Abstract
Purpose
Prior investigation has demonstrated that angioblasts are present in the inner retinas of human embryos and fetuses and that they differentiate and organize to form the primordial retinal vasculature. The purpose of this study was to characterize these angioblasts further and examine ligands that might control their migration and differentiation.
Methods
Immunohistochemistry was used to localize stroma-derived factor-1 (SDF-1), its receptor CXCR4, stem cell factor (SCF), and its receptor c-Kit on sections obtained from human eyes at from 6 to 23 weeks’ gestation (WG). Coexpression of CD39 (marker for retinal angioblasts and endothelial cells) and CXCR4 or c-Kit was investigated by confocal microscopy.
Results
SDF-1 was prominent in inner retina with the greatest reaction product near the internal limiting membrane (ILM). SCF immunoreactivity was also confined to the inner retina and increased significantly between 7 and 12 WG. The level of both ligands declined by 22 WG. A layer of CXCR4+ and c-Kit+ precursors, some of which coexpressed CD39, existed in the inner retina from 7 to 12 WG. With migration, c-Kit was downregulated, whereas CD39+ cells continued to express CXCR4 as they formed cords. With canalization, CXCR4 expression was downregulated.
Conclusions
Embryonic human retina has a pool of precursors (CXCR4+ and c-Kit+) that enlarged centrifugally during fetal development. From this pool emerges angioblasts, which migrate anteriorly into the nerve fiber layer where SDF-1 and SCF levels are highest. c-Kit expression declines with apparent migration, and CXCR4 expression declines with canalization of new vessels. Both SCF and SDF-1 are associated with the differentiation of retinal precursors into angioblasts and their migration to sites of vessel assembly.
The fetal human retina remains avascular until 14 weeks’ gestation (WG). Recent studies have demonstrated that the initial human retinal vasculature develops by vasculogenesis, the in situ differentiation and assembly of blood vessels from vascular precursors. In dogs1 and humans,2–4 the precursors that participate have been found to express CD39 (ecto-ADPase). This ectoenzyme is found on endothelial cells (ECs) in all vascular beds and is responsible for controlling the extracellular level of ADP, thus limiting platelet aggregation. Retinal angioblasts also express VEGFR-2; however, they lack other markers commonly used for ECs and hematopoietic precursors such as CD34 and CD31.4 The exact origin of retinal angioblasts and the cues and stimuli for recruitment, differentiation, and organization of these cells into developing blood vessels are unknown.
One possible stimuli for homing of vascular precursors to retina may be stroma-derived factor (SDF)-1, which stimulates homing of endothelial precursor cells (EPCs) to sites of injury and angiogenesis.5–8 SDF-1 is a CXC chemokine that stimulates chemotaxis but not proliferation of neuronal precursors and CD34+ bone marrow cells.9,10 SDF-1 also stimulates the migration and tube formation by ECs of many origins in vitro.11–13 In vivo, SDF-1 plays a central role in hematopoietic stem and progenitor cell mobilization from marrow and stem cell homing to bone marrow.14
Unlike other chemokines that can bind to several G-protein-coupled, seven-transmembrane–spanning receptors, SDF-1 binds to only one receptor, CXCR4. CXCR4 is expressed by retinal angioblasts in dogs15 and humans4 by embryonic stem cells,16 and by hematopoietic progenitors.17 Mice deficient in CXCR4 die perinatally of vascular defects.13,18 In a prior study, we found that CXCR4 was associated with CD39+ angioblasts in embryonic and fetal human retina, but expression was downregulated in ECs.4 CXCR4 is also expressed on adult ECs from several tissues in vitro10,15,19,20 and intestinal mucosal ECs in vivo and in vitro.13 It is important to note that intestinal mucosal ECs are considered physiologically inflamed and not quiescent. Intestinal ECs make SDF-1 and CXCR4 and migrate, proliferate, and form tubes in response to SDF-1.13 Stem cell factor (SCF), basic fibroblast growth factor (FGF2), and vascular endothelial cell growth factor (VEGF) regulate CXCR4 and SDF-1 expression, and so autocrine expression of these growth factors can affect the number and the cycling of CXCR4 receptors by ECs and hemangioblasts.12
Another factor affecting precursor cells is SCF, also called steel factor and Kit ligand. SCF increases cell surface expression of CXCR4 in hematopoietic (CD34+) stem cells (HSCs).14 It is a pluripotent growth factor involved in early stages of hematopoiesis and in the development and function of germ cells, melanocytes, and mast cells.21 SCF is the ligand for c-Kit receptor tyrosine kinase (CD117) or c-Kit proto-oncogene product, which is a marker for progenitor cells in yolk sac,22 neural stem cells in ciliary epithelium, hematopoietic precursors in adult marrow,23 and progenitor cells in mouse retina.24 c-Kit is also expressed by many types of ECs.25 SCF supports the survival, migration and tube formation by human umbilical vein endothelial cells (HUVECS).26 HUVECs also produce SCF, and so it may serve as an autocrine growth factor for this type of EC.27 Cells of bone marrow origin that express c-Kit and von Willebrand’s factor (vWf) contribute to new vessel formation in the diabetic rat mesentery.28
The intent of this study was to determine whether CXCR4 and c-Kit are expressed by retinal angioblasts that are CD39+. The association of these receptors with their ligands, SDF-1 and SCF, respectively, was also investigated to perhaps implicate these ligands in attracting angioblasts to inner retina where they will differentiate and develop a retinal vasculature.
Methods
Age Determination and Preparation of Tissue
Eighteen fetal human eyes from 18 fetuses, ranging in age from 6 to 23 WG, were used in the study (Table 1). Tissues were provided by Advanced Bioscience Resources, Inc. (Alameda, CA) after aspiration abortions, in accordance with the guidelines set forth in the Declaration of Helsinki, with the approval by the Joint Commission for Clinical Research at the Johns Hopkins University School of Medicine. The age of each fetus was determined by using the last menstruation date and/or ultrasonography and fetal foot length as a reliable indicator of gestational age.29 After enucleation, the eyes were immediately fixed at room temperature for 1 hour with 2% paraformaldehyde in 0.1 M sodium phosphate buffer pH 7.2 with 5% sucrose. The eyes then were washed in 0.1 M sodium phosphate buffer with 5% sucrose and were shipped overnight at 4°C. For older (> 12 WG) cryopreserved eyes, the anterior segments were removed on receipt, and the eyecups or whole eyes of young fetuses were washed (30 minutes/wash) at room temperature in 0.1 M sodium phosphate buffer with increasing concentrations of sucrose: a 2:1, a 1:1, and a 1:2 mixture of 5% sucrose-20% sucrose and then for 2 hours in 20% sucrose.30 The eyecups were dissected to make cryoblocks of full-thickness eye wall. For 9 WG and younger eyes, the globes were processed and frozen in toto. The tissue was placed in flat embedding molds with an embedding solution consisting of a 2:1 mixture of 20% sucrose in 0.1 M sodium phosphate buffer/OCT compound (Tissue-Tek; Baxter Scientific, Columbia, MD) and infiltrated for 30 minutes at room temperature. The quadrants then were frozen in isopentane cooled with dry ice. Cross-sections 8 μm thick were cut on a cryostat (Frigocut N; Reichert Jung, Deerfield, IL) at −25°C.
Table 1.
Age and Preparation of Ocular Tissue
| Age (WG) | Cryo* | Wholemount† |
|---|---|---|
| 6 | 1 | |
| 6.5 | 1 | |
| 7 | 2 | |
| 7.5 | 2 | 1 (CD39/CXCR4) |
| 8 | 2 (CD39/CXCR4) | |
| 8.5 | 2 | 1 (CD39/CXCR4) |
| 9 | 2 | |
| 9.5 | 1 (CD39/CXCR4) | |
| 12 | 2 | 1 (CD39/c-Kit) |
| 14 | 1 | |
| 16 | 1 | |
| 20 | 2 | 1 (CD39/c-Kit) |
| 22 | 1 | 1 (CD39/CXCR4) |
| 23 | 1 |
Number of cryopreserved eyes.
Number of eyes used for retinal wholemounts.
Alkaline Phosphatase Immunohistochemistry
Streptavidin alkaline phosphatase (APase) immunohistochemistry was performed on sections of cryopreserved tissue using a previously published nitro blue tetrazolium (NBT) system.31 The sections were incubated overnight at 4°C with one of the following primary antibodies: mouse anti-CD39 (1:400; Chemicon, Temecula, CA); mouse anti-CD31 (1:1000; Dako, Carpinteria, CA); goat anti-SDF-1 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-CXCR4 (1:5000; Novus, Littleton, CO); rabbit anti-SCF (1:100; Assay Designs, Ann Arbor, MI); and rabbit anti-c-Kit (1:2000; Laboratory Vision, Fremont, CA). After they were washed in Tris-buffered saline (TBS), the sections were incubated for 30 minutes at room temperature with the appropriate biotinylated secondary antibodies diluted 1:500 (Kirkegaard and Perry, Gaithersburg, MD). Finally, the sections were incubated with streptavidin APase (1:500; Kirkegaard and Perry) and the APase activity was developed with a 5-bromo-4-chloro-3-indoyl phosphate (BCIP)-NBT kit (Vector Laboratories, Inc., Burlingame, CA), yielding a blue immunoreaction product. Melanin in RPE and choroidal melanocytes was bleached as previously reported31 and coverslips mounted with Kaiser’s mounting medium without counterstaining.
All slides from each eye were cut as serial sections in one sitting, to standardize sectioning and section thickness. All slides with sections were boxed with desiccant and stored at −80°C, until all eyes for the study were cut. Then slides from all eyes were incubated with the same antibodies and reagents on the same days. Thus, all comparisons of relative reaction product described herein were made between eyes handled identically and incubated together identically for each antibody on the same day.
Double Labeling
Double-label immunofluorescence was performed on cryopreserved tissue sections as previously reported.4 The sections were incubated for 2 hours at room temperature by combining two of the following primary antibodies: mouse anti-CD39 (1:200)/rabbit anti-CXCR4 (1:1000); or mouse anti-CD39 (1:200)/rabbit anti-cKit (1: 200). After they were washed in TBS, the sections were incubated for 30 minutes at room temperature with the goat anti-mouse secondary antibodies diluted 1:500 conjugated with Cy3 and the goat anti-rabbit antibody diluted 1:100 conjugated with FITC. Finally, the sections were mounted in DAPI counterstaining medium (4′,6′-diamino-2-phenylindole; Vector Laboratories, Inc.) and observed with a confocal microscope (model 510 META; Carl Zeiss MicroImaging, Inc., Thornwood, NY) and the spectra of fluorescence were analyzed.
Wholemounts
The tissue was fixed for 1 hour with 2% paraformaldehyde in TBS and then shipped overnight at 4°C in TBS. The anterior segments were removed from the eyes and the retinas separated from RPE. The retinas were incubated with mouse anti-CD39 (1:50) and either rabbit anti-CXCR4 (1:250) or rabbit anti-c-Kit (1:50) for 72 hours at 4°C. After they were washed in TBS, the retinas were incubated with secondary antibodies for 48 hours at 4°C: goat anti-mouse secondary antibodies diluted 1:100 conjugated with Cy3 (Jackson ImmunoResearch, West Grove, PA) and goat-anti rabbit secondary antibodies diluted 1:50 conjugated with FITC (Jackson ImmunoResearch). Immunofluorescence in flatmounts was then imaged with a confocal microscope (510 META; Carl Zeiss MicroImaging) at 488 and 532 nm excitation (FITC and Cy3 staining, respectively).
Confocal Microscopy
Tissue sections with fluorescent secondary antibodies and DAPI counterstain were examined with the confocal microscope. Excitation wavelengths included 405, 488, and 532 nm for the DAPI, FITC, and Cy3, respectively. Multispectral confocal microscopy was used as a validation tool for intracellular localization of antigens. Since multiple fluorescent colors were used to label various antigens and nuclei, it was necessary to use this multispectral confocal microscope that could separate the spectral overlap to determine true colocalization as demonstrated previously.32
Results
Gestation of 6–7 Weeks
The hyaloid vasculature formed in the vitreous, while the retina was avascular in the 6- to 7-WG embryonic human eye (Fig. 1A). The retina had an inner and outer neuroblastic layer and single cells in the nerve fiber layer (NFL; Fig. 1B). SDF-1 immunoreactivity was prominent in the developing lens, present diffusely throughout the stroma of the choroid and sclera, and present in the developing choriocapillaris and embryonic hyaloid vasculature (Fig. 1C). There was a gradient of SDF-1 in the central inner retina, with the greatest level adjacent to the internal limiting membrane (ILM; Fig. 1C). Central retina is defined in the following observations as extending from adjacent to the optic nerve (peripapillary region) to the anatomic equator. Cells expressing CXCR4 formed a layer in the inner retina that mirrored the area with SDF-1 localization except slightly posterior to the greatest concentration of SDF-1 (Fig. 1D, arrow)—that is, the most prominent localization of SDF-1 was adjacent to the layer of CXCR4-expressing cells (compare Figs. 1C, 1D). At 7 WG, the layer of CXCR4+ precursors tapered to a single row of cells toward but not reaching the ora serrata. CXCR4+ cells that appeared to be migrating toward the ILM in the central retina (greatest SDF-1) also expressed CD39 (Figs. 2A–D). Although CD39+ cells were present randomly throughout the inner retina in the flatmounts, the CD39+/CXCR4+ cells appeared fusiform and aligned with nerve fibers near the optic nerve head (Figs. 2E, 2F).
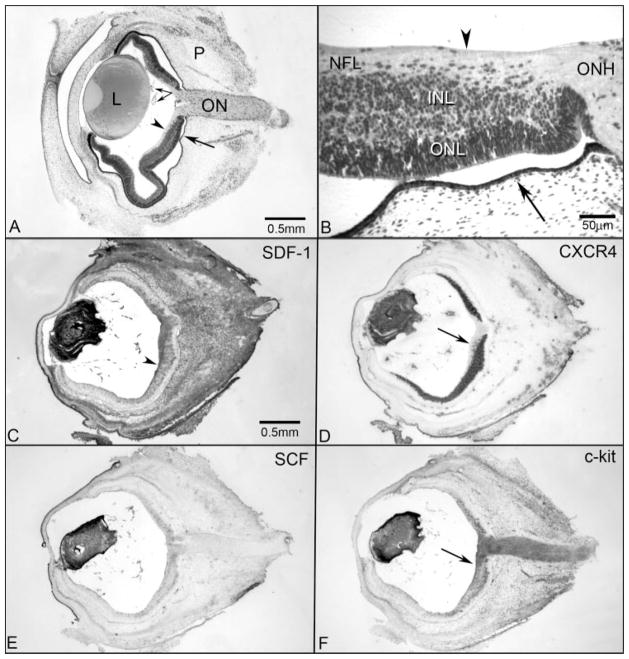
Figure 1.
Cross sections of 7-WG whole embryonic human eyes from a plastic-embedded eye (A, B) and from immunolabeled cryosections (C–F). (A) The transient fetal vasculature of the eye (paired arrows) was present in the vitreous and on the posterior surface of the lens (L). In this section, the retina was artifactually separated from the retinal pigment epithelium and choriocapillaris (arrow) except at the optic nerve (ON). The periocular mesenchyme (P) comprised the outer layer of the embryonic eye (retinal ILM; arrowhead). (B) Higher magnification of an adjacent plastic section showing the ILM (arrowhead), the NFL, the inner neuroblastic layer (INL), the outer neuroblastic layer (ONL), and the optic nerve head (ONH). Fusiform cells appeared to be migrating from the INL into the NFL in this region. (C) SDF-1 immunolabeling was intense in the lens, fetal vasculature, and a gradient of staining in the NFL (arrowhead) and INL of the retina was present that decreased toward the periphery. SDF-1 immunoreactivity was also intense in the periocular mesenchyme. (D) Adjacent cryosection immunolabeled with anti-CXCR4 demonstrated immunoreactivity in the lens and fetal vasculature and intense staining of cells in the INL. No cells in the ONL were labeled. Note that the fusiform cells that appeared to be migrating in the NFL were CXCR4+ (arrow). (E) SCF immunolabeling was intense in the lens and fetal vasculature and weaker and diffuse in the NFL and INL. No staining was observed in the ONL or ON. (F) c-Kit labeling in an adjacent cryosection showed immunoreactivity in the lens, the fetal vasculature, and the NFL and cells within the INL. This pattern appeared to mirror the staining of SCF in the retina. c-Kit was most prominent in the innermost retina, adjacent to the ILM (arrow), an area where most cells were CXCR4− (D, arrow). c-Kit was also expressed by cells in the ON. (A, B) hematoxylin and eosin; (C–F) APase/NBT blue reaction product after bleaching. Scale bar: (C) applies to (D–F).
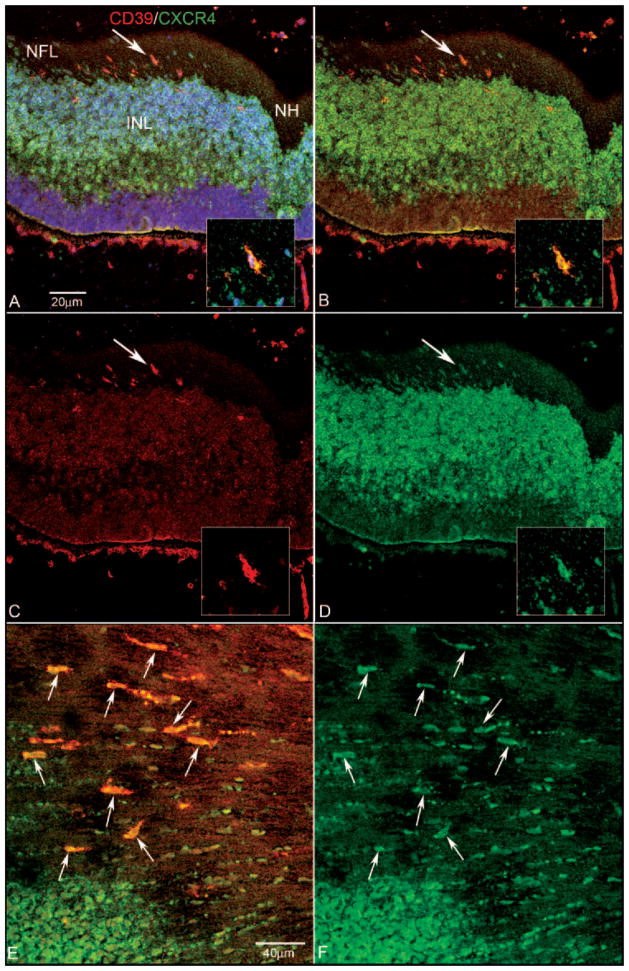
Figure 2.
Expression of CD39 and CXCR4 in retinal cross sections (A–D) and a retinal wholemount (E, F) at 7 WG. Blue: DAPI-counterstained nuclei; red: CD39; green: CXCR4. (A) Near the nerve head (NH), fusiform cells in the NFL were CXCR4+/CD39+ (A–D, arrow). Insets: higher magnification of double-labeled cell indicated by the arrow in (A–D). (B) The same section without the DAPI channel showing CXCR4-immunostained cells occupying the entire inner neuroblastic layer (INL) and some double-labeled cells in the NFL. A few double-labeled cells were near the inner portion of the INL. (C) Same section showing only the CD39 channel. (D) Same section showing only the CXCR4 channel. (E) Retinal wholemount showing fusiform cells (arrows) near the nerve head (to the right and out of the field) that expressed both CD39 and CXCR4. (F) Same region as (E) with only the CXCR4 channel shown.
SCF was prominent in corneal epithelium and in developing lens (Fig. 1E). The embryonic vasculature in vitreous and the inner retina also had SCF immunoreactivity. c-Kit-expressing cells in the retina formed a layer in the central inner retina similar in position to CXCR4-expressing cells except that the most prominent c-Kit+ cells were adjacent to the ILM in the central retina (most of these cells appeared to be CXCR4−; Figs. 1D, 1F, arrow) and at the posterior boundary of the precursor layer. A very notable difference between c-Kit and CXCR4 was that the entire optic nerve was positive for c-Kit but negative for CXCR4 (Figs. 1D, 1F). Some cells in the c-Kit+ layer also expressed CD39; however, the c-Kit in those cells was less than in adjacent c-Kit+/CD39− cells (Figs. 3A–D). Approximately half of the CD39+ cells in the precursor layer expressed c-Kit. Some fusiform CD39+ cells in the innermost central retina also had low levels of c-Kit. Although it is technically difficult to double-label sections for c-Kit and CXCR4 (both antibodies were raised in rabbit), it appeared from DAPI-counterstaining of the nuclei that almost all cells in the precursor pool expressed both receptors at some level (Figs. 2, 3). The only exception was cells immediately adjacent to ILM in the peripapillary retina (Figs. 1D, 1F, arrows).
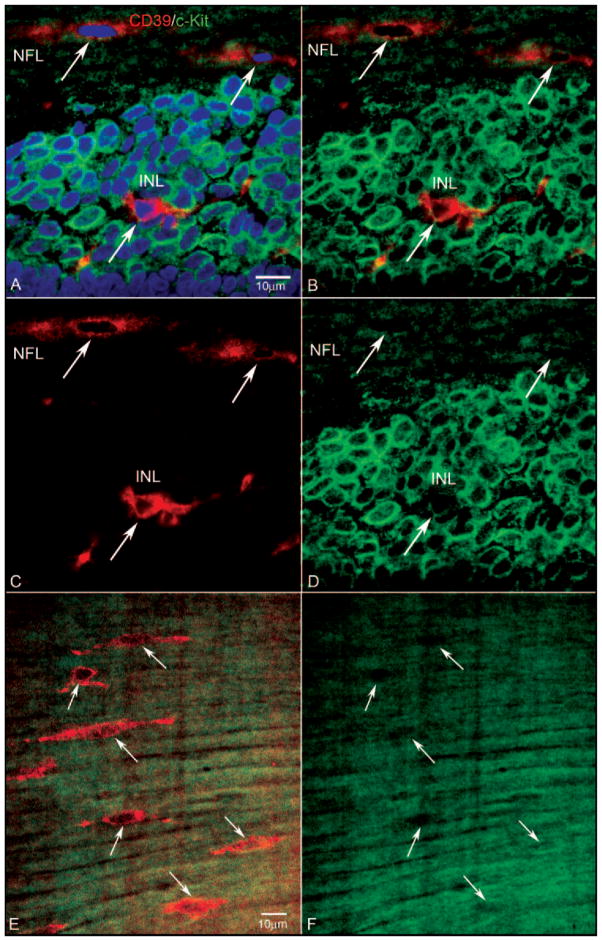
Figure 3.
Expression of CD39 and c-Kit in a retinal cross-section (A–D) and a retinal wholemount (E, F) from a 7-WG embryo. Blue: DAPI-counterstained nuclei; red: CD39; green: c-Kit. (A) Near the nerve head, fusiform cells in the NFL were CD39+/c-Kit−, whereas some cells in the inner neuroblastic layer (INL), were CD39+/c-Kit+ (A–D, arrow in INL). (B) Same section without the DAPI channel, showing that c-Kit+ cells occupied the entire region along with some double-labeled cells. The fusiform cells in the NFL appeared to be c-Kit−. (C) The same section showing only the CD39 channel. The CD39+ cell (arrow) in the INL had processes, suggesting it was migrating. (D) Same section showing only the c-Kit channel. The CD39+ cell in the INL appeared to be less c-Kit+ than did the c-Kit+/CD39− neighboring cells. (E) Retinal wholemount showing CD39+ fusiform cells near the nerve head (arrows) aligned with very linear cells expressing c-Kit. (F) Same region as shown in (E), with only the c-Kit channel showing that the cell expressing CD39 were negative for c-Kit. Scale bars: (A) applies to (B–D); (E) applies to (F).
Gestation of 12 Weeks
SDF-1 immunoreactivity was present in inner retina from disc to ora serrata at 12 WG, and the gradient of SDF-1 was still greatest at the ILM and reduced toward mid retina (Figs. 4A, 4C, 4E). The overall intensity was greater than at 7 WG, but at 12 WG, it was prominent from optic nerve head to ora serrata, which was one of the most intense areas in inner retina (Figs. 4A, 4C). CXCR4-expressing cells were present in the inner retina from the ILM in the central to mid retina but had not yet reached the ora serrata (Figs. 4B, 4D). In the peripheral retina, the greatest SDF-1 expression was toward the ora serrata where CXCR4+ cells were not yet present (Figs. 4A–D).
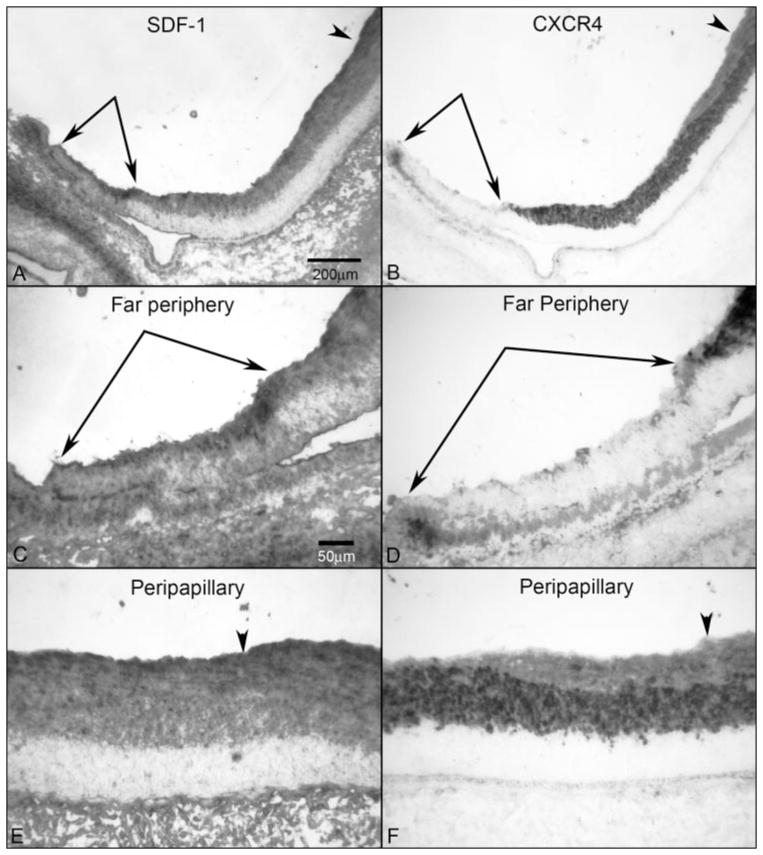
Figure 4.
SDF-1/CXCR4 axis in a 12-WG fetal eye. Low-magnification micrographs showing SDF-1 (A) and CXCR4 (B) from the peripapillary region adjacent to the optic nerve head (arrowhead) to the far periphery (paired arrows). In the far periphery, SDF-1 was more intense in the region where CXCR4+ precursors had not yet migrated (paired arrows). (C, D) Higher magnification of the peripheral retina showing more intense SDF-1 in advance of (paired arrows) CXCR4+ precursors. (E, F) Higher magnification of the peripapillary region showing less intense SDF-1 immunoreactivity than in the far periphery. CXCR4 immunoreactivity was present throughout the NFL at this age but was less intense than in the INL. Arrowhead: ILM. Stain: APase/NBT reaction product after bleaching. Scale bar: (A) applies to (B); (C) applies to (D–F).
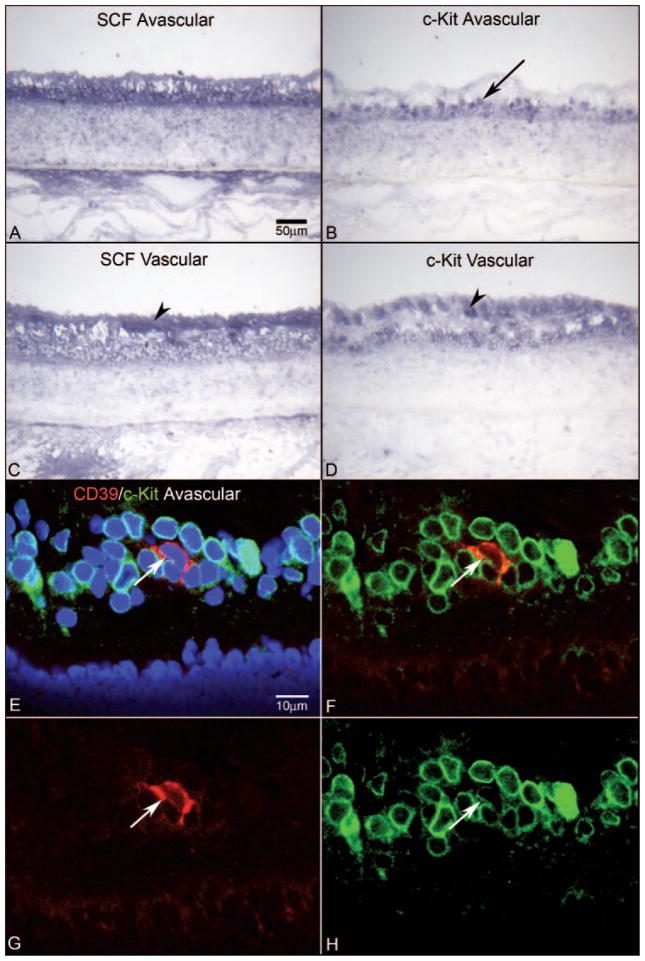
SCF levels were greatly increased at 12 WG compared with those at 7 WG. SCF was present in the inner retina from the optic nerve to the ora serrata, with the greatest intensity near the ILM (Figs. 5A, 5C). c-Kit expressing cells mirrored exactly the areas where the ligand SCF was present (Figs. 5B, 5D). It was intriguing that at the ora serrata the c-Kit+ layer of cells was in the outer retina and not adjacent to the ILM (Fig. 6A). Round c-Kit+ cells in the layer of precursors also expressed CD39 (Fig. 6). Some CD39+ cells that appeared to be migrating inward also expressed low levels of c-Kit. c-Kit/CD39 coexpression was greatest in the precursor layer and c-Kit expression diminished in CD39+ cells as they appeared to migrate inward toward the NFL (Figs. 6C–F).
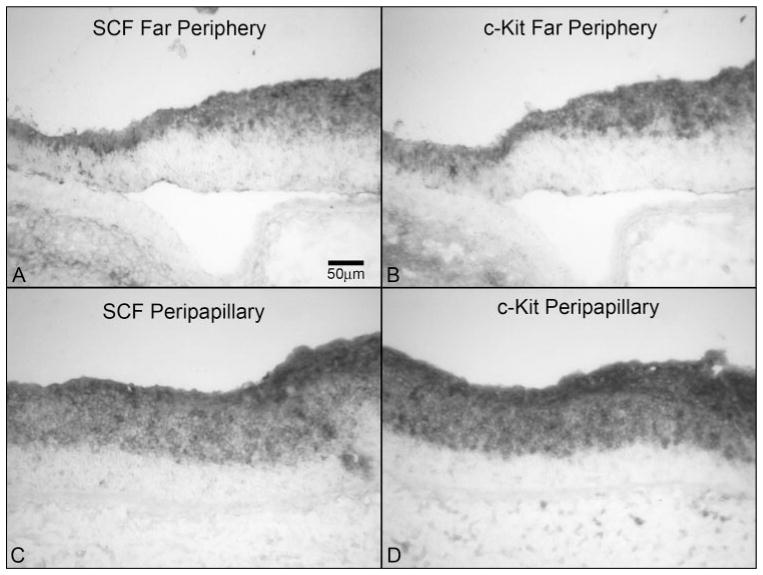
Figure 5.
SCF and c-Kit in a 12-WG fetal eye. (A, B) Comparison of SCF and c-Kit in the far periphery showing an almost identical pattern of immunoreactivity in the inner retina. (C, D) Peripapillary region from the same section showing an almost identical pattern and intensity of immunoreactivity for SCF and c-Kit, with no obvious difference, as seen in the far periphery. Stain: APase/NBT reaction product. Scale bar: (A) applies to all panels.
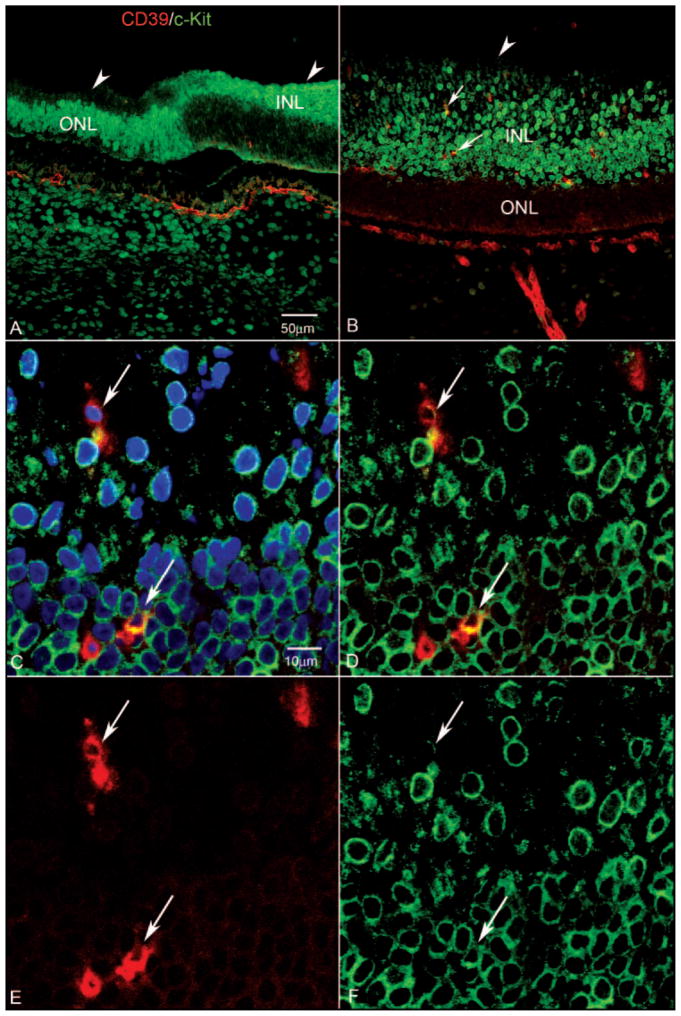
Figure 6.
Expression of CD39 and c-Kit in retinal cross section of a 12-WG fetal eye at low (A, B) and high (C–F) magnifications. Blue: DAPI-counterstained nuclei; red: CD39; green: c-Kit. (A) In the far periphery in this region of retina, c-Kit-expressing cells were present in the inner neuroblastic layer (INL) posteriorly and in the outer neuroblastic layer (ONL) more peripherally. Arrowheads: ILM. Very few CD39+ cells were evident in this region at this age. (B) Near nerve head, c-Kit+ cells occupied the entire extent of the INL. Some of these cells expressed CD39 (arrows). Arrowhead: ILM. (C) Higher magnification of the same cells indicated with the arrows in (B) showing that they were CD39+/c-Kit+ (arrows). (C–F) Arrows: same cells. (D) Same section without the blue DAPI channel, showing c-Kit-immunostained cells occupying the entire INL and some double-labeled cells in this region. (E) Same section showing only the CD39 channel. (F) Same section showing only the c-Kit channel. The CD39+ cell closest to the NFL (top arrow) appeared to have less c-Kit than did the deeper lying CD39+ cell. Scale bars: (A) applies to (B); (C) applies to (D–F).
Gestation of 14–16 Weeks
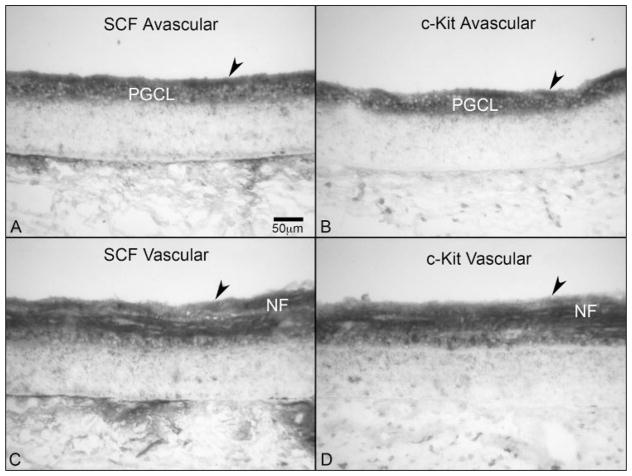
The retinal vasculature begins to form around 14 WG in the human4 and ECs in newly formed blood vessels express CD31 and CD39 (Figs. 7B, 7D). The 14 and 16 WG retinas were very similar in terms of location of the precursors and localization of the antibodies, so only the 16 WG is shown. In vascularized areas of retina at 16 WG, SDF-1 levels were greatly reduced compared with 12 WG, but it was still very prominent in the innermost region of the avascular retina (Figs. 7G, 7H). The layer of CXCR4+ cells was greatly reduced in size in central vascularized retina but a row of cells, perhaps ganglion cells, was present posterior to the forming retinal vasculature (Fig. 7F). Formed retinal blood vessels were negative for CXCR4 as shown in Figure 7F (arrowhead). In avascular areas (no CD31+ structures); however, there was still a substantial layer of precursors and individual CD39+ cells were still present (Figs. 7C, 7E). At the edge of the vasculature, CXCR4+/CD39+ cells were present in inner retina where they aggregate to form cords, anterior to the row of prominently labeled CXCR4+ cells (Fig. 8), but CXCR4 expression was absent in more mature blood vessels. Unlike SDF-1, SCF immunoreactivity was very prominent in the vascularized and avascular portions of the innermost retina at 16 WG (Fig. 9). Likewise, c-Kit-expressing cells were prominent in the same area, mirroring the localization of its ligand SCF (Fig. 9).
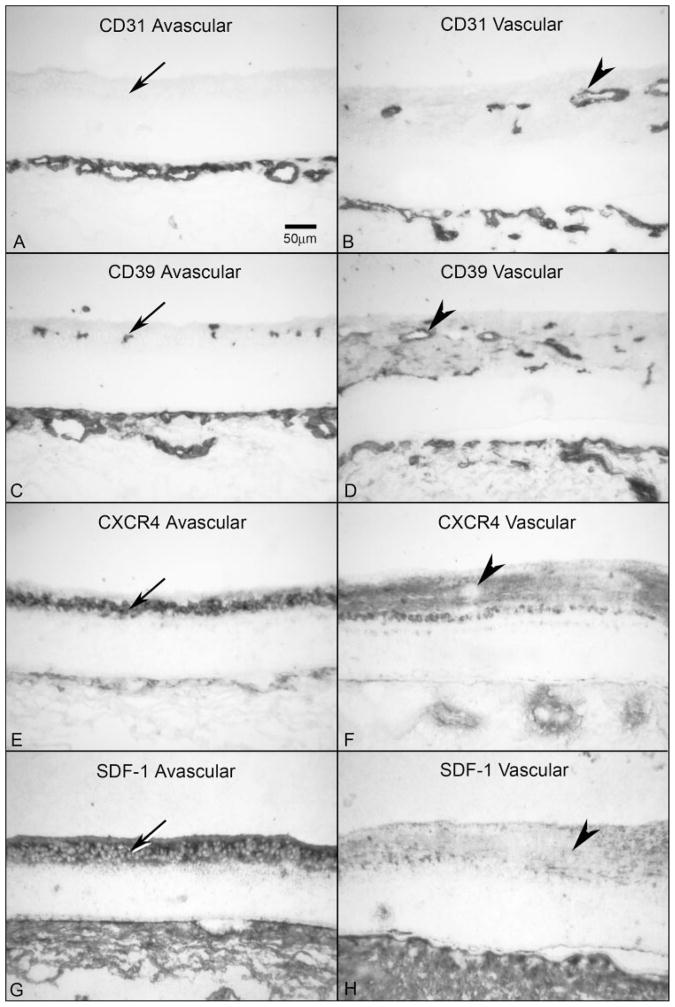
Figure 7.
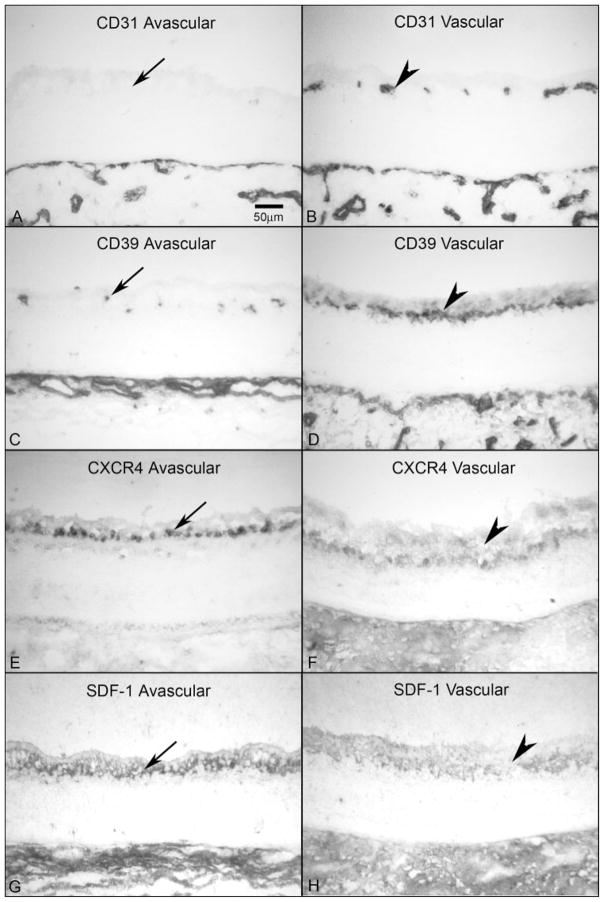
Comparison of CXCR4 and SDF-1 localization in avascular and vascularized retina from a 16-WG fetal eye. (A, B) CD31 was absent in avascular peripheral retina (A, arrow) but clearly labeled blood vessels in vascularized regions (B, arrowhead). The choriocapillaris is the layer of CD31+ blood vessels at the bottom in both panels. (C, D) CD39 was present on angioblasts in avascular retina (C, arrow) and in blood vessels and angioblasts in vascularized areas (D, arrowhead). (E, F) In avascular retina, CXCR4 labeling was intense in angioblasts within the PGCL (E, arrow), but was significantly reduced in areas that are vascularized and absent in retinal vessels (F, arrowhead). (G, H) SDF-1 immunoreactivity was much more intense in avascular inner retina (G, arrow) than in vascularized retina (H, arrowhead). Stain, APase/NBT blue reaction product. Scale bar: (A) applies to all panels.
Figure 8.
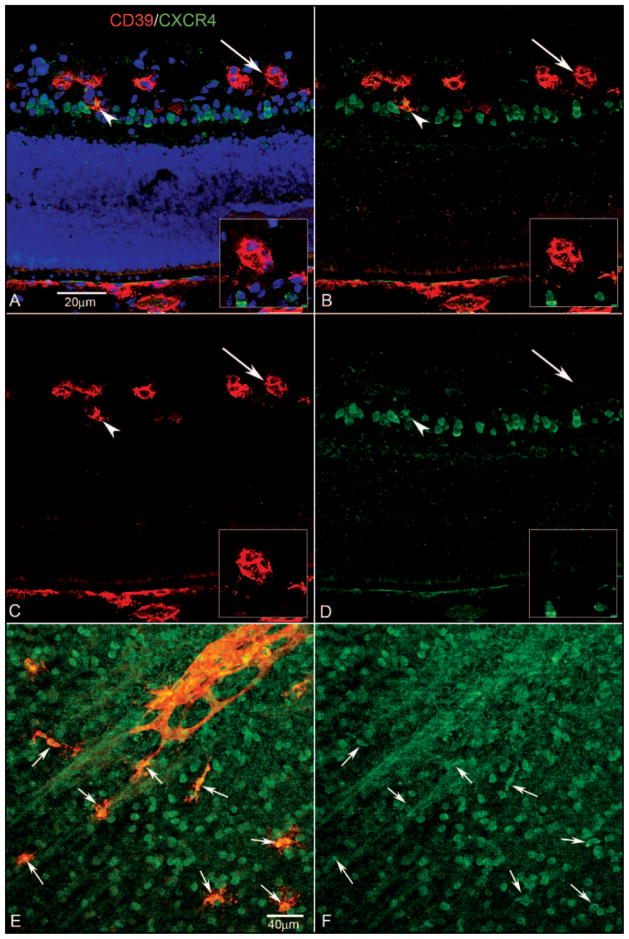
CD39 and CXCR4 at the edge of the developing vasculature at 16 WG. Blue: DAPI-counterstained nuclei; red: CD39; green: CXCR4. (A) CXCR4+ angioblasts within the PGCL began to express CD39 (arrowhead) and to migrate to join the tips of developing vessels, where they formed cords (arrow). Once canalized, CXCR4 expression was downregulated, but CD39 expression was retained. Insets: cord (areas indicated by arrows) at higher magnification. (B) Same section without the DAPI channel showing CXCR4+ cells occupying the PGCL and a double-labeled cell in this region (arrowhead) as well as at the tip of the forming capillary (arrow). (C) Same section showing only the CD39 channel. (D) Same section showing only the CXCR4 channel. Scale bar: (A) applies to all panels.
Figure 9.
SCF and c-Kit expression in avascular (A, B) and vascularized regions (C, D) of a 16-WG fetal retina. (A, B) SCF and c-Kit were both prominent throughout the inner retina of the avascular regions, were associated with nerve fibers just below the ILM (arrowhead) and were present diffusely within the PGCL. (C, D) In vascularized regions, NFs were positive for both SCF and c-Kit. Stain: APase/NBT blue reaction product. Scale bar: (A) applies to all panels.
Gestation of 20–23 Weeks
At 20 to 23 WG, both SDF-1- and CXCR4-expressing cells were very limited in areas with blood vessels (Figs. 10B, 10F, 10H). In these areas, CD39+ cells were present within and outside of the blood vessels (Figs. 10D, 11). Individual CD39+ cells also expressed CXCR4 in avascular areas (Figs. 11E, 11F), but CXCR4 expression declined as cords of CD39+ cells began to form lumens (Figs. 11A–D). SDF-1 expression was higher in avascular retina where individual CD39+ angioblasts were present (Figs. 10C, 10G). There was a row of CXCR4 expressing cells in avascular retina at the level of the ganglion cell layer (GCL) (Fig. 10E). SCF expression in the inner retina was prominent at the ILM in vascularized areas and still present at high levels in most of the inner retina in avascular areas of the retina (Figs. 12A, 12C). In vascularized retina, c-Kit appeared to be present in nerve fibers in the innermost retina and in the cells at the level of the putative ganglion cell layer (PGCL), but in avascular retina it was confined to cells in the putative ganglion cell layer (PGCL; Figs. 12A, 12B). The highest level of SCF was associated with the greatest density of c-Kit+ cells in vascular and avascular retina. CD39+ cells that appeared to be migrating in avascular retina had decreased expression of c-Kit (Figs. 12E, 12H) compared with neighboring cells that did not appear migratory.
Figure 10.
CXCR4 and SDF-1 in avascular and vascularized retina at 20 WG. (A, B) CD31 was absent in avascular retina (A, arrow) but was clearly present in blood vessels in vascularized regions (B, arrowhead). (C, D) CD39 was expressed by angioblasts in avascular retina (C, arrow) and blood vessels and angioblasts in vascularized areas (D, arrowhead). (E, F) In avascular retina, CXCR4 staining was intense in angioblasts within the PGCL (E, arrow) but was significantly reduced in areas that were vascularized and absent in retinal vessels (F, arrowhead). (G, H) SDF-1 immunoreactivity was much more intense in avascular inner retina at this age (G, arrow) than in vascularized retina (H, arrowhead). Stain, APase/NBT blue reaction product. Scale bar: (A) applies to all panels.
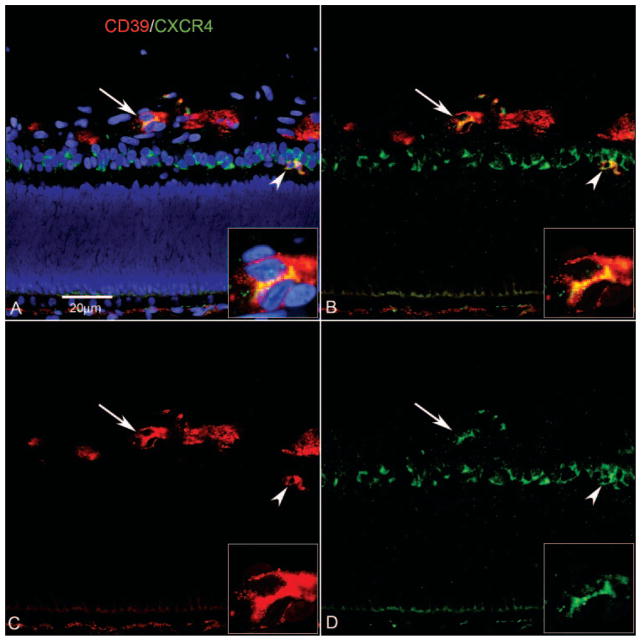
Figure 11.
Expression of CD39 and CRCR4 in a retinal cross section (A–D) and a retinal wholemount (E, F) at the edge of developing vasculature at 22 WG. Blue: DAPI-counterstained nuclei; red: CD39; green: CXCR4. (A) Once angioblasts aggregated and formed canalized blood vessels, CXCR4 expression was downregulated, but CD39 expression was retained (A–D, arrow). Inset: higher magnification of region indicated by arrows in (A–D). CXCR4+ angioblasts within the PGCL began to express CD39 (arrowhead) as they migrated toward the forming blood vessels. (B) Same section without the DAPI channel showing formed CD39+ blood vessels (arrow) and CXCR4+ cells occupying the PGCL, along with a double-labeled cell (arrowhead). (C) Same section showing how CD39 continued to be expressed in forming vessels. (D) Same section showing CXCR4 downregulation once the vessel had formed. (E) Retinal wholemount showing CD39+/CXCR4+ angioblasts in advance of the formed vasculature (arrows). Less differentiated angioblasts had a spherical morphology, whereas those with a fusiform morphology appeared to be aligning with the forming blood vessels. (F) Same field shown in (E) with only the CXCR4 channel visible. Scale bars: (A) applies to (B–D); (E) applies to (F).
Figure 12.
SCF and c-Kit in the avascular and vascularized retina (A–D) and the relationship of c-Kit and CD39 (E–H) in the avascular periphery of a 20-WG fetal retina. (E–H) Blue: DAPI counterstained nuclei; red: CD39; green: c-Kit. (A–D) APase/NBT blue reaction product. (B, D) C-Kit immunoreactivity was present in ganglion cells in the avascular inner retina (arrow) and vascular retina and in nerve fibers in vascular retina (arrowhead). (A, C) There was less SCF reaction product in vascularized retina than in avascular retina, and it appeared to be associated with nerve fibers. (E) In the far periphery, c-Kit+ precursors were located in the PGCL (as shown in B). As they began to express CD39 (arrow), c-Kit expression was downregulated. (F) Same section without the DAPI channel showed a CD39+/cKit+ cell (green/red) within the PGCL. (G) Same section showing only the CD39 channel. (H) Same section showing only the c-Kit channel. Note that the double-labeled cell had much less c-Kit expression than did the neighboring CD39−/c-Kit+ cells. Scale bars: (A) applies to (B–D); (E) applies to (F–H).
In the time period studied, SCF expression increased with age, finally declining at 20 to 23 WG. SDF-1, however, was prominently expressed earlier in development (6–9 WG) than SCF and declined with time until reaching a nadir at 20 to 23 WG.
Discussion
The initial retinal vasculature in humans develops by vasculogenesis, differentiation, and organization of angioblasts that are CD39+/VEGFR-2+/CD34−/CD31−.2,4 The origin of these vascular precursors is not known, but recent observations have demonstrated that they express CXCR4.4 The present study suggests that there is a pool of precursors that appear to be the origin of the CD39-expressing retinal angioblasts. The pool of precursors represents the anterior portion of the neuroblastic layer or inner neuroblastic layer of embryonic and early fetal retina and, from 6 to 12 WG, almost all cells in the layer expressed CXCR4 and c-Kit at some level (Figs. 2, 3, 6). The ultimate proof that the same cells have both receptors requires double labeling, which was technically difficult. Although there is proliferation in the outer portion of the neuroblastic layer, the precursors in inner retina do not proliferate.4
The present study suggests that SDF-1 and SCF is responsible for maintaining precursor cell populations and for potentiating the migration and differentiation of CXCR4+ and c-Kit+ precursors into retinal angioblasts (CD39+/CXCR4+) and perhaps into blood vessels. The appearance of CD39+/CXCR4+ angioblasts at the optic nerve head in flatmounts suggests that they migrate from the optic nerve head along the nerve fibers. However, in cross sections it appeared that they emerged from the layer of precursors in inner retina and then aligned along the nerve fibers (Fig. 2). We have previously observed that the round or nonmigratory CD39+/ADPase+ vascular precursors2,33 are in the precursor layer and now show that they express CXCR4 as well as c-Kit. They lose c-Kit but continue to express CXCR4, as they appear to migrate and then align with nerve fibers. They continue CXCR4 expression until they become ECs (CD31+, CD34+). This change in angioblast phenotype from nonmigratory to migratory during differentiation is well documented.34 Alternatively, CD39+/CXCR4+ cells may have migrated from adjacent mesoderm into the precursor pool. However, the presence of round, nonmigratory CD39+/CXCR4+ and CD39+/cKit+ cells in the precursor pool contradicts this possibility.
The pool of CXCR4+ and c-Kit+ precursors cells in fetal human inner retina is far more than needed for assemblage of the initial vasculature. It is possible that these progenitors are multipotent and give rise to nonvascular cells as well as vascular cells. However, this contradicts the current dogma that angioblasts are of mesodermal origin, and retinal neurons are from neuroectodermal progenitors. If the current dogma is correct, then it is more likely that vascular precursors and neuronal precursors express both c-Kit and CXCR4 in their undifferentiated states. CXCR4 regulates neuronal migration and axonal guidance in brain development.35–37 SDF-1 enhances migration and proliferation of cerebellar granule cells, chemoattracts microglia, and stimulates cytokine production and glutamate release by astrocytes.35–37 One study has shown that retinal ganglion cells express CXCR4 during development.38 SCF is a survival factor for neural crest stem cells39 but many reports suggest that its survival role is more critical in melanocyte precursor survival than in neuronal precursor.40 At 20 WG when the number of precursor cells has declined substantially, CXCR+ and c-Kit+ cells are still present at the level of the ganglion cell layer, and the cells are in a single row, suggesting that one fate for the cells from the precursor pool is certainly ganglion cells.
SDF-1 (CXCL-12) is a hypoxia-inducible CXC cytokine,41 and it is probable that embryonic and early fetal human inner retina is hypoxic. The level of SDF-1 declines as the retinal vasculature forms (compare Fig. 1 with Fig. 7), relieving the retina of physiological hypoxia.42–44 SDF-1 is a chemotactic factor for leukocytes, precursors, and ECs.10,11,45,46 It is responsible for homing of HSCs and EPCs to marrow and sites of injury or angiogenesis.5,7,8 SDF-1 not only stimulates migration of ECs but also tube formation and branching in vitro.12 There was a gradient of SDF-1 at 7 WG for inducing migration of CXCR4+ cells from the precursor pool (least SDF-1) to the NFL of the retina (greatest SDF-1 immunoreactivity), where the blood vessels form. There was also a gradient toward the ora serrata where CXCR4+ cells had not yet arrived at 12 and 16 WG, suggesting that the precursors may have migrated toward the higher concentration of SDF-1 in that region. Since proliferation is rare in the pool of precursors,4 their migration centrifugally in response to a chemoattractant is logical. In the adult retina, the most prominent SDF-1 immunoreactivity was in photoreceptor inner and outer segments, not in vasculature, where only a few vessels were positive.47
CXCR4 is the only known receptor for SDF-1 and is expressed by lymphocytes, monocytes, neutrophils, microglia, and ganglion cell precursors.38,48 It is also expressed by many types of ECs, HSCs, and EPCs.10,49,50 Its importance in vascular development is apparent, since CXCR−/− mice are defective in vascular development, hematopoiesis, and cardiogenesis.51 CXCR4 is expressed in dogs and humans on retinal angioblasts in vivo (Uno K, McLeod S, Lutty G, unpublished data in dogs, 2005) and in vitro (Uno K, McLeod S, Lutty G, unpublished data in humans, 2005).15 It seems inexplicable that CXCR4 expression would cease when the newly formed blood vessels are canalized in developing human retina, when it is present on some ECs in adult human retina and on retinal ECs in vitro.15,47 However, Salvucci et al.12 observed a decline and eventual loss in CXCR4 expression by ECs as they made tubelike formations on synthetic basement membrane matrix (Matrigel; BD Biosciences, Piscataway, NJ). Like SDF-1, CXCR4 expression is upregulated in hypoxia52,53 and in response to VEGF.12 ECs can produce SDF-1 and express CXCR4, suggesting that SDF-1 is an autocrine chemokine for ECs and precursors.12 SDF-1 and CXCR4 expression has also been observed in human ESC-derived embryonic EC differentiation from embryoid bodies.54 When AMD3100, a CXCR4 antagonist, was used in that study, EC outgrowth from the embryoid bodies and tube formation were inhibited.
SCF was prominent in inner retina throughout the period studied, but the level declined by 20 WG. SCF was first described as a multipotent growth factor involved in the early stages of hematopoiesis,55 as well as development and function of germ cells.56 A multitude of signaling pathways are activated by SCF resulting in chemotaxis, proliferation, differentiation, and survival, depending on the cell type and its environment.57 Although it has been extensively studied in mast cells, it has recently been shown that SCF promotes survival, migration, and tube formation by HUVECS.26 Sun et al.25 have demonstrated that SCF activates brain ECs in vitro and stimulates angiogenesis in normal brain and in brain tumors. In fetal human inner retina, SCF and its receptor c-Kit often appeared to be colocalized, which could suggest that it is an autocrine growth factor for the precursors. In fact, SCF has been demonstrated to be autocrine for both ECs and smooth muscle cells.58,59 However, autocrine production of SCF is unlikely if it is a stimulus for migration. It is more probable that diffusable SCF is present in the milieu and bound to its receptor on the precursors.
The receptor for SCF is c-Kit, which is expressed by HSCs, EPCs, mast cells, melanocytes, and germ cells.57 During embryonic life, SCF and c-Kit are expressed along migratory pathways and in destinations of primordial germ cells and melanocytes, in sites of hematopoiesis (yolk sac, fetal liver, and marrow), in gut, and in the central nervous system.55 Late-stage progenitor cells have c-Kit in mouse retina where its expression starts centrally and progresses centrifugally.24 c-Kit is expressed in EC precursors in the yolk sac of the mouse at embryonic day (E)10.5.22 In human placenta, c-Kit expression in ECs has been observed at 6 to 7 WG but expression declines by 12 to 14 WG.60 c-Kit was never observed in ECs in developing retina in the present study, but it is expressed by HUVECS in vitro and in vivo and in bovine brain and human dermal ECs in vitro.25,59 Sun et al.4 found that all three EC types proliferated in response to SCF. Proliferation was never observed in the precursor pool (CXCR4+ and c-Kit+ cells), angioblasts (CD39+/CXCR4+), nor ECs (CD31+/CD39+) in developing human retina between 6 and 23 WG.4
Although the two growth factors targeted in this study have unique roles in development, they also have many overlapping activities and can act in synergy. Both ligands are critical in hematopoiesis during development and adult life.55,61 SCF and SDF-1 enhance chemotaxis of neuronal progenitor cells and can act synergistically to stimulate focal adhesion formation and migration of CD34+ progenitors.9 As mentioned previously, both ligands can stimulate the migration of the ECs and play roles in recruiting precursors to sites of new blood vessel formation in pathologic events.62 Our study does not address the cells that produce SDF-1 nor SCF, but both are associated with nerve fibers and ganglion cells in fetal human inner retina. Our study also did not address whether the SDF-1 or SCF observed was active or whether it was free or bound to CXCR4 or c-Kit, respectively. Proteolysis can control the level of active ligands present in certain niches. MMP-9 and cathepsin K cleave both growth factors and this cleavage can inactivate either of the ligands.63
Chen et al.,54 in their study of sprouting of ECs from human embryoid bodies, concluded that the SDF-1/CXCR4 axis plays a critical role in regulating the initial vessel formation and function as a morphogen during embryonic blood vessel formation. Our observations suggest that the retinal vasculature may be a good example in vivo of the importance of this axis in human vascular development. We observed embryonic human retina has a large pool of precursors (CXCR4+ and c-Kit+ cells) that enlarges centrifugally during fetal development. From this pool emerges CD39+ cells, which will migrate anterior into the NFL where SDF-1 and SCF levels are highest. c-Kit expression declines with apparent migration. Cords, originally called primordial blood vessels,64 are eventually formed by the angioblasts (CXCR4+/CD39+) at the edge of the forming blood vessels. Once canalization occurs, CXCR4 expression in the fetal retinal vessels declines. In conclusion, SDF-1 may work in concert with SCF during early angioblast differentiation, whereas, as a morphogen, it continues to have an influence on cord formation.
Acknowledgments
Supported by National Eye Institute Grant EY09357 (GAL), EY01765 (Wilmer), and a gift from the Himmelfarb Family Foundation in the name of Morton Goldberg, MD. TH was a Bausch & Lomb Japan Research Fellow.
Footnotes
Disclosure: T. Hasegawa, None; D.S. McLeod, None; T. Prow, None; C. Merges, None; R. Grebe, None; G.A. Lutty, None
References
- 1.McLeod DS, Brownstein R, Lutty GA. Vaso-obliteration in the canine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37:300–311. [PubMed] [Google Scholar]
- 2.Chan-Ling T, McLeod DS, Hughes S, et al. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest Ophthalmol Vis Sci. 2004;45:2020–2032. doi: 10.1167/iovs.03-1169. [DOI] [PubMed] [Google Scholar]
- 3.Lutty GA, McLeod DS. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol. 1992;110:267–276. doi: 10.1001/archopht.1992.01080140123039. [DOI] [PubMed] [Google Scholar]
- 4.McLeod DS, Hasegawa T, Prow T, Merges C, Lutty GA. The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dyn. 2006;235:3336–3347. doi: 10.1002/dvdy.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler JM, Guthrie SM, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma. 2003;44:575–582. doi: 10.1080/1042819021000037985. [DOI] [PubMed] [Google Scholar]
- 7.Jin DK, Shido K, Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4(+) hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengupta N, Caballero S, Mames RN, Timmers AM, Saban D, Grant MB. Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci. 2005;46:343–348. doi: 10.1167/iovs.04-0153. [DOI] [PubMed] [Google Scholar]
- 9.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- 10.Feil C, Augustin HG. Endothelial cells differentially express functional Cxc-chemokine receptor-4 (CXCR-4/Fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun. 1998;247:38–45. doi: 10.1006/bbrc.1998.8499. [DOI] [PubMed] [Google Scholar]
- 11.Mirshahi F, Pourtau J, Li H, et al. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99:587–594. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- 12.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 13.Heidemann J, Ogawa H, Rafiee P, et al. Mucosal angiogenesis regulation by CXCR4 and its ligand Cxcl12 expressed by human intestinal microvascular endothelial cells. Am J Physiol. 2004;286:G1059–G1068. doi: 10.1152/ajpgi.00417.2003. [DOI] [PubMed] [Google Scholar]
- 14.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 15.Lutty GA, Merges C, Grebe R, Prow T, McLeod DS. Canine retinal angioblasts are multipotent. Exp Eye Res. 2006;83:183–193. doi: 10.1016/j.exer.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signaling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 17.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on Cd34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- 18.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 19.Pablos JL, Amara A, Bouloc A, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577–1586. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun HJ, Jo DY. Production of stromal cell-derived factor-1 (SDF-1) and expression of CXCR4 in human bone marrow endothelial cells. J Korean Med Sci. 2003;18:679–685. doi: 10.3346/jkms.2003.18.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327–340. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 22.Nadin BM, Goodell MA, Hirschi KK. Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood. 2003;102:2436–2443. doi: 10.1182/blood-2003-01-0118. [DOI] [PubMed] [Google Scholar]
- 23.Goolsby J, Marty MC, Heletz D, et al. Hematopoietic progenitors express neural genes. Proc Natl Acad Sci USA. 2003;100:14926–14931. doi: 10.1073/pnas.2434383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koso H, Satoh S, Watanabe S. c-Kit marks late retinal progenitor cells and regulates their differentiation in developing mouse retina. Dev Biol. 2007;301:141–154. doi: 10.1016/j.ydbio.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Hui AM, Su Q, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c-Kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem. 2004;279:18600–18607. doi: 10.1074/jbc.M311643200. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 28.Kelly DJ, Zhang Y, Gow RM, Itescu S, Gilbert RE. Cells expressing the stem cell factor receptor, c-Kit, contribute to neoangiogenesis in diabetes. Diab Vasc Dis Res. 2005;2:76–80. doi: 10.3132/dvdr.2005.013. [DOI] [PubMed] [Google Scholar]
- 29.Mhaskar R, Agarwal N, Takkar D, Buckshee K, Anandalakshmi Deorari A. Fetal foot length: a new parameter for assessment of gestational age. Int J Gynaecol Obstet. 1989;29:35–38. doi: 10.1016/0020-7292(89)90126-4. [DOI] [PubMed] [Google Scholar]
- 30.Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-B in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993;34:477–487. [PubMed] [Google Scholar]
- 31.Bhutto IA, Kim SY, McLeod DS, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:1544–1552. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa T, McLeod DS, Bhutto IA, et al. The embryonic human choroidal vasculature forms by hemo-vasculogenesis. Dev Dyn. 2007;236:2089–2100. doi: 10.1002/dvdy.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLeod DS, Lutty GA, Wajer SD, Flower RW. Visualization of a developing vasculature. Microvasc Res. 1987;33:257–269. doi: 10.1016/0026-2862(87)90021-5. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt AM, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–136. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- 35.Berger O, Li G, Han SM, Paredes M, Pleasure SJ. Expression of SDF-1 and CXCR4 during reorganization of the postnatal dentate gyrus. Dev Neurosci. 2007;29:48–58. doi: 10.1159/000096210. [DOI] [PubMed] [Google Scholar]
- 36.Stumm R, Höllt V. Cxc chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J Mol Endocrinol. 2007;38:377–382. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- 37.Stumm RK, Zhou C, Ara T, et al. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalasani SH, Baribaud F, Coughlan CM, et al. The chemokine stromal cell-derived factor-1 promotes the survival of embryonic retinal ganglion cells. J Neurosci. 2003;23:4601–4612. doi: 10.1523/JNEUROSCI.23-11-04601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sieber-Blum M. Growth factor synergism and antagonism in early neural crest development. Biochem Cell Biol. 1998;76:1039–1050. [PubMed] [Google Scholar]
- 40.Ito M, Kawa Y, Ono H, et al. Removal of stem cell factor or addition of monoclonal anti-c-Kit antibody induces apoptosis in murine melanocyte precursors. J Invest Dermatol. 1999;112:796–801. doi: 10.1046/j.1523-1747.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- 41.Ceradini DJ, Gurtner GC. Homing to hypoxia: Hif-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–428. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division: evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- 44.Patz A. Oxygen studies in retrolental fibroplasia: IV clinical and experimental observations. Am J Ophthalmol. 1954;38:291–307. doi: 10.1016/0002-9394(54)90845-4. [DOI] [PubMed] [Google Scholar]
- 45.Mohle R, Bautz F, Denzlinger C, Kanz L. Transendothelial migration of hematopoietic progenitor cells: role of chemotactic factors. Ann NY Acad Sci. 2001;938:26–34. doi: 10.1111/j.1749-6632.2001.tb03571.x. discussion 34–5. [DOI] [PubMed] [Google Scholar]
- 46.Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 47.Bhutto IA, McLeod DS, Merges C, Hasegawa T, Lutty GA. Localization of SDF-1 and its receptor CXCR4 in retina and choroid of aged human eyes and in eyes with age related macular degeneration. Br J Ophthalmol. 2006;90:906–910. doi: 10.1136/bjo.2006.090357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forster R, Kremmer E, Schubel A, et al. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–1531. [PubMed] [Google Scholar]
- 49.Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells: functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 50.Volin MV, Joseph L, Shockley MS, Davies PF. Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun. 1998;242:46–53. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- 51.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 52.Scheurer SB, Rybak JN, Rosli C, Neri D, Elia G. Modulation of gene expression by hypoxia in human umbilical cord vein endothelial cells: a transcriptomic and proteomic study. Proteomics. 2004;4:1737–1760. doi: 10.1002/pmic.200300689. [DOI] [PubMed] [Google Scholar]
- 53.Zagzag D, Lukyanov Y, Lan L, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 54.Chen T, Bai H, Shao Y, et al. SDF-1/CXCR4 signaling modifies the capillary-like organization of human embryonic stem cell-derived endothelium in vitro. Stem Cells. 2007;25:392–401. doi: 10.1634/stemcells.2006-0145. [DOI] [PubMed] [Google Scholar]
- 55.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 56.Sette C, Dolci S, Geremia R, Rossi P. The role of stem cell factor and of alternative c-Kit gene products in the establishment, maintenance, and function of germ cells. Int J Dev Biol. 2000;44:599–608. [PubMed] [Google Scholar]
- 57.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–2548. doi: 10.1007/s00018-004-4189-6. [DOI] [PubMed] [Google Scholar]
- 58.Hollenbeck ST, Sakakibara K, Faries PL, Workhu B, Liu B, Kent KC. Stem cell factor and c-Kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004;120:288–294. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi H, Ishii E, Saito S, et al. Umbilical vein endothelial cells are an important source of c-Kit and stem cell factor which regulate the proliferation of haemopoietic progenitor cells. Br J Haematol. 1996;94:606–611. doi: 10.1046/j.1365-2141.1996.d01-1855.x. [DOI] [PubMed] [Google Scholar]
- 60.Challier JC, Galtier M, Cortez A, Bintein T, Rabreau M, Uzan S. Immunocytological evidence for hematopoiesis in the early human placenta. Placenta. 2005;26:282–288. doi: 10.1016/j.placenta.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Juarez J, Bendall L. SDF-1 and CXCR4 in normal and malignant hematopoiesis. Histol Histopathol. 2004;19:299–309. doi: 10.14670/HH-19.299. [DOI] [PubMed] [Google Scholar]
- 62.Heissig B, Werb Z, Rafii S, Hattori K. Role of c-Kit/Kit ligand signaling in regulating vasculogenesis. Thromb Haemost. 2003;90:570–576. doi: 10.1160/TH03-03-0188. [DOI] [PubMed] [Google Scholar]
- 63.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 64.Ashton N. Retinal angiogenesis in the human embryo. Br Med Bull. 1970;26:103–106. doi: 10.1093/oxfordjournals.bmb.a070758. [DOI] [PubMed] [Google Scholar]