Abstract
Reports about ringworm in donkeys are scanty and refer to zoonotic agents such as Trichophyton mentagrophytes and Trichophyton verrucosum. Seventeen Asino Amiatina donkeys semi-extensively farmed in paddocks showed alopecic nummular, scaling areas mainly on head and neck. Microsporum racemosum cultivated from the lesions was identified by morphology and PCR. Affected animals healed spontaneously. The present record reports for the first time the occurrence of ringworm due to M. racemosum in naturally infected animals.
Keywords: Microsporum racemosum, Donkey, Equus asinus, Ringworm, Geophilic dermatophyte
1. Introduction
The present paper reports for the first time the occurrence of an outbreak of ringworm due to Microsporum racemosum in a donkey herd.
This geophilic fungal species was firstly isolated by Borelli [1] from a Rattus rattus in Venezuela, then from amazonian forest soil [2], from soil and animal hairs in Europe [3], [4]. Its perfect state was described by Rush-Munro et al. [2] and natural pathogenicity for humans has been reported [5], [6], [7], [8], [9]. Information about usual clinical presentation in animals is lacking, except for experimental infection of guinea pig, achieved using the clinical isolate of the second human case from the USA. In this animal species an erythematous, scaling lesion developed in a few days after the inoculation of spores obtained from culture [7], underlining the “highly pathogenic potential” of M. racemosum stated by Alteras and Evolceanu, [4]. In the present case donkeys showed scaling alopecic areas on head and neck.
2. Case
All the animals were semi-extensively reared in Tuscany (Central Italy), for milk production and onotherapy. The donkey herd consisted of 151 animals (8 jacks, 80 jennies, 63 foals) kept in 11 separate paddocks. In the summer of 2015 17 animals showed dermatological signs consisting of alopecic nummular, scaling areas mainly on head and neck (Fig. 1). The lesions had developed in about 2 weeks time. In detail symptomatic subjects were distributed as follows in separate paddocks: 1 stallion and a female for mating, 3 out of 19 lactating jennies, 7 out of 7 male foals and 5 out of 37 female foals. All the subjects present in the breed were regularly submitted to veterinary controls and appeared in general good health status. Because the clinical picture was suggestive of dermatophytosis, all symptomatic donkeys were sampled by Mc Kenzie brush technique and hairs were collected for microscopic direct examination. In addition a brush sampling was carried out in the 8 animals employed in onotherapy also, suspecting the possible occurrence of a zoonotic dermatophyte, even if no human skin lesions had been reported.
Fig. 1.

Alopecic areas on the neck of an infected donkey.
Hairs specimens were both clarified in KOH 10% for microscopic examination and cultured on Sabouraud Dextrose Agar (SDA) with cycloheximide 0.5 mg/mL and chloramphenicol 0.05 mg/mL (Liofilchem, Italy) and incubated at 25 °C for 15 days.
Microscopic examination revealed few arthrospores of about 5 µm in diameter, sparse on hairs (Fig. 2). All symptomatic animals and 5 out of 8 donkeys employed in onotherapy were culture positive for velvety colonies, rapidly spreading to powdery, with a surface cream color and a grape red reverse. Subcultures on Malt Extract Agar were achieved.
Fig. 2.
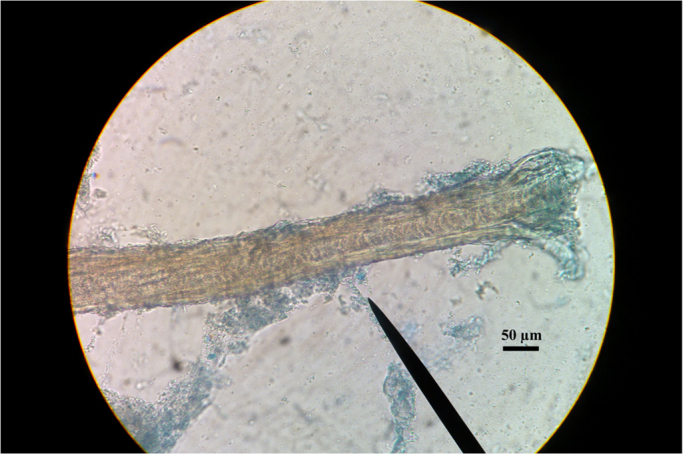
Arthrospores on infected hair (lactophenol cotton blue, 400× magnification).
Microscopic morphology consisted of several stalked macro and microconidia. Macroaleuriospores were large, echinulate, thin-walled and with more than 6 cells, often provided of terminal filament, while the club shaped microconidia were arranged in grape-like clusters (Fig. 3). The macro- and microscopic fungal morphology was consistent with M. racemosum. No other dermatophyte species was cultured. Considering the geophilic behavior of M. racemosum, cultures from soil of paddocks were achieved by hair bait technique, yielding ascigerous states of M. racemosum, Microsporum gypseum and Trichophyton ajelloi.
Fig. 3.
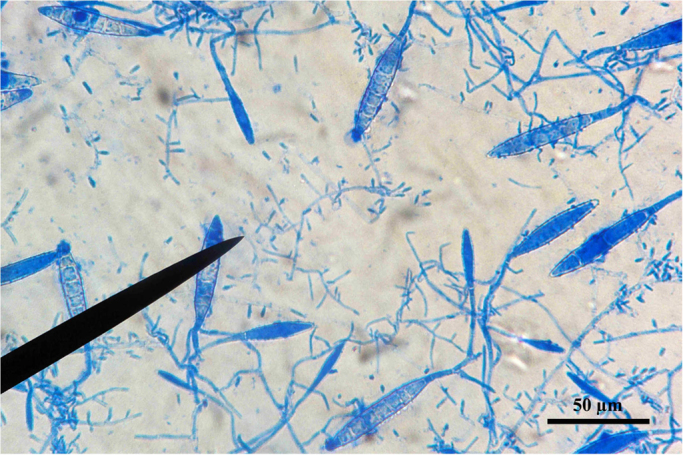
Microscopic morphology of isolate: microaleuriospores stalked and in racemes and large macroaleuriospores with terminal filaments (400× magnification).
Fungal isolates were send to the Centraalbureau voor Schimmelculture of the Netherlands for molecular confirmation. The identified strain (CBS accession N. 141511) was enlisted in the CBS culture collection.
Specific antimycotic drugs licensed for food producing animals are not available within the European Community, so the animals were not treated and spontaneous healing occurred in about three months. Further clinical cases were not registered during the following 8 months.
3. Discussion
Reports about ringworm in donkeys are scanty and refer to zoonotic agents such as Trichophyton mentagrophytes and Trichophyton verrucosum [10], [11]. Amiatina is an Italian native donkey (Equus asinus) breed classified by FAO as endangered [12]. Italian public institutions and private breeders associations made strong efforts to preserve this autochthonous breed [13], therefore the use of these animals in recreational activities (agritourism, hiking) and onotherapy (especially with children) as well as for meat (salami, stew) and milk (cosmetics industry, human nutrition) production has been promoted [12], [13]. Considering the close contact between donkeys and human beings, these latter sometimes have a developing and/or impaired immune system, such as patients affected by disability or discomfort and/or children, great attention should be paid to animals' health status to avoid spread of zoonotic infections.
Geophilic dermatophytes are keratinophilic fungi able to grow on keratin baits in soil. These fungal species represent the largest group among dermatophytes and are not frequently found to infect humans or animals [14], except for M. gypseum, occurring in about 0.1% of human patients [15], [16] and in 1.1% of dermatologically diseased dogs and cats [17]. Infections due to geophilic fungi usually do not occur simultaneously in several animals, although an outbreak of dermatophytosis caused by Microsporum nanum has been described in an extensive Iberian pig farm [18]. In this case 100% of lactating sows were involved, likely due to the immunomodulating effect of lactation. In the present report lactating jennies and foals aged from 1 to 4 years, appeared likely to develop skin lesions. Considering that 100% of foal males were symptomatic our findings could indicate that the stress of lactation on one hand, as well as to the biting and fighting habits of young adults, on the other hand, might have led to subtle immunosuppression which promoted clinical infection.
The results of the present study would suggest that other animal species, in addition to humans, are susceptible to infection with this fungus.
Conflict of interest
There are none conflict of interests.
Acknowledgments
The Authors thank Professor Sybren De Hoog for the molecular identification of fungal isolates.
References
- 1.Borelli D. Microsporum racemosum nova species. Acta Med. Venez. 1965;12:148–151. [Google Scholar]
- 2.Rush-Munro F.M., Smith J.M., Borelli D. The perfect state of Microsporum racemosum. Mycologia. 1970;62:856–859. [PubMed] [Google Scholar]
- 3.Ulfig K., Terakowski M., Płaza G. First isolation of Microsporum racemosum Borelli in Poland. Rocz. Panstw. Zakl. Hig. 1996;47:313–318. [PubMed] [Google Scholar]
- 4.Alteraş I., Evolceanu R. First isolation of Microsporum racemosum-Dante Borelli 1965 from Romanian soil (New data on its pathogenic properties) Mykosen. 1969;12:223–230. doi: 10.1111/j.1439-0507.1969.tb03465.x. [DOI] [PubMed] [Google Scholar]
- 5.Albornoz M.B. de, Lopez C.A., Afonzo N. Primero caso De Tinea corporis por EL Microsporum racemosum (Borelli 1965) Derm. Venez. 1972;2:310–318. [Google Scholar]
- 6.Daum V., McCloud D.J. Microsporum racemosum: first isolation in the United States. Mycopathologia. 1976;59:183–185. doi: 10.1007/BF00627882. [DOI] [PubMed] [Google Scholar]
- 7.Rippon J.W., Andrews T.W. Microsporum racemosum. Second clinical isolation from the United States and the Chicago area. Mycopathologia. 1978;64:187–190. doi: 10.1007/BF00576373. [DOI] [PubMed] [Google Scholar]
- 8.Rippon J.W. Forty four years of dermatophytes in a Chicago clinic (1944–1988) Mycopathologia. 1992;119:25–28. doi: 10.1007/BF00492226. [DOI] [PubMed] [Google Scholar]
- 9.García-Martos P., Gené J., Solé M., Mira J., Ruíz-Henestrosa R., Guarro J. Case of onychomycosis caused by Microsporum racemosum. J. Clin. Microbiol. 1999;37:258–260. doi: 10.1128/jcm.37.1.258-260.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali K.E., Abu-Samra M.T., Ibrahim A.M. Trichophyton mentagrophytes infection in the domestic donkey (Equus asinus asinus) Ann. Trop. Med. Parasitol. 1981;75:623–626. doi: 10.1080/00034983.1981.11687493. [DOI] [PubMed] [Google Scholar]
- 11.Abdalla W.G., Suliman E.A., Abdo El Gabbar N.A. A report on Trichophyton verrucosum in donkeys in the Sudan. Sudan J. Vet. Res. 2005;20:83–85. [Google Scholar]
- 12.W. Kugler, H.-P. Grunenfelder, E. Broxham, Donkey breeds in Europe: inventory, description, need for action, conservation. Report 2007/2008. Monitoring Institute for Rare Breeds and Seeds in Europe/SAVE Foundation, St Gallen, Switzerland.
- 13.Colli L., Perrotta G., Negrini R., Bomba L., Bigi D., Zambonelli P. Detecting population structure and recent demographic history in endangered livestock breeds: the case of the Italian autochthonous donkeys. Anim. Genet. 2013;44:69–78. doi: 10.1111/j.1365-2052.2012.02356.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi J.S., Gräser Y., Walther G., Peano A., Symoens F., de Hoog S. Microsporum mirabile and its teleomorph Arthroderma mirabile, a new dermatophyte species in the M. cookei clade. Med. Mycol. 2012;50:161–169. doi: 10.3109/13693786.2011.594456. [DOI] [PubMed] [Google Scholar]
- 15.Rezaei-Matehkolaei A., Rafiei A., Makimura K., Gräser Y., Gharghani M., Sadeghi-Nejad B. Epidemiological Aspects of Dermatophytosis in Khuzestan, southwestern Iran, an Update. Mycopathologia. 2016 doi: 10.1007/s11046-016-9990-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 16.Martinez E., Ameen M., Tejada D., Arenas R. Microsporum spp. onychomycosis: disease presentation, risk factors and treatment responses in an urban population. Braz. J. Infect. Dis. 2014;18:181–186. doi: 10.1016/j.bjid.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nardoni S., Mugnaini L., Papini R., Fiaschi M., Mancianti F. Canine and feline dermatophytosis due to Microsporum gypseum: a retrospective study of clinical data and therapy outcome with griseofulvin. J. Mycol. Med. 2013;23:164–167. doi: 10.1016/j.mycmed.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Sanchez A., Bazan J., de Mendoza J.H., Martinez R., Sanchez S., de Mendoza M.H. Outbreak of ringworm in a traditional Iberian pig farm in Spain. Mycoses. 2011;54:179–181. doi: 10.1111/j.1439-0507.2009.01776.x. [DOI] [PubMed] [Google Scholar]


