential case. For potential cases for which we were unable to obtain pertinent medical records or with insufficient information for adjudication (21%), case status was determined using a computer case definition (Appendix 5), derived from those cases with completed adjudication. The positive predictive value of the computerized case definition for serious cardiovascular events was 91% (Appendix 5).
Analysis
We calculated the hazard ratio for users of ADHD medications compared to nonusers from Cox regression models, using robust sandwich variance estimators to account for the matched study design and for persons entering the cohort multiple times.22 The hazard ratio was adjusted for both baseline characteristics and changes in characteristics that occurred during follow-up. We calculated the adjusted incidence of endpoints by multiplying the incidence rate in the nonusers by the hazard ratio.
Because the number of covariates reflecting baseline cohort characteristics was large relative to the number of endpoints, we adjusted for these covariates by including a site-specific propensity score in the regression models. The propensity score was defined as the probability that the patient was a current ADHD medication user on the first day of study follow-up, estimated for each site using logistic regression.23 The baseline variables in the propensity score included sociodemographic characteristics as well as information on medical care encounters consistent with psychiatric disorders, asthma and other respiratory illnesses, seizure and other neurologic disorders, unintentional injuries, cardiovascular diseases, and other diseases. For each site, we tested the adequacy of the propensity score models by calculating the propensity-score adjusted means of baseline variables for users and nonusers of ADHD medications; these were comparable (Appendix 6).
In our primary analysis, we adjusted for site, propensity score decile, and several time-dependent covariates (medical and psychiatric conditions, healthcare utilization, age, and calendar year) (Appendix 7). Additional analyses stratified by age (2–17 years, 18–24 years) and using alternative exposure groups, cohort inclusion criteria, and endpoint exclusions were performed to test key study assumptions. We performed all statistical analyses with SAS 9.1 (SAS Institute, Cary, North Carolina).
The study was planned by the authors. Data were gathered from each site and analyzed by the study biostatistician (PGA) who vouches for the data and the analysis along with the first author. The first author wrote the first draft and all authors participated in revision. The authors collectively decided to publish the paper.
Human Subjects Protection
The study was approved by the institutional review boards at each of the participating institutions, and the Food and Drug Administration Research in Human Subjects Committee. In addition, permission was obtained from the data sources for each site.
Results
The study cohort included 1,200,438 children and youth. The mean age of cohort members at baseline was 11.1 years, and ranged from 8.7 to 12.0 years at the study sites (Table 1). The mean length of follow-up for the cohort was 2.1 years, and ranged from 1.5–3.9 years at the study sites. Characteristics of current users and nonusers at baseline are shown in Table 2. Generally, current users had more evidence of healthcare utilization of all types. In addition, current users had greater prevalence of psychiatric comorbidities and greater use of psychotropic medications. Current users were also more likely to have asthma, seizures, and congenital heart defects. For both current users and nonusers, alcohol and drug use, as determined from medical care encounter records, were uncommon.
Table 1.
Study Cohort, by Site.
| Tennessee Medicaid |
Kaiser Permanente California* |
OptumInsight Epidemiology |
Washington Medicaid |
Total | |
|---|---|---|---|---|---|
| Study Period | 1986–2005 | 1999–2005 | 1998–2005 | 2000–2005 | 1986–2005 |
| Number in cohort | 200,198 | 191,772 | 692,187 | 116,281 | 1,200,438 |
| % Medicaid | 100.0 | 4.4 | 0 | 100.0 | 27.0 |
| Age in years, mean | 8.7 | 11.1 | 12.0 | 10.0 | 11.1 |
| First day of follow- up, mean |
1999.0 | 2002.1 | 2002.3 | 2002.2 | 2001.7 |
| Follow-up in years, mean |
3.9 | 2.6 | 1.5 | 2.1 | 2.1 |
Includes Kaiser Permanente Northern and Southern California regions.
Table 2.
Characteristics of Cohort Members, According to Use of ADHD Medications at Baseline.*
| Nonuser | Current User | |
|---|---|---|
| Demographic characteristics | ||
| Age in years, mean | 11.1 | 11.1 |
| Male, % | 70.9 | 71.1 |
| Non-white-, % | 50.5 | 36.8 |
| Reside in metropolitan area, % | 78.4 | 77.1 |
| Psychiatric conditions† | ||
| ADHD diagnosis, % | 1.3 | 57.4 |
| Major depression, % | 1.6 | 10.4 |
| Bipolar disorder, % | 0.2 | 2.1 |
| Psychosis, % | 0.1 | 0.5 |
| Autism, % | 0.2 | 1.4 |
| Mental retardation, % | 0.6 | 4.0 |
| Prior suicide attempt, % | 0.1 | 0.3 |
| Psychotropic medication use† | ||
| Antidepressants, % | 1.8 | 15.0 |
| Mood stabilizers, % | 0.5 | 4.2 |
| Antipsychotics, % | 0.4 | 5.2 |
| Benzodiazepines, % | 0.1 | 0.5 |
| Medical conditions† | ||
| Asthma, % | 16.1 | 22.1 |
| Seizures, % | 0.6 | 2.1 |
| Obesity, % | 0.9 | 1.2 |
| Major congenital heart defect, %‡ | 0.5 | 0.8 |
| Minor congenital heart defect, %‡ | 3.6 | 6.9 |
| Diabetes, % | 0.4 | 0.5 |
| Other serious health conditions, %§ | 0.9 | 1.3 |
| Alcohol and drug use† | ||
| Alcohol or drug use, % | 0.4 | 1.5 |
| Smoking, % | 0.6 | 0.9 |
| Use of health services† | ||
| Psychiatric hospitalization, % | 0.3 | 1.9 |
| Medical hospitalization, % | 2.5 | 4.1 |
| Medical emergency department visit, % | 12.9 | 15.8 |
| Any psychiatric care, % | 5.4 | 63.1 |
| Any cardiovascular care, % | 4.0 | 6.0 |
| Any outpatient visit, % | 75.1 | 92.9 |
| Any prescription, % | 22.0 | 31.7 |
Adjusted for age, sex, and site.
Measured from claims and medications used in the 365 days before study entry.
Major congenital heart defects included common truncus, transposition of the great vessels, Tetrology of Fallot, common ventricle, endocardial cushion defect, pulmonary atresia, tricuspid atresia, hypoplastic left heart syndrome, coarctation of the aorta, and total anomalous pulmonary venous return. Minor congenital heart defects included any other congenital heart anomaly.
Other serious health conditions included pneumonia, thyroid disease, and kidney disease.
The 2,579,104 person-years of follow-up included 373,667 person-years of follow-up for current use of ADHD medications, 607,475 person-years of follow-up for former use, and 1,597,962 years of follow-up for nonusers. There were 81 cohort members with serious cardiovascular events, or 3.1/100,000 person-years: 33 sudden cardiac deaths (1.3/100,000 person-years), 9 acute myocardial infarctions (0.3/100,000 person-years), and 39 strokes (1.5/100,000 person-years). Characteristics of the confirmed cases according to study drug exposure are shown in Appendix 8. In the multivariate model, older age, current antipsychotic use, major psychiatric illness, serious cardiovascular conditions, and chronic illness were associated with increased risk for serious cardiovascular events (Appendix 7).
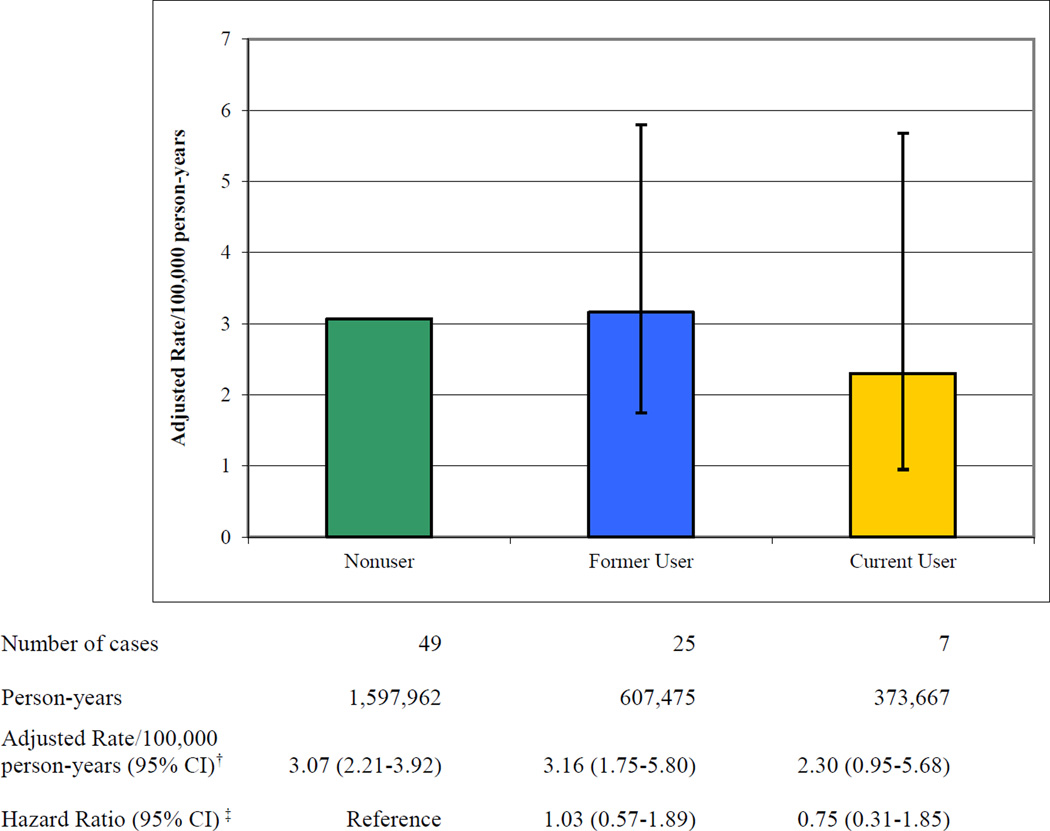
Current users of ADHD medications had an adjusted rate of serious cardiovascular events that was not statistically significantly different from that of nonusers (hazard ratio [HR] 0.75; 95% confidence interval [CI] 0.31 to 1.85) (Figure 1). The risk for former users did not differ materially from that for nonusers (HR 1.03; 95% CI 0.57–1.89). When former users served as the reference, which assessed the possible effect of unmeasured confounding, current users of ADHD medications had no increased risk of serious cardiovascular events (HR 0.70; 95% CI 0.29–1.72) (Appendix 9). There was also no evidence of increased risk for the individual endpoints of sudden cardiac death, acute myocardial infarction, or stroke (Table 3). We found no evidence of increased risk for methylphenidate (HR 0.96; 95% CI 0.31–2.97), the most frequently used ADHD medication (Appendix 10). Data were too sparse for other individual drugs to fit regression models.
Figure 1.
Adjusted Rates for Serious Cardiovascular Events According to Use of ADHD Medications.*
†Rates per 100,000 person-years were adjusted by multiplying the rate in the reference group by the hazard ratio for former and current users.
‡Hazard ratios were estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), age, and calendar year.
Table 3.
Adjusted Hazard Ratios for Individual Cardiovascular Endpoints, According to Use of ADHD Medications.
| ADHD medication use | Person- years |
Events | Rate/100,000 person-years |
Hazard Ratio† |
95% confidence interval |
|---|---|---|---|---|---|
| Sudden Cardiac Death | |||||
| Nonuser | 1,597,962 | 17 | 1.06 | 1.00 | Reference |
| Former user | 607,475 | 13 | 2.14 | 1.52 | 0.65–3.56 |
| Current User | 373,667 | 3 | 0.80 | 0.88 | 0.23–3.35 |
| Acute Myocardial Infarction† | |||||
| Nonuser | 1,597,962 | 6 | 0.38 | 1.00 | Reference |
| Former user | 607,475 | 3 | 0.49 | 0.88 | 0.16–4.71 |
| Current User | 373,667 | 0 | 0 | - | - |
| Stroke | |||||
| Nonuser | 1,597,962 | 26 | 1.63 | 1.00 | Reference |
| Former user | 607,475 | 9 | 1.48 | 0.80 | 0.33–1.96 |
| Current User | 373,667 | 4 | 1.07 | 0.93 | 0.29–2.97 |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), age, and calendar year. Because there were no events in the current user group, models were not calculated for acute myocardial infarction.
We performed several alternative analyses to test the robustness of study findings (Table 4). To assess possible bias from inclusion of persons who used ADHD medications before the beginning of follow-up,10 we restricted the current users of ADHD medications to new users (no ADHD medications during the 365 days preceding t0). Findings were essentially identical to those of the primary analysis (HR 0.73; 95% CI 0.24–2.10) (Appendix 11). When we included seven cases excluded from the primary analysis because they had evidence of severe underlying cardiac disease for which sudden cardiac death would not be unexpected, we found no increased risk for current users (HR 0.71; 95% CI 0.29–1.72) (Appendix 11). In analyses including only children 2–17 years of age, we found no association between ADHD medication use and serious cardiovascular events (HR 0.98; 95% CI 0.41–2.36) (Appendix 11). When children with evidence of serious psychiatric disease were excluded, we also found no association (HR 0.66, 95% CI 0.20–2.16) (Appendix 11).
Table 4.
Alternative Analyses, Adjusted Hazard Ratios for Serious Cardiovascular Events, According to Use of ADHD Medications.
| Analysis | Exposure | Reference | Hazard Ratio† | 95% Confidence Interval |
|---|---|---|---|---|
| Primary Analysis | Current User | Nonuser | 0.75 | 0.31–1.85 |
| Exposures were restricted to new ADHD medication users§ | New User | Nonuser | 0.73 | 0.24–2.10 |
| Cases included those with severe underlying cardiac disease for which sudden cardiac death would not be unexpected |
Current User | Nonuser | 0.71 | 0.29–1.72 |
| Restricted to children of age 2–17 years | Current User | Nonuser | 0.98 | 0.41–2.36 |
| Restricted to children without evidence of serious psychiatric disorders‡ |
Current User | Nonuser | 0.66 | 0.20–2.16 |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), age, and calendar year.
New users included individuals who had no ADHD medication use in the 365 days prior to t0.
This analysis excluded cohort members who had any of the following at baseline or during follow-up: use of psychotropic medications (antipsychotics, mood stabilizers or lithium), or evidence of treated mental illness (major depression, bipolar disorder, psychotic disorder, autism or hospitalization with a psychiatric diagnosis).
We also performed analyses to test other key study assumptions. A site-specific analysis (Appendix 12) suggested a potential difference between Medicaid and non-Medicaid sites, although numbers were very small. However, a pooled Medicaid versus non-Medicaid analysis found no statistical evidence of heterogeneity. Another analysis expanded the definition of current use to include the 89 days after the end of current use to account for possible exposure misclassification related to clinical use of ADHD medications or for medications stopped following prodromal symptoms of an endpoint (e.g. headache preceding stroke). Finally, we performed an analysis where time-dependent variables were fixed at baseline. The findings of these analyses were essentially identical to those reported here.
Discussion
Several regulatory and policy decisions resulted from the review of adverse event reports of serious cardiovascular events in ADHD medication users in Canada and the United States. In Canada, HealthCanada removed and then reinstated marketing of extended release mixed amphetamine salts.6, 7 In the United States, three different FDA advisory committees considered the issue and recommended a black box warning for stimulants, as well as a patient medication guide.24 In a controversial policy statement, the American Heart Association stated that screening electrocardiograms for children initiating ADHD stimulant therapy were “reasonable to obtain”,25 which was subsequently revised based on input from several pediatric organizations.24 This led to concern and confusion among healthcare providers, patients, and families about the risks of these medications.26 In this context, we studied the cardiovascular safety of ADHD medications in over 1.2 million children and youth from four geographically diverse health plans with >2.5 million person-years of follow-up. The point estimate of the relative risk provided no evidence that ADHD drugs increase risk of serious cardiovascular events, although the 95% confidence interval was consistent with up to a two-fold increased risk.
In the study population, which excluded children with possibly life-threatening illness, the incidence of serious cardiovascular events was 3.1/100,000 person-years, consistent with other studies.27–30 This limited study power, particularly for individual endpoints and drugs as well as for subgroups that might be particularly vulnerable to ADHD medication effects. We also had limited information for longer durations of use.
Could the study findings be the result of confounding? Comparison of current users and nonusers at baseline indicated a greater incidence of medical and psychiatric comorbidities in current users. We controlled for an extensive set of cardiovascular disease variables, which were included in site-specific propensity scores. Using this method we were able to account for many important risk factors for cardiovascular disease. However, differences in factors that we were unable to measure, such as adherence, differential prescribing of ADHD medications to children at lower risk of study outcomes, or illicit use of medications resulting in misclassification may have affected study findings.31, 32
We performed several alternative analyses to test the robustness of study findings. We used former users as the reference group, which could address many of the issues related to comparability between current users and nonusers. We performed an analysis restricted to new users to address bias that would be introduced from the inclusion of prevalent users in the cohort.10 Another analysis included cases excluded from the primary analysis because of preexisting severe cardiac disease for which sudden cardiac death would not be unexpected. We also performed analyses stratified by age. The findings from these additional analyses were essentially identical to our primary analysis.
Our findings of no increased risk of serious cardiovascular events in children and youth with ADHD medication use are consistent with some,33–36 but not all previous reports37 that have appeared since the FDA safety review of Adverse Event Reporting data for ADHD medications.6, 7 Importantly, our study included nearly twice the person-time of the combined person-time in four recent cohort studies and included several provisions to ensure accurate case ascertainment, including review of medical records and autopsies.
In conclusion, this population of children and youth with 2.5 million person-years of follow-up had 3.1 serious cardiovascular events per 100,000 person years. Although the point estimates of the relative risks for ADHD medications did not indicate increased risk, the upper bound of the 95% confidence interval indicates that up to a two-fold increased risk cannot be ruled out. However, the absolute magnitude of any increased risk would be low.
Supplementary Material
Acknowledgments
This project was funded in part under Contract Numbers HHSA290-2005-0042 (Vanderbilt) and HHSA290-2005-0033 (Harvard Pilgrim Health Care Institute) from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
The project was also funded by the Food and Drug Administration under the following contracts 223-2005-10100C (Vanderbilt), 223-2005-10012 (Kaiser Permanente Northern California), 223-2005-10006C (OptumInsight Epidemiology), and 223-2005-10012C (Harvard Pilgrim Health Care Institute).
We acknowledge the data partners who provided data needed to conduct the study: TennCare Bureau, Tennessee Department of Health, Washington State Health and Recovery Services Administration, Kaiser Permanente California (Northern and Southern regions), and OptumInsight Epidemiology.
We acknowledge the following individuals who provided assistance with the study:
Vanderbilt University School of Medicine: Patricia A. Gideon, Michelle DeRanieri, Leanne Balmer, Shannon D. Stratton, James R. Daugherty, Judith A. Dudley, Lynne Caples, Tracy Crowley, Ning Chen, Eli Poe
OptumInsight Epidemiology: Sherry Quinn, Eva Ng, Clorinda Hoffman
Kaiser Permanente Northern California: Connie Uratsu, Ninah Achacoso
Kaiser Permanente Southern California: Chantal Avila, Yan Luo
University of Washington: Li Zheng
Appendices: Attention Deficit-Hyperactivity Disorder Medications and Risk of Serious Cardiovascular Events in Children and Youth
These appendices provide supplementary material for the paper, including a more detailed presentation of several methodologic points and secondary analyses. They should be read in conjunction with the primary paper.
Table of Appendices
| Appendix 1 | Serious illness exclusions |
| Appendix 2 | Study person-time |
| Appendix 3 | Case definitions and identification |
| Appendix 4 | Medical record review |
| Appendix 5 | Computer algorithm for cases in which records were unavailable or had insufficient information |
| Appendix 6 | Site-specific cohort characteristics adjusted for propensity score |
| Appendix 7 | Full model |
| Appendix 8 | Clinical characteristics of confirmed cases |
| Appendix 9 | Analysis in which former users served as the reference |
| Appendix 10 | Adjusted rates of serious cardiovascular events for individual ADHD medications |
| Appendix 11 | Alternative analyses |
| Appendix 12 | Comparisons of Medicaid and Non-Medicaid sites |
Appendix 1
Serious Illness Exclusions
Children and youth with serious illnesses were excluded from the study because they were felt to have a substantially increased mortality risk. It would thus be inefficient to review deaths for these children as potential cases. It was also considered likely that the use of ADHD medications would be less frequent in this population. For example, none of the FDA cases of sudden cardiac death in persons under 25 years of age were reported to have these exclusion illnesses.6 Persons were thus excluded from the cohort if they had the following during the period 365 days prior to the qualifying date:
One inpatient claim with a diagnosis for the exclusion disease (Table A1.1), with the claim of interest appearing anywhere in the primary and secondary diagnoses; or,
Two outpatient claims separated by at least 30 days for the exclusion disease; or,
One prescription for a medication used to treat the exclusion disease; or,
One claim with a procedure for the exclusion disease.
Table A1.1.
Exclusion Illnesses
| Sickle cell disease |
| Cystic fibrosis |
| Cerebral Palsy |
| Cancer |
| Human Immunodeficiency Virus Infection |
| Organ transplant |
| Liver failure |
| Renal dialysis (except single inpatient episode) |
| Respiratory failure |
| Other potentially lethal diseases of childhood (metabolic diseases, aplastic anemia, congenital immune deficiencies, lethal chromosomal anomalies) |
Appendix 2
Study Person-time
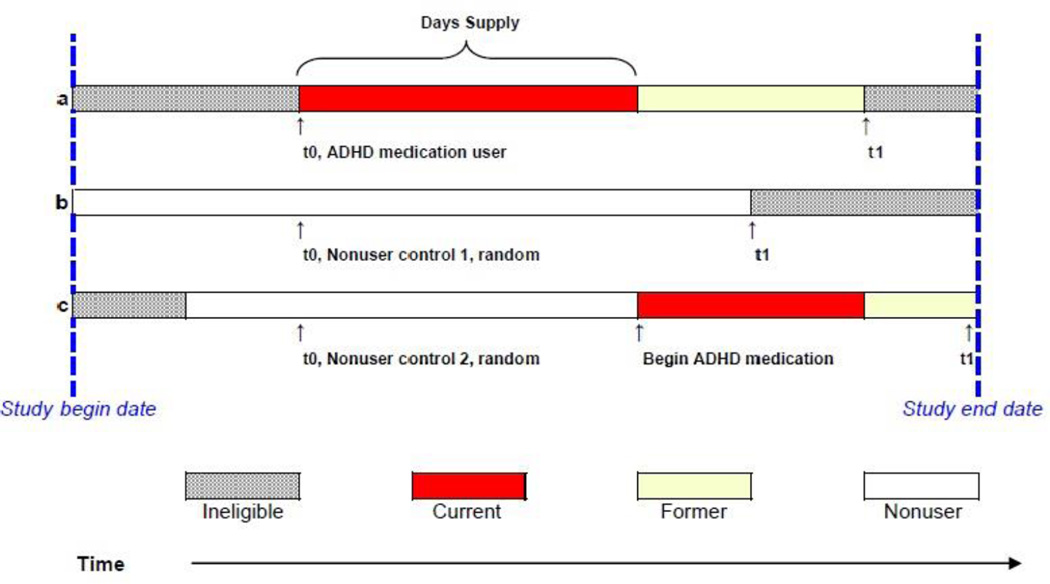
All study person-time was classified according to ADHD medication use as current, former, or nonuser. Figure A2.1 illustrates how this classification was performed.
Figure A2.1 Study Person-time.
To assemble the cohort, we first identified ADHD medication users who met study criteria (Figure A2.1, Person a). The first day of qualifying use was defined as t0. Study follow-up ended at the end of the study or when the person no longer met study criteria, defined as t1. For each ADHD user, we then randomly selected up to two control persons with no ADHD medication use on t0 (Figure A2.1, Persons b and c). Controls were from the same site’s health plan members enrolled on t0 matched for calendar year, age, and gender who also met the study inclusion criteria for users. Follow-up for nonusers began on t0 for the matched ADHD medication user and ended when the nonuser left the cohort, t1.
For each cohort member, every person-day during study follow-up was classified according to probable use of ADHD medications. Current use was defined as the period between the prescription start date and the end of the days of supply (including up to a 7-day carryover from previous prescriptions). Former use included person-time following current use through the end of study follow-up that was not classified as current use. Nonuse included person-time with no prescribed use of ADHD medications on these days or at any time in the past. Nonusers could become users of ADHD medications during follow-up (Person c), but they did not re-enter the cohort. Rather, their person-time was classified as described above.
Appendix 3
Case Definitions and Identification
Because we planned to review medical records for potential cases and anticipated that serious cardiovascular events in children and youth would be rare, initial definitions for potential cases selected for review and adjudication were intentionally broad to increase the sensitivity of our case finding. We first created a clinical definition for each endpoint (described in the Methods section) and then created a search definition of potential cases for review.
Sudden Cardiac Death
Potential sudden cardiac death (SCD) cases were identified from state death certificates (Tennessee, Washington State, Kaiser) or the National Death Index (Tennessee, Kaiser, OptumInsight Epidemiology). To ensure ascertainment of deaths occurring in youth 18 to 24 years of age (who may have moved away for college or early careers while still insured by a parent) for sites using state death certificates, we also performed National Death Index searches for any cohort member who was 18–24 years of age during follow-up, ended enrollment prior to another reason for end of follow-up, and had no evidence of being alive subsequently based on re-enrollment, other healthcare claims, or births. All deaths that were potential cases identified in the National Death Index search were already identified from state vital records.
We included the following underlying causes of death on death certificates and national death index searches: any cardiac system cause of death (ICD-9 390-459, ICD-10 I00-I99); congenital anomaly (ICD-9 740-759, ICD-10 Q00-89); diabetes (ICD-9 250, ICD10-E10-E14, collapse (ICD-9 780.2, ICD-10 R55); sudden death, unknown cause (ICD-9 798.0-798.9, ICD-10 R96); respiratory arrest (ICD-9 799.1, ICD-10 R09.2); death from ill-defined condition (ICD-9 799.8, ICD-10 R98); and unknown cause of death (ICD-9 799.9, ICD-10 R99). A secondary source was hospital discharge data, including Emergency Department (ED) records. We included the following primary diagnoses for hospitalizations with death: cardiac arrest (ICD-9 427.5), sudden death, unknown cause (ICD-9 798.0-798.9); respiratory arrest (ICD-9 799.1), and cardiac arrest due to a procedure (ICD-9 997.1).
Acute Myocardial Infarction
Potential cases of acute myocardial infarction were identified from principal hospital discharge diagnoses of acute myocardial infarction or cause of death from death certificates using the following codes: acute myocardial infarction (ICD-9 410, ICD-10 I21, I22), intermediate coronary syndrome (ICD-9 411.1, ICD-10 I20.0), acute coronary occlusion (ICD-9 411.8, ICD-10 I24), old myocardial infarction (ICD-9 412, ICD-10 I25.2), angina pectoris (ICD-9 413, ICD-10 I20.1, I20.8, I20.9), coronary atherosclerosis (ICD-9 414.0, ICD-10 I25.0, I25.1), aneurysm of heart (ICD-9 414.1, ICD-10 I25.3, I25.4), other specified forms of chronic ischemic heart disease (ICD-9 414.8, ICD-10 I25.5-I25.9), and sequelae of myocardial infarction (ICD-9 429.7, ICD-10 I23).
Stroke
Potential stroke cases were identified from principal hospital discharge diagnoses of stroke or cause of death from death certificates using the following codes: intracerebral hemorrhage (ICD-9 431, ICD-10 I61, I64), nontraumatic extradural hemorrhage, (ICD-9 432.0 ICD-10 I62.1), unspecified intracranial hemorrhage, (ICD-9 432.9, ICD-10 I62.0, I62.9), occlusion and stenosis of precerebral arteries, (ICD-9 433, ICD-10 I65), occlusion of cerebral arteries, (ICD-9 434, ICD-10 I63, I66), transient cerebral ischemia, (ICD-9 435, ICD-10 G45.9), acute, but ill-defined, cerebrovascular disease, (ICD-9 436, ICD-10 I67, I68), late effects of cerebrovascular disease, (ICD-9 438, ICD-10 I-69), hemiplegia, (ICD-9 342, ICD-10 G81), other paralytic syndromes, [ICD-9 344 (not 344.6), ICD-10 G83].
Appendix 4
Medical Record Review
Medical records were reviewed by two adjudicators at the lead site (two cardiologists for sudden death and acute myocardial infarction and two neurologists for stroke) based on clinical criteria for the outcome of interest and to exclude cases due to non-cardiac causes (e.g. overdose, other underlying illnesses). For the <5% of cases in which the adjudicators differed on any element of the adjudication [either whether the event was a case or the type of outcome (i.e. hemorrhagic stroke vs. thromboembolic stroke)], the study principal investigator met with the adjudicators for resolution. Final case status is shown below (Figure A.4.1).
Figure A.4.1. Identification of Cases and Medical Record Review.Exclusion of Potential Cases Based on Medical Record Review
Table A.4.1.
Reasons for Exclusion of Potential Cases.
| Reason | All | Sudden Cardiac Death |
Acute Myocardial Infarction |
Stroke |
|---|---|---|---|---|
| All | 254 | 149 | 28 | 77 |
| Syncope/Weakness/Dizziness only§ | 86 | 75 | 0 | 11 |
| Trauma | 34 | 14 | 0 | 20 |
| Evaluated and found not to have the condition |
26 | 7 | 12 | 7 |
| Miscode | 16 | 2 | 6 | 8 |
| Other diagnoses | 15 | 11 | 1 | 3 |
| Prior event | 10 | 0 | 0 | 10 |
| Suicide | 9 | 9 | 0 | 0 |
| Procedure Related | 9 | 4 | 5 | 0 |
| Spinal cord injury | 8 | 0 | 0 | 8 |
| Overdose | 8 | 6 | 1 | 1 |
| Gunshot wound | 7 | 6 | 1 | 0 |
| Seizure only | 5 | 2 | 0 | 3 |
| Drowning | 5 | 5 | 0 | 0 |
| Infection | 4 | 1 | 1 | 2 |
| Transient Ischemic Attack | 4 | 0 | 0 | 4 |
| Undetermined | 3 | 3 | 0 | 0 |
| House fire | 2 | 2 | 0 | 0 |
| Snake bite | 1 | 0 | 1 | 0 |
| Homicide | 1 | 1 | 0 | 0 |
| Choking | 1 | 1 | 0 | 0 |
In cases where the child/youth did not die.
Appendix 5
Computer algorithm for cases where medical records were unavailable or had insufficient information for adjudication
This appendix describes the computer algorithm developed for cases for which medical records were sought, but were not available or where the record was reviewed but had insufficient information for adjudication. The positive predictive value of the algorithm across all three endpoints was 91%.
Decision rule for sudden cardiac death
For sudden cardiac death, the computer-based definition was based on prior literature38 and the predictive value of codes in the present study, and included the following:
Evidence of death (death certificate or national death index), AND
No evidence of other explanatory cause in the causes of death [(i.e. motor vehicle collision, gunshot wound, drowning, suicide, post-operative death, or cocaine use/abuse (ICD-9 304.2, 305.6, 968.5)], AND
- Cause of death included any of the codes below38:
ICD9 ICD10 From previous literature38 401.9 Essential hypertension, NOS I10 Essential hypertension 402 Hypertensive heart disease, NOS I11.9 Hypertensive heart disease w/o heart failure 410 Acute myocardial infarction I21 Acute myocardial infarction I22 Subsequent myocardial infarction I23 Certain complications following AMI 411 Other acute/subacute ischemic heart disease I24 Other acute ischemic heart disease 412 Old myocardial infarction I25.2 Old myocardial infarction (incl. With I25) 413 Angina pectoris I20 Angina pectoris 414 Other forms of chronic ischemic heart disease I25, I25.1 Chronic ischemic heart disease 425.4 Primary cardiomyopathy, other I42, I42.9,I42.8 Cardiomyopathy, Not otherwise specified 427.5 Cardiac arrest I46 Cardiac arrest I47.0 Reentry ventricular arrhythmia 427.1 Paroxysmal ventricular tachycardia I47.2 Ventricular tachycardia 427.4 Ventricular fibrillation and flutter I49.0 Ventricular fibrillation and flutter 427.8 Arrhythmia, other but not specified I49.8 Other specified cardiac arrhythmias 427.9 Arrhythmia (cardiac), NOS I49.9 Cardiac arrhythmia, unspecified 429.2 Cardiovascular disease, unspecified I51.6 Cardiovascular disease, unspecified 429.9 Heart disease, unspecified I51.9 Heart disease, unspecified 440.9 Arteriosclerosis, NOS I70.9 Atherosclerosis, NOS 798.2 Death in <24 hours R96.1 Death in <24 hours 798.9 Unattended death R98 Unattended death From the present study 745.0 Common truncus Q20 Anomalies of cardiac chambers 745.1 Transposition of great vessels Q20.3 Transposition of great vessels 745.2 Tetrology of Fallot Q21.3 Tetrology of Fallot 745.3 Common ventricle Q20.0 Common ventricle 745.6 Endocardial cushion defects Q21.2 Endocardial cushion defects 746, 745 Other congenital anomalies of heart Q22, Q23 Other congenital anomalies of heart
Among 241 potential sudden cardiac deaths, records for 45 were unavailable or had insufficient information. Among these cases, one additional case met the computer algorithm definition and was included as a case in the analysis. The positive predictive value of the algorithm as applied to the found cases was 86%.
Decision rule for acute myocardial infarction
For acute myocardial infarction, the computer-based definition was based on prior literature39 and the predictive value of codes in the present study and included the following:
Hospitalization with at least 2 days stay (i.e. including at least three calendar days) OR Death, AND
Discharge diagnosis or Cause of Death = 410 (acute myocardial infarction).
Of 66 potential acute myocardial infarctions, records for 29 were unavailable or had insufficient information (mostly cases that were only treated in the emergency department and did not result in hospital admission or death). Of these, one additional case met the computer algorithm definition and was included as a case in the analysis. The positive predictive value of this algorithm as applied to cases where records were obtained and reviewed was 100%.
Decision rule for stroke
For stroke, the computer-based definition was based on prior literature39 and the predictive value of codes in the present study and included the following:
Hospitalization with at least 2 days stay (i.e. including at least three calendar days) OR death, AND
No other codes in the discharge or death records indicate an alternate explanation (i.e. trauma, gunshot wound), AND
- The following ICD-9 codes were included in the discharge listing or causes of death:
Description ICD-9 codes ICD-10 codes Intracerebral hemorrhage 431 I61, I64 Occlusion and stenosis of precerebral arteries 433 I65 Occlusion of cerebral arteries 434 (not 434.xo) I63, I66 Acute, but ill-defined, cerebrovascular disease 436 I67, I68
Of 147 stroke potential cases, records for 23 were unavailable or had insufficient information. Of these, 6 met the computer algorithm definition and were included as cases. The positive predictive value of this algorithm as applied to the found cases was 91%.
Records unavailable or had insufficient information, case included based on computer algorithm
| Endpoint* | ICD-9 code | Description | N cases with records unavailable or with insufficient information |
Estimated positive predictive value from found cases |
|---|---|---|---|---|
| AMI | 410.11 | Acute myocardial infarction with prolonged hospitalization |
1 | 100% |
| STK | 431 | Intracerebral hemorrhage and hospitalization |
3 | 92% |
| STK | 433.21 | Occlusion, vertebral arteries and hospitalization |
2 | 92% |
| STK | 434.91 | Occlusion, cerebral arteries and hospitalization |
1 | 92% |
| SCD | I49.9 | Death with cardiac arrhythmia as cause of death |
1 | 86% |
Records unavailable or had insufficient information, case excluded based on computer algorithm
| Endpoint* | ICD-9 code | Description | N cases with records unavailable or with insufficient information |
Estimated positive predictive value from found cases |
|---|---|---|---|---|
| AMI | 410 | Acute myocardial infarction, 1 day stay (no death) |
4 | 0% |
| AMI | 411.1 | Intermediate coronary syndrome | 2 | 0% |
| AMI | 413 | Angina pectoris | 7 | 0% |
| AMI | 414.00 | Coronary Atherosclerosis | 12 | 0% |
| AMI | 414.8 | Other ischemic heart disease | 2 | 0% |
| AMI | 429.71 | Sequelae of acute myocardial infarction | 1 | 0% |
| SCD | 427.5 | Cardiac arrest, no death, no hospitalization | 8 | 0% |
| SCD | 780.2 | Collapse | 28 | 3% |
| SCD | 799.1 | Respiratory arrest | 1 | 0% |
| SCD | 799.9 | Unknown cause of death | 1 | 25% |
| SCD | R99 | Other ill defined mortality | 4 | 26% |
| SCD | I80.2 | Phlebitis | 1 | 10% |
| SCD | I51.4 | Myocarditis | 1 | 0% |
| STK | 342 | Hemiplegia | 2 | 0% |
| STK | 344 | Other paralytic syndromes | 6 | 0% |
| STK | 431 | Intracerebral hemorrhage, no death, no hospitalization |
1 | 0% |
| STK | 432.0 | Extradural hemorrhage | 1 | 0% |
| STK | 432.9 | Unspecified cerebrovascular disease | 1 | 30% |
| STK | 433.10 | Occlusion carotid arteries | 1 | 33% |
| STK | 434.91 | Occlusion cerebral arteries, no hospitalization |
1 | 0% |
| STK | 435.9 | Transient ischemic attack | 2 | 0% |
| STK | 436 | Acute ill defined cerebrovascular disease, no death, no hospitalization |
1 | 0% |
| STK | I60.7 | Subarachnoid hemorrhage | 1 | 30% |
SCD=sudden cardiac death, AMI=acute myocardial infarction, STK=stroke
Appendix 6
Propensity Score Diagnostics
One important check of the specification of the propensity score model is whether or not, after adjustment for propensity score, the distribution of the covariates is balanced. We performed this check for the ADHD medication user propensity score40 using a variant of the inverse probability of treatment method described by Brenner.41, 42 The advantage of this method is that it standardizes the distribution of the nonuser group to that of the current user group, which is left unadjusted. The method of Brenner works as follows: for patient i, let ri be the variable value in the group providing the standard and si that in the group being standardized. Then the weight is defined as ri/si. Thus, for the nonuser:user propensity scores, considering the ith patient in the nonuser group, ri is the probability of treatment with ADHD medications, given a comparable covariate pattern. This is simply the propensity score for that patient. Similarly, si is the probability of being a nonuser, which is 1-propensity score. Table A.6 shows the covariate balance after adjusting the nonuser distribution. Unadjusted distributions of the study covariates by exposure group are shown in Table 2.
Table A.6.
Characteristics of nonusers and current users by study site, adjusted for propensity score.
| Tennessee Medicaid |
Kaiser Permanente Northern & Southern California |
OptumInsight Epidemiology |
Washington State Medicaid |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Nonuser | Current | Nonuser | Current | Nonuser | Current | Nonuser | Current |
| Demographic characteristics | ||||||||
| Age in years, mean | 8.8 | 8.7 | 11.2 | 11.1 | 12.5 | 12.0 | 10.1 | 10.0 |
| Male | 70.9% | 70.1% | 74.0% | 74.0% | 69.5% | 70.3% | 72.0% | 72.4% |
| Nonwhite | 26.1% | 29.8% | 50.0% | 56.4% | 0.0% | 0.0% | 16.2% | 16.5% |
| Reside in metropolitan area, % | 62.2% | 63.7% | 95.9% | 95.6% | - | - | 70.7% | 69.4% |
| Psychiatric conditions | ||||||||
| Major depression | 10.0% | 9.4% | 13.3% | 11.6% | 12.4% | 11.1% | 8.3% | 6.0% |
| Bipolar disorder | 2.0% | 2.2% | 1.7% | 1.7% | 1.9% | 2.2% | 1.7% | 1.8% |
| Psychosis | 1.1% | 1.0% | 0.6% | 0.4% | 0.5% | 0.4% | 1.1% | 0.6% |
| Autism | 1.0% | 0.9% | 2.3% | 2.5% | 1.0% | 1.2% | 1.5% | 1.2% |
| Mental Retardation | 5.9% | 5.4% | 1.2% | 2.1% | 2.3% | 4.2% | 3.5% | 3.6% |
| Prior suicide attempt | 0.4% | 0.4% | 0.3% | 0.2% | 0.3% | 0.3% | 0.1% | 0.1% |
| Psychotropic medication use | ||||||||
| Antidepressant | 16.5% | 17.5% | 17.8% | 14.7% | 17.2% | 13.8% | 18.5% | 18.5% |
| Mood stabilizers | 4.5% | 4.8% | 3.4% | 3.4% | 3.9% | 4.1% | 5.2% | 5.6% |
| Antipsychotics | 6.0% | 7.3% | 4.4% | 4.5% | 3.7% | 4.8% | 5.4% | 5.6% |
| Benzodiazepines | 0.5% | 0.4% | 0.4% | 0.3% | 0.7% | 0.6% | 0.4% | 0.2% |
| Medical Conditions | ||||||||
| Asthma | 31.1% | 27.6% | 24.8% | 21.1% | 26.5% | 21.4% | 23.0% | 18.4% |
| Seizures | 4.4% | 3.6% | 1.3% | 1.1% | 2.3% | 1.9% | 2.6% | 2.1% |
| Obesity | 1.4% | 1.3% | 3.4% | 3.1% | 0.8% | 0.8% | 0.5% | 0.5% |
| Major congenital heart disease‡ | 2.2% | 2.0% | 0.9% | 0.8% | 0.5% | 0.5% | 0.5% | 0.4% |
| Minor congenital heart disease‡ | 8.0% | 7.5% | 4.3% | 3.8% | 8.1% | 7.6% | 6.4% | 6.5% |
| Diabetes | 0.9% | 0.7% | 0.3% | 0.2% | 0.5% | 0.5% | 0.6% | 0.5% |
| Other serious health condition§ | 1.8% | 1.4% | 1.2% | 0.90% | 1.8% | 1.5% | 1.6% | 1.1% |
| Alcohol and drug use | ||||||||
| Alcohol or drug use | 1.2% | 1.1% | 1.5% | 1.5% | 0.9% | 1.0% | 2.1% | 1.2% |
| Smoking | 1.6% | 1.4% | 1.6% | 1.2% | 1.0% | 0.8% | 1.0% | 0.7% |
| Use of health services | ||||||||
| Psychiatric hospitalization | 3.3% | 3.2% | 2.1% | 1.5% | 1.9% | 1.7% | 2.3% | 2.1% |
| Psychiatric outpatient visits | 54.3% | 59.0% | 57.4% | 67.9% | 55.5% | 63.7% | 47.7% | 58.7% |
| Cardiovascular hospitalization | 0.5% | 0.4% | 0.4% | 0.3% | 0.2% | 0.2% | 0.3% | 0.3% |
| Cardiovascular ED Visit | 1.4% | 1.3% | 0.3% | 0.3% | 0.3% | 0.3% | 1.3% | 1.0% |
| Cardiovascular outpatient visits | 9.3% | 8.4% | 3.5% | 2.8% | 7.1% | 6.5% | 4.9% | 4.3% |
| Other outpatient visits | 95.9% | 95.4% | 93.7% | 91.8% | 92.8% | 92.8% | 90.3% | 91.2% |
| Any prescription | 37.9% | 33.8% | 28.1% | 24.7% | 40.5% | 34.4% | 20.8% | 23.5% |
| Propensity Score | ||||||||
| Site specific propensity score | 55.8% | 58.2% | 60.4% | 67.2% | 56.7% | 61.9% | 51.8% | 60.1% |
Adjusted for propensity score using the method of Brenner.41
Measured in the 365 days before study entry.
Major congenital heart defects included common truncus, transposition of the great vessels, Tetrology of Fallot, common ventricle, endocardial cushion defect, pulmonary atresia, tricuspid atresia, hypoplastic left heart syndrome, coarctation of the aorta, and total anomalous pulmonary venous return. Minor congenital heart defects included any other congenital heart anomaly.
Other serious health conditions included pneumonia, thyroid disease, and kidney disease.
Appendix 7
Serious Cardiovascular Events According to Use of ADHD Medications, Full Model
| Parameter | Chi Squared |
Hazard Ratio |
95% confidence interval |
|---|---|---|---|
| Age | <.0001 | 1.15 | 1.09–1.22 |
| Current antipsychotic use | 0.4271 | 1.52 | 0.54–4.24 |
| Major psychiatric illness | 0.0010 | 2.72 | 1.50–4.95 |
| Substance abuse | 0.5837 | 0.67 | 0.16–2.83 |
| Serious cardiovascular | 0.0001 | 5.36 | 2.26–12.71 |
| Serious chronic illness | 0.0002 | 5.12 | 2.19–11.93 |
| Medical hospitalization | 0.3421 | 0.64 | 0.25–1.61 |
| General medical care access | 0.4883 | 1.26 | 0.66–2.42 |
| Site Washington State | 0.2030 | 0.57 | 0.24–1.35 |
| Site Tennessee Medicaid | 0.8231 | 0.92 | 0.42–1.98 |
| Site OptumInsight Epidemiology | 0.0008 | 0.24 | 0.11–0.55 |
| Propensity Score Decile 9 | 0.1989 | 0.50 | 0.17–1.44 |
| Propensity Score Decile 8 | 0.0775 | 0.30 | 0.08–1.14 |
| Propensity Score Decile 7 | 0.2462 | 0.55 | 0.20–1.51 |
| Propensity Score Decile 6 | 0.1326 | 0.42 | 0.14–1.30 |
| Propensity Score Decile 5 | 0.7971 | 1.12 | 0.47–2.69 |
| Propensity Score Decile 4 | 0.7579 | 1.15 | 0.47–2.81 |
| Propensity Score Decile 3 | 0.1411 | 0.41 | 0.13–1.34 |
| Propensity Score Decile 2 | 0.4329 | 0.67 | 0.25–1.83 |
| Propensity Score Decile 1 | 0.3400 | 0.60 | 0.21–1.71 |
| Current user | 0.5342 | 0.75 | 0.31–1.85 |
| Former user | 0.8999 | 1.04 | 0.57–1.89 |
Appendix 8
Clinical Characteristics of Confirmed Serious Cardiovascular Events, According to Use of ADHD Medications
| Nonuser | Former ADHD Medication User |
Current ADHD Medication User |
|
|---|---|---|---|
| Sudden cardiac death† | |||
| Number of cases | 17 | 12 | 3 |
| Age, mean (standard deviation) |
14.6 (4.2) | 18.7 (4.3) | 14.0 (8.0) |
| Autopsy reviewed | 13 (76.5%) | 9 (75.0%) | 3 (100.0%) |
| Cardiac abnormalities found at autopsy |
Left ventricular hypertrophy (1) Hypertrophic Obstructive Cardiomyopathy (3) No abnormality (9) |
Left ventricular hypertrophy (1) Dilated cardiomyopathy (2) Tunneling of left anterior descending coronary artery (1) No abnormality (6) |
Dilated cardiomyopathy (1) Fibro-fatty change sino- atrial node (1) Dysplasia of atrioventricular node artery (1) |
|
Acute myocardial infarction† |
|||
| Number of cases | 6 | 2 | 0 |
| Age, mean (standard deviation) |
18.7 (2.7) | 18.0 (1.4) | - |
| ST segment elevation on electrocardiogram |
6 (100%) | 1 (50%) | - |
| Coronary artery occlusion noted at cardiac catheterization (among those who underwent the procedure) |
3 of 5 who had cardiac catheterization performed |
1 of 2 who had cardiac catheterization performed |
|
| Stroke† | |||
| Number of cases | 21 | 8 | 4 |
| Age, mean (standard deviation) |
14.9 (4.1) | 16.3 (2.2) | 14.5 (5.1) |
| Etiology, Number (%) | |||
| Hemorrhagic stroke | 15 (71.4%) | 2 (25.0%) | 2 (50.0%) |
| Stroke from vessel occlusion or vessel abnormality |
2 (9.5%) | 2 (25.0%) | 1 (25.0%) |
| Embolic stroke | 1 (4.8%) | 1 (12.5%) | 1 (25.0%) |
| Unknown despite imaging |
2 (9.5%) | - | - |
| Ischemic | 1 (4.8%) | 3 (37.5%) |
Cases where outcomes were validated with medical records. Note that this table excludes cases where medical records were not reviewed, including one sudden cardiac death, one acute myocardial infarction, and six strokes.
For sudden cardiac death, the mean age at time of death was comparable across the study medication groups. Autopsy reports were reviewed for 78.1% of the sudden cardiac death cases and revealed occasional structural abnormalities, including left ventricular hypertrophy, hypertrophic obstructive cardiomyopathy, coronary artery anomalies, and fibro-fatty changes of the sino-atrial node. For acute myocardial infarction, the mean age for cases was greater than that for sudden cardiac death. All but one of the cases of acute myocardial infarction (87.5%) had electrocardiogram ST segment elevation. Seven of the cases of acute myocardial infarction underwent cardiac catheterization and coronary vessel occlusion was noted in 4 (57%). For strokes, the mean age of cases across the drug exposure groups was comparable. Hemorrhagic strokes were the most common stroke type for all three groups.
Appendix 9
Analysis in Which Former Users Served As the Reference
In this analysis, former users of ADHD medications were the reference group to account for possible unmeasured confounding.
Table A.9.1.
Adjusted Hazard Ratios for Serious Cardiovascular Events, According to Use of ADHD Medications, Former Users as the Reference.
| ADHD medication use | Person- years |
Events | Rate/100,000 person-years |
Hazard Ratio† |
95% confidence interval |
|---|---|---|---|---|---|
| Former user | 607,475 | 25 | 4.12 | 1.00 | Reference |
| Nonuser | 1,597,962 | 49 | 3.07 | 1.24 | 0.73–2.08 |
| Current User | 373,667 | 7 | 1.87 | 0.70 | 0.29–1.72 |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), age, and calendar year.
Appendix 10
Adjusted Rates for Serious Cardiovascular Events According to Use of Individual ADHD Medications
| ADHD medication use | Person- years |
Events | Rate/100,000 person-years |
Hazard Ratio† |
95% confidence interval |
|---|---|---|---|---|---|
| Nonuser | 1,597,962 | 49 | 3.07 | 1.00 | Reference |
| Former user | 607,475 | 25 | 4.12 | 1.03 | 0.57–1.89 |
| Current User | 373,667 | 7 | 1.87 | 0.75 | 0.31–1.85 |
| Methylphenidate | 192,257 | 4 | 2.08 | 0.96 | 0.31–2.97 |
| Amphetamines‡ | 137,448 | 1 | 0.73 | - | - |
| Atomoxetine‡ | 29,330 | 1 | 3.41 | - | - |
| Pemoline‡ | 14,632 | 1 | 6.83 | - | - |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), age, and calendar year.
Because of low numbers of events for use of amphetamines, atomoxetine, and pemoline, regression models were not fit for these individual medications.
Appendix 11
Alternative Analyses
Alternative Analysis Addressing Exposure Group Definitions
In this analysis, we restricted the analysis to individuals who had no ADHD medication use in the 365 days prior to t0. Thus, covariates were measured at drug initiation.
Table A.11.1a.
Adjusted Hazard Ratios for Serious Cardiovascular Events, According to Use of ADHD Medications, Restricted to New Users of ADHD Medications.
| ADHD medication use | Person- years |
Events | Rate/100,000 person-years |
Hazard Ratio† |
95% confidence interval |
|---|---|---|---|---|---|
| Nonuser | 1,597,962 | 49 | 3.07 | 1.00 | Reference |
| Former user | 376,456 | 19 | 5.05 | 1.13 | 0.60–2.13 |
| Current User | 192,040 | 4 | 2.08 | 0.73 | 0.24–2.10 |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), age, and calendar year.
In this analysis, the analysis was restricted to new users and focused on the individual endpoints, sudden cardiac death, acute myocardial infarction, and stroke.
Table A.11.1b.
Adjusted Hazard Ratios for Individual Cardiovascular Endpoints, According to Use of ADHD Medications, Restricted to New Users of ADHD Medications.
| ADHD medication use |
Person- years |
Events | Rate/100,000 person-years |
Hazard Ratio† |
95% confidence interval |
|---|---|---|---|---|---|
| Sudden Cardiac Death | |||||
| Nonuser | 1,597,962 | 17 | 1.06 | 1.00 | Reference |
| Former user | 376,456 | 8 | 2.13 | 1.13 | 0.41–3.10 |
| Current User | 192,040 | 2 | 1.04 | 0.76 | 0.18–3.26 |
| Acute Myocardial Infarction | |||||
| Nonuser | 1,597,962 | 6 | 0.38 | 1.00 | Reference |
| Former user | 376,456 | 3 | 0.80 | - | - |
| Current User | 192,040 | 0 | 0 | - | - |
| Stroke | |||||
| Nonuser | 1,597,962 | 26 | 1.63 | 1.00 | Reference |
| Former user | 376,456 | 8 | 2.13 | 1.14 | 0.47–2.76 |
| Current User | 192,040 | 2 | 1.04 | 0.97 | 0.22–4.27 |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), age, and calendar year. Because there were no events in the current user group, acute myocardial infarction models were calculated for former users and nonusers only.
Alternative Analyses Addressing Case Definitions
The case definitions for sudden cardiac death excluded potential cases with severe underlying cardiac disease that would likely be the cause of any sudden death event rather than a medication exposure. In reviewing the clinical characteristics of cases excluded due to other cardiac disease, we found five patients with severe congestive heart failure, several who were awaiting heart transplant; 1 patient with an arrest event in whom a post-mortem discovered a ruptured aortic aneurysm, and 1 patient with a history of viral illness who collapsed while running and had confirmed viral myocarditis on post-mortem. In this alternative analysis, we included all of these cases as confirmed events.
Table A.11.2.
Adjusted Hazard Ratios for Serious Cardiovascular Events, According to Use of ADHD Medications, Including Cardiac Cases Excluded for Having Severe Underlying Cardiac Disease.
| ADHD medication use | Person- years |
Events | Rate/100,000 person-years |
Hazard Ratio† |
95% confidence interval |
|---|---|---|---|---|---|
| Nonuser | 1,597,962 | 54 | 3.38 | 1.00 | Reference |
| Former user | 607,475 | 27 | 4.44 | 1.01 | 0.58–1.78 |
| Current User | 373,667 | 7 | 1.87 | 0.71 | 0.29–1.72 |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), age, and calendar year.
Alternative Analyses Addressing Age
These analyses were stratified by age 2–17 years and age 18–24 years.
Table A.11.3.
Adjusted Hazard Ratio for Serious Cardiovascular Events, According to Use of ADHD Medications, Stratified by Age.
| ADHD medication use | Person- years |
Events | Rate/100,000 person-years |
Hazard Ratio† |
95% confidence interval |
|---|---|---|---|---|---|
| Age 2–17 years | |||||
| Nonuser | 1,516,662 | 45 | 2.97 | 1.00 | Reference |
| Former user | 576,553 | 21 | 3.64 | 1.03 | 0.55–1.95 |
| Current User | 355,360 | 7 | 1.97 | 0.98 | 0.41–2.36 |
| Age 18–24 years | |||||
| Nonuser | 81,300 | 4 | 4.92 | 1.00 | Reference |
| Former user | 30,922 | 4 | 12.94 | 0.92 | 0.14–6.24 |
| Current User | 18,307 | 0 | 0 | - | - |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), psychiatric conditions (major psychiatric illness, substance abuse, and antipsychotic use), utilization variables (medical hospitalization and general medical care access), and calendar year. Because there were no events in the current user group for cohort members of age 18–24 years, full models were calculated for age 2–17 years and models for former users only for age 18–24 years.
Alternative Analyses Excluding Children with Serious Psychiatric Illness
These analyses excluded children with evidence of serious psychiatric illness, defined as use of psychotropic medications (antipsychotics, lithium or mood stabilizers) or claims evidence of major psychiatric illness (depression, bipolar disorder, psychotic disorder, autism, or psychiatric hospitalizations for any psychiatric diagnosis in the past 365 days) at baseline or children who developed evidence of these conditions during follow-up, who were excluded from the date that they met evidence of serious psychiatric illness through the end of their follow-up.
Table A.11.4.
Adjusted Hazard Ratio for Serious Cardiovascular Events, According to Use of ADHD Medications, Excluding Children with Serious Psychiatric Illness.
| ADHD medication use | Person- years |
Events | Rate/100,000 | Hazard Ratio† |
95% confidence interval low |
95% confidence interval high |
|---|---|---|---|---|---|---|
| Non-user | 1,534,206 | 43 | 2.80 | 1.00 | Ref | Ref |
| Former user | 457,171 | 10 | 2.19 | 0.82 | 0.36 | 1.86 |
| Current user | 280,306 | 3 | 1.07 | 0.66 | 0.20 | 2.16 |
Hazard ratios estimated with Cox regression models which included site-specific propensity score decile, site, medical conditions (serious cardiovascular disease, serious chronic illness), utilization variables (medical hospitalization and general medical care access), and calendar year.
Appendix 12
Comparison of outcomes by site and Medicaid vs. Non-Medicaid enrollment
We compared the occurrence of serious cardiovascular events for the exposure groups (ADHD medication nonusers and current users) according to individual site. Given the rarity of these events and the small numbers of events for the individual sites, these data are unadjusted. For those sites that had at least one case in each of the exposure groups, we calculated the unadjusted incidence rate-ratios (IRRs) and 95% confidence intervals (CIs), using as the estimated variance of the log (IRR) the square root of the sum of the reciprocals of the numbers of exposed and unexposed cases.
For those sites for which there were no cases in one of exposure groups, we calculated the difference in unadjusted incidence (RD) between current users and nonusers. The 95% CI for the RD was calculated using a test-based method. The statistical test was a standard chi-square test for heterogeneity, calculated as follows:
where
N0, N1 are the numbers of cases in ADHD medication nonusers and current users
L0, L1 are the corresponding person-years of exposure
I is the pooled incidence in the nonusers and current users.
The square-root of the chi-square statistic (1 degree of freedom), or z, is the absolute value of a standard normal random variable with mean 0 and standard deviation 1. The test-based 95% confidence interval is thus:
The data presented here should be interpreted as a qualitative evaluation of potential differences between the sites. There are two factors that limit precision. First, these data are unadjusted for potential differences between the ADHD medication exposure groups. Second, for both the IRR and the RD, the accuracy of the 95% CIs requires an adequate number of events. The standard criterion is that there should be at least 5 events expected in each group. For some of the sites, this criterion was not met. For these sites, the 95% confidence intervals presented here, for both the IRR and the RD, are likely to be too narrow.
The total number of cases at the individual sites was small, ranging from 32 (Tennessee Medicaid) to 6 (Washington Medicaid). All of the sites had events in the nonuser group. However, two of the sites (Kaiser, I3) had no events among current users; thus, IRRs could not be calculated for these sites.
For three of the sites, the 95% confidence intervals for the RD included 0, indicating no difference in the occurrence of serious cardiovascular events between ADHD nonusers and current users. The 95% confidence interval for the RD in Washington Medicaid did not include zero; nor did it overlap with those for Kaiser and I3. However, given the small numbers, this nominal confidence interval for Washington is likely to be too narrow.
Given that the incidence of serious cardiovascular events among ADHD users was higher in the Medicaid sites than in the non-Medicaid sites, we conducted a post-hoc analysis of a potential interaction between Medicaid: non-Medicaid sites. This analysis pooled the data according to type of site and is unadjusted. Given that there were no cases in ADHD current users for the non-Medicaid sites, the IRR could not be calculated. With regard to the RD, the 95% confidence interval for the pooled Medicaid sites includes the RD for the pooled other sites, thus indicating absence of heterogeneity.
Table A.12.1.
Comparison of outcomes by site and Medicaid vs. Non-Medicaid enrollment
| Nonuser | Current User | Incidence Rate Ratio | Incidence Difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Person Years |
I/105 | Events | Person Years |
I/105 | IRR | 95% CI | RD | 95% CI | |||
| Tennessee | 29 | 470,853 | 6.16 | 3 | 77,541 | 3.87 | 0.63 | 0.19 | 2.06 | −2.29 | −8.09 | 3.51 |
| Kaiser | 10 | 329,872 | 3.03 | 0 | 77,773 | 0.00 | n/a | n/a | n/a | −3.03 | −6.90 | 0.84 |
| I3 | 11 | 651,489 | 1.69 | 0 | 176,264 | 0.00 | n/a | n/a | n/a | −1.69 | −3.61 | 0.23 |
| Washington | 2 | 145,748 | 1.37 | 4 | 42,088 | 9.50 | 6.93 | 1.27 | 37.86 | 8.13 | 2.00 | 14.26 |
| Medicaid | 31 | 616,601 | 5.03 | 7 | 119,629 | 5.85 | 1.16 | 0.51 | 2.64 | 0.82 | −3.61 | 5.25 |
| Other | 21 | 981,361 | 2.14 | 0 | 254,037 | 0.00 | n/a | n/a | n/a | −2.14 | −3.94 | −0.34 |
I=Incidence
PY=Person years
IRR=Incidence rate ratio
CI=confidence interval
RD=Rate difference
Footnotes
This is equivalent to the standard formula (N0−I*L0)2/(N*L0*L1/L2), where N is the total of events and L total person-years, see, for example, Modern Epidemiology
Reference List
- 1.Centers for Disease Control and Prevention. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children - United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep. 2010;59(44):1439–1443. [PubMed] [Google Scholar]
- 2.Brown RT, Amler RW, Freeman WS, et al. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics. 2005;115(6):e749–e757. doi: 10.1542/peds.2004-2560. [DOI] [PubMed] [Google Scholar]
- 3.Buck ML. A monthly newsletter for health care professionals from the children's medical center at the University of Virginia. Pediatric Pharmacotherapy. 2002;8(3):1–4. [Google Scholar]
- 4.Elia J, Ambrosini PJ, Rapoport JL. Treatment of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(10):780–788. doi: 10.1056/NEJM199903113401007. [DOI] [PubMed] [Google Scholar]
- 5.Rappley MD. Clinical practice. Attention deficit-hyperactivity disorder. N Engl J Med. 2005;352(2):165–173. doi: 10.1056/NEJMcp032387. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Drug Safety and Risk Management Advisory Committee Meeting. 2006 [Google Scholar]
- 7.Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med. 2006;354(14):1445–1448. doi: 10.1056/NEJMp068049. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RE, Vollmer WM. Comparing sources of drug data about the elderly. J Am Geriatr Soc. 1991;39(11):1079–1084. doi: 10.1111/j.1532-5415.1991.tb02872.x. [DOI] [PubMed] [Google Scholar]
- 9.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129(4):837–849. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- 10.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 11.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142(10):1103–1112. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 12.Marcus FI, Cobb LA, Edwards JE, et al. Mechanism of death and prevalence of myocardial ischemic symptoms in the terminal event after acute myocardial infarction. Am J Cardiol. 1988;61(1):8–15. doi: 10.1016/0002-9149(88)91295-7. [DOI] [PubMed] [Google Scholar]
- 13.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65(3):457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 14.Siscovick DS, Raghunathan TE, Psaty BM, et al. Diuretic therapy for hypertension and the risk of primary cardiac arrest. N Engl J Med. 1994;330(26):1852–1857. doi: 10.1056/NEJM199406303302603. [DOI] [PubMed] [Google Scholar]
- 15.Albert CM, Hennekens CH, O'Donnell CJ, et al. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279(1):23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AE, Carlson MD, Fogoros RN, Higgins SL, Venditti FJ., Jr Classification of death in antiarrhythmia trials. J Am Coll Cardiol. 1996;27(2):433–442. doi: 10.1016/0735-1097(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 17.Gillum RF, Fortmann SP, Prineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108(1):150–158. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 18.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44(3):E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Mahaffey KW, Roe MT, Kilaru R, et al. Characterization of myocardial infarction as an end point in two large trials of acute coronary syndromes. Am J Cardiol. 2005;95(12):1404–1408. doi: 10.1016/j.amjcard.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349(11):1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Reeder B, Shuaib A, Mazagri R. Validity of stroke diagnosis on hospital discharge records in Saskatchewan, Canada: implications for stroke surveillance. Cerebrovasc Dis. 1999;9(4):224–230. doi: 10.1159/000015960. [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med. 2010;152(6):337–345. doi: 10.1059/0003-4819-152-6-201003160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 24.Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451–453. doi: 10.1542/peds.2008-1573. [DOI] [PubMed] [Google Scholar]
- 25.Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407–2423. doi: 10.1161/CIRCULATIONAHA.107.189473. [DOI] [PubMed] [Google Scholar]
- 26.Wilens TE, Prince JB, Spencer TJ, Biederman J. Stimulants and sudden death: what is a physician to do? Pediatrics. 2006;118(3):1215–1219. doi: 10.1542/peds.2006-0942. [DOI] [PubMed] [Google Scholar]
- 27.Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334(16):1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 28.Mahle WT, Campbell RM, Favaloro-Sabatier J. Myocardial infarction in adolescents. J Pediatr. 2007;151(2):150–154. doi: 10.1016/j.jpeds.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 29.Schoenberg BS, Mellinger JF, Schoenberg DG. Cerebrovascular disease in infants and children: a study of incidence, clinical features, and survival. Neurology. 1978;28(8):763–768. doi: 10.1212/wnl.28.8.763. [DOI] [PubMed] [Google Scholar]
- 30.Broderick J, Talbot GT, Prenger E, Leach A, Brott T. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol. 1993;8(3):250–255. doi: 10.1177/088307389300800308. [DOI] [PubMed] [Google Scholar]
- 31.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366(9502):2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 32.Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- 33.Schelleman H, Bilker WB, Strom BL, et al. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics. 2011;127(6):1102–1110. doi: 10.1542/peds.2010-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winterstein AG, Gerhard T, Shuster J, Johnson M, Zito JM, Saidi A. Cardiac safety of central nervous system stimulants in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2007;120(6):e1494–e1501. doi: 10.1542/peds.2007-0675. [DOI] [PubMed] [Google Scholar]
- 35.Winterstein AG, Gerhard T, Shuster J, Saidi A. Cardiac safety of methylphenidate versus amphetamine salts in the treatment of ADHD. Pediatrics. 2009;124(1):e75–e80. doi: 10.1542/peds.2008-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy S, Cranswick N, Potts L, Taylor E, Wong IC. Mortality associated with attention-deficit hyperactivity disorder (ADHD) drug treatment: a retrospective cohort study of children, adolescents and young adults using the general practice research database. Drug Saf. 2009;32(11):1089–1096. doi: 10.2165/11317630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Gould MS, Walsh BT, Munfakh JL, et al. Sudden death and use of stimulant medications in youths. Am J Psychiatry. 2009;166(9):992–1001. doi: 10.1176/appi.ajp.2009.09040472. [DOI] [PubMed] [Google Scholar]
- 38.Chung CP, Murray KT, Stein CM, Hall K, Ray WA. A computer case definition for sudden cardiac death. Pharmacoepidemiol Drug Saf. 2010;19(6):563–572. doi: 10.1002/pds.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choma NN, Griffin MR, Huang RL, et al. An algorithm to identify incident myocardial infarction using Medicaid data. Pharmacoepidemiol Drug Saf. 2009;18(11):1064–1071. doi: 10.1002/pds.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sturmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005;161(9):891–898. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosner B. Fundamentals of Biostatistics. 6th. Belmont, CA: Duxbury; 2006. [Google Scholar]
- 42.Brenner H, Arndt V, Gefeller O, Hakulinen T. An alternative approach to age adjustment of cancer survival rates. Eur J Cancer. 2004;40(15):2317–2322. doi: 10.1016/j.ejca.2004.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.