Abstract
The development and evolution of the inner ear sensory patches and their innervation is reviewed. Recent molecular developmental data suggest that development of these sensory patches is a developmental recapitulation of the evolutionary history. These data suggest that the ear generates multiple, functionally diverse sensory epithelia by dividing a single sensory primordium. Those epithelia will establish distinct identities through the overlapping expression of genes of which only a few are currently known. One of these distinctions is the unique pattern of hair cell polarity. A hypothesis is presented on how the hair cell polarity may relate to the progressive segregation of the six sensory epithelia. Besides being markers for sensory epithelia development, neurotrophins are also expressed in delaminating cells that migrate toward the developing vestibular and cochlear ganglia. These delaminating cells originate from multiple sites at or near the developing sensory epithelia and some also express neuronal markers such as NeuroD. The differential origin of precursors raises the possibility that some sensory neurons acquire positional information before they delaminate the ear. Such an identity of these delaminating sensory neurons may be used both to navigate their dendrites to the area they delaminated from, as well as to help them navigate to their central target. The navigational properties of sensory neurons as well as the acquisition of discrete sensory patch phenotypes implies a much more sophisticated subdivision of the developing otocyst than the few available gene expression studies suggest.
Keywords: ear development, ear sensory neurons, sensory neuron migration, sensory neuron projection
Introduction
Systems biology of the inner ear development is just coming of age with the identification of rudimentary gene networks involving specific genes (e.g., bHLH, Pax, Wnt, Forkhead), which play fundamental roles in the fate assignment and subsequent clonal expansion of these basic cellular building blocks (reviewed in Fritzsch and Beisel, 2001; Fekete and Wu, 2002). Recently, we have hypothesized that the development of the neurosensory and nonsensory epithelium of the vertebrate inner ear can be interpreted as transformation of the basic building blocks of the sensory ciliated neuronal structures of C. elegans and Drosophila. In contrast, formation of the sensory neurons appears to be a de novo formation peculiar to vertebrates. The mechanisms by which these epithelia form the complex three-dimensional structure and their interactions with sensory neurons are just now becoming understood. We will present herein new ideas and data concerning the formation of the distinct endorgans of the inner ear and their innervation patterns. In particular, we will explore the current molecular understanding of endorgan formation, separation, and transformation to present a model of saccular transformation into the cochlea. In principle, the well-known molecular multiplication and diversification model will be followed (Venter et al., 2001). We propose that enlargement of a given sensory epithelium, through enhanced proliferation followed by segregation and development of novel, functionally relevant modifications, is at the core of inner ear sensory patch evolution.
Evolution has transformed a simple ear with only two canals and a single macula communis found in ancestral vertebrates into a complex three-dimensional structure that has up to nine distinct endorgans (Fig. 1) in derived vertebrates (Lewis et al., 1985; Fritzsch, 1987; Fritzsch and Wake, 1988). Previous work on the evolution of these various sensory epithelia suggests specific relationships. For example, it has been proposed that the saccule gives rise to the basilar papilla or cochlea of land vertebrates (Fritzsch, 1992). In keeping with this suggestion, recent developmental evidence has lent itself to the idea of evolutionarily progressive segregation of sensory epithelia through unknown processes to form distinct rather than confluent sensory patches (Norris, 1892; Fritzsch and Wake, 1988; Cantos et al., 2000; Farinas et al., 2001; Fritzsch et al., 2001). However, almost none of these articles have actually taken a closer look as to what kind of transformation is occurring to turn a saccule-like anlage into a mammalian cochlea and how this segregation relates to the formation of discrete innervation patterns.
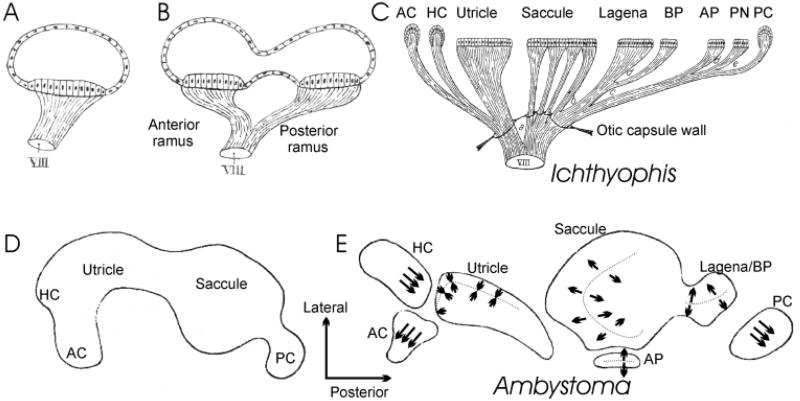
Figure 1.
These images show the conjecture of de Burlet (1934) about the evolutionary similarities of endorgan and innervation multiplication from two hypothetical ancestors (A,B) to the vertebrates with the largest number of sensory epithelia, Southeast Asian caecilians (C). In his accompanying text, de Burlet pointed this out explicitly, but without proposing any mechanism. Conceivably, two mechanisms are apparent. In one mechanism there is lineage relationship between hair cells and sensory neurons. Thus whenever hair cell precursors are split their innervation will split. Alternatively, there is no relationship at all between hair cells and sensory neurons, and the multiplication of innervation comes about through the selective attraction of sensory afferents to newly formed endorgans. In the latter scenario both central and peripheral specifications of the sensory projections need to be accomplished independently of the changes in hair cell specification. In the lineage relationship scenario, any alteration in the specification of hair cells would also affect the information conveyed by sensory cells, thus allowing for a rapid coevolution of hair cell and sensory neuron fate specification. The work of Norris (1892) on salamanders indicated that the above-presented evolutionary scenario might actually be recapitulated during development. He depicted that a single prosensory anlage splits over time into the different sensory organs (D,E). It is unclear at which point in development polarity of hair cells is established. We tentatively superimposed the known adult polarity distribution in salamanders (Lewis et al., 1985) onto the almost completely segregated patches of forming sensory epithelia. Note that all canal cristae have only one hair cell polarity, that the utricle has only polarity toward the striola (indicated by the dotted line), and that the saccule and sensory epithelia derived from the saccule are all polarized away from a dividing line in many salamanders. Abbreviations: AC, anterior crista; AP, amphibian papilla; BP, basilar papilla; HC, horizontal crista; PC, posterior crista; PN, papilla neglecta. Modified after Norris (1892), de Burlet (1934), and Lewis et al. (1985).
For example, as distinct sensory patches have developed, neuronal projections to these patches have apparently also coevolved. In order to accommodate a divergent functionality of each endorgan, there exists a need for afferents to segregate from existing projections. This separation is crucial to ensure that the novel information gathered by the new endorgan is reaching the brainstem in a distinct conduit for further specific processing. Nineteenth century anatomists suggested a parallel evolution between the progressive segregation of each sensory epithelium with a concurrent segregation of sensory projections to these newly evolved endorgans. Retzius (1884) and the brothers Sarasin (1892) were the first to recognize this trend, and Retzius (1884) used this idea to develop a cladistic relationship among extant vertebrates. de Burlet (1934) tried to further formalize this idea of conservation and transformation of the inner ear innervation by suggesting a progressive split of both innervation and sensory epithelia (Fig. 1).
In contrast to this apparent evolutionary segregation of peripheral innervation, it has been hypothesized that the central projection of vestibular and cochlear afferents may originally overlap (Larsell, 1967). Such overlap in “ancestral” vertebrates appears to precede the segregated projections observed in the more recently derived vertebrates (Dickman and Fang, 1996; McCormick, 1999). It has only recently been recognized that during inner ear development, innervation is initially fairly precise. For example, central projections will undergo only limited reorganization of terminals due to continuing progressive segregation (Maklad and Fritzsch, 2002). Likewise, developmental analyses of inner ear afferent innervation suggest an early and precise segregation of afferent projections to their respective sensory epithelia (Fritzsch et al., 1995; Huang et al., 2001; Kim et al., 2001). It is fair to say that hardly any information exists about the molecular mechanism that specifies either central or peripheral projections to and from the ear.
We will provide evidence that suggests a developmental link between sensory patch formation and formation of novel sensory neurons. In addition, data will also be presented suggesting that spatio-temporal expression of neurotrophins in sensory epithelia is a critical mediator for the proper segregation of afferent projection to specific endorgans.
Segregation of Endorgans: an Evolutionary Perspective
In general, evolution of the ear is based on multiplication of existing sensory patches. These modifications are likely followed by functional diversification through creation of modified, unique structures that allow transduction of a previously unexplored property of the mechanical energy that reaches the ear.
The simplest ear to be found in extant vertebrates is the hagfish ear. This ear has only three sensory epithelia, one macula communis, and two crista organs (Lewis et al., 1985; Fritzsch, 2001a,b). The largest number of sensory patches in the ear is found in certain species of limbless amphibians (Fig. 1), which have nine different sensory patches: three canal cristae, utricle, saccule, lagena, papilla neglecta, papilla basilaris, and papilla amphibiorum (Sarasin and Sara-sin, 1892; Fritzsch and Wake, 1988). Descriptive developmental evidence has long suggested that the evolution of multiple sensory epithelia came about through developmental splitting of a single sensory anlage (Norris, 1892; Fritzsch et al., 1998). Moreover, it appears that organs such as the lagena may have evolved three times independently in vertebrates, each time by splitting off from the saccule (Fritzsch, 1992). In contrast, other organs such as the basilar papilla may have evolved only once, namely during the segregation of the lagena from the saccule in the tetrapod ancestors (Fritzsch, 1987).
Such segregation and functional diversification are best documented for the papilla neglecta/papilla am-phibiorum system. It appears that the location of each of the two sensory patches to either the utricle or the saccule and association with the perilymphatic sound conduction system may determine the future function as a sound pressure receiver or as an additional vestibular receptor (Fritzsch and Wake, 1988; Brichta and Goldberg, 1998). Another well-documented example is the utricle in some fishes (herring) that forms three distinct sensory patches. While two of these patches retain their ancestral function as gravistatic receptors, one patch has acquired a novel function as a sound pressure receiver by association with the perilymphatic sound conduction system (Lewis et al., 1985; Fritzsch, 2001a).
Development and Evolution of a Third Sensory Patch for Angular Velocity Reception
Comparison of craniate vertebrates suggests that two canals with their associated sensory epithelia is a primitive feature retained in lampreys and hagfish. In contrast, jawed vertebrates have modified this primitive ear by adding a third canal, the horizontal canal (Lewis et al., 1985). Detailed analysis of the organization of the canal sensory epithelium shows distinct differences between hagfish, lampreys, and jawed vertebrates that implies a morphocline toward formation of a crista (Fritzsch, 2001a).
Recently the homeobox gene orthodenticle (Otx) was identified as being important for the formation of the horizontal canal in jawed vertebrates. Otx is a highly conserved gene that delineates the anterior part of the central nervous system in vertebrates and invertebrates (Reichert and Simeone, 1999). Jawed vertebrates have evolved two Otx genes, Otx1 and Otx2. Both show partially overlapping expression in the brain and the ear (Morsli et al., 1999; Cantos et al., 2000). In contrast, Otx is not expressed in the lamprey ear (Tomsa and Langeland, 1999). Confirming the suggested role of Otx1 is the absence of the horizontal (lateral) canal in Otx1 null mutant mice (Morsli et al., 1998). Moreover, Otx1 can be substituted for Otx2 in the forebrain but not in the ear (Acampora et al., 2000). Thus, it can be speculated that the entire horizontal canal system depends on Otx1 (Mazan et al., 2000). However, closer examination of the Otx1 null phenotype has revealed that some parts of the horizontal crista appear to form initially (Morsli et al., 1999) and may remain as displaced patches of hair cells (Fritzsch et al., 2001). Moreover, sensory fibers were found to extend to those displaced patches of hair cells (Fritzsch et al., 2001).
Indeed, close comparison of jawed vertebrates with lampreys suggests certain similarities with the dorsal papilla, a small patch of sensory hair cells of lampreys. These similarities in distribution and pattern of innervation of the dorsal papilla of lampreys and the displaced horizontal crista of Otx1 null mutant mice suggest a two-step evolution of the horizontal canal system. First, through molecularly unknown mechanisms, a multiplication of hair cells and a segregation of the dorsal sensory patch occurred in the common ancestor of lampreys and jawed vertebrates. This patch has a separate innervation and thus would be able to transmit specific information only gathered by this sensory patch to the brain. This scenario predicts the existence of distinct genes that are essential for formation of the horizontal crista but have little or no effect on horizontal canal formation.
Further evolution has refined this central projection into two discrete patterns, an elasmobranch pattern and a pattern for all other jawed vertebrates. In each of these two lineages the horizontal canal system is linked with eye movement control to form a unique output system (Fritzsch, 1998). Evolution of the third, horizontally positioned canal allowed a direct coding of angular movement in all three cardinal planes for fast activation of eye and postural control movements without additional computational delay in the hindbrain. This advantage was evolutionarily stabilized and thus extant vertebrates have three patterns of eye muscles and eye muscle innervation (Fritzsch, 1998). Unfortunately, the pattern of horizontal crista/dorsal papilla projection as compared with the vertical cristae still needs more detailed analyses in lampreys, elasmobranches, and other jawed vertebrates. The above outlined scenario of horizontal canal evolution predicts that these three vertebrate lines will each have a unique pattern of central projections of these probably homologous sensory epithelia.
Development of the Cochlea
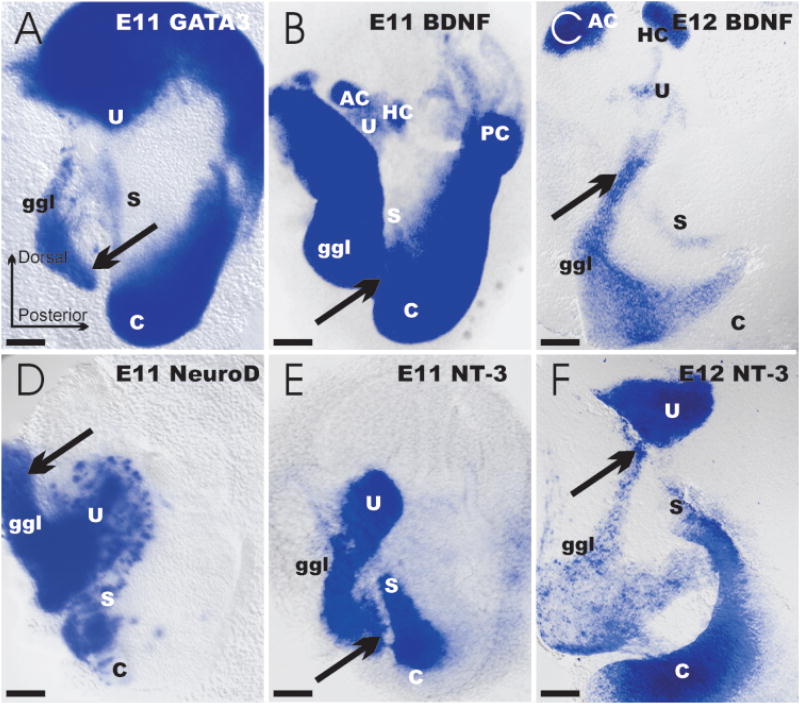
An evolutionary scenario of developmental segregation of the tetrapod cochlea from the saccule was proposed some time ago (Norris, 1892; Fritzsch, 1992). However, initial molecular analysis of chick ear development showed that each sensory patch appeared as a discrete entity instead of through progressive segregation from a single, molecularly recognizable anlage (Wu and Oh, 1996). These data support a boundary model (Brigande et al., 2000; Cantos et al., 2000; Fekete and Wu, 2002). This model proposes that a given sensory receptor develops initially at the intersection of specific polarity coordinates that reflect expression domains of specific genes in the ear. However, more recent data on chicken (Cole et al., 2000) as well as on mouse inner ear development (Morsli et al., 1998) have re-established the possibility for the progressive segregation of the cochlear anlage from the saccule during development. Studying the in situ expression for bone morphogenetic protein-4 (BMP-4), Serrate 1, and lunatic fringe, Cole et al. (2000) suggested the existence of a sensory competent region that encompasses all presumptive sensory organs. Likewise, using in situ hybridization, Morsli et al. (1998) described a single patch of lunatic-fringe-positive cells that underwent progressive segregation into the utricle, saccule, and cochlea during development of the mouse ear. These findings were confirmed through the analyses of the developmental expression dynamics of neurotrophins (Farinas et al., 2001). Specifically, the lacZ reporter for brain-derived neurotrophic factor (BDNF) expression suggests an early formation of the three canal cristae [embryonic day 12 (E12); Fig. 2]. Each epithelium is uniquely characterized by exclusive expression of BDNFlacZ. In contrast, neurotrophin-3 (NT-3lacZneo) is initially up-regulated as early as E9.5 in a single, anteroventral patch. In the course of the next 4 days, this single patch elongates and gives rise to the utricle (E11.5) followed by a segregation of the saccule (Fig. 2), and, finally, cochlea by E13.5 (Farinas et al., 2001) and older stages (Fig. 3).
Figure 2.
These wholemounted ears show the expression of various genes as revealed with a lac-Z marker inserted into GATA3, BDNF, NeuroD, and NT-3 genes (A,B,D,E). At embryonic day 11 (E11) distinct patterns of expression are apparent. Note that the distribution of GATA3 and BDNF has some similarities, but also show clear differences. In contrast, similarities between the NeuroD and NT-3 expression in the otocyst are obvious in the area of the future utricle and saccule. Note that each of these four genes is expressed both in restricted areas of the ear and in cells of the forming vestibulo-cochlear ganglion. LacZ-positive cells (arrows) can be seen to delaminate from the otocyst in all four cases. Delamination and cellular migration to the forming vestibulo-cochlear ganglion is even more obvious at later stages [E12 (C,F)] where lacZ- positive cells can be traced from equally lacZ-positive areas of the otocyst. Bar indicates 100 μm.
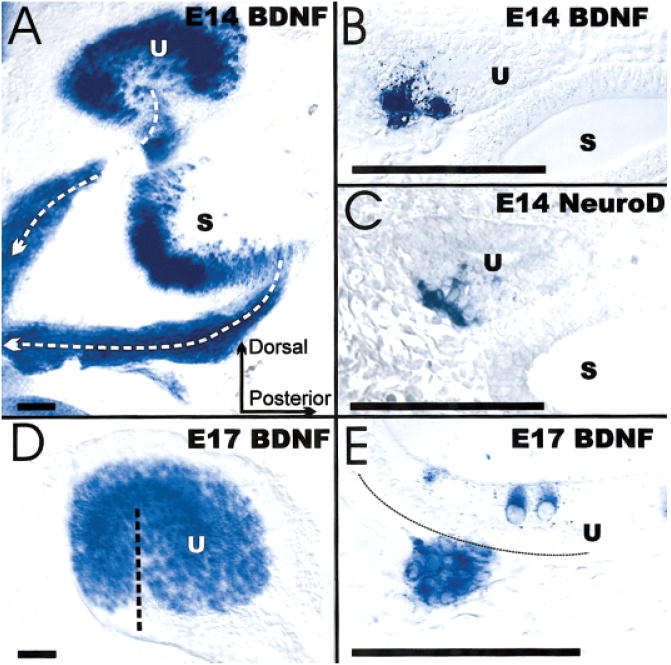
Figure 3.
These images show the distribution of lacZ-labeled cells in histological sections (B,C,E), wholemounts (A,D), BDNF (A,B,D,E), and NeuroD (C). These images show the similarities in distribution of NeuroD and BDNF inside the utricle as well as in delaminating cells (B,C). The wholemounts show the distribution of BDNF in forming hair cells inside the saccule and utricle as well as the delaminating cells converging from specific areas of these sensory epithelia toward the forming vestibular ganglion (A). Such cells delaminate as late as E17 from the hair-cell-free hilus region of the utricle that is filled with hair cells only after completion of this delamination (D,E). Bar indicates 100 μm.
It needs to be stressed that the use of the lacZ reporter for BDNF and NT-3 expression to identify sensory patch anlage is justified by the restricted expression of these neurotrophins in hair cells (BDNF) and supporting cells (NT-3). The expression patterns of most of the other developmental markers, such as lunatic fringe or BMP-4, have yet to be clarified in the ear in regards to their functional relationship with sensory patch development (Fekete and Wu, 2002). For example, BMP-4 predominantly inhibits neural differentiation in early development. In the brain, fibroblast growth factors (FGFs) typically counterbalance BMP-4 activity (Diez del Corral and Storey, 2001; Scully and Rosenfeld, 2002), and the alleged role of BMP-4 in dorsal interneuron specification was recently shown to be mediated by Wnt genes (Muroyama et al., 2002). It is, however, fair to say that the role of BMP-4 can change in later development to be more proneural, but this role has not yet been explored in detail in the ear owing to the early lethality of BMP-4 null mutant mice. Conditional null mice specific for the ear are needed to further clarify the role of BMP-4.
Interestingly, expression of BDNFlacZ initially parallels closely with the expression domain of BMP-4 in the abneuronal region of the cochlea (Morsli et al., 1998; Farinas et al., 2001), whereas NT-3 expression closely corresponds to lunatic fringe expression patterns. While BDNF expression is early in development of the three canal cristae, BDNF expression is delayed until E12.5 in the utricle and saccule and until E13.5 in the cochlea (Fig. 2). Closer examination of inner ear sections and wholemounts shows that full segregation of the three NT-3lac-Zneo-positive patches coincides with the morphogenetic separation of each sensory epithelium (Figs. 2 and 3). Thus, the initial separation of utricle and saccule parallels with the formation of the utriculo-saccular foramen. If the formation of this foramen is even only partially disabled, as in Otx-1 null mutant mice, both epithelia remain confluent (Morsli et al., 1999; Fritzsch et al., 2001). The segregation of the saccule and cochlea concurs with the formation of the ductus reuniens. Interestingly, in this case the expression of BDNF simply stops in the connecting nonsensory epithelium between the saccule and the cochlea at E13.5 (Farinas et al., 2001).
In summary, both older descriptive as well as more recent molecular analyses suggest that possibly a single patch of sensory organ competence is divided by unknown mechanisms into the sensory patches of a specific vertebrate. Development appears to recapitulate this aspect of ear evolution surprisingly unaltered.
Establishing Hair Cell Polarity in Sensory Epithelia
If developmental segregation of sensory patches is accepted as a major driving force in ear evolution, then the differential polarity of hair cells within and between endorgans must be addressed. A plausible mechanism(s) must be detailed on how such transformations in hair cell polarity might have occurred. These variations in polarity permit differential mechanical stimulation to be recognized by one sensory epithelium versus another. For example, the utricle has two opposing polarity orientations of the hair cells in the epithelium (Fig. 1), while the canals have only one polarity of hair cells (Lewis et al., 1985). Derivation of a canal from the utricle requires that segregation occurs from only one area of polarity of hair cells. Indeed, developmental studies (Figs. 1 and 2) suggest that the horizontal crista may derive only from the abneuronal polarity patch (Farinas et al., 2001; Fritzsch et al., 2001). Interestingly, the horizontal crista has a similar polarity compared to this part of the utricle (Lewis et al., 1985). In contrast, hair cells in the evolutionarily and developmentally (Morsli et al., 1998) earlier derived anterior and posterior crista have a polarity away from the utricle. In order to resolve this issue, more data on early markers around E11–12 are needed to demonstrate more exactly where each of the two vertical canal crista are derived from in the utricle.
Like the canal cristae, the mammalian cochlea has hair cells of only one polarity. If one extends the line of polarity of the saccule across the ductus reuniens one ends up with the striola of the saccule roughly equal to the divide between the greater and lesser epithelial ridge. The greater epithelial ridge would be equivalent to the neuronal half of the saccule whereas the lesser epithelial ridge would be equivalent to the abneuronal half of the saccule. The recently reported finding of hair cell differentiation of cells of the greater epithelial ridge through expression of mouse atonal homologue 1(Math1) (Zheng and Gao, 2000) would be in line with such an interpretation. These data imply that the greater epithelial ridge has retained the capacity to form hair cells, but is not normally doing so owing to some changes in the up-stream regulation of hair-cell-specific gene expression.
Interestingly, this suggests, similar to the relationship of canals and saccule, that polarity is conserved away from the striola/greater epithelial ridge. It thus appears that the cochlea of mammals is nothing but an elongated and highly modified abneuronal half of the saccule. Clearly, the development of expression domains of both lunatic fringe (Morsli et al., 1998) and NT-3 (Farinas et al., 2001) support this possibility by showing a progressive segregation of a single patch into a saccule and the cochlea.
BDNF shows an initial up-regulation in the striola region (Fig. 2) of both utricle and saccule (Farinas et al., 2001). In the cochlea, the up-regulation of BDNF sweeps from inner hair cells to outer hair cells, indicating a conservation of spatio-temporal patterning between the utricle, saccule, and cochlea. The main problem with such a scenario is the need to explain the loss of hair cell differentiation in the ductus reuniens and in the neuronal part of the cochlea, the largest part of the greater epithelial ridge (GER). However, the data on Math1 expression effects clearly show the potential of this area to generate hair cells (Zheng and Gao, 2000). Likewise, the formation of extra hair cells in the GER in Hes5 null mutants (Zine et al., 2001) shows the latent capacity of the GER to form hair cells. In addition, those extra GER hair cells have a polarity opposite to the cochlear hair cells (A. Zine, personal communication). Those sets of experimental data are compatible with a cochlear origin out of the saccular anlage through selective suppression of hair cell formation in the GER. Clearly, neurotrophins, while representing suitable sensory epithelia markers, will in themselves not determine development of the sensory epithelia, as is apparent by the normal development in neurotrophin null mutants (Fritzsch et al., 1999). Further molecular understanding of neurotrophin expression regulation in the ear is needed to relate the expression of these markers to sensory patch differentiation.
Hair cell polarity is essential for each sensory patch. For example, turning the hair cell polarity of the cochlea by 90° would render the cochlea essentially unresponsive to sound. Despite this importance, next to nothing is known about how polarity is achieved at a cellular level. However, some of the molecular players of denticle polarity formation in Drosophila parasegment development have been identified in the developing ear, for example, Serrate (Morsli et al., 1998; Hatini and DiNardo, 2001). It remains to be shown how many of the other molecular players of this developmental module will be found in the ear and whether their interaction is important for the polarity formation of hair cells during sensory patch development.
Evidence for Cell Fate Links between Sensory Neuron and Hair Cell Precursors
In order to comprehend the change in cell fate acquisition to form the three-dimensional cochlea, we need to understand how sensory neuron development relates to hair cell development. In general, sensory neurons and hair cell formation each depend on a single proneuronal gene, neurogenin 1(ngn-1), and Math1, respectively. Ngn-1, a bHLH gene, is essential for formation of all sensory neurons in the ear (Ma et al., 2000). Similarly, Math1 has been shown to be essential for hair cell development (Bermingham et al., 1999). If the sensory neurons and hair cells have a common precursor, then alternations in ngn-1 and/or Math1 may affect generation of these two cell types. In this context, loss of ngn-1 also results in reduction in the sensory epithelia. In particular the saccule and the cochlea show severe reductions in hair cell formation (Ma et al., 2000). This suggests that there are interactions between ngn-1 and Math1 dependent precursors. Two possibilities exist for such an interaction. One of these is that some clones are first ngn-1 dependent and subsequently switch to Math1, shutting down ngn-1 through unspecified intracellular signaling pathways (Fritzsch and Beisel, 2001; Gowan et al., 2001). This does not necessarily mean that sensory neurons and hair cells are clonally related as this could also come about through a transient and independent expression of ngn-1 in presumptive sensory patches without forming sensory neurons. Alternatively, ngn-1 and Math1 exist in distinct clones but the clones are linked through an extracellular suppressor system such as the well-known delta/notch system (Lanford et al., 2000). At any rate, ngn-1 null mutants show a massive effect of ngn-1 on hair cells.
Spatial Origin of Sensory Precursors in Mice
To explore the validity of the proposed scenario for sensory patch formation and its hypothetical link to sensory neuron formation, we will revisit the recent evidence characterizing specific areas of precursor delamination as revealed through expression patterns of neurotrophins (Farinas et al., 2001). In vitro experiments have shown that a large proportion of sensory neurons delaminate from the anteroventral quadrant of the otocyst (Van de Water, 1983). This is observed in a number of vertebrates as demonstrated by various staining techniques to label delaminating cells (Cole et al., 2000; Fekete and Wu, 2002). However, none of these studies has actually performed a three-dimensional mapping to demonstrate how stable or mobile the area(s) of delamination is over the rather protracted period of sensory neuron proliferation, which lasts several days in mice (Ruben, 1967). Those areas of delamination correspond to either part of the sensory patches (Figs. 2 and 3) or are adjacent to sensory patches (Farinas et al., 2001). These data suggest that delamination of cells actually occurs in spatially restricted areas of the ear.
The BDNFlac Z reporter system has been used to demarcate neuronal cellular delamination in the inner ear. The utricle, more specifically the hilus region, gives rise to delaminating cells from E10.5 to E16.2 (Figs. 2 and 3). These cells express BDNF and NT-3, and the delaminating cell types derive from the same restricted area on the neuronal side of the utricle. In the later stages there is a mutual exclusion of BDNF-positive hair cells and delaminating cells (Fig. 3). Only after all delamination has stopped at E16.5 is there an addition and filling in of the hilus region by hair cells. Indeed the hilus appears to be an area that produces in temporal sequence a mixture of delaminating cells and hair cells (Fig. 3). Again, this spatio-temporal pattern is consistent with the possibility that expression of Math1 (hair cells) and ngn-1 (precursors) does not occur in the same cell.
The pattern of delamination around the saccule is different from that in the utricle. It appears that all BDNFlac Z- and NT-3lac Zneo-positive delaminating cells are derived from the expanding region between the cochlear base and the saccule (Figs. 2, 3, 4). This region is initially free of BDNF expression but shows a subsequent up-regulation of BDNF in these putative precursors. The appearance of these cells is not due to a generalized retention of the lacZ marker. NT-3 expression will be down-regulated in this area between E12.5 and E13.5. This area will not generate hair cells but will turn into the ductus reuniens connecting the cochlear duct with the saccule. It thus appears that the connecting area between saccule and cochlea transforms into a domain of delaminating precursor cells at the expense of hair cell formation. It is interesting in this context that in ngn-1 null mutants the reduction in size and hair cell number was largest in the saccule (Ma et al., 2000). Essentially, in the ngn-1 null mutant saccular development is arrested at the level of an E12.5 day embryonic mouse ear.
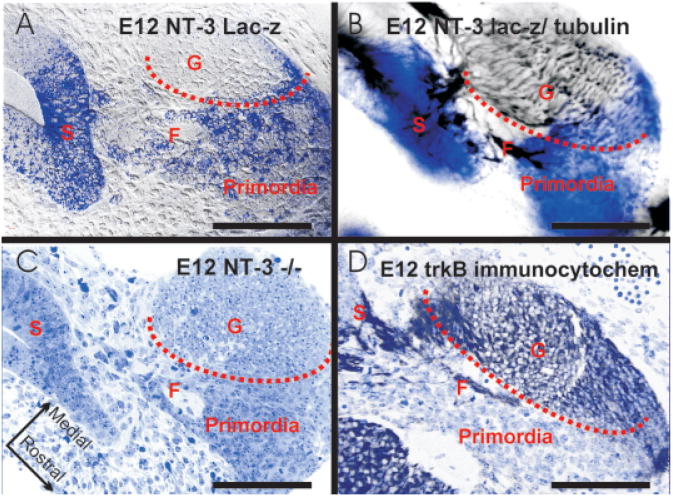
Figure 4.
Horizontal sections show the distribution of delaminating saccular cells and various markers. Note that the NT-3lac Zneo-positive cells (A,B) emigrate from the saccular anlage (S) to aggregate near the forming cochleo-vestibular ganglion (G). The sensory neurons have a distinct morphology (C) and are positive for α-acetylated tubulin (B) and trkB (D). In contrast, the delaminating cells (labeled “primordia”) are positive for NT-3lac Zneo (A,B) but not for tubulin (B) or trkB (D). Fibers to the saccule are passing between these cells in a fascicle (F) surrounded by lacZ-positive precursors. Bar indicates 100 μm.
Most interesting is the pattern of sensory precursor delamination in the cochlea. Presumably, a large fraction of delaminating cells from the area of the future ductus reuniens provides saccular and perhaps also basal turn spiral sensory neurons. Two to three additional areas of delaminating NT-3-positive, BDNF-negative cells were found near the base and middle turn (Farinas et al., 2001). Even more intriguing, a very large group of delaminating BDNFlac Z-positive cells was found only near the apex. However, no NT-3-positive cells were ever detected delaminating from the apex. The cochlear apex is thus unique in that it behaves like canal cristae with respect to delaminating cells and early up-regulation of BDNF in hair cell precursors.
Unfortunately, our data on delaminating cells from canal cristae are not as clear. At least the anterior and horizontal cristae apparently give rise to some BDN-Flac Z-positive precursors between E10–11 (Fig. 2). Data for the posterior cristae are even ambiguous. However, judging from other markers such as NeuroD (Figs. 2, 3, and 5) it is possible that precursors that do not express BDNFlac Z delaminate from those presumptive sensory epithelia. Judging from sections (Liu et al., 2000) and wholemounts (Kim et al., 2001) the area of NeuroD-positive sensory precursor delamination is larger than the areas delineated by sensory patch markers such as NT-3 (Figs. 2 and 5). Moreover, some delaminating NeuroD-positive neuronal precursors appear to be also positive for either BDNF or NT-3 (Fig. 5). A more detailed correlation of neurotrophin-positive delaminating cells with cells carrying other markers such as neurogenic differentiation 1(NeuroD) (Figs. 2 and 5) or brain factor 1(BF1) (Hatini et al., 1999) and a clonal analysis of their fate are needed to establish how many of these cells are actually turning into neurons.
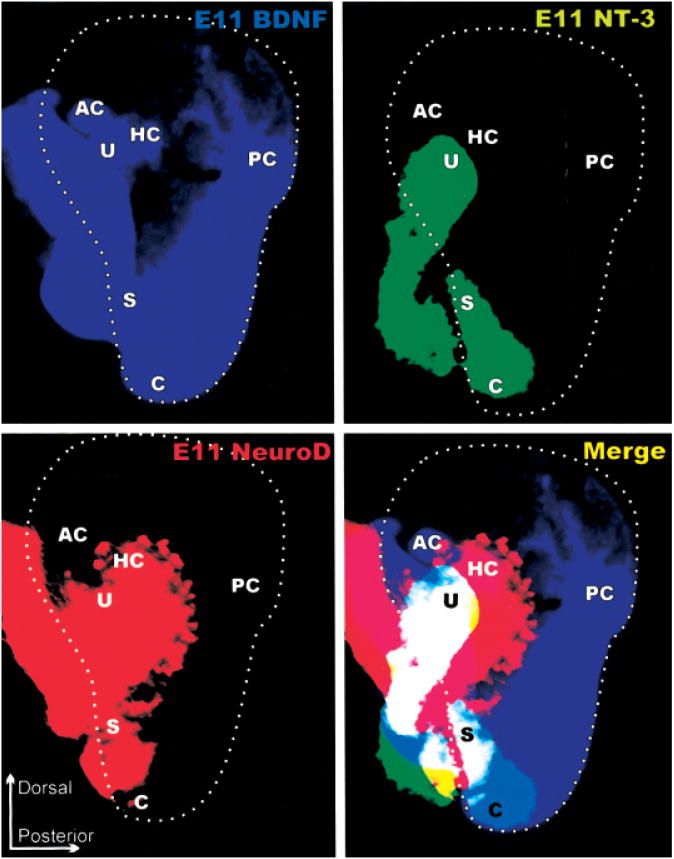
Figure 5.
The distribution of BDNF, NT-3, and NeuroD expression in E11 ears. Each image is a computer generated color-coded image of the lacZ expression shown in Figure 2. The panel labeled “merge” shows the computer generated superposition of these expression domains to highlight the relative level of overlap of the three genes. Note that the area delineated by the delaminating sensory neuron precursors that are NeuroDlacZ positive comprises at this stage areas of what has been delineated as sensory epithelia primordia based on neurotrophin lacZ expression. The only exception from this expression of NeuroDlacZ is the posterior crista, which is already fully innervated at this stage in development, suggesting an even earlier delamination of sensory precursors. These data also demonstrate that the ear consists already at this early stage of a mosaic of variously overlapping gene expression domains.
Overall, the evidence of delaminating cells, as revealed with BDNF and NT-3 lacZ markers, suggests a much richer variety of origins of delaminating cells in the ear than previously anticipated. This varies from cells expressing BDNFlac Z alone (canal cristae) to a mix (and possibly coexpression) of BDNFlac Z- and NT-3lac Zneo-positive cells (utricle and saccule) to a patchy delamination of either BDNFlac Z-positive cells (apex) or NT-3lacZneo-positive cells (base) in the cochlea. Closer examinations using acetylated tubulin or trkB or trkC antibodies suggest that those proliferating precursors lose neurotrophin expression when they start to differentiate into sensory neurons (Fig. 4). In addition, neurites of differentiating sensory neurons extend toward the utricle and saccule surrounded by delaminating neurotrophin-positive cells (Fig. 4). In an analogous system, the epibranchial placode-derived sensory neurons, neural crest cells, appear to serve as guidance structures for sensory afferents to reach the brain (Begbie and Graham, 2001). We would like to suggest that the delaminating cells provide guidance cues for the neurites to navigate toward specific sensory epithelia.
Quantitative Relationship of Hair Cells and Sensory Neurons
It has long been known that the afferent to hair cell ratio is much higher in auditory than in vestibular sensory organs (Lewis et al., 1985; Will and Fritzsch, 1988). Mice are no exception to this rule and show multiple type I afferent converging onto a single inner hair cell. In contrast, most innervation to vestibular hair cells and outer hair cells of the cochlea is by divergence of a single afferent fiber onto multiple hair cells. In fact, assuming that 90% of the 32,000 spiral sensory neurons in humans are type I and 10% are type II (Lewis et al., 1985), this suggests a ratio of 29,000:3,500 = 8:1 for the inner and of 3,000:14,000 = 1:5 for the outer hair cells. Interestingly, the ratio of type II neurons to OHCs is not unlike the ratio of vestibular sensory neurons to vestibular hair cells (1:5 for saccule, 1:5 for mammalian utricle, 1:3–1:4 for canal cristae; Lewis et al., 1985). This suggests that indeed there is tremendous increase in the number of sensory neurons per inner hair cell, and this increase decreases in a base to apical gradient (Ryugo, 1992).
We propose that the formation of these extra sensory neurons is at the expense of hair cells. More precisely, the formation of the ductus reuniens as well as the greater epithelial ridge reflects the transformation of pluripotent precursors into precursors that give rise only to sensory neurons. Having a null mutation for the bHLH gene responsible for this transformation would result in a loss of all precursors and, if Math1 -dependent clones are downstream of these ngn-1 -dependent clones, in a subsequent reduction of hair cell formation. Detailed measurements of the ductus reuniens area in ngn-1 null mutants compared to control animals will be necessary to verify this possibility.
There is no doubt that sensory neuron lineage markers such as ngn-1 and NeuroD appear in both the otocyst and in delaminating sensory neuron precursors (Liu et al., 2000; Ma et al., 2000; Kim et al., 2001). ngn-1 is upstream of NeuroD in these precursors. NeuroD appears to be upstream of Brn3a (i.e., POU domain, class 4, transcription factor 3, Pou4F3) (Kim et al., 2001), which in turn is upstream of trkC(neurotrophic tyrosine kinase, receptor, type 3) (Huang et al., 2001). A result of the shared gene network, commonalties in the NeuroD- and Brn3a-null phenotype are found, such as basal turn neuronal loss and aborted growth of sensory afferents to the posterior crista (Huang et al., 2001; Kim et al., 2001). Delaminating precursors also appear to undergo further cell division after delaminating from the ear. It remains to be determined how the total number of precursors relates to the total number of postmitotic sensory neurons and how this is regulated. The final ratio is rather unlikely to be regulated by the neurotrophins as a single hair cell can support up to 10 sensory neurons (inner hair cells in the cochlea) or is only one supporter for a diverging fiber that innervates several hair cells (outer hair cells and vestibular system). Indeed, branching patterns in neurotrophin mutants suggest that competition between fibers to access hair cells is more important than neurotrophin levels. Conditional delayed elimination of neurotrophins in the ear is needed to further test this hypothesis.
Establishing Topology of Sensory Neuron Identity
It is fair to say that we do not know how sensory neurons establish their identity. Sensory neurons develop precise initial projections of their axons into the CNS (Maklad and Fritzsch, 2002) and of their dendrites to the various endorgans of the ear (Fritzsch et al., 1995; Huang et al., 2001). The presence of such precise initial projections suggests that developing sensory neurons have an identity at the time their processes extend toward the CNS or reach the ear. No distinct molecular marker unique to even a subset of sensory neurons in the ear is known (Fekete and Wu, 2002), except for the apparently selective GATA enhancer-binding protein 3 (GATA3) expression (Fig. 2) in the developing spiral sensory neurons (Rivolta and Holley, 1998; Karis et al., 2001; Lawoko-Kerali et al., 2002). GATA3 and other zinc finger proteins are known to participate in establishing cellular identity. In other GATA3-expressing neurons, absence of GATA3 causes misrouting of fibers (Karis et al.,2001). However, whether GATA3 affects the precision of spiral sensory neuron projection is unknown as spiral sensory neurons are lost in GATA3 null mutants.
Several possible venues for generation of fiber diversity are suggested by other neurosensory systems. Recent work has shown that the vestibular sensory neurons that innervate specific sensory epithelia are not clustered together in the vestibular ganglion but are interspersed with neurons projecting to other epithelia (Maklad and Fritzsch, 1999). This specification of sensory neuron identity is unlikely to be caused by molecular gradients within the ganglion. Such a scenario would, comparable to positional identity acquisition in the retina, require that neighbors project to adjacent targets. Instead, neighbors can project to rather different targets such as a canal crista and the saccule (Maklad and Fritzsch, 1999) or different parts of the vestibular nuclei (Maklad and Fritzsch, 2002). In the olfactory sensory neurons, odorant coding receptor genes regulate axonal guidance so that all sensory neurons expressing the same odorant receptor converge onto one or a few glomeruli of the olfactory bulb (Wang et al., 1998). Such a solution seems to be unlikely for the inner ear sensory neurons. They appear to establish almost simultaneously their peripheral connections and their central connections, probably as a consequence of the identity acquisition process.
The topological differences in delaminating cells of the inner ear (Figs. 2, 3, and 5) suggest another possibility. Many cells delaminating from various areas of the developing ear appear to be neuronal precursors as indicated by the expression of NeuroD (Figs. 2 and 5). As they delaminate, the identity of their topological origin is retained. With the extension of their dendrites prior to, during, or after delamination (Rubel and Fritzsch, 2002), these neuronal precursors may project back into the same region of the otocyst from which they originally delaminated. Growing dendrites toward the sensory epithelia along the newly delaminating cells (Fig. 4) would enhance the precision of the targeting. If this scenario is plausible, it would require a much finer quilted patterning of the otocyst (Fig. 5) by multiple overlapping or bordering gene expression domains to provide information for at least six distinct topologies. Comparable to the increasing complexity of molecular cell fate determination by nested gene expression domains in the CNS (Qian et al., 2001), the ear might also present a case of early cell fate assignment through nested gene expression domains. In this context it is gratifying that many members of the FGF family of ligands appear to be expressed in the ear (Pickles, 2001). FGF genes are known to play an important role in ear induction (Fekete and Wu, 2002) as well as in neuronal identity. In analogy to their role in the CNS, FGFs may set up gradients that specify sensory neuron identity (Scully and Rosenfeld, 2002).
Conclusion
In this review we concentrated on later stages of ear development that lead to the formation of the sensory epithelia and the cell fate determination of sensory neurons. Using neurotrophin expression patterns as markers we show progressive segregation of a single NT-3lac Zneo-positive patch into the utricle, saccule, and cochlea sensory epithelia. We also show how the three canal cristae emerge as discrete BDNFlac Z-positive patches. Besides being markers for sensory epithelia development, neurotrophins are also expressed in delaminating cells that migrate toward the developing vestibular and cochlear ganglia. These delaminating cells originate from multiple sites at or near the developing sensory epithelia, and at least some are neuronal precursors as evidenced by the expression of NeuroD. Most importantly, this differential origin of precursors raises the possibility that the precursors of sensory neurons acquire positional information before delamination from the ear. In addition, these delaminating sensory neuron precursors may use this information to navigate to the area they delaminated from. This proposal implies a much more sophisticated subdivision of the developing otocyst than that suggested by the few available gene expression studies. Limited evidence, based on RT-PCR analysis for isoform expression of the large FGF family consisting of at least 23 genes, demonstrates the expression of numerous members in an as yet unknown pattern in the developing inner ear. We suggest that evolution of the ear comes about by slightly modifying this pattern. This is achieved through addition of new genes, or multiplication and functional diversification of existing genes, to modify the general hair cell and sensory neuron fate acquisition program to develop the six specific, functionally distinct endorgans in the mammalian inner ear and their innervation.
Acknowledgments
Parts of this article were presented at the Sixth International Congress of Vertebrate Morphology, July 21–26, 2001, in Jena, Germany. This symposium was supported by DRF.
Contract grant sponsor: NIDCD; contract grant number: 2P01 DC00215 (B.F.)
Contract grant sponsor: NIDCD; contract grant number: R01 DC04279 (K.W.B.)
References
- Acampora D, Gulisano M, Simeone A. Genetic and molecular roles of Otx homeodomain proteins in head development. Gene. 2000;246:23–35. doi: 10.1016/s0378-1119(00)00070-6. [DOI] [PubMed] [Google Scholar]
- Begbie J, Graham A. Integration between the epibranchial placodes and the hindbrain. Science. 2001;294:595– 598. doi: 10.1126/science.1062028. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. The papilla neglecta of turtles: a detector of head rotations with unique sensory coding properties. J Neurosci. 1998;18:4314–4324. doi: 10.1523/JNEUROSCI.18-11-04314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigande JV, Kiernan AE, Gao X, Iten LE, Fekete DM. Molecular genetics of pattern formation in the inner ear: do compartment boundaries play a role? Proc Natl Acad Sci USA. 2000;97:11700–11706. doi: 10.1073/pnas.97.22.11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantos R, Cole LK, Acampora D, Simeone A, Wu DK. Patterning of the mammalian cochlea. Proc Natl Acad Sci USA. 2000;97:11707–11713. doi: 10.1073/pnas.97.22.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J Comp Neurol. 2000;424:509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- de Burlet HM. Vergleichende Anatomie des statoakustischen Organs. a) Die innere Ohrsphäre. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden Anatomie der Wirbeltiere. Berlin: Urban and Schwarzenberg; 1934. pp. 1293–1432. [Google Scholar]
- Dickman JD, Fang Q. Differential central projections of vestibular afferents in pigeons. J Comp Neurol. 1996;367:110–131. doi: 10.1002/(SICI)1096-9861(19960325)367:1<110::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Storey KG. Markers in vertebrate neurogenesis. Nat Rev Neurosci. 2001;2:835–839. doi: 10.1038/35097587. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The inner ear of the coelacanth fish Latimeria has tetrapod affinities. Nature. 1987;327:153–154. doi: 10.1038/327153a0. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The water-to-land transition: Evolution of the tetrapod basilar papilla, middle ear and auditory nuclei. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer-Verlag; 1992. pp. 351–375. [Google Scholar]
- Fritzsch B. Evolution of the vestibulo-ocular system. Otolaryngol Head Neck Surg. 1998;119:182–192. doi: 10.1016/S0194-5998(98)70053-1. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The morphology and function of fish ears. In: Ostrander G, editor. The Laboratory fish Exeter. Academic Press; 2001a. pp. 250–259. [Google Scholar]
- Fritzsch B. The cellular organization of the fish ear. In: Ostrander G, editor. The Laboratory fish Exeter. Academic Press; 2001b. pp. 480–487. [Google Scholar]
- Fritzsch B, Barald K, Lomax M. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. New York: Springer-Verlag; 1998. pp. 80–145. [Google Scholar]
- Fritzsch B, Beisel KW. Evolution and development of the vertebrate ear. Brain Res Bull. 2001;55:711–721. doi: 10.1016/s0361-9230(01)00558-5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001;211:388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Smeyne R, Fagan AM, Bar-bacid M. Reduction and loss of inner ear innervation in trkB and trkC receptor knockout mice: A whole mount DiI and scanning electron microscopic analysis. Audit Neurosci. 1995;1:401–417. [Google Scholar]
- Fritzsch B, Wake MH. The inner ear of gymnophione amphibians and its nerve supply: a comparative study of regressive events in a complex sensory system. Zoomorphol. 1988;108:210–217. [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Hatini V, DiNardo S. Divide and conquer: pattern formation in Drosophila embryonic epidermis. Trends Genet. 2001;17:574–579. doi: 10.1016/s0168-9525(01)02448-9. [DOI] [PubMed] [Google Scholar]
- Hatini V, Ye X, Balas G, Lai E. Dynamics of placodal lineage development revealed by targeted transgene expression. Dev Dyn. 1999;215:332–343. doi: 10.1002/(SICI)1097-0177(199908)215:4<332::AID-AJA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421– 2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsell O. Anura. In: Jansen J, editor. The Comparative Anatomy and Histology of the Cerebellum from Myxinoids through Birds. Minneapolis: The University of Minnesota Press; 1967. pp. 163–178. [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–391. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The vertebrate inner ear. Boca Raton: CRC Press; 1985. p. p 248. [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839– 2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Incomplete segregation of endorgan-specific vestibular ganglion cells in mice and rats. J Vestib Res. 1999;9:387–399. [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. The developmental segregation of posterior crista and saccular vestibular fibers in mice: A carbocyanine tracer study using confocal microscopy. Dev Brain Res. 2002;135:1–17. doi: 10.1016/s0165-3806(01)00327-3. [DOI] [PubMed] [Google Scholar]
- Mazan S, Jaillard D, Baratte B, Janvier P. Otx1 gene-controlled morphogenesis of the horizontal semicircular canal and the origin of the gnathostome characteristics. Evol Dev. 2000;2:186–193. doi: 10.1046/j.1525-142x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- McCormick CA. Anatomy of the Central Auditory Pathways of Fish and Amphibians. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. New York: Springer-Verlag; 1999. pp. 155–217. [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126:2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris HW. Studies on the development of the ear in Amblystoma. I. Development of the auditory vesicle. J Morphol. 1892;7:23–34. [Google Scholar]
- Pickles JO. The expression of fibroblast growth factors and their receptors in the embryonic and neonatal mouse inner ear. Hear Res. 2001;155:54–62. doi: 10.1016/s0378-5955(01)00247-7. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert H, Simeone A. Conserved usage of gap and homeotic genes in patterning the CNS. Curr Opin Neurobiol. 1999;9:589–595. doi: 10.1016/S0959-4388(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Retzius G. Das Gehörorgan der Wirbeltiere: II Das Gehororgan der Amnioten. Stockholm: Samson und Wallin; 1884. p. 345. [Google Scholar]
- Rivolta MN, Holley MC. GATA3 is downregulated during hair cell differentiation in the mouse cochlea. J Neurocytol. 1998;27:637–647. doi: 10.1023/a:1006951813063. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory System Development: Primary Auditory Neurons and Their Targets. Ann Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;(220):221–244. [PubMed] [Google Scholar]
- Ryugo DK. The auditory nerve; peripheral innervation, cell body morphology, and central projections. In: Webster DB, Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neuroanatomy. New York: Springer-Verlag; 1992. pp. 23–65. [Google Scholar]
- Sarasin P, Sarasin F. Über das Gehororgan der Caeciliiden. Anat Anz. 1892;7:812–815. [Google Scholar]
- Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- Tomsa JM, Langeland JA. Otx expression during lamprey embryogenesis provides insights into the evolution of the vertebrate head and jaw. Dev Biol. 1999;207:26–37. doi: 10.1006/dbio.1998.9163. [DOI] [PubMed] [Google Scholar]
- Van de Water TR. Embryogenesis of the inner ear: “In vitro studies”. In: Romand R, editor. Development of auditory and vestibular systems. New York: Academic Press; 1983. pp. 337–374. [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. Erratum in 2001 Science 292: 1838. [DOI] [PubMed] [Google Scholar]
- Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Will U, Fritzsch B. The octavus nerve of amphibians: Patterns of afferents and efferents. In: Fritzsch B, Ryan M, Wilczynski W, Hetherington T, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. New York: Wiley and Sons; 1988. pp. 159–184. [Google Scholar]
- Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]