Abstract
Context
The arteriovenous fistula is the preferred type of vascular access for hemodialysis because of lower thrombosis and infection rates and lower health care expenditures compared with synthetic grafts or central venous catheters. Early failure of fistulas due to thrombosis or inadequate maturation is a barrier to increasing the prevalence of fistulas among patients treated with hemodialysis. Small, inconclusive trials have suggested that antiplatelet agents may reduce thrombosis of new fistulas.
Objective
To determine whether clopidogrel reduces early failure of hemodialysis fistulas.
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled trial conducted at 9 US centers composed of academic and community nephrology practices in 2003–2007. Eight hundred seventy-seven participants with end-stage renal disease or advanced chronic kidney disease were followed up until 150 to 180 days after fistula creation or 30 days after initiation of dialysis, whichever occurred later.
Intervention
Participants were randomly assigned to receive clopidogrel (300-mg loading dose followed by daily dose of 75 mg; n = 441) or placebo (n = 436) for 6 weeks starting within 1 day after fistula creation.
Main Outcome Measures
The primary outcome was fistula thrombosis, determined by physical examination at 6 weeks. The secondary outcome was failure of the fistula to become suitable for dialysis. Suitability was defined as use of the fistula at a dialysis machine blood pump rate of 300 mL/min or more during 8 of 12 dialysis sessions.
Results
Enrollment was stopped after 877 participants were randomized based on a stopping rule for intervention efficacy. Fistula thrombosis occurred in 53 (12.2%) participants assigned to clopidogrel compared with 84 (19.5%) participants assigned to placebo (relative risk, 0.63; 95% confidence interval, 0.46–0.97; P = .018). Failure to attain suitability for dialysis did not differ between the clopidogrel and placebo groups (61.8% vs 59.5%, respectively; relative risk, 1.05; 95% confidence interval, 0.94–1.17; P = .40).
Conclusion
Clopidogrel reduces the frequency of early thrombosis of new arteriovenous fistulas but does not increase the proportion of fistulas that become suitable for dialysis.
Trial Registration
clinicaltrials.gov Identifier: NCT00067119
Approximately 470 000 Americans have end-stage renal disease, and most are treated with hemodialysis.1 A major challenge in caring for patients undergoing hemodialysis is maintaining a functioning vascular access, which is essential for performing the dialysis procedure. The effect of vascular access dysfunction is substantial—it is a leading reason for hospitalization among patients with end-stage renal disease and has associated annual costs in the United States that exceed $1 billion.2,3
The arteriovenous fistula is the preferred type of vascular access because thrombosis rates, infection rates, access-related expenditures, and total health care expenditures all are lower for patients with fistulas than for those with either synthetic arteriovenous grafts or central venous catheters.4 Recognition of these advantages is reflected in the clinical practice guidelines of several professional societies and has triggered a major initiative by the Centers for Medicare & Medicaid Services to increase the prevalence of fistulas.4–6 However, the advantages of fistulas are counterbalanced by the substantially higher proportion of fistulas than grafts that are never able to be used for dialysis because of failure to mature adequately to support effective hemodialysis.7 Early fistula failure is the major barrier to increasing fistula prevalence and, in many patients, leads to prolonged use of central venous catheters, which are the least desirable type of vascular access because of their high rates of catheter-associated bacteremia and inadequate solute clearance.
Several small placebo-controlled trials observed lower rates of early fistula thrombosis with short-term use of antiplatelet agents in the postoperative period.8–15 However, none of these studies had adequate power to demonstrate statistically significant differences between the treatment groups and none reported the proportion of fistulas that ultimately matured into a functional vascular access. To further evaluate the effect of platelet inhibition on fistula thrombosis and maturation failure, we performed a multicenter, randomized, double-blind, placebo-controlled trial of clopidogrel administered following fistula creation in a large group of patients with advanced chronic kidney disease or end-stage renal disease.
METHODS
Participants
The study design has previously been reported in detail.16 Briefly, participants were enrolled from 9 centers in the United States. Individuals undergoing creation of a new upper extremity fistula were eligible for enrollment if they were receiving maintenance treatment with hemodialysis or were expected to begin maintenance hemodialysis within 6 months. Major exclusion criteria included active bleeding or bleeding events requiring red blood cell transfusions within the previous 12 weeks, a platelet count less than 75 × 103/µL, known coagulopathy, acute ulcer disease, systolic blood pressure higher than 200 mm Hg or diastolic blood pressure higher than 115 mm Hg, advanced liver disease, inability to discontinue antiplatelet or anticoagulant therapy including aspirin during the study drug administration period, pregnancy, and current substance abuse. Unless there was a history of myocardial infarction or cerebrovascular accident within the previous 12 months, the use of antiplatelet agents did not preclude enrollment if the investigator and the patient’s physicians thought their discontinuation for 7 weeks was medically safe. Race/ethnicity data were collected based on patient self-report. The institutional review board at each center approved the protocol and all participants provided written informed consent.
Study Design and Procedures
Participants were assigned in equal proportions to receive clopidogrel or placebo using a computer-generated permuted block randomization with stratification by location of the fistula (forearm vs upper arm) and by center. Randomization was performed within 1 calendar day after fistula creation surgery following confirmation that a fistula was created and that it was patent by physical examination. Participants and members of the study team were blinded to treatment assignment. Study drug administration began immediately after randomization. Clopidogrel was administered orally, with a loading dose of 300 mg on day 1 followed by 75 mg each day thereafter for an additional 41 days. Clopidogrel and matching placebo tablets were provided by Sanofi-Synthelabo (Ambares, France) and packaged by Fisher Clinical Services (Allentown, Pennsylvania). Participants receiving antiplatelet or anticoagulant agents prior to enrollment discontinued such treatment for at least 7 days prior to surgery. Resumption of these medications was permitted after the 6 weeks of study drug administration. Adherence to study medication was assessed by pill counts and expressed as: [(number of pills dispensed − number of pills returned)/number of pills prescribed × 100%.
Decisions regarding fistula creation, initiation of use of the fistula, and procedures performed to enhance fistula maturation were made by the participant’s physicians and not dictated by the study protocol. Data collection was performed at baseline, 6 weeks after fistula creation, and monthly thereafter until ascertainment of fistula suitability. Adverse events were recorded until 30 days after discontinuation of the study medication. Study medication was discontinued before 6 weeks in the event of fistula thrombosis confirmed by the participant’s vascular surgeon or the investigator.
Outcomes
The primary outcome was thrombosis (ie, patency failure) 6 weeks after fistula creation. The fistula was classified as patent if a bruit was audible with a stethoscope throughout systole and diastole at least 8 cm proximal to the arteriovenous anastomosis. Fistula patency was assessed by trained study personnel.
The major secondary outcome was failure to attain suitability for dialysis. Fistula suitability was defined as the ability to use the fistula for dialysis with 2 needles and maintain a dialysis machine blood flow rate adequate for optimal dialysis (≥300 mL/min) during 8 of 12 dialysis sessions occurring during a 30-day suitability ascertainment period. For participants receiving maintenance hemodialysis at the time of enrollment and for participants who started maintenance hemodialysis within 120 days after fistula creation, the suitability ascertainment period began between 120 and 150 days after fistula creation surgery. The start of the suitability ascertainment period was the first dialysis session within days 120 and 150 during which fistula cannulation was performed. If dialysis initiation occurred after day 120, the ascertainment period began at dialysis initiation. During each dialysis session of the suitability ascertainment period, all blood flow measurements recorded after the first hour and before the last 15 minutes of the dialysis session were included in the determination of the suitability outcome. If a fistula was not being used during the fistula suitability ascertainment period, the fistula was classified as not suitable, even if it had been successfully used prior to the ascertainment period.
Bleeding was classified as minor, intermediate, major, life-threatening, or fatal, as previously described.16 The classifications of major, life-threatening, and fatal bleeding were reviewed by a quality control committee consisting of a subset of the investigators, but the categorization made by the clinical center investigator was maintained for analyses.
Statistical Analysis
The primary and secondary outcomes in the 2 treatment groups were compared using the Mantel-Haenszel χ2 test stratified according to clinical center and the location of the fistula (forearm or upper arm).17 Treatment effects were expressed as a weighted average of the strata-specific relative risks. Poisson regression models were used to test for differences in the relative risks (interactions) among the clinical center and fistula location strata.18
The comparisons of the primary and secondary outcomes were based on the participants’ randomized treatment assignments, irrespective of adherence to the intervention. However, participants who could not have patency or suitability ascertained were censored from the analyses of these outcomes. In sensitivity analyses performed to address missing data, bounds on the minimum and maximum relative risks for the primary outcome were obtained by imputing all missing outcomes in 1 treatment group as “patent” and all missing outcomes in the other treatment group as “not patent.” For sensitivity analyses of the secondary outcome, suitability was imputed for participants without suitability assessments based on vital status and the 6-week patency outcome.
A data and safety monitoring board (DSMB) approved the protocol prior to implementation, reviewed adverse events every 6 months, and reviewed the interim analyses of efficacy. A formal stopping guideline based on a Lan-DeMets spending function was used to approximate the O’Brien-Fleming boundary for 4 interim efficacy analyses. The stopping guideline also included a boundary for early termination in the absence of trends for a treatment effect at the third or fourth interim analysis. The majority of the total type I error (α) was allocated to the later interim analyses. At the actual interim analyses, the estimated information fractions (defined as the proportion of total projected patency assessments completed at each analysis) were 0.241, 0.479, 0.733, and 0.813, with corresponding nominal 2-sided α levels of <.00001, .0015, .020, and .024. The P values and 95% confidence intervals (CIs) for the primary analysis were adjusted for interim monitoring using the repeated CI approach.19 All other CIs and 2-sided P values are reported on a comparison-wise basis without adjustment for interim analyses or multiple comparisons. Statistical analyses were performed using SAS software, version 9.1 (SAS Institute Inc, Cary, North Carolina).
Sample Size
The trial was designed to enroll a total of 1284 participants (642 in each treatment group). This sample size would have provided 85% power to detect a relative reduction in the fistula thrombosis rate of 30% in the clopidogrel group at a 2-sided α level of .05, assuming a thrombosis rate of 25% in the placebo group. We based our prediction of the thrombosis rate in the placebo group on a pooled analysis of previously published placebo-controlled trials evaluating antiplatelet agents on thrombosis of new fistulas.20 We viewed an effect size of 30% as clinically compelling, and it was also consistent with the findings of the previous small trials of antiplatelet therapy. The power calculation incorporated a 3% rate of treatment drop-in (ie, initiation of antiplatelet therapy by treating physicians to participants randomized to placebo) and a 3% rate of treatment dropout (ie, discontinuation of study drug among participants randomized to clopidogrel), a loss to follow-up prior to the 6-week patency assessment of 5%, and an upward adjustment of 2.7% to account for the O’Brien-Fleming stopping rule. The target sample size also provided 81% power at a 2-sided α level of .05 to detect a 20% relative reduction in failure to attain fistula suitability for dialysis, assuming a failure rate of 40% in the placebo group.21
RESULTS
Early Termination of Enrollment
At the recommendation of the DSMB, enrollment was terminated on October 24, 2006, after the fourth interim analysis, when the study information fraction had reached 0.813. The recommendation for termination was based on the prespecified stopping rule for efficacy of the intervention on the primary end point. Recognizing the importance of determining the effect of clopidogrel on the secondary outcome (fistula suitability), the DSMB considered conditional power calculations for suitability before making the decision to terminate enrollment. These calculations indicated that continuing the trial could not have shown a statistically significant benefit of clopidogrel on the suitability outcome and was unlikely (conditional probability <.05) to yield a relative risk in a qualitatively different direction than that observed at the fourth interim analysis.
For participants randomized prior to the early termination date, study drug administration continued for a full 6 weeks, and follow-up continued until the earlier of the following: ascertainment of the suitability outcome or June 18, 2007, the date investigators were unblinded to the trial results and the reason for early termination.
Patients
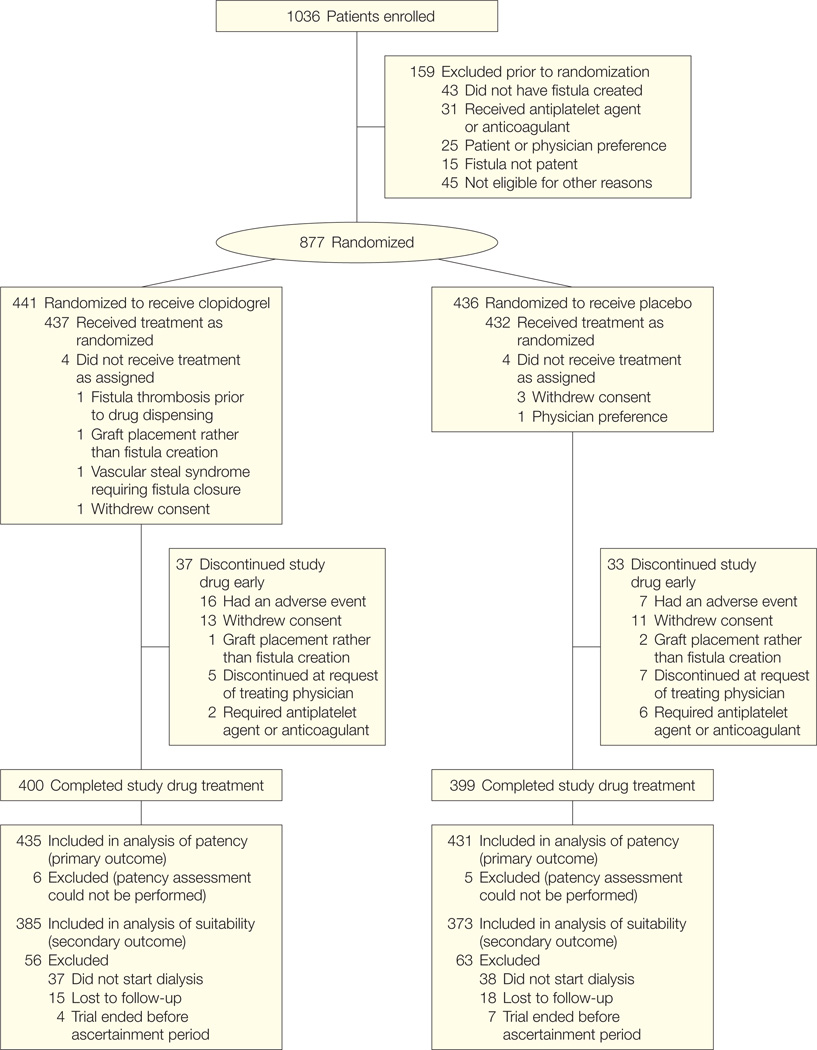
Between January 7, 2003, and October 24, 2006, 1036 participants consented and 877 were randomized (Figure). Four hundred forty-one participants were randomized to receive clopidogrel and 436 to receive placebo. Two participants randomized to the clopidogrel group and 2 participants randomized to the placebo group had placement of a synthetic graft rather than creation of a fistula. These participants discontinued study medication immediately after it was recognized that a fistula was not created. Fistula surgeries were performed at 27 hospitals by 71 surgeons, and dialysis was performed at 125 facilities affiliated with the 9 clinical centers. The median time from surgery to study drug administration was 1.5 hours (10th–90th percentile, 0.2–17 hours). Adherence to study medication was greater than 90% for 87% of participants assigned to clopidogrel and for 86% of participants assigned to placebo.
Figure.
Participant Flow Through the Trial
As shown in Table 1, baseline characteristics of the participants were similar in the 2 treatment groups. Fifty-four percent of the participants received a forearm fistula. Among the upper arm fistulas, 69% were created by an anastomosis between the brachial artery and cephalic vein and 25% were created using the brachial artery and a transposed basilic vein.
Table 1.
Baseline Participant Characteristicsa
| Characteristics | Clopidogrel (n = 441) |
Placebo (n = 436) |
|---|---|---|
| Age, mean (SD), y | 52.7 (14.7) | 54.5 (14.4) |
| Male | 273 (61.9) | 275 (63.1) |
| Black | 221 (50.1) | 201 (46.1) |
| Body mass index, mean (SD)b | 30.2 (8.6) | 29.3 (7.5) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 141.0 (21.4) | 139.9 (21.4) |
| Diastolic | 78.8 (13.4) | 78.7 (14.3) |
| Diabetes mellitus | 217 (49.2) | 205 (47.0) |
| Cardiovascular diseasec | 110 (24.9) | 107 (24.5) |
| Cerebrovascular diseased | 23 (5.2) | 31 (7.1) |
| Peripheral arterial diseasee | 16 (3.6) | 12 (2.7) |
| Venous thromboembolic diseasef | 12 (2.7) | 15 (3.4) |
| Aspirin use | 103 (23.4) | 102 (23.4) |
| ACE inhibitor or ARB use | 246 (55.8) | 262 (60.1) |
| Statin use | 164 (37.2) | 171 (39.2) |
| Current tobacco use | 91 (20.6) | 81 (18.6) |
| Hemoglobin, mean (SD), g/dL | 11.6 (1.8) | 11.6 (1.7) |
| Serum albumin, mean (SD), g/dL | 3.7 (0.6) | 3.7 (0.6) |
| Preoperative vascular mapping | 330 (75.9) | 318 (73.8) |
| Previous arteriovenous access | 79 (17.9) | 81 (18.6) |
| Hemodialysis initiated before fistula creation | 239 (54.2) | 233 (53.4) |
| Study fistula | ||
| Forearm | 233 (52.8) | 238 (54.6) |
| Upper arm | 208 (47.2) | 198 (45.4) |
| Radial artery–cephalic vein (forearm)g | 209 (47.4) | 209 (47.9) |
| Brachial artery–cephalic vein (upper arm)h | 142 (32.2) | 138 (31.7) |
| Brachial artery–basilic vein (upper arm)h | 53 (12.0) | 47 (10.8) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
SI conversions: To convert hemoglobin to g/L, multiply by 10. To convert serum albumin to g/L, multiply by 10.
Data are expressed as No. (%) of participants unless otherwise noted. Comparisons were made using t tests for continuous variables and χ2 tests for categorical variables. For all comparisons, P > .05.
Body mass index was calculated as weight in kilograms divided by the height in meters squared.
Includes history of myocardial infarction, angina, coronary artery angioplasty, coronary artery bypass surgery, or congestive heart failure.
Includes history of stroke, transient ischemic attack, or carotid endarterectomy.
Includes history of nontraumatic amputation, lower extremity angioplasty or bypass surgery, or claudication.
Includes history of deep venous thrombosis or pulmonary embolism.
Other types of forearm anastomoses included ulnar artery–cephalic vein and radial artery–basilic vein.
Other types of upper arm anastomoses included proximal radial artery–cephalic vein and brachial artery–median cubital vein.
Thirty-seven participants (8.4%) in the clopidogrel group and 33 participants (7.6%) in the placebo group discontinued the study medication early. The reasons for early discontinuation of study medication did not differ between treatment groups (Figure). Assessment of fistula patency was performed in 435 participants (98.6%) and 431 participants (98.9%) in the clopidogrel and placebo groups, respectively (Figure).
Fistula Thrombosis at 6 Weeks
Among the 866 participants who had patency assessed, the primary outcome of fistula thrombosis at 6 weeks occurred in 53 participants (12.2%) in the clopidogrel group compared with 84 participants (19.5%) in the placebo group (relative risk, 0.63; 95% CI, 0.46–0.97; P = .018) (Table 2). Ten participants in the placebo group and 7 participants in the clopidogrel group had a surgical or percutaneous intervention to restore patency or promote maturation before 6 weeks. There was no significant interaction between fistula location (forearm vs upper arm) and treatment assignment (P = .15). Similarly, there was no significant interaction between clinical center and treatment assignment (P = .57). In sensitivity analyses evaluating extreme best- and worst-case imputation scenarios for the 11 participants (1.3%) without patency assessments, the lower and upper bounds on the possible relative risk associated with clopidogrel ranged between 0.63 and 0.69.
Table 2.
Fistula Thrombosis
| No. (%) of Patients | Relative Risk (95% Confidence Interval)b |
||
|---|---|---|---|
| Clopidogrel (n = 435)a |
Placebo (n = 431)a |
||
| Thrombosis at 6 wk (all patients) | 53 (12.2) | 84 (19.5) | 0.63 (0.46–0.97)c |
| By location | |||
| Forearm fistula | 31 (12.9) | 60 (24.7) | 0.53 (0.36–0.77) |
| Upper arm fistula | 22 (11.3) | 24 (12.8) | 0.89 (0.52–1.53) |
Six of the 441 patients randomized to clopidogrel and 5 of the 436 patients randomized to placebo were not included because patency was not evaluated.
Relative risks were stratified for fistula location and center.
The 95% confidence interval reported is the repeated confidence interval adjusted for interim monitoring. The repeated P value adjusted for interim monitoring is .018.
Fistula Suitability for Dialysis
Fistula suitability was assessed in 758 randomized patients (86.4%) and in 95.8% of those who initiated dialysis soon enough to have suitability assessed (Figure). Of the fistulas assessed for suitability, the percentage with suitability failure did not differ between the clopidogrel group and the placebo group (61.8% vs 59.5%; relative risk, 1.05; 95% CI, 0.94–1.17; P = .40) (Table 3). The estimated relative risk of 1.05 was unchanged in the sensitivity analyses that incorporated imputation for the missing suitability outcomes. Similarly, there was no difference between groups in the percentage with suitability failure when fistulas that were treated with a percutaneous or surgical intervention because of poor maturation were classified as suitability failures (67.5% vs 65.4%; relative risk, 1.04; 95% CI, 0.94–1.15; P = .42).
Table 3.
Fistula Suitability Failure
| No. (%) of Patients | Relative Risk (95% Confidence Interval)b |
||
|---|---|---|---|
| Clopidogrel (n = 385)a |
Placebo (n = 373)a |
||
| Suitability failure (all patients) | 238 (61.8) | 222 (59.5) | 1.05 (0.94–1.17)c |
| By location | |||
| Forearm fistula | 144 (66.9) | 137 (64.0) | 1.05 (0.92–1.20) |
| Upper arm fistula | 94 (55.3) | 85 (53.4) | 1.05 (0.87–1.27) |
| By failure reason | |||
| Fistula abandoned with no expectation of future use |
115 (29.9) | 134 (35.9) | 0.85 (0.69–1.03) |
| Fistula not yet in use despite treatment with dialysis |
57 (14.8) | 47 (12.6) | 1.17 (0.83–1.66) |
| Fistula in use during ascertainment period but failed to meet suitability criteria |
66 (17.1) | 41 (11.0) | 1.56 (1.08–2.24) |
Fifty-six of the 441 patients randomized to clopidogrel and 63 of the 436 patients randomized to placebo were not included because suitability was not ascertained (Figure).
Relative risks were stratified for fistula location and center.
P = .40.
In both treatment groups, most of the fistula suitability failure outcomes were due to lack of use of the fistula during the ascertainment period, either because the fistula had already been abandoned or because the treating physician thought that it had not yet matured adequately (Table 3). Because of the possibility that our criteria for fistula suitability were too stringent, as a sensitivity analysis we removed the dialysis machine blood flow criteria from the definition of suitability and classified fistulas as suitable solely on the basis of use during 8 dialysis sessions during the suitability ascertainment period. Using this modified definition, the overall fistula suitability failure rate was 49.9% and there was no difference between treatment groups (47.8% in the clopidogrel group vs 52.1% in the placebo group; relative risk, 0.92; 95% CI, 0.81–1.07; P = .30).
Adverse Events
Adverse events were similar in the 2 treatment groups (Table 4). In particular, neither the frequency nor the severity of bleeding events was greater among participants treated with clopidogrel than among those who received placebo.
Table 4.
Adverse Eventsa
| Events | No. (%) of Patients | P Valueb | |
|---|---|---|---|
| Clopidogrel (n = 441) |
Placebo (n = 436) |
||
| Any serious adverse event | 67 (15.2) | 81 (18.6) | .20 |
| Bleeding | 13 (2.9) | 12 (2.8) | .84 |
| Minor | 0 | 0 | >.99 |
| Intermediate | 6 (1.4) | 5 (1.2) | >.99 |
| Major | 3 (0.7) | 3 (0.7) | >.99 |
| Life-threatening | 4 (0.9) | 4 (0.9) | >.99 |
| Fatal | 0 | 0 | >.99 |
| Hospitalization | 64 (14.5) | 77 (17.7) | .16 |
| Ischemic heart disease | 3 (0.7) | 7 (1.6) | .22 |
| Congestive heart failure | 6 (1.4) | 9 (2.1) | .45 |
| Arrhythmia | 1 (0.2) | 7 (1.6) | .04 |
| Hypertension | 0 | 5 (1.1) | .03 |
| Cerebrovascular disease | 2 (0.5) | 1 (0.2) | >.99 |
| Infection | 4 (0.9) | 3 (0.7) | >.99 |
| Vascular access event: study fistula | 5 (1.1) | 6 (1.4) | .77 |
| Vascular access event: nonstudy access | 15 (3.4) | 12 (2.8) | .70 |
| Death | 4 (0.9) | 4 (0.9) | >.99 |
Adverse events were collected until 30 days after the last dose of study medication.
Comparisons were made using the Fisher exact test.
COMMENT
We found that clopidogrel reduced the frequency of early thrombosis of new arteriovenous fistulas. The drug was well-tolerated and did not increase bleeding events during a 6-week administration period. The beneficial effect of clopidogrel on thrombosis was not accompanied by an increase in the proportion of fistulas that were suitable for dialysis, the secondary outcome of the trial.
The results of our study are similar to those of the largest previous study of antiplatelet therapy for new fistulas.15 In that trial of 258 participants, early thrombosis occurred in 19% of patients treated with placebo compared with 12% of those treated with ticlopidine. In contrast with our study, that prior study did not report the number of fistulas that were suitable for dialysis.
To be used for dialysis, a newly created fistula must mature; that is, the artery and vein must undergo dilation and remodeling to accommodate a markedly increased blood flow. Maintenance of patency is necessary for fistula maturation and restoration of patency is rarely possible if thrombosis occurs within the first several weeks after fistula creation. Thus, we hypothesized that prevention of early thrombosis would be associated with a higher rate of fistula maturation. Our finding of a beneficial effect of clopidogrel on fistula patency but not on suitability is important to the evolving understanding of the pathophysiology of fistula maturation and failure, and suggests that early patency is necessary but not sufficient for fistula maturation. Processes such as development of stenosis of the draining vein or poor arterial inflow may limit successful fistula maturation despite platelet inhibition and prevention of thrombosis. The discrepancy between the effects of clopidogrel on fistula patency and fistula suitability suggests that thrombosis may be a manifestation rather than a cause of maturation failure. Future work directed at determining the contributions to fistula maturation of underlying vascular function, vascular anatomy, and surgical technique may identify better targets for interventions to improve fistula outcomes.
The proportion of fistulas with suitability failure was substantial and higher than we anticipated.16 Previous studies, mostly from single centers, have reported fistula maturation failure rates ranging from 18% to 53%.21 During the period in which the trial was conducted there was an increased emphasis in clinical practice guidelines and by regulatory agencies on creating fistulas rather than synthetic grafts.4,6 It is likely that efforts by treating physicians at the participating centers to increase fistula use led to liberalization of selection criteria for fistula creation. We speculate that these changes in criteria for attempting fistula creation contributed to the high rate of fistula suitability failure observed in our trial.
We considered the possibility that our criteria for fistula suitability were too stringent and that a benefit of clopidogrel on suitability would have been apparent had we used a different definition of suitability. However, in a sensitivity analysis that eliminated the dialysis machine blood flow criteria, the suitability failure rate remained high and the proportions of participants with suitability failure remained similar in the 2 treatment groups. Thus, it is unlikely that the definition of suitability used in the trial masked a benefit of clopidogrel.
It is possible that clopidogrel could indirectly improve fistula suitability by maintaining patency long enough to enable the performance of maturation-enhancing interventions such as percutaneous angioplasty of vessel stenosis or surgical revision of the arteriovenous anastomosis.22–24 Although such procedures were permitted in the trial at the discretion of the treating physicians, they were performed on only a small proportion of fistulas; thus, it is possible that a modest beneficial effect of clopidogrel on fistula suitability would have been observed had a more aggressive approach to repairing anatomic lesions been taken.
Our trial has several strengths. It is the first large, multicenter trial evaluating an intervention to improve outcomes of new fistulas and the first trial that includes fistula suitability for dialysis as an outcome. The participants were enrolled from both urban and rural settings and from both academic and community practices in multiple geographic regions within the United States. The treatment groups were balanced with respect to baseline characteristics. Adherence to study medication was good and the primary outcome was assessed in nearly all of the participants.
Our trial has some limitations. We excluded patients taking antiplatelet agents or anticoagulants if they were unable to discontinue the medications during the study drug administration period. This exclusion, while motivated by safety considerations, would be expected to restrict the study population to those with a lower burden of vascular disease and, perhaps, a lower risk of fistula maturation failure, potentially limiting the ability to generalize the findings of the trial to the general hemodialysis population. However, despite the lower frequency of cardiovascular disease in the study participants compared with the US end-stage renal disease population,1 the high rate of fistula suitability failure that we observed suggests that the study population was, in fact, at high risk of maturation failure. A second limitation of the trial is that the fistula suitability end point could not be ascertained in participants who had not initiated dialysis treatment by the end of the study. Incomplete ascertainment of suitability was anticipated and was one of the reasons that fistula suitability was the secondary, rather than the primary, study outcome.16 The statistical power of the study to detect an effect of clopidogrel on the suitability outcome was limited because the achieved sample size of 877 was 32% smaller than the target sample size. However, the 95% CI for the suitability outcome suggests that, at best, clopidogrel might have reduced the risk of suitability failure by 6%. Thus, it is unlikely that a clinically important benefit of clopidogrel on fistula suitability was missed because of the reduction in power that resulted from the early termination of enrollment.
In conclusion, clopidogrel reduces the incidence of early thrombosis of new arteriovenous fistulas but does not increase the proportion that become suitable for dialysis. The high rate of fistula suitability failure observed in this large trial conducted at centers with a particular interest in hemodialysis vascular access provides a compelling argument for additional efforts to identify mechanisms underlying fistula maturation failure, criteria for selecting suitable candidates for fistula creation, and interventions to enhance fistula maturation.
Acknowledgments
Dr Dember reports having received consulting fees from Proteon Therapeutics. Dr Allon reports having received consulting fees from Arrow International. Dr Dixon reports having received consulting fees from Proteon Therapeutics and Pervasis Therapeutics. Dr Greenberg reports having received consulting fees from Sanofi-Synthelabo. Dr Kaufman reports having received consulting fees from Proteon Therapeutics. Dr Kusek reports that he owns stock in Pfizer, Lilly, and Decode Genetics. Dr Feldman reports having received grant support from Amgen, Hoffman La Roche, General Electric, and Watson Pharmaceuticals; having received consulting fees from Kirin Pharmaceuticals; and having provided expert testimony for General Electric.
Funding/Support: The trial was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grants U01DK058986, U01DK058982, U01DK058968, U01DK058978, U01DK058981, U01DK058985, U01DK058966, and U01DK058973). Study drug was donated by Sanofi-Synthelabo, Ambares, France.
Role of the Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases project officers (Drs Meyers and Kusek) worked collaboratively with the investigators and members of the data coordinating center in designing the study, monitoring the study performance, interpreting data, and preparing the manuscript. The drug manufacturer had no involvement in designing or conducting the study, analyzing or interpreting the data, or preparing the article.
Additional Contributions: We are grateful to the participating patients, to our colleagues who referred patients for enrollment, and to the dialysis unit staff members who facilitated conduct of the trial.
Dialysis Access Consortium Study Group
Boston University Medical Center: L. Dember, J. Kaufman, M. Hawley, A. Lauer, P. LeSage, R. Nathan, E. Holmberg; Baystate Medical Center: G. Braden, M. Ryan, A. Berkowitz; Duke University Medical Center: A. Greenberg, M. Berkoben, E. Kovalik, J. Lawson, J. Middleton, S, Schwab, D. Schumm, S. Adams, K. Gitter, T. Cantaffa, A. Quarles; Maine Medical Center: J. Himmelfarb, J. Whiting, J. Kane, S. Freedman, R. Violette, H. Cyr-Alves, K. Garrison; University of Alabama at Birmingham: M. Allon, M. Robbin, M. Lockhart, B. Casey, J. Newsome; University of Iowa: B. Dixon, B. Franzwa, L. Hunsicker, J. Hoballah, D. Katz, W. Sharp, T. Kresowik, Y. Wu, S. Rayhill; Renal Core Associates (Peoria, Illinois): T. Pflederer, K. DuPage, K. Welch, F. Darras, B. Banqero, B. Ketel, A. Wounded Arrow, C. Grant, J. Deep, L. Pyszka; University of Texas-Southwestern: M. Vazquez, I. Davidson, R. Toto, L. Littmon, C. Ying, T. Lightfoot, H. Quinones, R. Saxena, P. Clagett, J. Valentine, B. Dolmatch, J. Thompson; Baylor University Medical Center: A. Fenves, G. Pearl; Vanderbilt University Medical Center: T. Ikizler, P. Egbert; Wake Forest University: M. Rocco, P. Daeihagh, A. Tuttle, V. Mauck, T. Hoosier, D. McBride; Washington University in St Louis: J. Delmez, D. Windus, D. Coyne, M. Rothstein, S. Shenoy, R. Creaghan, B. Lluka; National Institute of Diabetes and Digestive and Kidney Diseases: J. Kusek, C. Meyers; Steering Committee Chair: H. Feldman (University of Pennsylvania); Data Coordinating Center (Cleveland Clinic Foundation): G. Beck, J. Gassman, T. Greene, B. Hu, S. Bi, A. Liu, M. Radeva, L. Tuason, B. Weiss; Data and Safety Monitoring Board: N. Levin (chair), A. Besarab, G. Chertow, M. Diener-West, T. Louis, W. McClellan, C. Stehman-Breen.
Footnotes
Author Contributions: Dr Beck had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dember, Beck, Allon, Delmez, Dixon, Himmelfarb, Vazquez, Gassman, Greene, Radeva, Davidson, Kaufman, Meyers, Kusek, Feldman.
Acquisition of data: Dember, Beck, Allon, Delmez, Dixon, Greenberg, Himmelfarb, Vazquez, Braden, Davidson, Ikizler, Kaufman, Meyers.
Analysis and interpretation of data: Dember, Beck, Allon, Delmez, Dixon, Greenberg, Himmelfarb, Gassman, Greene, Radeva, Braden, Ikizler, Rocco, Kaufman, Meyers, Kusek, Feldman.
Drafting of the manuscript: Dember, Beck.
Critical revision of the manuscript for important intellectual content: Dember, Beck, Allon, Delmez, Dixon, Greenberg, Himmelfarb, Vazquez, Gassman, Greene, Radeva, Braden, Ikizler, Rocco, Davidson, Kaufman, Meyers, Kusek, Feldman.
Statistical analysis: Beck, Gassman, Greene, Radeva.
Obtained funding: Dember, Beck, Allon, Delmez, Dixon, Greenberg, Himmelfarb, Vazquez, Meyers, Kusek.
Administrative, technical, or material support: Dember, Beck, Delmez, Dixon, Greenberg, Davidson, Meyers, Kusek, Feldman.
Study supervision: Dember, Beck, Allon, Delmez, Dixon, Greenberg, Himmelfarb, Vazquez, Gassman, Greene, Radeva, Braden, Ikizler, Rocco, Davidson, Kaufman, Meyers, Kusek, Feldman.
Financial Disclosures: No other disclosures were reported.
Previous Presentation: The findings of this trial were presented at the annual meeting of the American Society of Nephrology; November 7, 2007; San Francisco, California.
REFERENCES
- 1.US Renal Data System. 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 2.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7(4):523–535. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 3.US Renal Data System. 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2005. [Google Scholar]
- 4.Vascular Access 2006 Work Group. NKF-KDOQI clinical practice guidelines for vascular access, update 2006. Am J Kidney Dis. 2006;48(suppl 1):S176–S276. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Ohira S, Naito H, Amano I, et al. 2005 Japanese Society for Dialysis Therapy guidelines for vascular access construction and repair for chronic hemodialysis. Ther Apher Dial. 2006;10(5):449–462. doi: 10.1111/j.1744-9987.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 6.Fistula First National Vascular Access Improvement Initiative. Change concepts. [Accessed January 2, 2008]; http://www.fistulafirst.org. [Google Scholar]
- 7.Lacson E, Jr, Lazarus JM, Himmelfarb J, Ikizler TA, Hakim RM. Balancing fistula first with catheters last. Am J Kidney Dis. 2007;50(3):379–395. doi: 10.1053/j.ajkd.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Andrassy K, Malluche H, Bornefeld H, et al. Prevention of p.o. clotting of AV cimino fistulae with acetylsalicyl acid: results of a prospective double blind study. Klin Wochenschr. 1974;52(7):348–349. doi: 10.1007/BF01468835. [DOI] [PubMed] [Google Scholar]
- 9.Michie D, Wombolt D. Use of sulfinpyrazone to prevent thrombus formation in arteriovenous fistulas and bovine grafts of patients on chronic hemodialysis. Curr Ther Res. 1977;22:196–204. [Google Scholar]
- 10.Albert F. Prevention of early thrombus formation in arteriovenous fistulae. Dial Transplant. 1981;10:167C–167D. [Google Scholar]
- 11.Gröntoft KC, Mulec H, Gutierrez A, Olander R. Thromboprophylactic effect of ticlopidine in arteriovenous fistulas for haemodialysis. Scand J Urol Nephrol. 1985;19(1):55–57. doi: 10.3109/00365598509180223. [DOI] [PubMed] [Google Scholar]
- 12.Fiskerstrand CE, Thompson IW, Burnet ME, Williams P, Anderton JL. Double-blind randomized trial of the effect of ticlopidine in arteriovenous fistulas for hemodialysis. Artif Organs. 1985;9(1):61–63. doi: 10.1111/j.1525-1594.1985.tb04349.x. [DOI] [PubMed] [Google Scholar]
- 13.Janicki K, Dmoszynska A, Janicka L, Stettner S, Jesipowicz J. Influence of antiplatelet drugs on occlusion of arteriovenous fistula in uraemic patients. Int Urol Nephrol. 1992;24(1):83–89. doi: 10.1007/BF02552122. [DOI] [PubMed] [Google Scholar]
- 14.Janicki K, Janicka L, Dmoszynska A, Marczewski K, Smarz I. Effects of ticlopidine on platelet activity and occlusion of arteriovenous fistulas in IPD patients. Dial Transplant. 1994;23:576–579. [Google Scholar]
- 15.Gröntoft KC, Larsson R, Mulec H, Weiss LG, Dickinson JP Fistula Study Group. Effects of ticlopidine in AV-fistula surgery in uremia. Scand J Urol Nephrol. 1998;32(4):276–283. doi: 10.1080/003655998750015458. [DOI] [PubMed] [Google Scholar]
- 16.Dember LM, Kaufman JS, Beck GJ, et al. Design of the Dialysis Access Consortium (DAC) clopidogrel prevention of early AV fistula thrombosis trial. Clin Trials. 2005;2(5):413–422. doi: 10.1191/1740774505cn118oa. [DOI] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 18.Gart JJ. Statistical analyses of the relative risk. Environ Health Perspect. 1979;32:157–167. doi: 10.1289/ehp.7932157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennison CTB. Group Sequential Methods With Applications to Clinical Trials. Boca Raton, FL: Chapman & Hall/CRC; 2000. [Google Scholar]
- 20.Kaufman JS. Antithrombotic agents and the prevention of access thrombosis. Semin Dial. 2000;13(1):40–46. doi: 10.1046/j.1525-139x.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 21.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62(4):1109–1124. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 22.Turmel-Rodrigues L, Mouton A, Birmele B, et al. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant. 2001;16(12):2365–2371. doi: 10.1093/ndt/16.12.2365. [DOI] [PubMed] [Google Scholar]
- 23.Beathard GA, Arnold P, Jackson J, Litchfield T. Aggressive treatment of early fistula failure. Kidney Int. 2003;64(4):1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 24.Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol. 2006;17(5):807–813. doi: 10.1097/01.RVI.0000217928.43396.35. [DOI] [PubMed] [Google Scholar]