Abstract
Objectives
To determine the relative levels of 3 potent inhibitors of angiogenesis (endostatin, pigment epithelium–derived factor, and thrombospondin 1) in the retinal pigment epithelium–Bruch’s membrane–choriocapillaris complex in the submacular region in aged control eyes and eyes with age-related macular degeneration (AMD).
Methods
Immunohistochemical analysis with antibodies against endostatin, pigment epithelium–derived factor, and thrombospondin 1 was performed on the macular region of aged control donor eyes (n=8; mean age, 79.8 years) and eyes with AMD (n=12; mean age, 83.9 years). Three independent masked observers scored the reaction product (scored from 0–7). Mean scores from the control eyes and the eyes with AMD were analyzed using 1-way analysis of variance and unpaired t test.
Results
In control eyes, strong immunoreactivity of all 3 inhibitors was observed in the retinal pigment epithelium–Bruch’s membrane–choriocapillaris complex. Immunoreactivity for endostatin, pigment epithelium–derived factor, and thrombospondin 1 in Bruch’s membrane was significantly lower in eyes with AMD compared with aged control eyes (analysis of variance, P=.003, P = .009, and P< .001, respectively). In the choriocapillaris, a significant reduction was observed in endostatin (analysis of variance, P=.02) and thrombospondin 1 (analysis of variance, P=.005) in eyes with AMD.
Conclusions
These findings suggest that endogenous angiogenesis inhibitors in the retinal pigment epithelium–Bruch’s membrane–choriocapillaris complex may provide a biochemical barrier for choroidal neovascular invasion.
Clinical Relevance
Decreased levels of angiogenic inhibitors at the retinal pigment epithelium–Bruch’s membrane–choriocapillaris complex in eyes with AMD make Bruch’s membrane vulnerable to choroidal neovascularization.
Choroidal Neovascularization (CNV) is a major cause of vision loss in patients with age-related macular degeneration (AMD).1 Because the exact mechanism underlying the pathogenesis of CNV is still poorly understood, identifying risk factors and preventive strategies is important to decrease the effect and burden of blindness from this condition. Several theories of pathogenesis have been proposed, including retinal pigment epithelium (RPE) dysfunction, alterations in Bruch’s membrane, oxidative stress, genetic defects, ocular perfusion abnormalities, inflammatory processes, and ischemia.2,3
Bruch’s membrane is a stratified extracellular matrix complex that functions as a physical as well as biochemical barrier for normal physiologic processes and pathological processes like CNV. Disruption of or damage to this barrier often results in the growth of CNV into the sub-RPE and/or subretinal spaces. Age-related changes in Bruch’s membrane have been studied to ascertain those factors that determine progression to AMD in some individuals. At present, the determinants of Bruch’s membrane changes that predispose to CNV are unclear. New vessel formation is thought to occur as a consequence of an imbalance in the stimulating and inhibiting influences of growth factors, and any disruption to growth factor diffusion through Bruch’s membrane to the choroid could alter this balance. Evidence has been found for a similar mechanism in laser-induced CNV, supporting the hypothesis that an imbalance between angiogenic stimulators and inhibitors is a cause of pathological neovascularization.4
Angiogenesis, as occurs in CNV, is tightly controlled by a dynamic balance between positive and negative regulators. In most quiescent healthy tissues, inhibitory influences predominate and vessels remain stable. In contrast, in a variety of pathological states such as neovascular AMD, neovascularization occurs because of decreased production of inhibitors and/or increased production of angiogenic stimulators.5 There is considerable evidence that vascular endothelial growth factor A is a prime regulator of angiogenesis.6 Endogenous negative regulators have been identified as well, including thrombospondin 1 (TSP-1), angiostatin and endostatin, and pigment epithelium–derived factor (PEDF).7–9
We have previously described the immunohistochemical localization of 3 endogenous angiogenesis inhibitors (endostatin, PEDF, and TSP-1) individually in submacular choroids of eyes with AMD.10–12 These inhibitors are predominantly extracellular proteins that are part of the matrix or bind to the matrix. The purpose of this study was to collectively determine whether the relative levels of these 3 potent inhibitors were significantly changed in individual subjects with AMD as well as in eyes with AMD as a group. We have primarily focused on the sub-macular RPE–Bruch’s membrane–choriocapillaris (CC) complex, which represents the extracellular matrix environment for the basal surface of RPE cells and functions as a physical as well as biochemical barrier for normal physiologic and pathological processes.
METHODS
DONOR EYES
Eight eyes from human aged donors (age range, 75–86 years; mean age, 79.8 years) with no evidence or clinical history of age-related changes in the macula and 12 eyes from donors with early and late AMD (age range, 61–105 years; mean age, 83.9 years) were used. Human donor eyes were obtained with the help of Janet Sunness, MD (Greater Baltimore Medical Center, Baltimore, Maryland), Carol Applegate (Wilmer Ophthalmological Institute, Baltimore), and the National Disease Research Interchange (Philadelphia, Pennsylvania). Donor eyes were shipped to the Wilmer Ophthalmological Institute in wet gauze sponges at 4°C. Table 1 shows the characteristics of each human donor subject used in this analysis. All of the donors were white. The diagnosis of AMD was made by reviewing ocular medical history (if available) and the postmortem gross examination results of the posterior eyecup. The protocol of the study adhered to the tenets of the Declaration of Helsinki regarding research involving human tissue and was approved by the Johns Hopkins Medicine Institutional Review Boards.
Table 1.
Characteristics of Human Donor Cryopreserved Eyes
| Subject No./Sex/Age, ya | Time, h
|
Primary Cause of Death | Medical History | Ocular Diagnosis | |
|---|---|---|---|---|---|
| Death to Enucleation | Death to Fixation | ||||
| 1/F/75 | 2.5 | 33 | Heart disease | Healthy | |
| 2/F/76 | 7 | 27 | Lung cancer | HTN | Healthy |
| 3/M77 | 1 | 26 | COPD | HTN | Healthy |
| 4/M/80 | 2.5 | 28 | COPD | Healthy | |
| 5/M/80 | 7.15 | 28 | Intracranial hemorrhage | HTN | Healthy |
| 6/M/82 | 3 | 15 | Metastasis brain cancer | Healthy | |
| 7/M/83 | 3 | 16 | Cardiac respiratory arrest | Healthy | |
| 8/F/86 | 5 | 26 | Respiratory failure | Healthy | |
| 9/M/61 | 3.5 | 34 | Metastasis esophageal cancer | AMD, early | |
| 10/M/74 | 4 | 33 | Prostate cancer | AMD, early | |
| 11/M/79 | 3 | 33 | Pneumonia | HTN, asthma | AMD, early |
| 12/F/81 | 5 | 29 | Myocardial infarction | HTN | AMD, early |
| 13/M/83 | 3 | 12 | Prostate cancer | DM, HTN | AMD, early |
| 14/F/98 | 2 | 33 | Old age | AMD, early | |
| 15/F/69 | 3.5 | 40 | Subarachnoid hemorrhage | Pulmonary fibrosis | AMD (GA), late |
| 16/M/75 | 7 | 30 | Aspiration pneumonia | AMD (GA), late | |
| 17/F/93 | 4 | 20 | Multisystem failure | DM, HTN | AMD (disciform scar), late |
| 18/M/94 | 3 | 36 | Cardiac failure | AMD (disciform scar), late | |
| 19/M/95 | 3.5 | Unknown | Cardiomyopathy | AMD (disciform scar), late | |
| 20/M/105 | 4.5 | 11 | COPD | AMD (disciform scar, GA), late | |
Abbreviations: AMD, age-related macular degeneration; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; GA, geographic atrophy; HTN, hypertension.
All of the donors were white.
TISSUE PREPARATION
The posterior eyecups were fixed in 2% paraformaldehyde in 0.1M sodium phosphate buffer (pH 7.4) with 5% sucrose at room temperature for 1 hour. The tissue was cut into culottes of the vitreous–retina–choroid complex and cryopreserved as previously described.13 Serial 8-μm cryosections were cut from the inferior macula, collected in duplicate on glass slides coated with Vectabond (Vector Laboratories, Inc, Burlingame, California), dried, and stored at −80°C.
ALKALINE PHOSPHATASE IMMUNOHISTOCHEMICAL ANALYSIS
Streptavidin alkaline phosphatase immunohistochemical analysis was performed on cryosections using a nitroblue tetrazolium development system as previously described.10 The sections were incubated overnight at 4°C with one of the following primary antibodies: goat antihuman endostatin (dilution, 1:4000; R&D Systems, Minneapolis, Minnesota), rabbit antihuman recombinant PEDF (dilution, 1:60 000; graciously provided by Patrick Tong, MD, PhD, at Wilmer Ophthalmological Institute), and mouse antihuman TSP-1 (dilution, 1:100; Abcam, Cambridge, Massachusetts). Blood vessels were immunolabeled in adjacent sections with mouse antihuman CD34 (dilution, 1:800; Signet Laboratory, Dedham, Massachusetts). Alkaline phosphatase was developed with a 5-bromo-4-chloro-3-indoyl phosphate–nitroblue tetrazolium kit (Vector Laboratories, Inc), yielding a blue immunoreaction product. To demonstrate the validity of antibody binding, antiendostatin and anti-PEDF antibodies were preincubated with a 200-fold excess of peptide or human serum albumen overnight at 4°C before use.10,11 As a negative control, the primary antibodies were omitted or a nonimmune IgG was used at the same protein concentration as the primary antibody.12 Melanin pigment in RPE and choroidal melanocytes was bleached as described previously.10 Hematoxylin and eosin staining was used to examine the morphology of the choroid in aged control eyes and eyes with AMD.
Three independent masked observers (I.A.B., K.U., and G.A.L.) scored the relative intensity of the immunoreactivity for each antibody in choroidal structures using a previously described 7-point grading system.14,15
STATISTICAL ANALYSIS
Statistical analysis was performed using InStat version 2.0 software (GraphPad Software, San Diego, California) and SAS version 9 commercial statistical software (SAS Institute, Inc, Cary, North Carolina). A mean (standard deviation) score for each group (aged control, early AMD, and late AMD) was determined from the scores of all of the graders for each choroidal structure. The P values were determined by comparing mean scores from the aged control eyes with scores from eyes with AMD using 1-way analysis of variance, and P<.05 was considered statistically significant. A post hoc t test with Bonferroni adjustment was used to compare mean scores from each group whenever applicable.
RESULTS
IMMUNOLOCALIZATION OF ANGIOGENESIS INHIBITORS IN THE RPE–BRUCH’S MEMBRANE–CC COMPLEX OF AGED CONTROL CHOROID
The immunostaining for TSP-1, endostatin, and PEDF was present in the RPE–Bruch’s membrane–CC complex, including the RPE basal lamina, intercapillary septa, and choroidal stroma in each healthy aged control donor subject. Bruch’s membrane had the most prominent TSP-1 (Figure 1B and C, subject 4), endostatin (Figure 2A and B), and PEDF (Figure 3B and C) immunoreactivity. At higher magnification, the basal lamina of RPE cells was intensely labeled for endostatin (Figure 2B) and PEDF (Figure 3C). Strong immunoreactivity for endostatin (Figure 2A and B) and PEDF (Figure 3B and C) was also observed in the CC. Large choroidal blood vessels, intercapillary septa, and the choroidal stroma had moderate labeling for endostatin and PEDF, but TSP-1 labeling was very weak in these structures. The TSP-1 immunoreactivity had a uniform distribution in each aged control donor tissue. However, immunostaining for endostatin and PEDF was not uniform but rather heterogeneous. Staining with CD34 was localized in endothelial cells of the CC and large choroidal vessels (Figure 3A). Hematoxylin and eosin staining showed normal morphological features of the choroid (Figure 1A and Figure 2C).
Figure 1.
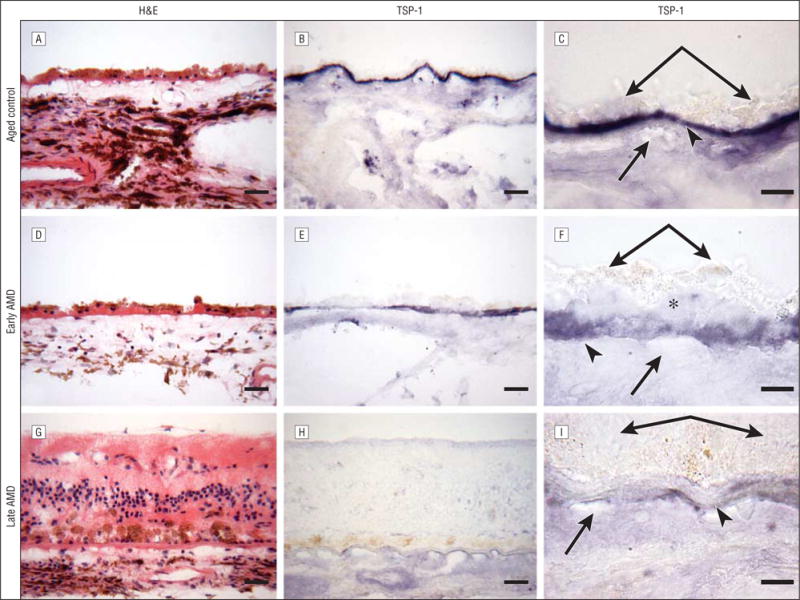
Immunostaining for thrombospondin 1 (TSP-1). Submacular choroid from a healthy aged control eye (subject 4) (A–C), an eye with early age-related macular degeneration (AMD) (subject 10) (D–F), and an eye with late AMD (subject 19) (G–I). A, D, and G, Hematoxylin and eosin (H&E) staining shows morphological features of the choroid. B and C, In the aged control eye, TSP-1 immunoreactivity is intense in Bruch’s membrane. In Bruch’s membrane, TSP-1 immunoreactivity is weaker in the eye with early AMD (E and F) compared with the aged control eye (B and C) and is greatly reduced in the eye with late AMD (H and I) compared with both the aged control eye (B and C) and the eye with early AMD (E and F). C, F, and I are high-magnification photographs of B, E, and H, respectively. Double arrows indicate retinal pigment epithelium; arrowheads, Bruch’s membrane; single arrows, choriocapillaris; and asterisk, basal laminar deposits. Bars indicate 30 μm in A, B, D, E, G, and H and 10 μm in C, F, and I.
Figure 2.
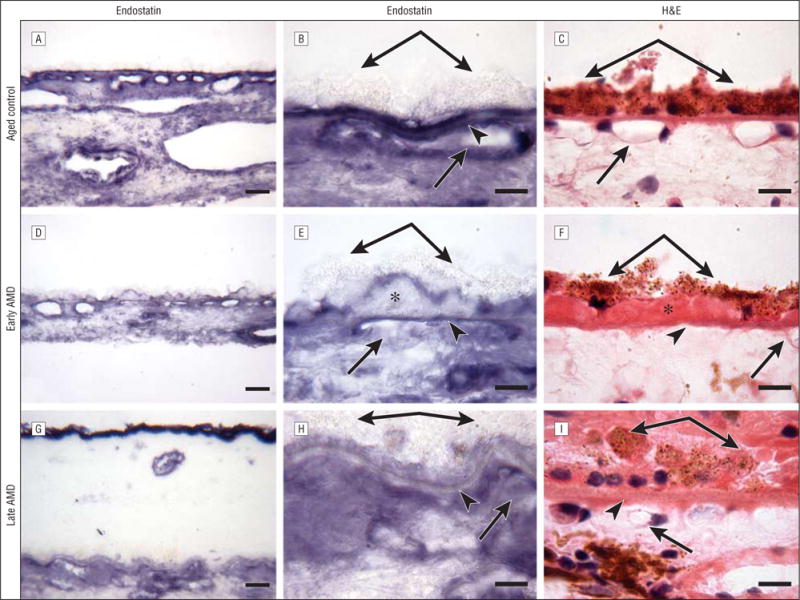
Immunostaining for endostatin. An aged control eye (subject 4) (A–C), an eye with early age-related macular degeneration (AMD) (subject 10) (D–F), and an eye with late AMD (subject 19) (G–I). C, F, and I, Hematoxylin and eosin (H&E) staining demonstrates the morphological changes in the retina and choroid, such as migration of retinal pigment epithelial cells into the retina in late AMD (I). F, Eosin stains the basal laminar deposits intensely pink in the eye with early AMD. A and B, In the aged control eye, endostatin is prominent in the retinal pigment epithelium basal lamina, Bruch’s membrane, and choriocapillaris. The pattern of immunostaining of endostatin appeared similar between the eye with early AMD (D and E) and the aged control eye (A and B), but the endostatin immunoreactivity in Bruch’s membrane is weaker in the eye with early AMD (D and E) compared with the aged control eye (A and B). The expression of endostatin is greatly reduced in Bruch’s membrane in the eye with late AMD (G and H) compared with the aged control eye (A and B), and the reaction product of endostatin appears more diffuse in the choroidal stroma. B, E, and H are high-magnification photographs of A, D, and G, respectively. Double arrows indicate retinal pigment epithelium; arrowheads, Bruch’s membrane; single arrows, choriocapillaris; and asterisks, basal laminar deposits. Bars indicate 30 μm in A, D, and G and 10 μm in B, C, E, F, H, and I.
Figure 3.
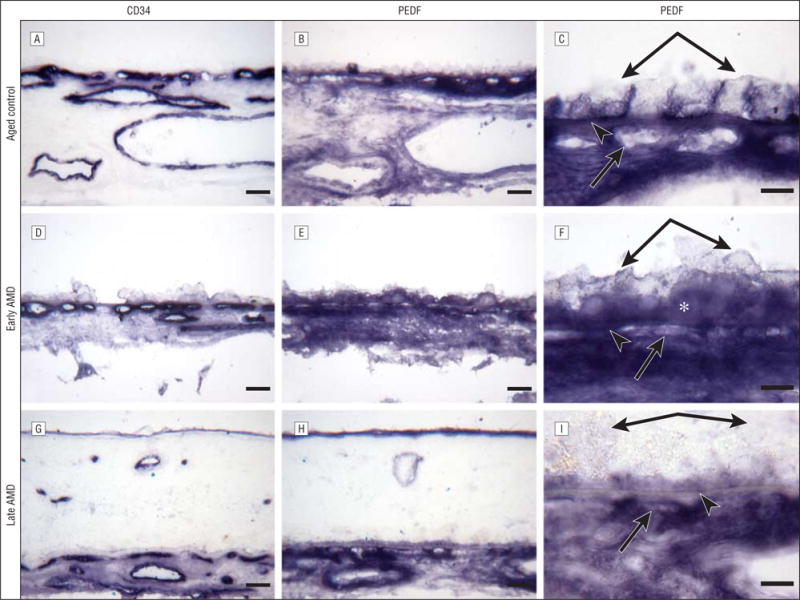
Immunostaining for pigment epithelium–derived factor (PEDF). Frozen sections of choroid from an aged control eye (subject 4) (A–C) and an eye with early age-related macular degeneration (AMD) (subject 10) (D–F) and of a retina and choroid from a subject with late AMD (subject 19) (G–I). A, D, and G, Staining with CD34 is associated with the choroidal blood vessels. The choriocapillaris appears normal by CD34 localization in the aged control eye (A) and the eye with early AMD (D), whereas CD34 localization demonstrates a highly constricted choriocapillaris in some areas with some lumens not positive for CD34, suggesting no viable endothelial cells, in the eye with late AMD (G). B and C, Pigment epithelium–derived factor intensely stains the retinal pigment epithelium basal lamina, Bruch’s membrane, and choriocapillaris in the aged control eye. In the eye with early AMD (E and F), the pattern of immunostaining of PEDF appears similar to that in the aged control eye (B and C), and the reaction product is more diffuse in the choroidal stroma. F, Note that the basal laminar deposits (asterisk) are labeled with PEDF antibody. In the eye with late AMD (H and I), PEDF immunoreactivity is prominent but weaker in Bruch’s membrane compared with that in the aged control eye (B and C) and the eye with early AMD (E and F). C, F, and I are high-magnification photographs of B, E, and H, respectively. Double arrows indicate retinal pigment epithelium; arrowheads, Bruch’s membrane; and single arrows, choriocapillaris. Bars indicate 30 μm in A, B, D, E, G, and H and 10 μm in C, F, and I.
IMMUNOLOCALIZATION OF ANGIOGENESIS INHIBITORS IN THE CHOROID OF EYES WITH AMD
Eyes With Early AMD
Representative examples of several characteristics of eyes with early and late AMD are shown in Figure 1 and Figure 2. Drusen and basal laminar deposits were routinely observed in eyes with AMD (Figure 2F), and the CC lumens appeared constricted and irregular (Figure 3G). In the sections stained with hematoxylin and eosin, the eosin stained the basal laminar deposits intensely pink in eyes with early AMD (Figure 2F). In a representative eye with early AMD (subject 10), weak TSP-1 (Figure 1E and F) and endostatin (Figure 2D and E) expressions were observed in the RPE–Bruch’s membrane–CC complex, whereas the pattern and intensity of PEDF immunostaining appeared comparable to the healthy aged control choroid (Figure 3E and F). The scores for TSP-1, endostatin, and PEDF were significantly lower in Bruch’s membrane in eyes with early AMD compared with aged control eyes (Figure 4). However, the endostatin immunoreactivity score in Bruch’s membrane was not significantly different in eyes with early AMD compared with eyes with late AMD. In the RPE basal lamina, such reduction was observed in endostatin only (Figure 4). In the CC, immunostaining for TSP-1 and endostatin was significantly weaker in eyes with early AMD compared with aged control eyes. Basal laminar deposits were intensely immunoreactive with PEDF (Figure 3E and F). Four of 6 eyes with early AMD had lower scores for all of the 3 inhibitors in Bruch’s membrane than the corresponding mean score of the aged control eyes. In 1-way analysis of variance, comparison of the RPE–Bruch’s membrane–CC complex scores between aged controls, eyes with early AMD, and eyes with late AMD showed a statistically significant difference among the 3 groups in all of the parameters except the RPE basal lamina (Table 2).
Figure 4.
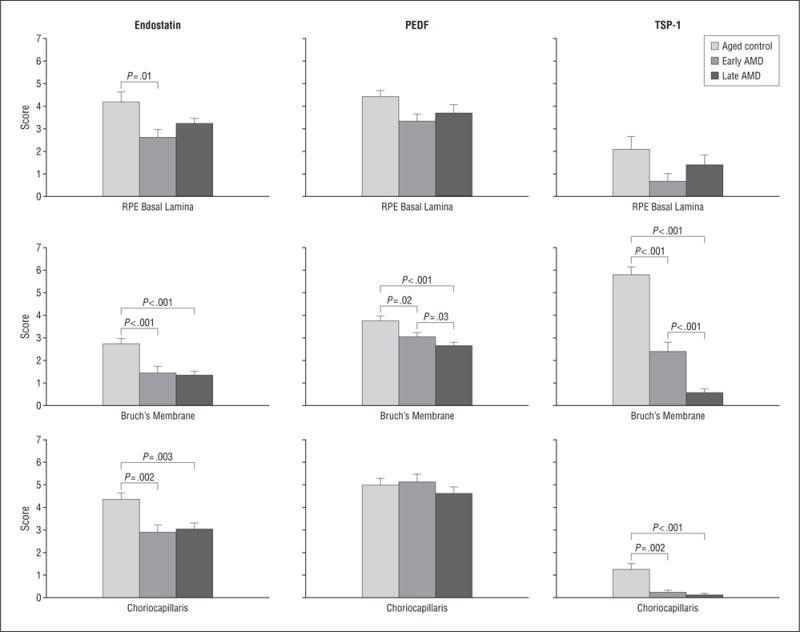
The mean scores of staining with endostatin, pigment epithelium–derived factor (PEDF), and thrombospondin 1 (TSP-1) between aged control eyes, eyes with early age-related macular degeneration (AMD), and eyes with late AMD. The significance of the difference between the groups by t test is indicated. RPE indicates retinal pigment epithelium; error bars, standard deviation.
Table 2.
Comparison of the Retinal Pigment Epithelium–Bruch’s Membrane–Choriocapillaris Complex Parameters in 8 Aged Control Eyes, 6 Eyes With Early Age-Related Macular Degeneration, and 6 Eyes With Late Age-Related Macular Degeneration
| Factor | Parameter | Immunoreactivity Scores, Mean (SD)
|
P Valuea | ||
|---|---|---|---|---|---|
| Aged Control Eyes | Eyes With Early AMD | Eyes With Late AMD | |||
| Endostatin | Bruch’s membrane | 2.750 (1.0) | 1.444 (1.1) | 1.333 (0.7) | .01 |
| RPE basal lamina | 4.188 (2.1) | 2.611 (1.5) | 3.222 (1.0) | .19 | |
| CC | 4.354 (1.4) | 2.889 (1.4) | 3.028 (1.2) | .06 | |
| PEDF | Bruch’s membrane | 3.875 (0.9) | 3.167 (0.8) | 2.556 (0.7) | .01 |
| RPE basal lamina | 4.583 (1.4) | 3.583 (1.3) | 3.556 (1.8) | .36 | |
| CC | 5.354 (1.4) | 5.333 (1.4) | 4.611 (1.1) | .46 | |
| TSP-1 | Bruch’s membrane | 5.792 (1.6) | 2.389 (1.7) | 0.556 (0.7) | < .001 |
| RPE basal lamina | 2.083 (2.7) | 0.667 (1.4) | 1.389 (1.8) | .51 | |
| CC | 1.250 (1.2) | 0.222 (0.4) | 0.111 (0.3) | .02 | |
Abbreviations: AMD, age-related macular degeneration; CC, choriocapillaris; PEDF, pigment epithelium–derived factor; RPE, retinal pigment epithelium; TSP-1, thrombospondin 1.
P values from 1-way analysis of variance.
Eyes With Late AMD
In the example of the choroid in an eye with late AMD (subject 19) (Figure 1), negative to very weak TSP-1 (Figure 1H and I), endostatin (Figure 2G and H), and PEDF (Figure 3H and I) staining was observed in Bruch’s membrane. The immunoreaction for endostatin and PEDF appeared more diffuse in the choroidal stroma (Figure 2H and Figure 3I). Hematoxylin and eosin staining demonstrated the morphological changes in the retina and choroid, such as migration of RPE cells into the retina in late AMD (Figure 1G and Figure 2I). Localization of CD34 demonstrated a highly constricted CC in some areas with some lumens not positive for CD34, suggesting no viable endothelial cells (Figure 3G).
The eyes with late AMD had a significantly lower score for endostatin, PEDF, and TSP-1 in Bruch’s membrane when compared with the healthy aged control eyes (Figure 4). In the CC, a significant reduction was observed in endostatin and TSP-1. In the RPE basal lamina, immunoreactivity for the 3 inhibitors appeared not quite significant in eyes with late AMD compared with aged control eyes. With increasing severity of AMD (late AMD vs early AMD), the scores for PEDF and TSP-1 in Bruch’s membrane significantly declined in the eyes with late AMD compared with the eyes with early AMD. However, the differences in the RPE basal lamina and CC were not significant between the eyes with late and early AMD when the post hoc test (Bonferroni) was applied. Mean immunoreactivity scores for the RPE–Bruch’s membrane–CC complex of eyes with AMD vs aged control eyes are shown in the eFigure (available at http://www.archophthalmol.com).
When we compared the mean scores of the 3 groups (aged control, early AMD, and late AMD) by analysis of variance, all of the 3 inhibitors (endostatin, PEDF, and TSP-1) in all of the 3 groups were statistically significantly different from each other in Bruch’s membrane, whereas endostatin and TSP-1 were significantly lower in the CC (Table 2).
Considering the immunoreactivity scores for the 3 inhibitors in the RPE–Bruch’s membrane–CC complex in the eyes with early and late AMD, 67% (4 of 6) of the eyes with early AMD and 100% (6 of 6) of the eyes with late AMD had lower scores for all of the 3 inhibitors in Bruch’s membrane. Fifty percent (6 of 12) of eyes with early and late AMD had lower scores for all of the 3 inhibitors in the RPE basal lamina and the CC.
COMMENT
In this study, our immunostaining analysis revealed that the levels of 3 potent angiogenic inhibitors (endostatin, PEDF, and TSP-1) were significantly reduced in Bruch’s membrane in eyes with AMD. The 3 molecules are distinctly different from each other in structure, relationship to extracellular matrix, and mechanism of inhibition, yet all are potent inhibitors of angiogenesis. Reduced levels of angiogenic inhibitors may make Bruch’s membrane more vulnerable to the invasion of CNV.
Bruch’s membrane assumes importance in the physiology of the eye by virtue of its strategic location. It is interposed between the metabolically active photoreceptors and RPE and their major source of nutrition and oxygen, the CC. In addition to acting as a support element and an attachment site for the RPE, Bruch’s membrane also provides a semipermeable filtration barrier through which major metabolic exchange takes place and it functions as a physical barrier to the egress of cells and blood vessels from the choroid into the sub-RPE and subretinal spaces. Disruption of or damage to this physical barrier often results in the growth of CNV into the sub-RPE and/or subretinal spaces. The fact that Bruch’s membrane has substantial levels of 3 antiangiogenic factors suggests that it is a biochemical barrier as well. There was a significant decline in the levels of all of the 3 factors in Bruch’s membrane during AMD, and the magnitude of the decline in the levels of PEDF and TSP-1 in Bruch’s membrane paralleled the severity of AMD. The decline with the severity of AMD was not true for the RPE and CC. The difference between the results for these structures may be that PEDF and TSP-1 are matrix bound. With gradual deterioration of Bruch’s membrane in AMD, PEDF and TSP-1 may be released from that matrix into the milieu that includes the RPE and CC.
Various proteins that promote the regression of new vessels, including endostatin, PEDF, TSP-1, and others, are localized in the eye. We have focused on only 3 inhibitors, but in a recent review, Folkman16 discussed the numerous antiangiogenic agents that have been found to date, many of which may be present in the eye. The Angiogenesis Foundation17 currently lists 28 antiangiogenic molecules that are known, and several are being evaluated for treatment of CNV. It is possible that some of these antiangiogenic molecules exist in the RPE–Bruch’s membrane–CC complex as well and prevent CNV invasion, but these molecules have not yet been investigated in this tissue. Viral vector–mediated delivery of antiangiogenic molecules, such as endostatin18 and PEDF,19 has successfully diminished CNV in rodent models.20 Potential clinical applications for PEDF have included systemic administration to prevent ischemia-induced retinopathy in a murine model,21 and a phase 1 clinical trial using a PEDF vector delivered intraocularly to treat macular degeneration is under way.22 Intraocular injection of viral vectors that express PEDF or injection of recombinant PEDF suppresses retinal neovascularization or CNV.23,24
Pigment epithelium–derived factor, a 50-kDa secreted glycoprotein isolated from RPE cells,25 was shown to be a potent inhibitor of angiogenesis.19 It is a multifunctional protein with demonstrable neurotrophic, neuroprotective, gliastatic, antitumorigenic, antiangiogenic, and anti-vasopermeability properties.26,27 It blocks endothelial cells from forming new vessels by inducing apoptosis,21 thus inhibiting neovascularization of certain tumors. Different parts of the PEDF molecule mediate these various activities, and peptides corresponding to these regions retain the corresponding activity of native PEDF.
Thrombospondin 1 is a large (450-kDa) matricellular glycoprotein secreted by many cell types that binds to different matrix proteins and cell-surface receptors.28 This ability accounts for the multifunctional nature and sometimes contradictory functions of TSP-1. Thrombospondin 1 is both a stimulator and an inhibitor of angiogenesis. These contradictory functions demonstrate its ability to maintain a dynamic balance in the regulation of angiogenesis.28 Therefore, the potential role of TSP-1 as a therapeutic agent remains uncertain.29 It inhibits adhesion of vascular endothelial cells30,31 and cell invasion as well as tube formation by vascular endothelial cells.32–34 In human eyes, TSP-1 was localized in the RPE layer and Bruch’s membrane12,35,36 and in epiretinal membranes in several diseases.37 It also plays a role in ocular vascular homeostasis, and its absence contributes to vascular dysfunction associated with diabetes.38 Thrombospondin 1 knockout mice show increased vascular density during retinal vascular development.39
Endostatin, a 20-kDa C-terminal fragment of collagen XVIII, has been identified as an endogenous angiogenesis inhibitor.18 Collagen XVIII is the core protein of a heparan sulfate proteoglycan in vascular and epithelial basement membranes.40 Some proteases, such as matrix metalloproteinases, can cleave a proteinase-sensitive hinge so that endostatin can be released from collagen XVIII and become available.41,42 Marneros et al43 demonstrated that the extracellular matrix component of collagen XVIII or endostatin is essential for maintenance of the RPE. Aged Col18a1−/− mice show massive accumulation of electron-dense amorphous material with membranous debris between the RPE and Bruch’s membrane that is similar in appearance and composition to basal laminar deposits in early AMD44 and contains excess basement membrane material. This suggests that the absence of collagen XVIII or endostatin leads to altered properties of Bruch’s membrane that either cause the RPE to produce excess basement membrane material or interfere with the clearance of such basement membrane material, eventually resulting in a progressive accumulation of basal laminar deposit–like material under the RPE with age. Endostatin has been shown to inhibit the angiogenic activities45,46 and known to up-regulate anti-angiogenesis genes; therefore, it is likely to also be an endogenous inhibitor of CNV.47 Intravitreous or intravenous delivery of endostatin by viral vectors was shown to inhibit diabetic retinopathy and CNV in experimental studies.48,49
Whatever the initial stimulus for CNV formation and invasion, it is clear that angiogenic factors are involved, as CNV membrane and RPE cells have been shown to be immunoreactive for various angiogenic factors. All of the aforementioned antiangiogenic factors (endostatin, PEDF, and TSP-1) seem to have well-defined roles in inhibiting the angiogenic activities; therefore, all of the 3 are likely to be endogenous inhibitors of angiogenesis in the choroid. In this study, expression of 3 potent inhibitors decreased in Bruch’s membrane with severity of AMD. These data support the hypothesis that the balance between angiogenic and antiangiogenic factors may be altered in AMD by declining levels of inhibitors. We recently observed no significant difference in relative vascular endothelial growth factor immunoreactivity levels in the RPE–Bruch’s membrane–CC complex between aged subjects and subjects with AMD,11 suggesting that a decline in antiangiogenic agents and not an increase in angiogenic factors may upset the balance that is normally present. Recent studies also have clarified the importance of a balance between local inhibitory and stimulatory factors in pathological angiogenesis.50 When there is a shift toward more of the positive regulators and/or less of the negative regulators, this likely would favor a proangiogenesis state leading to the progression of pathological disorders associated with excessive angiogenesis. In the case of AMD, the antiangiogenic barrier of Bruch’s membrane is compromised.
CONCLUSIONS
We conclude that the potent endogenous angiogenesis inhibitors are constitutively localized in the RPE–Bruch’s membrane–CC complex and may provide a biochemical barrier to prevent CNV formation and progression. The significantly reduced levels of 3 endogenous angiogenesis inhibitors in Bruch’s membrane of eyes with AMD may make Bruch’s membrane vulnerable to invasion by CNV. These angiogenesis inhibitors may have distinct mechanisms of action or molecular targets, but all are associated with Bruch’s membrane. Combinations of 2 or more angiogenic inhibitors with different molecular mechanisms or targets may achieve synergistic effects on CNV as demonstrated recently for retinal neovascularization.51 Decreased levels of endogenous angiogenesis inhibitors at the Bruch’s membrane–RPE complex in the macular region of eyes with AMD have not only opened a new field for investigation into the pathogenesis of CNV but also revealed a new target for pharmacological interventions.
Acknowledgments
Funding/Support: This work was supported by grants EY-01765 (Wilmer Ophthalmological Institute) and R01-EY016151 (Dr Lutty) from the National Institutes of Health and by grants from Research to Prevent Blindness (Wilmer Ophthalmological Institute), the Foundation Fighting Blindness (Dr Lutty), and the Altsheler Durell Foundation.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: We thank the eye donors and their relatives for their generosity. Janet Sunness, MD, Greater Baltimore Medical Center, and Carol Applegate, Wilmer Ophthalmological Institute, helped in acquiring eyes from subjects with age-related macular degeneration.
Additional Information: The eFigure is available at http://www.archophthalmol.com.
References
- 1.Green WR, Key SN. Senile macular degeneration: a histopathological study. Trans Am Ophthalmol Soc. 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- 2.Spaide RF, Armstrong D, Browne R. Continuing medical education review: choroidal neovascularization in age-related macular degeneration: what is the cause? Retina. 2003;23(5):595–614. doi: 10.1097/00006982-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 4.Ogata N, Wada M, Otsuji T, et al. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43(4):1168–1175. [PubMed] [Google Scholar]
- 5.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston: clinical applications of research on angiogenesis. N Engl J Med. 1995;333(26):1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 7.Chader GJ. PEDF: raising both hopes and questions in controlling angiogenesis. Proc Natl Acad Sci U S A. 2001;98(5):2122–2124. doi: 10.1073/pnas.061024098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talks KL, Harris AL. Current status of anti-angiogenic factors. Br J Haematol. 2000;109(3):477–489. doi: 10.1046/j.1365-2141.2000.01864.x. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 10.Bhutto IA, Kim SY, McLeod DS, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(5):1544–1552. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]
- 11.Bhutto IA, McLeod DS, Hasegawa T, et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82(1):99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uno K, Bhutto IA, McLeod DS, et al. Impaired expression of thrombospondin-1 in eyes with age-related macular degeneration. Br J Ophthalmol. 2006;90(1):48–54. doi: 10.1136/bjo.2005.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutty GA, Merges CA, Threlkeld AB, et al. Heterogeneity in localization of isoforms of TGF-b in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993;34(3):477–487. [PubMed] [Google Scholar]
- 14.McLeod DS, Lefer DJ, Merges CA, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995;147(3):642–653. [PMC free article] [PubMed] [Google Scholar]
- 15.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141(3):673–683. [PMC free article] [PubMed] [Google Scholar]
- 16.Folkman J. Angiogenesis: organizing principle for drug discovery. Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 17.Angiogenesis Foundation. About angiogenesis: understanding angiogenesis. http://www.angio.org/understanding/understanding.html. Accessed May 2007.
- 18.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 19.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285(5425):245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 20.Reich SJ, Bennett J. Gene therapy for ocular neovascularization: a cure in sight. Curr Opin Genet Dev. 2003;13(3):317–322. doi: 10.1016/s0959-437x(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 21.Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular anti-angiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci U S A. 2001;98(5):2593–2597. doi: 10.1073/pnas.031252398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen H, Chu KW, Campochiaro P, et al. Clinical protocol: an open-label, phase I, single administration, dose-escalation study of ADGVPEDF.11D (ADPEDF) in neovascular age-related macular degeneration (AMD) Hum Gene Ther. 2001;12(16):2029–2032. [PubMed] [Google Scholar]
- 23.Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43(3):821–829. [PubMed] [Google Scholar]
- 24.Mori K, Gehlbach P, Ando A, et al. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002;43(7):2428–2434. [PubMed] [Google Scholar]
- 25.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53(3):411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 26.Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol Med. 2002;8(7):330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 27.Becerra SP. Focus on Molecules: pigment epithelium-derived factor (PEDF) Exp Eye Res. 2006;82(5):739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Esemuede N, Lee T, Pierre-Paul D, et al. The role of thrombospondin-1 in human disease. J Surg Res. 2004;122(1):135–142. doi: 10.1016/j.jss.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Ambati J, Ambati BK, Yoo SH, et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 30.Lahav J. Thrombospondin inhibits adhesion of endothelial cells. Exp Cell Res. 1988;177(1):199–204. doi: 10.1016/0014-4827(88)90037-7. [DOI] [PubMed] [Google Scholar]
- 31.Vogel T, Guo NH, Krutzsch HC, et al. Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type I repeats of thrombospondin. J Cell Biochem. 1993;53(1):74–84. doi: 10.1002/jcb.240530109. [DOI] [PubMed] [Google Scholar]
- 32.Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111(2):765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolsma SS, Volpert OV, Good DJ, et al. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122(2):497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell SC, Volpert OV, Ivanovich M, Bouck NP. Molecular mediators of angiogenesis in bladder cancer. Cancer Res. 1998;58(6):1298–1304. [PubMed] [Google Scholar]
- 35.He S, Incardona F, Jin M, et al. Thrombospondin-1 expression in RPE and choroidal neovascular membranes. Yan Ke Xue Bao. 2006;22(4):265–274. [PubMed] [Google Scholar]
- 36.Miyajima-Uchida H, Hayashi H, Beppu R, et al. Production and accumulation of thrombospondin-1 in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(2):561–567. [PubMed] [Google Scholar]
- 37.Hiscott P, Larkin G, Robey HL, et al. Thrombospondin as a component of the extracellular matrix of epiretinal membranes: comparisons with cellular fibronectin. Eye. 1992;6(pt 6):566–569. doi: 10.1038/eye.1992.123. [DOI] [PubMed] [Google Scholar]
- 38.Sheibani N, Sorenson CM, Cornelius LA, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemia. Biochem Biophys Res Commun. 2000;267(1):257–261. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Wu Z, Sorenson CM, et al. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228(4):630–642. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 40.Zatterstrom UK, Felbor U, Fukai N, Olsen BR. Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct Funct. 2000;25(2):97–101. doi: 10.1247/csf.25.97. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki T, Fukai N, Mann K, et al. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998;17(15):4249–4256. doi: 10.1093/emboj/17.15.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreras M, Felbor U, Lenhard T, et al. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486(3):247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 43.Marneros AG, Keene DR, Hansen U, et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004;23(1):89–99. doi: 10.1038/sj.emboj.7600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Schaft TL, Mooy CM, de Bruijn WC, Bosman FT, de Jong PT. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232(1):40–46. doi: 10.1007/BF00176436. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi N, Anand-Apte B, Lee M, et al. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J. 1999;18(16):4414–4423. doi: 10.1093/emboj/18.16.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eriksson K, Magnusson P, Dixelius J, et al. Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett. 2003;536(1–3):19–24. doi: 10.1016/s0014-5793(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 47.Abdollahi A, Hahnfeldt P, Maercker C, et al. Endostatin’s anti-angiogenic signaling network. Mol Cell. 2004;13(5):649–663. doi: 10.1016/s1097-2765(04)00102-9. [DOI] [PubMed] [Google Scholar]
- 48.Mori K, Ando A, Gehlbach P, et al. Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol. 2001;159(1):313–320. doi: 10.1016/S0002-9440(10)61697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auricchio A, Behling KC, Maguire AM, et al. Inhibition of retinal neovascularization by intraocular viral-mediated delivery of anti-angiogenic agents. Mol Ther. 2002;6(4):490–494. doi: 10.1006/mthe.2002.0702. [DOI] [PubMed] [Google Scholar]
- 50.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21(7):1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 51.Kramerov AA, Saghizadeh M, Pan H, et al. Expression of protein kinase CK2 in astroglial cells of normal and neovascularized retina. Am J Pathol. 2006;168(5):1722–1736. doi: 10.2353/ajpath.2006.050533. [DOI] [PMC free article] [PubMed] [Google Scholar]


