Abstract
Background. Hepatitis C virus (HCV) treatment access among human immunodeficiency virus (HIV)/HCV-coinfected people who inject drugs is poor, despite a high burden of disease in this population. Understanding barriers and facilitators to HCV treatment uptake is critical to the implementation of new direct-acting antivirals.
Methods. We conducted in-depth interviews with patients, physicians, and social workers at an HIV treatment facility and methadone maintenance treatment centers in Guangzhou, China to identify barriers and facilitators to HCV treatment. We included patients who were in various stages of HCV treatment and those who were not treated. We used standard qualitative methods and organized data into themes.
Results. Interview data from 29 patients, 8 physicians, and 3 social workers were analyzed. Facilitators and barriers were organized according to a modified Consolidated Framework for Implementation Research schematic. Facilitators included patient trust in physicians, hope for a cure, peer networks, and social support. Barriers included ongoing drug use, low HCV disease knowledge, fragmented reimbursement systems, HIV exceptionalism, and stigma.
Conclusions. Expanding existing harm reduction programs, HIV treatment programs, and social services may facilitate scale-up of direct-acting antivirals globally. Improving integration of ancillary social and mental health services within existing HIV care systems may facilitate HCV treatment access.
Keywords: China, direct-acting antivirals, HCV, HIV, people who inject drugs
Although hepatitis C infection treatment regimens have rapidly advanced, these benefits have yet to translate into improvements in clinical outcomes and systems of care [1]. Up to 30% of chronically infected hepatitis C virus (HCV) patients will progress to cirrhosis within 20 years, leading to end-stage liver disease and hepatocellular carcinoma [2, 3]. Hepatitis C virus has surpassed human immunodeficiency virus (HIV)-related deaths in several high-income nations [4].
Direct-acting antivirals (DAAs) have achieved over 90% sustained virological response (SVR) rates across all genotypes [5, 6]. Short duration, interferon-free regimens are now possible even for patients with HIV coinfection [7]. Direct-acting antivirals will not only improve SVR rates, but they also may reduce dependence on laboratory testing and subspecialists [8]. The World Health Organization now recommends DAAs as standard of care for HCV [9] and that all HCV-positive adults, including people who inject drugs (PWID), should be assessed for treatment regardless of symptoms, ongoing drug use, or perceived disease severity [9]. Access to DAAs is an important global health priority for low- and middle-income countries, which continue to rely on interferon-based regimens [9]. In one European modeling study, a modest increase in HCV treatment rates from 8 to 15 per 1000 PWID could halve HCV prevalence within 15 years [10].
Hepatitis C virus/HIV-coinfected individuals have a 50% higher risk of mortality than HIV monoinfected patients and should be prioritized for treatment [9, 11]. It is unfortunate that coinfected PWID also have the worst rates of treatment access [12, 13]. In one study of 845 coinfected patients in the United States, <35% of patients eligible for treatment were referred for HCV care, and only 0.7% of the full cohort achieved SVR [14]. Even if cost is addressed, significant social and structural barriers hinder implementation of effective care for HCV among PWID [15–19]. Unless these barriers are reduced, scale up of DAAs is unlikely to be successful.
National leaders have prioritized improving access to HCV treatment in China [11], home to 30 million people living with HCV infection. Two thirds of PWID are HCV positive [20], and an estimated 6% of patients enrolled in methadone treatment are HIV/HCV coinfected (approximately 80 000 individuals) [21]. China's large methadone treatment system and HIV care system provide an opportunity to examine facilitators and barriers to HCV treatment access among coinfected PWID. The purpose of this study was to explore social and structural factors affecting HCV treatment access in Guangzhou, China in order to inform the development of strategies for expanding access.
METHODS
Site
Our research sites included the outpatient HIV clinic at the Guangzhou Eighth People's Hospital (a high-volume referral center for HIV management and treatment within the province), the adjacent inpatient hospital, and 2 district-based methadone clinics. Twenty-seven patients were recruited from clinic, and 2 patients were admitted to the hospital for treatment of opportunistic infections and interviewed on the ward. Field observation at clinics took place for 1 month before interviews.
Subject Recruitment
A purposive sample of HCV/HIV-coinfected PWID were recruited and interviewed from October 2013 to March 2014. We included physicians and social workers to gain a better understanding of the local social environment, but we focused on patient narratives to develop a nonmedical perspective. Verbal consent was obtained from all participants. All participants received a phone card worth 100 RMB (approximately 16 US Dollars [USD]) for their participation.
Interviews
A semistructured, in-depth interview guide (Supplementary 1) was field tested among 8 patients, 2 inpatient physicians, and 3 key informants. Trained interviewers fluent in the local language (Cantonese or Mandarin) conducted interviews in a private location. Interview duration was 30 to 120 minutes. Interviews were audio-recorded if consent to record was provided; if not, field notes were taken. The interview guide included questions regarding the patients' experience with HCV treatment, HCV/HIV clinical history, injection drug use habits, and sociodemographic information. Emergent themes were explored further in subsequent interviews until thematic saturation was reached. We defined thematic saturation as the point at which further interviews did not yield new themes or trends.
Analysis
Our methodological approach was inductive in which themes were derived from empirical data, influenced by discourse analysis and grounded theory [22, 23] Interview transcripts were primary sources and were translated from Chinese to English. Transcripts were analyzed using Atlas.ti (version 7; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany). Initial coding was undertaken by 2 independent authors after examining 5 transcripts separately and developing a list of preliminary themes. These themes were discussed at length between the 2 researchers; any discrepancies were discussed with a third, senior investigator. The themes were then organized into a hierarchy based on their relationships with one another (Supplementary 2). These themes were also tested in subsequent participant interviews for validity. All transcribed data was examined based on the coding book.
We analyzed the preliminary themes using an adapted version of the Consolidated Framework for Implementation Research (CFIR) [24]. The CFIR domains included the following: (1) intervention characteristics, (2) individuals involved, (3) internal environment, and (4) external environment [24]. Intervention characteristics include cost, complexity, adaptability, sources, and evidence strength and quality. The individuals involved embody the knowledge and beliefs about intervention, self-efficacy, individual stage of change, identification with an organization, and other personal attributes. The inner environment includes structural characteristics, culture, and the implementation climate. Finally, the outer environment describes patient needs and resources, peer pressure, and external policies and incentives. We classified the barriers and facilitators to HCV treatment into this framework to present the hierarchy and interrelatedness of our findings.
Institutional Review Board
This study was approved by the University of North Carolina, Chapel Hill Institutional Review Board and the Guangzhou Eighth People's Hospital Institutional Review Board.
RESULTS
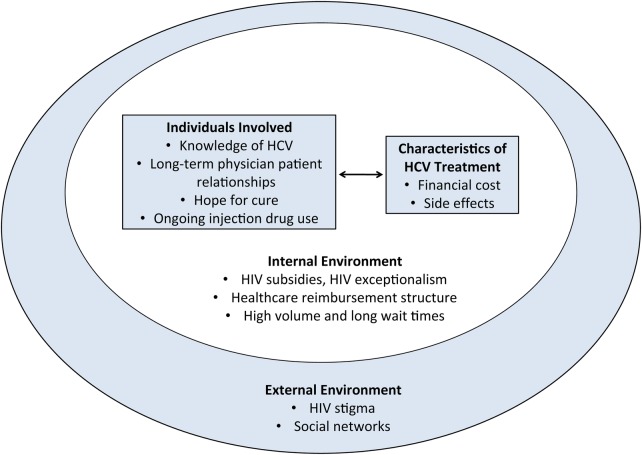
Interview transcripts from 29 patients, 8 physicians, and 3 social workers were analyzed (Table 1). Four patients (14%) had undergone interferon-based HCV treatment. No patients had received DAAs. Factors associated with HCV treatment access were organized based on (1) individuals involved, (2) intervention characteristics, (3) inner environment, and (4) outer environment (Figure 1). Physician and social worker data generally corroborated and extended patient perspectives.
Table 1.
Patient Population and Demographic Data
| Age (Years) | 27–53 |
|---|---|
| Gender | 28 M, 1 F |
| Enrolled in methadone treatment | 12 (41%) |
| Guangzhou resident | 17 (59%) |
| Inpatient participant | 2 (6%) |
| Highest education level attained | Elementary: 9 (31%) Middle: 13 (45%) High School: 6 (21%) College: 1 (3%) |
| History of HCV treatment | 4 (14%) |
| Unemployment rate | 13 (45%) |
| Average income (USD/year) | $3150 |
| History of detention | 22 (76%) |
Abbreviations: HCV, hepatitis C virus; USD, US Dollars.
Figure 1.
Factors impacting hepatitis C virus (HCV) treatment among people who inject drugs in Guangzhou, China. HIV, human immunodeficiency virus.
Individuals Involved
Positive Patient-Physician Relationships Facilitated Treatment
Long-term patient-physician relationships facilitated engagement in care. Human immunodeficiency virus patients formed closer relationships with infectious disease specialists and ancillary staff due to a model of HIV care that involved a multidisciplinary care team, frequent follow up, and continuity of care. As a result, HIV physicians played an important role in recommending HCV treatment and setting expectations for treatment outcomes, side effects, and adherence.
Later, my [HIV] doctor told me that my HCV viral load had reached more than 10 million [and] that hepatitis C could be treated […] He started prescribing the medication and I have been on hepatitis C treatment for more than a month.
– Patient, 39M, treated
Social acceptance of patients by infectious disease and substance abuse specialists also increased trust and willingness to accept treatment.
The infectious diseases hospitals are much better, just like the methadone clinic. The physicians there are closer to our type of patients.
– Patient, 38M, treated
The Hope for Cure Was a Strong Motivator for Treatment
Patients were willing to bear the financial and physical cost of treatment for a chance to achieve a SVR. Unlike HIV treatment, the HCV treatment course seemed finite and allowed patients to feel hopeful about being cured. Patients were able to plan ahead, both financially and emotionally.
If you want to be cured, there are no real problems, you just need to look at your attitude. Adherence is a bit inconvenient, but you just need to make sure you want to be cured. If you have a ‘waiting to die’ attitude, the outcomes would be very different.
– Patient, 39M, treated
Ongoing Injection Drug Use Was a Perceived Disincentive for Treatment
Providers and patients believed that ongoing drug use would have a significant negative impact on medication compliance and clinic follow up.
How would they have the money to get hepatitis C treatment? His first priority is drugs. These people simply do not work. If they do not work, where does the money come from? Even if they earn a bit, they would spend it on drugs. How can they treat hepatitis C?
– Patient 39M, untreated
Some providers considered patients' personal financial and social circumstances before recommending treatment, omitting a discussion about HCV entirely if they thought their patient was poor. Furthermore, PWID actively using drugs were mistrustful of the medical system for fear of being identified, arrested, and detained; these individuals were unlikely to seek care even if diagnosed.
Characteristics of Hepatitis C Virus Treatment
The Most Pervasive Barrier to Hepatitis C Virus Treatment Access Was Financial Cost
It's not that people don't want to get treated. It's that they don't have the financial means, so they are forced to give up.
– Patient, 39M, untreated
Personal poverty was a common theme, related to a history of injection drug use, prior incarceration, HIV coinfection, and lack of employment. Patients often relied on the government subsistence allowance of 500–600 RMB per month (approximately 80 to 100 USD) to meet their basic living needs. In comparison, the cost of a full 48-week course of peg-interferon and ribavirin cost 60 000 RMB (approximately 9600 USD).
90% of the people who got our kind of disease are relatively poor. In general, our type of people [PWID] do not have work for long periods of time […] I have no guarantee of basic needs such as three meals a day. How could I afford this treatment?
– Patient, 39M, untreated
Although HIV care was provided for free for all patients, HCV testing and treatment were not free. Supplemental insurance (a quarterly allowance for inpatient and outpatient health expenditures) was available only to patients registered at an urban address. Patients often delayed treatment as they saved money to support treatment. Many patients borrowed from families to meet the high cost of HCV treatment.
We need to wait until we save enough money. You can't start treatment for one month and then stop for a month, it won't work … but I'm worried about my liver getting worse.
– Patient, 43M, untreated
Inner Environment
Structural Barriers in the Health System Affected Access to Hepatitis C Virus Treatment
Fragmented reimbursement systems resulted in healthcare disparities in which tests and treatments were covered for some patients but not for others. This resulted in delays in both diagnosis and seeking care.
In an urban city, residents are allowed to have a free blood test once a year. Not in rural areas. People in rural areas usually don't choose to go to hospital until they have a severe problem.
– Patient, 47M, untreated
Furthermore, outpatient visits ranged from 3 to 8 minutes each, with long patient wait times. A single provider could see from 30 to 100 patients a day. Patient visits were often focused on HIV first; discussions of HCV and treatment options were rare.
Human Immunodeficiency Virus Coinfection Often Competed With Hepatitis C Virus Care
At times, HIV infection was a direct impediment to HCV treatment. National treatment guidelines stated that coinfected patients should have a CD4 count >200 to be eligible for HCV treatment, which excluded many PWID [9, 25]. Human immunodeficiency virus was also given higher priority than HCV, such that healthcare delivery, funding streams, and social services were focused on HIV, reflecting HIV exceptionalism [26]. As a result, healthcare needs related to HCV were often overshadowed, with much less disease knowledge and prioritization for HCV.
I thought HCV, in contrast to HIV, was not a big problem. I thought HIV treatment was more important. The fear of HIV is much greater than HCV […] there is not much publicly available medical information.
– Patient, 40M, untreated
Furthermore, HIV-coinfected patients were seen exclusively by HIV doctors, whereas HCV-monoinfected patients were typically seen by hepatologists. As a result, there were systematic differences in the quantity and quality of HCV care. There were instances where patients reported that physicians did not discuss their HCV-positive diagnosis and focused solely on HIV management. From the perspective of the physicians, some felt that their patients would not be able to afford, tolerate, or adhere to HCV treatment and therefore withheld the discussion.
To be honest, for those who had HIV, which is already a heavy burden, HCV treatment will cost several tens of thousands of yuan a year. Some people have the will but not the ability. It all depends on your circumstances.
– Patient, 42M, treated
Human immunodeficiency virus exceptionalism resulted in lack of information and familiarity with HCV infection. There was pervasive uncertainty about the long-term health consequences of chronic HCV infection, treatment length, anticipated side effects, and chances for treatment success. Some coinfected patients were not aware of their HCV status and therefore did not seek treatment.
I had no idea about my HCV, I just knew that most drug addicts have it.
– Patient, 41M, untreated
Decreased public awareness resulted in ambivalence about the disease.
People don't think treating HCV is important. They believe HCV won't cause harm for a long time and rarely results in cirrhosis … most patients are unwilling to treat HCV because they don't know about it.
– Social worker, 25M
Human Immunodeficiency Virus Coinfection Also Facilitated Access to Resources and Support
Patients with HIV infection could qualify for additional healthcare funds for medications and laboratory testing, as well as social support from nonprofit social work organization focused on HIV. This funding offset the cost of HCV treatment and was an important facilitator for treatment. Additional funding could be obtained for inpatient services for HIV-infected patents, and physicians would sometimes admit patients to the hospital to reduce out of pocket HCV treatment costs.
[HCV treatment] feels a bit expensive, but I am relatively lucky, the “Red Ribbon Organization” gives me 600 RMB subsidy every month, 3 months is 1800 RMB. Last time I saw the doctor, I spent all of it.
– Patient, 39M, treated
Human immunodeficiency virus diagnosis was also an entry point into the healthcare system, which led to an HCV diagnosis. Patients hospitalized with opportunistic infections were routinely tested for HCV. A positive diagnosis meant that some patients who became medically eligible for HCV treatment could receive it.
I thought I was young and robust, so I did not know about the HCV until my HIV was diagnosed.
– Patient, 40M, untreated
Outer Environment
Pervasive Human Immunodeficiency Virus Stigma Impacted Access to Hepatitis C Virus Treatment
We are the marginalized people, the most marginalized of society.
– Patient, 29M, untreated
Stigma was often tied to HIV status and history of injection drug use. Patients from rural areas reported pervasive stigma, even refusal of medical treatment, at their local hospitals, requiring them to travel long distances to find providers willing to take HIV-positive patients. Once outside of their area of residence, however, their HCV treatment was no longer covered, and financial challenges prevailed. Stigma against HIV-infected individuals reflected a moralistic attitude toward the disease and drug use. This was often internalized into a tainted sense of self.
If you were walking in the street and a normal person told you that he used to be a drug user, you would turn around and leave, because deep down you would think, he must be a bad guy. Even if you changed to a good guy, you are still bad.
– Patient, 29M, untreated
Stigma impacted disclosure, increased the sense of vulnerability, and rendered patients hesitant to discuss HCV with providers.
Talking about my HCV will do little help. If I talk about it aren't I just exposing myself?
– Patient, 40M, untreated
Fear of disclosure among peers perpetuated ongoing HCV transmission and decreased interest in HCV treatment, particularly if storing and injecting interferon would expose their disease status.
Social Support Promoted Awareness About Hepatitis C Virus Treatment
Patients found support among peers in the HIV clinic or the methadone clinic. A common identity resulted in greater trust. A peer-to-peer network, sometimes supported by formal community-based organization (CBO) activities, was important for disseminating information about treatment. Friends were important resources for identifying which hospitals and providers would provide compassionate care for the patient population. Friends and family were also important sources of moral support.
My friend is hopeful [about being cured of HCV] because his friends have also been through treatment and they support him.
– Patient, 39M, treated
Social workers played a critical role as well. They provided individual and group counseling to support medication adherence, abstinence from injection drug use, and daily attendance at methadone clinics. Social workers also provided job counseling to ease reintegration into employment. Social workers also played a role in connecting previously treated patients with patients currently contemplating treatment. However, they were also severely limited by time constraints and often traveled long distances to cover multiple sites.
DISCUSSION
This study presents the social and structural challenges to HCV treatment access among coinfected PWID. Other studies have demonstrated that HCV treatment outcomes improve when social factors unique to PWID are addressed [6–8, 10]. Our study favors acknowledgement of these social factors among PWID in a densely populated Chinese city. These factors also play a critical role in the management of other chronic diseases among this population, particularly in a primary care setting [27].
We found that social and peer networks facilitated HCV treatment access. These networks filled important roles with counseling, emotional support, linkage to community resources, and addiction recovery. This is consistent with literature showing that nonjudgmental treatment settings with peer-led support groups reduce stigma, facilitating treatment access and adherence [28]. Prior studies have demonstrated the effectiveness of using multidisciplinary team including peers to enhance community-based treatment programs [17, 29]. The importance of peers in contributing to HCV treatment access suggests that involving PWID CBOs and related groups may improve access.
Our research suggests that HCV treatment access is facilitated by integration with existing health delivery systems serving coinfected PWID. Other studies have demonstrated success with HCV treatment delivery in HIV clinics, prison health services, and methadone maintenance programs [17, 30, 31]. These are settings in which PWID at high risk for both HIV and HCV are most likely to be identified, and targeted interventions may be helpful [32]. Our research is also consistent with existing outcomes and cost-effectiveness literature showing that integration of HCV and HIV services may facilitate psychosocial support, financial subsidies, community health worker networks, and early monitoring of adverse effects for patients [33].
Our finding that patient-physician trust facilitated HCV treatment access suggests that interpersonal relationships play an important role in treatment decisions. Our interviews with physicians corroborated this finding that longitudinal relationships were important in facilitating acceptance and adherence to medical treatment. This is consistent with research from Australia demonstrating that trusting relationships between HCV-positive patients and their health workers can reduce stigma, increase healthcare service utilization, and reduce risk behaviors [29]. Long-term physician patient relationships allow expression of caring, concern, and compassion, which are important aspects of trust and treatment decisions [34]. Thus, providing the time and opportunity to nurture physician-patient relationships may contribute to increased rates of HCV treatment.
We found that poverty and high drug costs limited HCV treatment access. Hepatitis C virus treatment costs (both DAAs and interferon-based regimens) are prohibitive in the absence of generic formulations and reimbursement reform. A 12-week course of sofosbuvir currently costs approximately 84 000 USD in China, approximately 70 times the subsistence allowance provided over the same period of time [35]. Discussions have begun to include DAAs in the list of essential medicines, and Gilead has announced generic licensing and tiered pricing strategies to remove barriers for treatment implementation in 91 developing countries [36, 37]. However, China, Brazil, Russia, and other middle-income countries with high burden of HCV have been excluded from this list [35].
Finally, we found that hopefulness about cure increases motivation for testing and treatment. The development of DAAs provides an opportunity to mobilize communities, improve screening, and increase health-seeking behaviors. This is consistent with findings from qualitative research showing that hopefulness for an HIV cure may increase uptake of HIV testing [38]. As increasing numbers of HCV-positive PWID become interested in DAA treatment, linkage to care and retention will become critical priorities [39].
The strengths of this fieldwork are its location and in-depth focus on PWID, a large but marginalized population heavily affected by HCV. Our study site is also a strength—Guangzhou is a rapidly growing economy that has prioritized infectious disease research and healthcare reform, and progress here may be emblematic of large-scale change in China. This study may provide insight into HCV treatment implementation in other middle-income countries. Our empiric findings may inform harm reduction and HCV treatment programs in the United States, where PWID continue to be marginalized, integration of mental health and social interventions is poor, HIV coinfection is prevalent, and morbidity and mortality from HCV are on the rise.
Our study has multiple limitations. First, this is a single qualitative study that used purposive sampling. Broader inferences made from these data should be made with caution. Second, we were only able to interview 1 woman, and there may be sex differences in treatment access [16, 40]. Third, all HCV treatment provided to patients was interferon-based, which may not be transferrable to DAAs. Well tolerated oral regimens with very high rates of SVR and cure may mitigate some of the barriers identified in our study, including concerns about side effects, need for frequent follow up (and geographic limitations), and feelings of hopelessness. When asked, all patients unanimously agreed that a pill (ie, ribavirin) was much preferred to a needle (ie, interferon) as a route of drug administration, especially when needles were associated with intravenous drug abuse. By extension, DAAs may have higher acceptability and adherence rates.
However, changing the drug itself will not effect change on systems level—cost, stigma, and marginalization of PWID will continue to be an enormous barrier for DAAs. Middle-income countries such as China, Brazil, Russia, Ukraine, Philippines, and Mexico are seen as emerging markets for DAAs and were excluded from generic licensing agreements. A report from Médecins Sans Frontières' estimated that 49 million individuals living with HCV in middle-income countries were excluded from these agreements [41].
CONCLUSIONS
Direct-acting antivirals are currently our greatest tool for controlling the global HCV epidemic. This feat will be possible only by combining highly efficient, well tolerated drug combinations, active screening strategies, and improved access to care for PWID. This study provides in-depth study of the factors impacting access to current HCV treatment regimens. These findings may be transferrable to other settings, and they have been corroborated by other studies in low-, middle-, and high-income countries. Ongoing qualitative evaluation of DAA access in PWID is critical for guiding interventions. Future policy priorities should be focused on addressing the specific needs of PWID and improving linkage and retention in healthcare systems.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We acknowledge Drs. Faye Hu, Qingyan Ma, Christopher Stewart, and Joel Palefsky for their mentorship and support.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013; 10:553–62. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis C. (Fact sheet no. 164); 2014. Available at: http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed 19 April 2016.
- 3.Lavanchy D. The global burden of hepatitis C. Liver Int 2009; 29:74–81. [DOI] [PubMed] [Google Scholar]
- 4.Ly KN, Xing J, Klevens RM et al. . The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012; 156:271–8. [DOI] [PubMed] [Google Scholar]
- 5.Feeney ER, Chung RT. Antiviral treatment of hepatitis C. BMJ 2014; 348:g3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 2014; 146:1176–92. [DOI] [PubMed] [Google Scholar]
- 7.Naggie S, Sulkowski MS. Management of patients coinfected with HCV and HIV: a close look at the role for direct-acting antivirals. Gastroenterology 2012; 142:1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano V, Labarga P, Fernández-Montero JV et al. . The changing face of hepatitis C in the new era of direct-acting antivirals. Antiviral Research 2013; 97:36–40. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection, 2014. Available at: http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed 19 April 2016. [PubMed]
- 10.Martin NK, Vickerman P, Grebely J et al. . Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale‐up in the age of direct‐acting antivirals. Hepatology 2013; 58:1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Zhu H, Wu Y et al. . HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010–12: a retrospective observational cohort study. Lancet Infect Dis 2014; 14:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papatheodoridis G, Tsochatzis E, Hardke S, Wedemeyer H. Barriers to care and treatment for patients with chronic viral hepatitis in Europe: a systematic review. Liver Int 2014; 34:1452–63. [DOI] [PubMed] [Google Scholar]
- 13.Beisel C, Heuer M, Otto B et al. . German cohort of HCV mono-infected and HCV/HIV co-infected patients reveals relative under-treatment of co-infected patients. AIDS Res Ther 2014; 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta SH, Lucas GM, Mirel LB et al. . Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS 2006; 20:2361–9. [DOI] [PubMed] [Google Scholar]
- 15.Treloar C, Rance J, Backmund M. Understanding barriers to hepatitis C virus care and stigmatization from a social perspective. Clin Infect Dis 2013; 57:S51–5. [DOI] [PubMed] [Google Scholar]
- 16.Swan D, Long J, Carr O et al. . Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS 2010; 24:753–62. [DOI] [PubMed] [Google Scholar]
- 17.Treloar C, Newland J, Rance J, Hopwood M. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J Viral Hepat 2010; 17:839–44. [DOI] [PubMed] [Google Scholar]
- 18.Harris M, Rhodes T, Martin A. Taming systems to create enabling environments for HCV treatment: negotiating trust in the drug and alcohol setting. Soc Sci Med 2013; 83:19–26. [DOI] [PubMed] [Google Scholar]
- 19.Coupland H, Maher L. Notions of injecting drug users' candidacy for hepatitis C treatment: conflicting provider, patient, and public health perspectives. Contemp Drug Probl 2010; 37:549–73. [Google Scholar]
- 20.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–42. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhang D, Chen W et al. . High prevalence of HIV, HCV and tuberculosis and associated risk behaviours among new entrants of methadone maintenance treatment clinics in Guangdong Province, China. PLoS One 2013; 8:e76931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. St. Louis: Transaction Publishers, 2009. [Google Scholar]
- 23.Potter J, Wetherell M. Discourse and Social Psychology: Beyond Attitudes and Behaviour. New York: Sage, 1987. [Google Scholar]
- 24.Damschroder LJ, Aron DC, Keith RE et al. . Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009; 4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor LE, Swan T, Mayer KH. HIV coinfection with hepatitis C virus: evolving epidemiology and treatment paradigms. Clin Infect Dis 2012; 55:S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayer R. Public health policy and the AIDS epidemic: an end to HIV exceptionalism? N Engl J Med 1991; 324:1500–4. [DOI] [PubMed] [Google Scholar]
- 27.Nambiar D, Stoove M, Dietze P. A cross-sectional study describing factors associated with utilisation of GP services by a cohort of people who inject drugs. BMC Health Serv Res 2014; 14:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis 2013; 57:S56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alavi M, Grebely J, Micallef M et al. . Assessment and treatment of hepatitis C virus infection among people who inject drugs in the opioid substitution setting: ETHOS study. Clin Infect Dis 2013; 57:S62–9. [DOI] [PubMed] [Google Scholar]
- 30.Post JJ, Arain A, Lloyd AR. Enhancing assessment and treatment of hepatitis C in the custodial setting. Clin Infect Dis 2013; 57:S70–4. [DOI] [PubMed] [Google Scholar]
- 31.Gunn RA, Lee MA, Callahan DB et al. . Integrating hepatitis, STD, and HIV services into a drug rehabilitation program. Am J Prev Med 2005; 29:27–33. [DOI] [PubMed] [Google Scholar]
- 32.Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy 2007; 18:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford N, Singh K, Cooke GS et al. . Expanding access to treatment for hepatitis C in resource-limited settings: lessons from HIV/AIDS. Clin Infect Dis 2012; 54:1465–72. [DOI] [PubMed] [Google Scholar]
- 34.Blendon RJ, Benson JM, Hero JO. Public trust in physicians—U.S. medicine in international perspective. N Engl J Med 2014; 371:1570–2. [DOI] [PubMed] [Google Scholar]
- 35.Forette C. Gilead's License on Hepatitis C Drugs, Sofosbuvir and Ledipasvir: A Fool's Bargain - Myths and Facts, 2014. Available at: http://www.hepcoalition.org/IMG/pdf/countries_excluded_gilead_s_vl.pdf. Accessed 19 April 2016.
- 36.Gilead. Chronic Hepatitis C Treatment Expansion: Generic Manufacturing for Developing Countries. Available at: http://www.gilead.com/~/media/Files/pdfs/other/HCV%20Generic%20Agreement%20Fast%20Facts%203215.pdf. Accessed 19 April 2016.
- 37.Jayasekera CR, Barry M, Roberts LR, Nguyen MH. Treating hepatitis C in lower-Income countries. N Engl J Med 2014; 370:1869–71. [DOI] [PubMed] [Google Scholar]
- 38.Chu C, Wu F, He X et al. . Exploring the social meaning of curing HIV: a qualitative study of people who inject drugs in Guangzhou, China. AIDS Res Hum Retroviruses 2015; 31:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong VW, Wong GL, Chim AM et al. . Targeted hepatitis C screening among ex-injection drug users in the community. J Gastroenterol Hepatol 2014; 29:116–20. [DOI] [PubMed] [Google Scholar]
- 40.Crockett B, Gifford SM. “Eyes Wide Shut”: narratives of women living with hepatitis C in Australia. Women Health 2004; 39:117–37. [DOI] [PubMed] [Google Scholar]
- 41.Strategies to Secure Access to Generic Hepatitis C Medicines. MSF 2015. Available at: https://www.msfaccess.org/sites/default/files/MSF_assets/HepC/Docs/HepC_brief_OvercomingbarriersToAccess_ENG_2015.pdf. Accessed 19 April 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.