Abstract
Metastatic deposits to the oral cavity are exceptionally rare. The commonest tumor types metastasizing to the oral cavity include lung and breast carcinoma. Renal cell carcinoma is believed to be the third most common infra clavicular tumor to metastasize to the head and neck. We report a case where an oral cavity deposit was the initial presentation for an occult clear cell renal carcinoma. Additional therapeutic options, including immunotherapy, tyrosine kinase inhibitors, and participation in a clinical trial, should be discussed with the patient despite the poor overall prognosis.
Key words: Renal cell cancer, oral cavity, gingival metastases
Introduction
Metastatic deposits to the oral cavity are exceptionally rare. The commonest tumor types metastasizing to the oral cavity include lung and breast carcinoma.1 Renal cell carcinoma is believed to be the third most common infra clavicular tumor to metastasize to the head and neck.1 In the majority of cases identified in the literature, the primary tumor was known and patients often had advanced metastatic disease. We report a case where an oral cavity deposit was the initial presentation for an occult clear cell renal carcinoma.
Case Report
A 60-year old gentleman presented to his dentist with a 5-month history of an enlarging gingival mass. He was fit and well with no significant co-morbidity, he has a 40-year history of smoking. On presentation, he had a nodular fungating mass of (6×7 cm) originating from the inferior alveolar arch gingiva extending anteriorly causing displacement of adjacent teeth (Figure 1). The lesion was painless but was interfering with swallowing and speech, he also complained of drooling of saliva, which was problematic. Systemic examination yielded no other significant abnormality. Blood tests including complete blood count, liver function test and renal function were normal.
Figure 1.
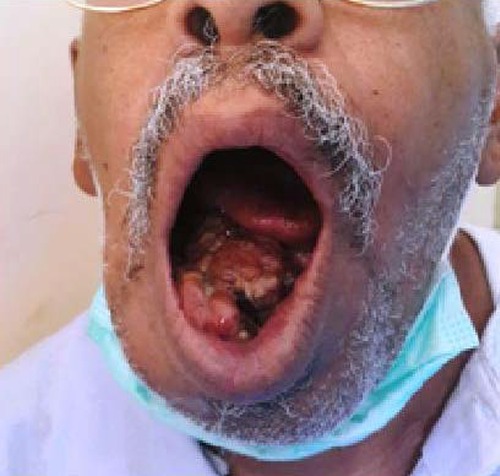
Large irregular intra-oral mass lesion.
The initial working diagnosis was a fibroma but it was felt that a squamous cell carcinoma should be excluded. A biopsy was performed. This demonstrated malignant epithelial cells of clear cell type suspicious for a renal origin.
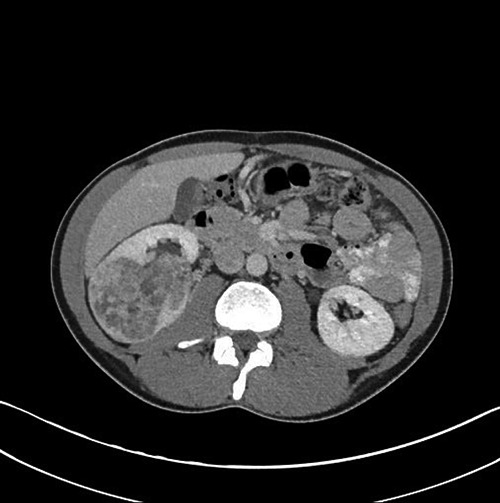
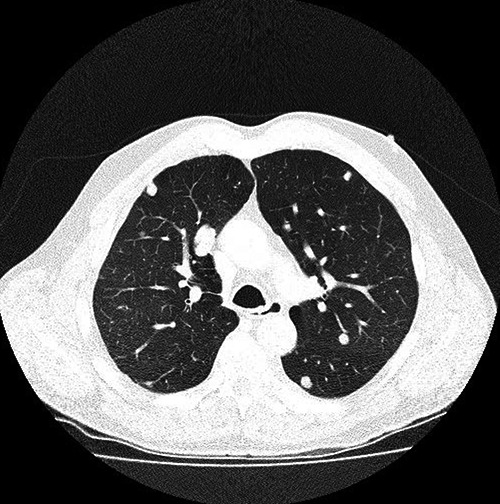
Patient underwent a full body staging computed tomography (CT) which identified a large (9.5 cm) exophytic mass arising from the lower pole of the right kidney (Figure 2), CT guided biopsy positive for clear cell renal cancer, CT chest showed multiple pulmonary nodules of variable sizes consistent with pulmonary metastases (Figure 3). CT of the head and neck showed a large soft tissue mass centered on the right inferior alveolar arch with associated erosive bone changes of the adjacent right mandibular body, no significant lymphadenopathy or other sites of distant disease were identified.
Figure 2.
Computed tomographic image showing multiple pulmonary metastases.
Figure 3.
Contrast enhanced computed tomographic image showing a large irregular right renal mass.
Discussion
Renal cell carcinoma accounts for approximately (3%) of all adult cancers. Clear cell renal cell carcinoma (CCRCC) is a subtype of renal cell carcinoma typically characterized by malignant epithelial cells with clear cytoplasm. CCRCCs have higher preference for vascular invasion than for lymphatic invasion.2 This results in higher incidence of renal vein involvement with CCRCC. This is associated with increased likelihood for distant metastasis rather than locoregional lymphatic spread which is the common pattern of spread for other subtypes of renal cell carcinoma. Most common sites for distant metastases from CCRCC include lung (33-72%), infra-abdominal lymph nodes (3-35%), bone (21-25%), brain (7-13%) and liver (5-10%).3 Hirschberg et.al review of the literature identified the gingiva and alveolar mucosa as the commonest site for intra-oral metastasis (54.8%) followed by the tongue (27.4%).4
Metastasis to the oral cavity generally is not common. It forms about 1-3% of all malignant oral neoplasms. The common primary tumors are breast, lung and kidney. The mandible is the commonest involved site.5,6 Metastatic involvement of the gingiva can be a challenging diagnosis to be made at initial presentation due to the vast spectrum of mass forming/ulcerating lesions that are known to affect the gingiva. Therefore thorough clinical assessment should be made to try and differentiate a malignant lesion/deposit from the more frequently observed benign pathologies like giant cell granuloma and fibroma. This highlights the difficulties encountered at the initial stage of clinical diagnosis. Knowledge of a preexisting primary malignancy, particularly of the lung, breast or renal cell carcinoma should prompt the consideration of intra-oral metastases in cases of atypical mucosal lesions. Following histological confirmation, further tests may be required to localize the primary tumor, if not known at presentation, and to formally stage the disease. In our case a full body contrast enhanced staging CT successfully identified the large renal primary. In addition to this numerous pulmonary nodules of varying sizes were also demonstrated in keeping with pulmonary metastases. No enlarged upper abdominal lymph nodes were identified. Some authors have suggested that intra-oral metastases should be considered as a bad prognostic feature signaling widespread hematological spread of the primary tumor.5 The best treatment approach will depend on the stage of disease and other tumor and patient related factors. Due to the nature of intra-oral metastases and associated high risk of ulceration, bleeding and disturbance of function, many authors believe local excision if possible, to be the best treatment regardless of disease stage.7 Renal cell carcinoma is known to be largely radio-resistant. The usefulness of radiotherapy in the management of intra-oral renal cell metastases is not fully established.8
This patient was given external radiotherapy first (40 Gy/20 fractions), using two lateral wedged fields, by Cobalt-60 due to the mass’s inoperability. In addition the patient also received subcutaneous alpha interferon (6 million units three times/week for 2 month). The treatment was limited by poor clinical response and poor tolerability due to grade 2 hematological toxicity and grade 3 vomiting and flu like illness.
The patient was considered a candidate for tyrosine kinase inhibitor and sorafenib was chosen following discussion with the patient.
He was started on sorafenib 400 mg bid, dramatic response were achieved in form of complete disappeared of the oral mass within 3 months (Figure 3), but partial response of the lung secondaries, He remains on sorafenib 400 mg pending progression or anticipated toxicity.
In summary, intra-oral metastases from renal cell carcinoma is a rare but recognized clinical entity. It is often seen in patients with advanced metastatic disease but can also be the presenting abnormality. Local excision is felt to be the treatment of choice offering both disease volume reduction with the potential of cure in the case of oligometastasis and excision is also considered to be the best form of palliation as oral metastases are likely to be problematic as they advance.
Conclusions
RCC has been shown to metastasize to the head and neck region in rare instances. Therefore, the work-up of a new oral or neck lesion in light of a history of RCC should include metastatic RCC as part of the differential diagnosis.
It is vital to perform immunohistochemical staining to differentiate between metastatic RCC and primary head and neck tumors.
Additional therapeutic options, including immunotherapy, tyrosine kinase inhibitors, and participation in a clinical trial, should be discussed with the patient despite the poor overall prognosis.
Figure 4.
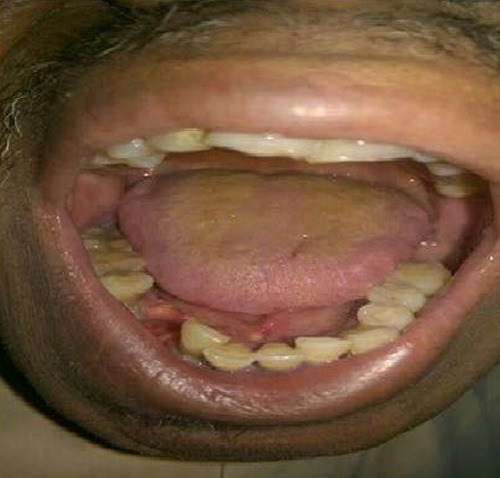
Patient’s response after sorafenib.
References
- 1.Maestre-Rodríguez Ó, González-García R, Mateo-Arias J, et al. Metastasis of renal clear-cell carcinoma to the oral mucosa, an atypical location. Med Oral Patol Oral Cir Bucal 2009;14:e601-4. [DOI] [PubMed] [Google Scholar]
- 2.Bonsib SM. Renal lymphatics and lymphatic involvement in sinus vein invasive (pT3b) clear cell renal cell carcinoma: a study of 40 cases. Mod Pathol 2006;19:746-53. [DOI] [PubMed] [Google Scholar]
- 3.Mai KT, Landry DC, Robertson SJ, et al. A comparative study of metastatic renal cell carcinoma with correlation to subtype and primary tumor. Pathol Res Pract 2001;197:671-5. [DOI] [PubMed] [Google Scholar]
- 4.Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med 1993;22:385-90. [DOI] [PubMed] [Google Scholar]
- 5.Nishinura Y, Ykata H, Kawsaki T. Metastatic tumor of the mouth and jaws, a review of the Japanese literature. J Maxillofacial Surg 1982;10:353-8. [DOI] [PubMed] [Google Scholar]
- 6.Kumar GS, Minijunta BS. Metastatic tumors of the jaws and the oral cavity. J Oral Maxillofacial Pathol 2013;17:71-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis GL, Jensen JL, Reingold IM, Barr RJ. Malignant neoplasms metastatic to gingivae. Oral Surg Oral Med Oral Pathol 1977;44:238-45. [DOI] [PubMed] [Google Scholar]
- 8.Azam F, Abobaker M, Golins S. Tongue metastases as an initial presentation of a case of RCC and review of literature. J Med Case Rep 2008;2:249. [DOI] [PMC free article] [PubMed] [Google Scholar]


