Abstract
Carbon nanotubes (CNTs) have numerous exciting potential applications and some that have reached commercialization. As such, quantitative measurements of CNTs in key environmental matrices (water, soil, sediment, and biological tissues) are needed to address concerns about their potential environmental and human health risks and to inform application development. However, standard methods for CNT quantification are not yet available. We systematically and critically review each component of the current methods for CNT quantification including CNT extraction approaches, potential biases, limits of detection, and potential for standardization. This review reveals that many of the techniques with the lowest detection limits require uncommon equipment or expertise, and thus, they are not frequently accessible. Additionally, changes to the CNTs (e.g., agglomeration) after environmental release and matrix effects can cause biases for many of the techniques, and biasing factors vary amongst the techniques. Five case studies are provided to illustrate how to use this information to inform responses to real-world scenarios such as monitoring potential CNT discharge into a river or ecotoxicity testing by a testing laboratory. Overall, substantial progress has been made in improving CNT quantification during the past ten years, but additional work is needed for standardization, development of extraction techniques from complex matrices, and multi-method comparisons of standard samples to reveal the comparability of techniques.
Keywords: carbon nanotubes, CNTs, characterization, complex environmental matrices, soil, surface waters, sediment, wastewater, analytical chemistry
Graphical abstract
Introduction
The steady increase in potential applications1 and production1,2 of carbon nanotubes (CNTs) and their inevitable release during the life cycle of products has raised questions regarding their potential impact on humans and the environment.3,4 CNTs can be conceptually understood as rolled up graphitic sheets of hexagonally arranged carbon atoms with sp2 hybridization. These materials have exceptional mechanical strength as well as thermal and electrical conductivity properties that make them ideal for a myriad of potential applications (e.g. construction, environmental, optical, electronic, and biomedical).5–8 The annual production capacity of CNTs reached 2 × 106 kg (2.25 ktons) yr−1 in 2011 with an estimated production capacity of 5 × 106 kg (4.5 ktons) yr−1; this change was a 10-fold increase since 2006.1 With increasing production volume, it is important to determine the potential for biological exposures to CNT during the production, usage, and disposal of CNT-enabled products. The necessary linchpin to quantifying potential CNT exposure, and any risks from it, is the availability of robust analytical methods for quantifying CNTs in complex environmental matrices.9 These methods are critical for the assessment of potential CNT exposure, toxicity testing on the potential risks that may occur after exposure, and determination of the environmental fate of CNTs.10
Analytical techniques to quantify CNTs usually rely on unique physicochemical properties of CNTs that differentiate them from other compounds in relevant media. These approaches leverage the structural, thermal, and electrical properties of CNTs and include spectroscopic,11,12,13,14,15 optical,16,17 and thermal16,14,18 techniques used individually or in combination.9,15 Importantly, techniques used for analysis of traditional organic and inorganic toxic chemicals are often not applicable for the following reasons: a) unlike most organic pollutants, CNTs have a distribution of lengths and diameters rather than a single molecular structure and, therefore, mass spectrometry methods, a key tool in current organic analytical methods, generally cannot be used; the large molecular weight of CNTs could potentially challenge mass spectrometric methods too; b) most techniques cannot distinguish between CNTs and naturally occurring black carbon allotropes (e.g., soot or charcoal), which are present at much higher concentrations in the environment than those modeled for CNTs19; c) several other carbon forms are often present in samples (e.g., natural organic matter; NOM) which may interfere with CNT quantification in the sample matrix; and d) the wide range of shapes, sizes, diameters, functional groups, and agglomeration states make it difficult to develop a universal analytical method for quantifying all types of CNTs. In addition, commercially manufactured CNTs may also contain substantial concentrations of metal catalysts, amorphous carbon, and graphitic (non-CNT) nanoparticles (NPs) which may cause biases with some analytical techniques, but are essential for other techniques.20–22
While there have been numerous analytical techniques used to quantify CNTs in various matrices,4,14–16,23–38 for each technique there have only been a limited number of studies, often made by a single laboratory, and thus the robustness of the methods is unknown. In particular, relevant experimental parameters including comprehensive characterization of the CNTs and quantities used for testing and calibration procedures are not always reported. Moreover, failed attempts to apply new methods and techniques or to replicate approaches described in previous studies are often not published, and thus, the limitations of each technique such as potential biases for various matrices (e.g., water or soil with natural (NOM) or soil organic matter (SOM)) are often unclear. Overall, while some recent review papers have focused in part on CNT quantification,4,39,40 many critical topics (e.g., interferences in key matrices (environmental, biological, synthetic polymers), and the potential biases with CNT quantification from changes to the CNTs (e.g., oxidation)) related to the development of robust, precise, and reproducible CNT quantification methods have not yet been critically evaluated.
This manuscript reviews CNT quantification techniques and evaluates their applicability for different key matrices (water, soil/sediment, tissue) and different types of CNTs (i.e., single-wall carbon nanotubes (SWCNTs) or multiwall carbon nanotubes (MWCNTs)). We report a critical evaluation and comparison among the advantages and limitations of each technique including biases for relevant matrices, biases from physicochemical changes to CNTs in those matrices (i.e., oxidation/degradation, wrapping with organic molecules, and agglomeration), detection limits in various matrices, the potential for standardization, and the types of CNTs that can be analyzed. In addition, methods for extraction or separation of CNTs from different matrices, which may be necessary for sample preparation for some techniques, are enumerated. These quantification, separation, and extraction techniques may also be relevant for quantifying CNT loading in consumer products but the focus of this paper will be on scenarios relevant for assessing the potential environmental risks and fate of CNTs. For example, potential quantification techniques for representative scenarios related to environmental release and potential ecotoxicological effects are discussed. Future research topics to elucidate and improve the analytical performance of these techniques and CNT quantification in general are also highlighted. This paper is intended to serve as a reference to guide scientists in the area of CNT quantification through the selection of an appropriate technique given a type of CNT, sample matrix, and CNT concentration. Given the substantial literature on physicochemical properties and characterization of CNTs,41,42 basic background information on these subjects is not provided. While CNTs are also widely known to cause artifacts in many nanotoxicology assays such as by adsorbing key reagents,26,43–45 this manuscript will focus on biases related to quantification of CNTs and not biases in the measurements of their potential toxicological effects.
Extraction and Separation Procedures for CNTs
Numerous techniques have been investigated to extract or separate CNTs from different matrices to overcome quantification limits in complex biological and environmental media (Table 1). In this manuscript, we define “extraction” as the isolation of analytes from a matrix by their physical transition from one phase into another. In contrast, separation means the isolation of analytes from themselves (e.g. differently sized CNTs), or from a matrix within a given phase (e.g. a mobile phase in chromatography or field flow fractionation). Successful extraction methods usually involve the suspension of CNTs in a specific media in which interfering compounds are less soluble, but the converse approach can also be utilized: removing the matrix while leaving the CNTs. However, most reported separation or extraction methods have only been used by a single research group in one or a small number of studies to partly or fully separate CNTs from an environmental matrix (e.g., asymmetric flow field flow fractionation (AF4), matrix digestion, and sonication with surfactants).15,23,46 Other techniques have not yet been utilized with environmental and biological matrices (e.g., density gradient centrifugation, gel permeation chromatography, capillary electrophoresis, two-polymer phase extraction), but instead have been successfully applied to simpler matrices (e.g., deionized water) or have been used for CNT purification.47–49 These techniques may be valuable for use with environmental and biological matrices and are also listed in Table 1. Conversely, there has been more progress with extraction and analysis of fullerenes, another carbon nanomaterial, from complex matrices.50–56
Table 1.
Extraction and separation techniques to isolate CNTs from environmental and biological matrices
| Technique | Overview | Strengths | Limitations |
|---|---|---|---|
| Asymmetric Flow Field-Flow Fractionation 46,47,130–133 | Flow-assisted separation technique based on particle diffusion against a hydrodynamic field in the absence of a stationary phase | Enables separation of well dispersed CNTs by length, reduces sample polydispersity, possibility for online/offline coupling with a variety of analytical techniques can yield complementary information | Time-consuming and laborious operation/method development, high sample dilution during the analysis, possibility of strong particle-membrane interactions may result in low recoveries, separation less efficient (low number of theoretical plates) than with e.g., capillary electrophoresis |
| Capillary Electrophoresis134–137 | Based on the different electrophoretic mobilities of the species (on the basis of their charge/size ratio) through an electrolyte contained in a fused silica capillary when an electrical field is applied; a suspension of CNTs, usually in a surfactant, is subjected to an electric current | Potential separation of bulk samples of CNTs according to the charge/size ratio, length sorting and separation according to bundled/non-bundled can occur; high theoretical plate number, thus potentially superior resolution power, due to the plug-like flow of the electroosmotic flow | Laborious sample preparation for controlled experiments, several important challenges still remain, including limited sensitivity, non- quantitative recoveries, and reproducibility problems; micellular electrokinetic chromatography cannot be used, as CNTs are too large to reside in the intramicellular region |
| Centrifugation138 | Large suspended particles are removed first on basis of difference in sedimentation velocities | Potential isolation of CNTs from matrix, either in sediment or supernatant | Protocol will depend on CNT and matrix, further separation of the fraction is challenging without disturbing neighboring fractions |
| Density gradient centrifugation139,140 | Particles will equilibrate to their isopycnic (equal bouyancy point) in a density gradient at sufficiently high applied acceleration | Can enable extraction of specifically modified subpopulations, resolves aggregate states | Low processing quantity, kinetic and transport non-idealities can occur, different aggregation states have different buoyant densities. |
| Size exclusion chromatography48,141–147 | A chromatographic method that separates analytes based on their size and shape by differential exclusion from the pores of the stationary phase; no interactions must exist between CNTs and the stationary phase | Relatively simple and inexpensive, good size separation for SWCNTs within a certain length limit and shape | It has mainly been used for short single-walled carbon nanotubes; it is unclear if this technique can separate larger SWCNTs or MWCNTs; prefiltration might be needed; agglomerates can get trapped within the chromatographic column or the prefilter; well dispersed suspensions are required; only for qualitative analysis; no environmental samples have been tested |
| Matrix Digestion23 | Different chemicals or solutions are used to dissolve the matrix (e.g., tissues) to facilitate subsequent analytical techniques | Lowers detection limits and removes potential biases for many techniques | Different approaches will likely need to be developed for each type of matrix (e.g., tissue vs. sediment) and may need to be developed for different types of tissues |
| Micro- nanofiltration13,148 | Use of micro and nanopore- sized filters to separate analytes based on their size | Very simple and inexpensive; at low CNT concentrations, can treat larger volumes than other techniques | Mainly used for CNT suspensions with very little interferences and at low concentrations to avoid clogging the filters; it is difficult to regenerate the CNT suspension for further characterization/quantification; there might be sample losses and filter interferences |
| Selective Oxidation14,18,149 | Use of thermal or chemical oxidation to separate more refractory carbon fractions (CNTs) from more labile organic carbon | Allows for a cleaner (and easier) subsequent characterization or quantification | Not very reliable in the presence of interfering material or when the oxidation is not complete (e.g. coals, very rich organic carbon environments); recoveries might vary between different types of CNTs |
| Sonication with surfactant15 | Use of a surfactant to create a stable CNT suspension that can then be separated from the remaining non CNT material that settles down at a different speed | Can extract CNTs with varying surface chemistry from sediment, no special equipment is necessary | Recoveries vary among SWCNTs with no recognizable pattern; repeatability varies, surfactants may interfere with quantitation procedure |
Currently, many challenges remain in CNT extraction and separation strategies. First, it is unclear to what extent many of these techniques would be applicable for both MWCNTs and SWCNTs given the different properties of these two classes of CNTs, as most methods have only been applied to one or the other. This thought may be extended beyond the number of walls, to include any change in physicochemical properties (e.g., length, internal or external diameter, number of walls, or functional groups). Nevertheless, we expect that separation and extraction techniques may have to be tailored for a specific physicochemical property. For example, a method that can isolate short CNTs from a matrix could be ineffective when used against a population of long, highly entangled CNTs. Second, separation or extraction methods have not yet been applied to CNTs as utilized in potential consumer applications such as in polymer nanocomposite matrices. Given that CNTs will be released into the environment from consumer products, it is important to quantify the release of CNTs from these products after environmental stresses. It may also be important to quantify the concentration of CNTs in the consumer products, such as CNT-containing nanocomposites, to determine the potential quantity that could be released. Given challenges related to collecting and quantifying CNTs released from polymeric nanocomposites, one approach to estimate the quantity of CNTs released is to use a mass balance approach by quantifying the CNT concentration in a product before and after environmentally relevant degradation processes. For example, established methods are needed to extract CNTs from CNT-containing nanocomposites before and after the weathering and degradation processes (e.g., due to UV degradation and abrasion) to enable quantification of CNT concentrations.57–60 This will allow scientists to more fully address the complete life cycle of nano-enabled consumer products. Finally, extraction or separation procedures may change the physicochemical properties of the CNTs, potentially impacting the reliability of results from analytical methods. One such example is the matrix digestion approach described by Doudrick et al.,23 which was suitable for subsequent analysis using thermal optical transmittance (TOT), but is potentially unsuitable for spectroscopic quantification by Raman scattering, because of concerns that the Raman spectra (e.g., ratio of D to G band) may be altered by the digestion procedure. Overall, although encouraging results have been obtained for a limited number of studies, the overall development of extraction and separation methods for CNTs from matrices for quantitative analyses is still a relatively new area of research.
Quantification techniques
A broad range of techniques have been developed to quantify or identify CNTs in environmentally and biologically relevant matrices (Table 2). In general, the techniques can be sorted into four groups: those that rely on the unique spectroscopic and thermal characteristics of the CNTs (that enable them to be distinguished from the matrix), those that utilize the presence of metal catalyst impurities (associated with the CNTs from the synthesis process), those that require isotopically enriched or depleted CNTs (e.g., with carbon-14 or carbon-13), and finally, microscopic techniques. There are large differences in the sensitivities and applicability of these techniques. Some thermal processes produce detectable gases (CO, CO2), while others measure radiative heating of a sample. For example, the microwave method involved irradiating CNT containing samples with microwave radiation, wherein the carbon nanotubes absorb the microwave radiation, and the increase in temperature is proportional to the CNT concentration for a given matrix.61,62 When comparing different studies, even those using the same quantification technique, there is substantial diversity in the characteristics of the CNTs utilized.
Table 2.
Selected techniques for CNT Quantitation
| Method | Overview | Strengths | Limitations |
|---|---|---|---|
| Spectroscopic | |||
| Absorbance65,109,150,151 | Measures absorbance of aqueous sample; can include ultraviolet, visible, or near infrared wavelengths | Readily available in many environmental laboratories | Interference from other sample components, relatively high detection limit, only applicable for aqueous samples |
| Near infrared fluorescence (NIRF) 15,29,31,152 | A specific emission spectra can be used as an identification tool of SWCNTs; the intensity of the fluorescence signal can be used for quantification of SWCNTs | Quantification/Detection at very low limits of detection | Limited to non-functionalized SWCNTs; semi-conducting SWCNTs but not metallic SWCNTs can be detected |
| Raman13,122,123,153–164 | Measures radial breathing (SWCNT), G, D and G′ vibrational bands in dry and various solvent suspended samples, tissues | Minimal sample preparation, enables CNT characterization, compatible with in vitro and in vivo samples, can be used with a microscope, low detection limits achieved using resonance Raman conditions | Some matrices may produce interferences, sensitive to laser power, requires calibration for quantitative analysis |
| Spectrometric | |||
| Inorganic Element Analysis29,32 | Measures trace catalytic metallic elemental impurities intercalated in the CNT structure (Cr, Co, Cu, Fe, Mo, Ni, Y, Zn), analysis of bulk metal content; the applicability of this approach could be impacted by removal of the metal catalysts by purification but catalysts located within the CNTs often remain after purification processes | Multi-elemental capability and extreme sensitivity of ICP-MS allow an accurate and selective determination of metal impurities of CNT in a wide range of matrices at ngL−1 or sub ngL−1 levels, the rapid sample throughput of this method is attractive for routine screening | Carbon is generally not detectable with standard ICP-MS methods, quantitative sample dissolution is required prior to analysis; incomplete sample digestion, release of metal ions from the CNTs in the sample matrix, or elemental contamination from the sample digestion steps could lead to an important bias in the bulk metal content determination; the feasibility of using this technique could depend partly on if the metal contents of the CNTs are known a priori |
| Single particle inductively coupled plasma-mass spectrometry (spICP-MS)22 | Metal catalyst impurities are used as proxies to detect and quantify CNTs; the applicability of this approach could be impacted by removal of the metal catalysts by purification but catalysts located within the CNTs often remain after purification processes | Potential capability for the size, size distribution, and particle number concentration determination of CNT; high selectivity to differentiate CNT at extremely low concentrations from naturally occurring carbon- containing species (i.e. cells, organic detritus, humic acid); very low detection limit | Size/length estimation requires the invalid assumption that metal content is homogeneous among the CNTs, very small particles cannot be separated from the background, leaching of catalysts in the sample matrix prior to spICP-MS analysis can bias the result, only applicable for aqueous samples; the feasibility of using this technique could depend partly on if the metal contents of the CNTs are known a priori |
| Microscopic | |||
| Atomic Force Microscopy109,165 | Measure the surface features of a sample by dragging a cantilever over the sample; the length of identifiable tubes can be determined by the movements of the cantilever | Most trusted technique for determining number and length | Deposition bias, measurement bias, and detection errors are all possible in most samples |
| Hyperspectral Imaging166,167 | Measures reflectance spectra of NPs in a darkfield (visual near infrared/short-wave infrared spectral range), resulting in 2D-optical images with full spectral information that contain a full spectrum (400 nm to 1000 nm or 900 nm to 1700 nm, respectively) in each pixel; CNTs appear bright against a dark background | Easy sample preparation, provides optical (i.e. differentiation between single nanotube and nanotube- agglomerate) and spectral information, allows spatial localization of particles, can provide semi-quantitative information, short-wave infrared spectral range could be applicable for detection of SWCNTs | Currently long analysis times, visual near infrared not specific for CNTs, many potential analysis artifacts |
| Photoacoustic (PA)24,168–170 | PA measures the acoustic response to the rapid volume change resulting from the absorption of an optical pump beam and the transfer of heat to the surrounding environment | Suitable for detection in liquids such as water and complex media such as plants, minimal sample preparation, can be quantifiable, excellent penetration depth enables samples > 100 μm, works equally well with metallic and semiconducting SWCNTs and MWCNTs, label free, unaffected by some complex media issues including carbon-on-carbon | Signal is dependent on absorption and heat transfer to material surrounding the CNTs, can be 10x lower sensitivity than PT, medium surrounding CNTs must be transparent to the beams, heating laser must overlap with absorbance of the CNTs, signal scales with size of CNT cluster, non-transparent media may cause detection issues, quantification may require diameter and length distributions |
| Photothermal (PT)24,168,169 | PT measures the optical scattering response of a probe beam to the change in local environment refractive index that results from the absorption of an optical pump beam and the transfer of heat to the surrounding environment | Suitable for detection in liquids such as water and complex media such as plants, minimal sample preparation, can be quantifiable, penetration depth can handle samples up to 10 μm, works equally well with metallic and semiconducting SWCNTs and MWCNTs, label free, unaffected by some complex media issues including carbon-on-carbon, sensitivity down to single particle sensitivity, lower LOD than absorbance-based measurements | Same as Photoacoustic plus is limited to thin samples (< 100 μm) |
| Scanning Electron Microscopy and Scanning Transmission Electron Microscopy | Measures the interaction of a finely focused electron beam with the CNTs; secondary electrons, and transmitted electrons can be used for image formation | Provides detailed morphological properties (length, width, shape) of individual CNTs; individual CNTs can be localized in complex matrices based on morphological criteria | Labor intensive, often only qualitative information |
| Transmission Electron Microscopy (TEM)27,66 | Illuminates a selected sample area (parallel electron beam) and detects the transmitted electron after passing through the samples | Provides detailed morphological properties (length, width, shape) of individual CNTs; high resolution can be used to distinguish between SWCNTs and MWCNTs; CNTs can be identified in energy filtered TEM images | Challenging sample preparation for tissues; it may be very hard to detect NPs in complex samples at low concentrations; low contrast (conventional TEM) due to reduced interactions between CNTs at the electron beam at high acceleration voltages |
| Thermal | |||
| CTO-37518 | Quantification of carbon that remains after combustion at 375 °C for 24 h under excess air sample and subsequent chemical oxidation | Particularly good for complex matrices such as soil and sediment | Not fully tested for suspensions, requires high concentrations of CNTs and low concentrations of interferences (e.g., soot interfering with MWCNTs or graphene with SWCNTs) |
| Thermal Gravimetric Analysis (TGA) 20,171,172 | Quantification of mass percentage of phases with distinct thermal stabilities under a variety of reactive atmospheres (usually air) and relatively rapid temperature programs (e.g., heating rates of 5 C/min to 20 C/min,; room temperature- ca. 950 °C); each sample takes 1 h to 2 h total | A rapid technique that allows the quantification of multiple phases in a single sample, good for complex matrices, no special sample preparation needed | Effect of thermal ramp rate and reactive atmospheres on apparent phase distribution is not well understood (and is largely ignored), detection limits are relatively high for solid matrices, potential for interferences between sample matrix (e.g., other carbon nanomaterials, soot, or black carbon) and CNT decomposition temperatures |
| Thermal Gravimetric Analysis-Mass Spectrometry (TGA-MS) 14 | TGA coupled with mass spectrometric detection of evolved gas fragments, typically in the 2 to 300 m/z range | Mass fragments can give insight into the chemical structure of the source material (e.g., C/H/O ratios or unique evolved fragments) | Current mass spectrometers have poor mass resolution (ca. 1 amu), relatively high detection limits, and low sampling rates relative to the chamber flush rate (i.e., consequently, only a small portion of the evolved mass is transferred to the MS); all reduce identification accuracy and increase detection limit |
| Total Organic Carbon (TOC) Analysis71 | TOC analysis can be conducted on water or soil samples by oxidizing (chemical, heated catalyst, UV) carbon to carbon monoxide or dioxide which is detected by infrared or other detectors | TOC analysis of waters has been used to measure CNTs in stock solutions in water | Very little optimization of temperature or catalytic conditions have been examined; its application to CNT stock solutions have been consistent with prepared masses; any organics, such as natural organic matter, in solution or soils would interfere; this is a non-specific method and thus matrices that contain sufficiently high concentrations of other carbon nanomaterials (e.g., graphene), soot, or black carbons would impact the technique |
| Thermal Optical Transmittance (TOT) 16,23 | As the sample is analyzed under programmed temperature, the volatilized and combusted carbon travels to an oxidizing oven, where it is transformed into carbon dioxide (CO2); the amount of elemental carbon is determined based on the CH4 signal measured using a flame ionization detector; sample is first heated under inert conditions to remove volatile organic carbon, then oxidizing carrier gas is used for elemental carbon; the portion of TC that is organic carbon or elemental carbon is defined by the method, which determines where the organic carbon-elemental carbon split is placed post-analysis; this split can be automatic on the basis of automatic optical correction; the optical transmittance or reflectance is observed throughout analysis, and the split is placed where the transmittance/ reflectance returns to the initial reading; for samples in which optical correction does not work, a manual split defined by the analyst should be used | Very reliable technique for detecting elemental carbon in environmental matrices, this technique could differentiate between types of CNTs based on their thermal stability | Too much organic carbon in a sample causes peak overlapping between elemental and organic carbon which affects the accuracy; similar carbonaceous materials such as graphene and fullerene will be counted in the CNT peak if they exist in the sample; unless the peak from CNT is far enough from other carbonaceous material, it is difficult to exclude the other carbonaceous materials but adjusting the temperature program might help to some extent |
| Isotopic labeling | |||
| Carbon-13 Labelling21,32,78 | A measure of the ratio of 13C to 12C, applicable for all CNTs but works best for isotopically enriched or depleted CNTs | Instrumentation is readily available in many environmental laboratories | Highly dependent on matrix and large variability may be observed for CNTs that are not specifically 13C enriched |
| Carbon-14 Labelling15,26,30,99,124,152,173–181 | Measures beta emissions from carbon-14 emissions, can be used to quantify liquids after mixing with scintillation cocktail or any matrix after combustion in a biological oxidizer, autoradiography can provide spatial distribution of radioactivity | Provides definitive quantification of CNTs in complex matrices, can be used as an orthogonal technique to develop other analytical techniques, can be used to identify degradation products | High cost to synthesize radioactively labeled CNTs, safety concerns, limited availability of radioactively labeled CNTs |
| Other radioactive isotopes96–98 | Measures release of emissions from a radioactive isotope that is associated (e.g., attached to a polymer wrapping the CNT) with the CNT | This approach can enable extremely low detection limits, can be used with a range of CNT surface functionalizations, non-destructive sample is possible for gamma emitters | Artifacts are possible if the radioactive isotope becomes separated from the CNT, it may be challenging or impossible to determine if this occurred in complex matrices without orthogonal CNT quantitation techniques |
| Additional Techniques | |||
| Analytical Ultracentrifugation (AUC) 60,165,182–184 | Measurement of sedimentation velocity distribution, can be used to determine particle density or size/shape distribution | Can measure entire CNT population via absorbance or interference measurement, high resolution, little size bias | Finicky technique that requires well understood and controlled samples for robust analysis |
| Gravimetric185 | The CNT concentration in suspension is estimated by drying a fraction of the suspension and weighing it, or by determining the fraction of CNTs not suspended during the dispersion process (e.g., by sonication) by weighing the mass of CNT particles at the bottom of the container | Uses readily available equipment | Limited to high CNT concentrations, only applicable for aqueous suspensions |
| Microwave Method62,125,186,187 | Measures the temperature rise of a sample at a specific microwave energy within a specific timeframe | Straightforward method for CNT detection and quantification in biological tissue, low cost | Not commercially available; it still remains to be investigated for environmental samples if interferences arise from other carbon allotropes with similar behavior in the microwave field (e.g., carbon black, soot)188 |
| aF4-MALS46 | Measures a shape factor (ρ=radius of gyration/hydrodynamic radius) of particles present in a complex liquid sample (e.g. surface water, leachate, soil and sediment extract), which is indicative of the particle aspect ratio; comparing these results to a CNT-free sample can then be used for CNT detection | Allows for CNT detection in water, soils, and sediments; may be useful in exposure studies | Need for the baseline of a CNT-free sample, full quantitative use currently not straightforward, often low CNT recoveries for aF4 |
Abbreviations: Asymmetric flow field flow fractionation with multi-angle light scattering (aF4-MALS), analytical ultracentrifugation (AUC), carbon nanotube (CNT), chemothermal oxidation at 375 °C (CTO-375), inductively coupled plasma-mass spectrometry (ICP-MS), near infrared fluorescence (NIRF), multiwall carbon nanotube (MWCNT), photoacoustic (PA), photothermal (PT), single particle inductively coupled plasma-mass spectrometry (spICP-MS), single-wall carbon nanotube (SWCNT), transmission electron microscopy (TEM), thermal gravimetric analysis (TGA), thermal gravimetric analysis-mass spectrometry (TGA-MS), total organic carbon (TOC), thermal optical transmittance (TOT).
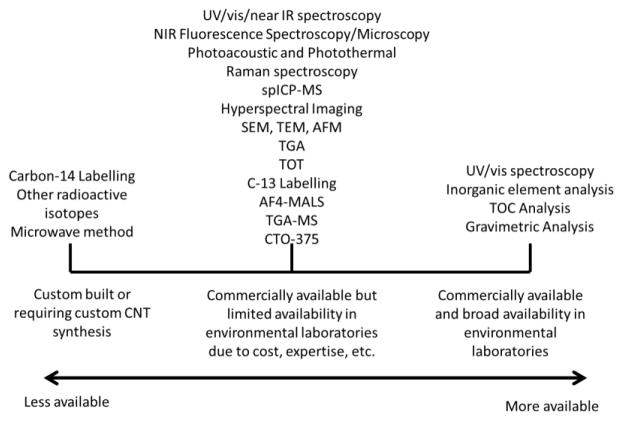
It is evident from Figure 1 that, while some instruments used in the CNT quantification techniques are commercially available (e.g., UV/vis/NIR spectroscopy and Raman spectroscopy), most of the techniques require uncommon equipment that need to be partially or wholly custom built (e.g., microwave method, photoacoustic and photothermal imaging) or expertise that is not readily available. The use of uncommon instruments in these techniques also poses challenges for commercial ecotoxicity testing facilities to fulfill guidelines for standard methods related to maintaining a consistent exposure concentration.63 While some analytical instruments that can be used to quantify CNTs are widely available (e.g., UV/vis spectrophotometry), some of them have significant potential interferences as will be discussed in detail in subsequent sections. To provide one example, challenges related to the use of UV/vis spectrophotometry have recently been described including absorption coefficients dependent on the CNT structure distribution and dispersion method, as well as decreasing absorption coefficients with CNT agglomeration and uncertainty in determining non-CNT from CNT contributions.64,65 The lack of robust and widely available analytical methods likely contributes to the exclusive use of nominal concentrations to describe the exposure concentration and the absence of reported changes in CNT concentrations during experiments in many nanoecotoxicology studies.
Figure 1. Availability of CNT quantification techniques.
Abbreviations: Asymmetric flow field flow fractionation with multi-angle light scattering (AF4-MALS), analytical ultracentrifugation (AUC), chemothermal oxidation at 375 °C (CTO-375), near infrared fluorescence (NIR), single particle inductively coupled plasma-mass spectrometry (spICP-MS), thermal gravimetric analysis (TGA), thermal gravimetric analysis-mass spectrometry (TGA-MS), total organic carbon (TOC), thermal optical transmittance (TOT), scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM).
Microscopic techniques can provide unambiguous identification of the CNTs in a complex matrix (e.g., transmission electron microscopy (TEM) analysis using electron energy loss spectroscopy or high resolution TEM ),27,66 but low or uneven distributions of CNTs on microscopy samples hamper the conversion of the number of CNTs detected on (several) images to the number/mass concentration of CNTs in a sample. These limitations can be overcome, for matrices without substantial interferences, by using a centrifugation-based method to capture the CNTs from a known volume onto a microscopy sample holder (e.g. TEM grid). Under these conditions, frequency data (number of CNTs per area) can be converted into particle number and mass concentration metrics.67–69 However, when one considers projected environmentally relevant concentrations of CNTs (typically ng to μg kg−1 solids),70 the likelihood that one captures a CNT onto a microscopy grid with μg-sized environmental samples is exceedingly small. Overall, due to limitations related to the sample preparation issues (low CNT concentration especially compared to other solids, overlapping particles, and uneven distribution of CNTs onto the sample holders), results from electron microscopic techniques remain mainly on a qualitative level, and are currently of limited utility for quantitation.
While electron microscopic techniques are very helpful to confirm the identity of CNTs in a matrix if the CNT loading is sufficiently high, reliable controls of the sample matrix without CNTs, the CNTs alone, the sample holder, and any other interferences are needed to avoid false positive or false negative results, but these controls are rarely available for environmental samples. In addition, the amount of time required for sample preparation depends on the samples matrix and greatly varies among techniques. For example, obtaining TEM images suitable for automated image analyses may require that individual CNTs are evenly distributed on a TEM grid and do not overlap with other particles. This often requires elaborate and tailored extraction, dispersion and deposition techniques that are very time intensive to develop. In contrast, sample preparation for hyperspectral imaging microscopy is usually very fast, as liquid samples can be directly cast onto a microscopy slide and subsequently imaged. However, the current commercial setup lacks the possibility for automated image acquisition as well as suitable measures to determine the deposited sample volume, which hampers its quantitative capabilities.
Due to the similarities between CNT structure and that of atmospheric soot or carbon black, many analytical techniques that have been used for their extraction or isolation from air, soil, or sediment have been also used to quantify CNTs (e.g., thermal optical transmittance (TOT), chemothermal oxidation at 375 °C (CTO-375), thermogravimetric analysis (TGA), and total organic carbon (TOC)).14,16,18,71 While TOT can measure CNTs, custom temperature ramping programs are required for CNTs that differ from standard National Institute for Occupational Safety and Health (NIOSH) methods used for soot analysis on atmospheric samples.16 Similar modifications may also help improve CNT quantification by other thermal techniques such as CTO-375. Sampling of soot in air requires separation from the air, and usually involves filters, impactors or centrifugal separation. Airborne CNTs would likely also be captured by these techniques.72–76
All of the quantification techniques are critically assessed in subsequent sections for the potential impact of matrix interferences or interferences from changes that may occur to the CNT in different test systems or the natural environment. For example, the impact of CNT degradation, as has been shown to occur enzymatically and due to interactions with cells and bacteria,77–83 and oxidation on the performance of different analytical methods are evaluated. In addition, the limits of detection (LODs) for these techniques in different media are compared and used to assess the potentially relevant techniques for five case study scenarios. The potential for these techniques to be standardized, a critical issue for regulatory agencies, is also discussed.
Evaluation of potential matrix interferences for quantification procedures
Perhaps the principal reason that quantification of CNTs in environmentally relevant matrices is challenging is because of matrix interferences, namely difficulties associated with detecting carbon in a carbon background, especially at modeled average environmental CNT concentrations.70,84–86 The matrix characteristics that are most likely to cause interferences are described in detail in Table S1. Overall, natural waters and cell media (e.g., in studies with fish or human cells) have significantly fewer matrix interferences compared to biological tissues, soil/sediment, and released material from nanocomposites. For most spectroscopic measurements, while molecules and suspended particles in natural waters and cell media can potentially scatter incoming/outgoing light thus potentially biasing measurements, methods that account for these effects are generally available; in contrast, separation from the matrix is often needed prior to CNT quantification in tissues, soil/sediment, and fragments released from nanocomposites. For inorganic elemental analysis, having a constant and relatively low background metallic content of the matrix of the same element as the catalyst(s) of the CNTs is most important for all relevant matrices to achieve a low LOD and accuracy. Additionally, the multi-isotopic capability of the inorganic elemental analysis may enable qualitative and/or quantitative isotopic analysis when the isotopic ratios of the catalyst particles differ from those typically observed in the environmental matrix. For single-particle inductively coupled plasma-mass spectrometry (spICP-MS) analysis of CNTs, the background metallic content in nanoparticulate form in matrices is similarly important with regards to the accuracy of the measurement, while low background metallic content in dissolved form is necessary for achieving a low LOD. However, spICP-MS instruments operating at microsecond dwell times can only perform nanoparticle isotopic analysis for detection of two elements, a capability which nevertheless can be used to distinguish naturally occurring NPs from their engineered counterparts.87 While spICP-time of flight (ToF)-MS has recently shown the capacity for multi-element analysis,88,89 the size limit of detection was larger for gold and silver NPs compared to quadrupole-based instruments.89 Given the expected small amounts of the catalysts associated with individual CNTs and challenges associated with determining the background cut off level for SWCNT analysis using spICP-MS,22 it is unclear if spICP-ToF-MS will work well for CNT quantification.
Thermal techniques often do not show interferences with natural waters and cell media, although there were technique-specific chemicals in these matrices (e.g., peptone in the media for TGA analysis)90 that could impact the results. Two key considerations for many of the thermal techniques are whether components in the matrix can change the thermal stability of the CNTs and if there is the potential for overlap in the oxidation temperatures of CNTs and combustible components of the matrix. Thermal techniques could generally work in all matrices but the detection limit will be higher in matrices with more interferences as will be discussed in a subsequent section. Lower LODs may be achievable by first extracting the CNTs or decreasing the bias from other forms of organic carbon.
Quantification of CNTs (and other carbon nanomaterials91–95) via isotopic labelling generally has fewer interferences than the other techniques, but obtaining isotopically enriched CNTs is typically challenging and/or expensive. Furthermore, this approach is only relevant for laboratory studies, not for detecting CNTs released into the environment. A related strategy, labeling CNTs with coatings containing a radioisotope, was used in many early biodistribution studies in the biomedical field,96–98 but has not been used in environmental or ecotoxicological studies. The challenge with this approach is that the accuracy of any measurement is contingent upon the radioactive tracer remaining associated with the CNTs.
Natural abundance, stable isotopic measurements (e.g., carbon-13)21 face similar limitations in that they require a CNT-free sample to which one can compare the isotopic composition in order to deploy the technique quantitatively. In laboratory studies, this is possible and more economically viable than radiolabeling techniques, but one has to carefully select CNT-free controls for quantifying CNTs in environmental samples. Furthermore, while the initial label is more expensive, the analytical techniques required to trace a carbon-14 label (i.e., liquid scintillation counting) are facile compared to the expert preparatory and analytical equipment required to trace natural-abundance isotopes (i.e., much lower levels of either carbon-14 or carbon-13 require accelerator mass spectrometers and isotope ratio mass spectrometers, respectively, and each with closed-tube-combustion preparation upstream). Nevertheless, the carbon source for SWCNTs produced using the high pressure carbon monoxide (HiPco) process is usually biomethane,21 which has a strong naturally depleted carbon-13 signature, and such CNTs would be good candidates for using natural abundance, stable isotopic measurements.78
Evaluation of potential bias from changes to the CNTs
In addition to interferences from different environmentally and biologically relevant matrices, changes that may occur to CNTs while in these matrices can also cause interferences for many of the quantification techniques (Table S2). The extent to which agglomeration, degradation, and wrapping by other molecules occurs depends on the physicochemical properties of the CNTs and of the matrix. It is well known that CNTs will agglomerate in waters with sufficient ionic strength if they are not stabilized through, for example, a surfactant and that CNTs have a large capacity to adsorb natural organic matter.99–102 With regards to CNT agglomeration, while most techniques are sensitive to this change (e.g., most thermal techniques, Raman, NIRF, UV/vis/NIR absorbance, and spICP-MS), some are not impacted by it (e.g., inorganic element analysis) or may even be enhanced (e.g., hyperspectral imaging). Potential interference from CNT agglomeration may result in, for example: a) changes to the intensity or peak wavelengths in the spectrophotometry signals; b) shifts in the thermal stability of the CNTs, which could prevent separation from other components in the matrix, such as black carbon soot; or c) hindering uniform distribution on a filter prior to analysis by TOT. Agglomeration may also increase the heterogeneity and affect representativeness of the subsamples in a matrix, which could lead to increased uncertainty. However, larger subsamples could help lower the uncertainty when feasible.
The literature shows variable results on the degradation of CNTs in environmental matrices. In some studies, degradation of carbon-14 labeled CNTs by enzymes or bacteria has been shown to be slow or not detectable 77,78,103 except under specific situations with a special microbial consortium.77 In contrast, studies assessing the degradation of non-carbon-14 labeled CNTs have often shown substantial degradation.82 The cause of this discrepancy is unclear. Studies on the photodegradation of CNTs have shown significant modifications to their surface structure or the loss of fluorescence under some experimental conditions.104,105 Thus, it is reasonable to assume that some degree of degradation could occur with CNTs in surface waters if they stay suspended for a sufficiently long period. Almost all quantification techniques are sensitive to CNT degradation and oxidation, although the degree of oxidation needed before it impacts quantification varies among techniques. One exception is carbon-14 analysis, which is not impacted by oxidation. In contrast, the degree of oxidation can directly impact CNT thermal properties and potentially the capacity to differentiate between CNTs and other forms of carbon present in the matrix using many of the thermal based techniques.
Wrapping of organic molecules around CNTs, such as proteins or NOM, may also impact most quantification techniques. Many of the potential changes that could cause biases, such as decreased signal intensity of a spectroscopic measurement or a change in the thermal stability of CNTs for thermal measurements, are similar to those discussed for degradation. However, the reason behind these changes is from the impact of the coating on the CNT properties rather than a change to the core CNT material itself as would occur during degradation. One challenge in discussing the potential bias from organic molecules wrapping around CNTs, and also agglomeration and oxidation/degradation, is that the magnitude of the bias relates partly to the degree of agglomeration, oxidation, and the quantity of organic molecules associated with the CNTs. It is possible to foresee examples when these changes in the environmental matrices could have a bias, but it is challenging to quantify the magnitude of the expected bias without information about the sample system (e.g., aqueous phase NOM concentrations can range between 5 mg/L and 50 mg/L) or the extent of oxidation. This information about the sample system or magnitude of likely changes could allow one to account for biases.
Being aware of the potential biases present in a sample from these changes to the CNTs and/or carrier matrix will support researchers in determining to what extent these factors may impact their measurements. However, it might be challenging to get this kind of information from samples with low CNT concentrations when there is a low signal to noise ratio. Environmentally-relevant information on the rate of CNT modifications (e.g., oxidation) by environmental processes is limited,77,103,106–108 and systematic studies of those processes would be an enormous benefit to parallel efforts to quantify CNTs in the environment. While leaching of metal catalysts from the CNTs in environmental matrices is not explicitly covered in the above changes to the CNTs, it could dramatically impact analyses using spICP-MS or elemental analysis. The potential for changes in the catalyst particles associated with the CNTs in environmental matrices is the primary reason that these techniques are not more broadly used despite their low LODs.
Detection limits of quantification techniques
The LOD for CNT quantification is one of the most critical performance metrics required to compare the various techniques. However, the definition of the LOD depends partly on how the CNT mass in a given sample is determined. The most common approach is for the whole sample, including CNTs, catalyst particles, and any carbonaceous impurities, to be included in the CNT mass used. It is possible instead to only use the CNTs themselves, at least for SWCNTs where, after purification procedures, the properties are more clearly distinguishable and high quality separation techniques exist.109 While additional metrics such as number or surface area concentrations are highly desired,63,110 the LOD values provided here are for mass concentrations.
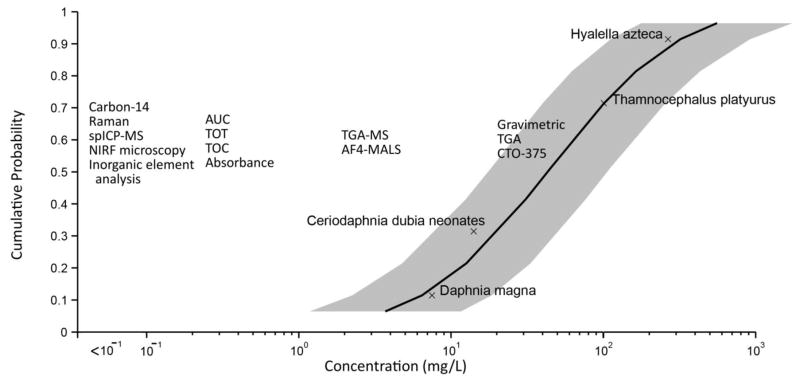
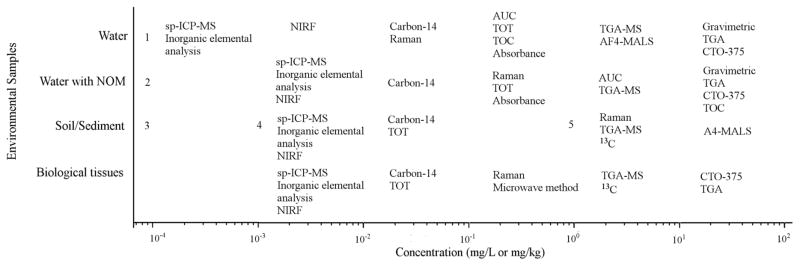
There are two different approaches for determining the necessary LOD for quantifying contaminants in the environment. The first requires that the LOD is adequate for quantification of the contaminant at concentrations that may have harmful effects. An alternative requirement is for the analytical techniques to quantify the contaminant at the concentration that it is determined or estimated to be present in the environment. We have compared the LODs for the various analytical techniques using both approaches through comparing the LODs to a species sensitivity distribution for CNT acute toxicity to pelagic organisms (Figure 2) and to modeled environmental concentrations (Figure 3). Several trends are evident from reviewing these figures. First, the LODs in water span several orders of magnitude with some techniques only capable of quantifying CNTs in samples with concentrations greater than 10 mg/L (e.g., gravimetric measurements), while the most sensitive techniques can detect concentrations between 0.1 μg/L and 1 μg/L (e.g., spICP-MS) (Figure 3). Second, the lowest LOD values are for pristine water samples and increase with higher amounts of potential interferences in the matrix. Higher LODs are observed when NOM is present in waters, and even higher LODs are typically achieved when using CNT quantification techniques in soils, sediments, and biological tissues. Third, multiple techniques appear capable of quantifying CNTs at concentrations relevant for stock suspensions (e.g., 10 mg L−1 to 100 mg L−1) that could be used for pelagic aquatic toxicity testing (Figure 2). As discussed in more depth in a case study, some techniques could also be used to quantify the initial exposure concentration for ecotoxicity testing and the concentration after the experiment concludes. Fourth, the LODs are often orders of magnitude higher than the average modeled environmental concentration, but some are within the range of modeled sediment concentrations despite the lower LODs for CNT quantification in sediments. This suggests that it may be feasible to quantify CNTs in the environment under certain conditions. Overall, these figures can be used to assess which methods may offer suitable techniques for an intended purpose, as is described in more detail in the case studies. Alternatively, extraction or separation techniques (see above) may be necessary to selectively isolate and concentrate the CNTs prior to analysis.
Figure 2.
Comparison between detection limits for analytical techniques in a water-only media under optimal conditions juxtaposed with a species sensitivity distribution for CNTs for acute toxicity testing of pelagic organisms. For the species sensitivity distribution, the 95 % confidence for the LC50 values is shown by the gray shaded area around the curve. The detection limits for the techniques span a range of one order of magnitude (e.g., 1 mg/L to 10 mg/L). This figure is modified with permission from Garner et al.189
Abbreviations: Asymmetric flow field flow fractionation with multi-angle light scattering (aF4-MALS), analytical ultracentrifugation (AUC), chemothermal oxidation at 375 °C (CTO-375), near infrared fluorescence (NIRF), single particle inductively coupled plasma-mass spectrometry (spICP-MS), thermal gravimetric analysis (TGA), thermal gravimetric analysis-mass spectrometry (TGA-MS), total organic carbon (TOC), thermal optical transmittance (TOT).
Figure 3.
Detection limits for analytical techniques in various media under optimal conditions and modeled environmental concentrations (184, 285, 386, 470, 519); modeled environmental concentrations are not available for biological matrices. The detection limits for individual techniques span a range of one order of magnitude (e.g., 1 mg/L to 10 mg/L)
Abbreviations: Asymmetric flow field flow fractionation with multi-angle light scattering (aF4-MALS), analytical ultracentrifugation (AUC), chemothermal oxidation at 375 °C (CTO-375), near infrared fluorescence (NIRF), single particle inductively coupled plasma-mass spectrometry (spICP-MS), thermal gravimetric analysis (TGA), thermal gravimetric analysis-mass spectrometry (TGA-MS), total organic carbon (TOC), thermal optical transmittance (TOT).
Potential for standardization
There are numerous reference materials (RM; e.g., UV/vis spectroscopy calibration standards) and standard methods that can support the standardization of CNT quantification techniques (Table S3). In addition, there are multiple CNT RMs and representative test materials (Table S4); RMs have assigned values for certain properties, whereas representative test materials are only guaranteed to be stable and homogeneous with respect to one or more specified properties but may be used in the development of test methods which assess properties other than those for which stability and homogeneity have been shown.111 Currently, three RMs are available for SWCNTs, while MWCNTs are only available as representative test materials. The careful characterization of the CNT RMs may be useful for the standardization of numerous techniques, given the wide range of properties that have been certified (i.e., the sources of uncertainty are thoroughly understood and the certified values have meaningful metrological traceability) or for which information values are provided (i.e., the sources of uncertainty are not fully understood or a limited number of analyses were performed). Standardized methods are also already available for characterization of CNTs (e.g., Raman spectroscopy and NIR fluorescence characterization) which could be modified to develop standard methods for CNT quantitation.42,112–118 In addition, a modified version of a NIOSH standard method for use of TOT for elemental carbon analysis (NIOSH Method 5040) could potentially be used for CNT quantification. However, the robustness of this method for CNTs will still need to be evaluated for different matrices. Extraction and separation procedures also need to be standardized but are not addressed in this section due to the limited number of studies on this topic. Research topics that would support the standardization of these techniques are described in the Future Research Topics section.
Case studies
In this section, five case studies will be used to illustrate how the quantitative methods described in this manuscript could be utilized to address hypothetical situations requiring CNT quantitation. The scenario for the first two case studies is that scientists are asked to determine whether the concentration of CNTs in a stream receiving effluent from a treatment plant where CNTs may be released is above 500 μg L−1; this concentration was chosen because it is approximately 50 % of the lowest LC50 value of the species sensitivity distribution shown in Figure 2. This scenario will be discussed in the context of whether the CNT characteristics (e.g., SWCNT or MWCNT, catalyst materials, and thermal properties) are known a priori or not. In the third case study, scientists will be trying to measure the exposure concentration to organisms during a laboratory ecotoxicity experiment in a water only system with an organism that has an EC50 value (the concentration at which 50 percent of the organisms are affected) of 10 mg L−1 and the lowest concentration tested is 1 mg L−1. In the fourth case study, CNTs with known characteristics are accidentally released into a lake, and scientists are asked to determine the concentration in the lake sediment. In the fifth case study, “OECD Test 305: Bioaccumulation in fish: aqueous and dietary exposure” is performed using a known type of CNTs and the scientists need to quantify the concentration in the fish tissues.
Case I: CNTs with known characteristics are released into a river
First, identify the techniques that may have LODs better than 500 μg L−1 using Figure 3: UV/vis spectroscopy, inorganic elemental analysis, spICP-MS, NIRF, Raman spectroscopy, TOT, and carbon-14 labeling. Electron microscopy should, in principle, be able to detect CNTs at these concentrations, but it may be challenging to identify CNTs amidst the other particulate matter, and quantification will be challenging as discussed above. Of particle risk is the ability to collect a representative sample where the TEM thin section actually contains a statistically significant number of CNTs. Nevertheless, electron microscopy could be used for a qualitative assessment or to confirm the presence/absence of CNTs based on results from the quantitative analysis. Among the quantitative techniques, the choice of which technique to employ first would depend on numerous factors such as their availability and if the unique properties of the CNTs of interest may eliminate some of the analytical techniques from consideration (e.g., quality assurance (QA), techniques only applicable for SWCNTs would not be relevant for MWCNT quantification). For example, carbon-14 labeling would not be relevant for field measurements, while NIRF would only be applicable for SWCNTs.15 In addition, Raman spectroscopy analysis would require preconcentration of the sample to yield the desired LOD which may be challenging.13 Next, the properties of the river water prior to the discharge location (e.g., thermal profile, elemental composition and organic matter concentration of the water) could be evaluated to assess what biases may be encountered during CNT quantification for various techniques. If it is possible to obtain the CNTs of interest, a next step would be to prepare a CNT dispersion, mix the dispersion with stream water prior to the location of discharge, and then analyze the water using the quantification technique(s) to determine relevant QA/quality control (QC) characteristics such as the LOD, reproducibility, bias, signal to noise ratio, and linearity of calibration curve. It may also be important to test the stability of the CNT in the water prior to the discharge location to assess if agglomeration or oxidation of the CNT could cause a bias; if agglomeration causes a significant bias, it may be possible to disperse the samples such as by adding a surfactant or sonicating the sample. If the QA/QC characteristics are sufficient to provide the needed level of statistical significance for the quantification measurement, the final step would be to analyze the test samples.
Case II: CNTs with unknown characteristics are released into a river
The process is substantially more complicated if characteristics of the CNT to be detected are unknown. First, it would be helpful to obtain water samples before and after the point source discharge location. It would then be possible to do some measurements to try to determine if characteristics of the river water reflective of CNT characteristics are changed. For example, an elemental analysis or spICP-MS analysis of the river waters could be conducted to assess if uncommon elements (e.g., yttrium) or ratios of elements (e.g., cobalt to molybdenum) often used for CNT catalysts are present at different concentrations before and after the location of discharge; measuring these samples before and after filtering could reveal if the metals are associated with particles such as CNTs. One distinct advantage of the metal analysis techniques is that the LODs for many of these elements are orders of magnitude better than the limit of detection needed for the CNTs (Figure 3). This information supported by other characterization techniques (e.g., TEM analysis to assess if SWCNTs or MWCNTs can be identified) could help determine the type of CNT being used. An alternate first step would be to obtain a sample directly at the discharge location and conduct these analyses. The advantage of this approach is that there would not be dilution of the CNTs, but the matrix may be substantially more complex (e.g., wastewater treatment plant effluent). A next step is to spike known concentrations of the specific CNT if identified, or alternatively RM SWCNTs and representative test material MWCNTs, into the river water prior to the discharge location and determine the QA/QC characteristics for the selected techniques and the extent to which agglomeration or oxidation could influence the results. If acceptable results can be obtained with the specific CNT (if identified) or the RM CNTs, then analysis can be conducted on the river sample after the location of discharge.
Case III: Laboratory Ecotoxicity Study
The third case study involves a laboratory ecotoxicity experiment during which the concentration remaining suspended during the experiment needs to be quantified. Depending upon what organism is tested, there may be interferences such as algae or bacteria which remain suspended and have CNTs associated with them. If it is straightforward to separate the test organisms from the media with suspended CNTs, numerous techniques may be applicable for quantifying the initial CNT concentration in suspension (≥1 mg L−1) (see Figure 2). The techniques available to determine the change in concentration during the experiment depend on the LOD needed for these measurements. For example, if it is unlikely that the CNT will settle during the experiment, numerous techniques would enable measurements to show that the concentration remained within 20 % of the initial concentration, the desired maximum concentration loss indicated in many OECD tests.63 However, if substantial settling occurs, it is necessary to determine the lowest detection limit needed (e.g., 0.1 mg L−1 to quantify a loss in concentration of 90 % of the initial concentration). When measuring the CNT concentration dispersed in tests with suspended unicellular organisms or small multicellular organisms (e.g., Tetrahymena thermophila), the cells themselves may cause biases or require the extraction of the CNTs. It is also unclear if CNTs that are suspended but associated with cells should be counted as part of the total suspended concentration. Nevertheless, many techniques could likely still be used to quantify the total suspended concentration but control experiments to test for potential biases from the cells and the matrix would need to be conducted prior to starting the experiment.
Case study IV: Quantification of CNTs with known characteristics in lake sediment
Quantifying CNTs in sediments is substantially more difficult than in water samples. As shown in Figure 3, the LODs for most techniques are at least an order of magnitude higher in soils and sediments compared to in waters. To quantify CNTs in sediments, a first step would be to obtain “clean” sediment from another water body ideally with similar sediment characteristics. Because the CNT type is known in this case study, it is possible to spike this clean sediment with CNTs and then assess the quality of the analytical results (e.g., linearity, LOD, etc.). The suitable techniques for this analysis will depend upon instrument availability, the type of CNT (e.g., NIRF after CNT extraction has been shown to be a valuable technique for analysis of SWCNTs in sediments15 but is not applicable to MWCNTs), and the estimated range of probable CNT concentrations in the sediment. If satisfactory LODs are not available for the available techniques in the reference sediment, it may be necessary to investigate extraction or separation methods to decrease the LOD (e.g., 15,46). Given the low detection limits obtained using NIRF after extraction (62 μg/kg),15 challenges with obtaining a better LOD are likely only to be problematic for MWCNTs unless the SWCNTs are oxidized or modified to the extent that NIRF is not applicable or NIRF is not available for sample analysis.
Case study V: Quantification of CNT in fish after a standard toxicity test
Assessing potential bioaccumulation of chemicals in organisms is an important component of risk assessment of chemicals. One frequently used test is OECD method 305: Bioaccumulation in fish: aqueous and dietary exposure.119 Again, the LODs for quantifying CNTs in organism tissues are greater than those in water, yet similar to the LODs for soils and sediments (Figure 3). While the whole fish is usually analyzed in this method, it may be beneficial to test the CNT biodistribution in addition to the total concentration in the fish. This is important because CNT translocation across the gut tract is rarely observed in ecotoxicological studies.27,31,120,121 If the biodistribution of SWCNTs is evaluated, then the technique with the best LOD is NIRF microscopy which has been reported to detect individual SWCNTs.31,121 If this instrument is not available, Raman microscopy and electron microscopy can be used to assess biodistribution of CNTs in organisms although it is important to carefully avoid artifacts;27,43,122,123 however, one should note that G/D ratios are strongly influenced by any sp2 or sp3 hybridized carbons present in the organism for Raman microscopy analysis. Other microscopic approaches such as photothermal/photoacoustic imaging have also been successfully used to assess the distribution of CNTs in plants, yet are infrequently available (Figure 1).24 To quantify the total concentration of CNTs in the fish, it is possible to use NIRF microscopy for SWCNTs,31 but extraction from the fish tissue will likely be needed for MWCNTs. An extraction procedure has been published for MWCNTs in rat lungs followed by quantification using TOT,23 but this approach has not yet been used in tandem with other quantification techniques or with fish tissues. If carbon-14 labeled CNTs are available, assessing uptake by and biodistribution in fish through carbon-14 labeling is a viable approach.124 The microwave method has also shown promise for detecting MWCNTs in biological samples (e.g., earthworms) but requires custom built equipment.62,125
Future Research Topics
The analysis that we present here on the current state of the science with regards to quantification of CNTs in matrices relevant for nanotechnology environmental health and safety measurements also reveals several key future research topics to move this field forward. First, most of the quantification techniques developed for aqueous environments will have potential biases or a higher LOD in complex matrices such as soils and biological tissues. Thus, the continued development of CNT extraction and separation procedures for environmental and biological matrices is a critical topic for additional research. Nevertheless, addressing the quality of the CNT separation depends in part on the robustness and precision of the subsequent analytical techniques, which also need to be improved. Second, sensitivity analyses of techniques can provide relevant information regarding the robustness of an experimental procedure to minor changes to a protocol and the contributions of various steps to the total uncertainty of the result. This approach and related approaches such as cause-and-effect analysis can highlight which steps of a protocol need to be carefully followed to ensure a reliable result and which steps are less critical.126 Third, interlaboratory comparisons, where multiple laboratories use the same protocol, are needed to standardize the more mature techniques and extraction and separation procedures. While it is necessary to assess many topics related to analytical precision of a single laboratory (e.g., within and between operator variability, instrument to instrument variability, day-to-day variability, all contributing to the within-laboratory repeatability), interlaboratory comparisons can provide unique information about the comparability of results among laboratories (i.e., between-laboratory reproducibility) and potential factors in the protocols that need to be controlled to standardize the procedure. Such information is needed to provide estimates of the bias and precision of an analytical method. Fourth, analyzing an individual or set of homogenized test samples using multiple techniques will be helpful in highlighting method specific biases and the comparability of results among methods (e.g., similarly to a black carbon quantification ring trial127). This differs from interlaboratory comparisons in that a single sample is analyzed by multiple techniques, as opposed to different laboratories using the same technique and test method. Similar results among orthogonal techniques would lead to greater confidence in the results of the methods while different results could yield insights into biases, strengths, and limitations of different methods. For example, in a recent study on the fate of SWCNTs in a mesocosm, an experimental setup designed to simulate the natural environment that often includes multiple species and which has been used in several nanotoxicity studies,128,129 both NIRF and elemental analysis were used on the same samples.29 The agreement among these methods suggested that elemental analysis may be a useful approach in these complex matrices if the catalysts used to synthesize the CNTs are of an element with low concentrations in the matrix (e.g., Mo).29 A similar approach could be used to compare among different extraction or separation techniques with a single sample. Fifth, isotopically enriched or depleted CNTs21,78 could be used to help develop other orthogonal techniques given that isotopic techniques often have the fewest biases for many of the matrices and changes that could occur to the CNTs in these matrices. Such an approach was used by Schierz et al. to develop the NIRF technique for quantification of SWCNTs in sediments after extraction by also testing the extraction procedure with carbon-14 labeled SWCNTs.15 Sixth, using extraction and/or separation techniques in combination such as AF4 followed by capillary electrophoresis could be another promising avenue for future research. Lastly, almost all quantitative techniques require known CNTs to yield information about their characteristic information (e.g., thermal profile, metal catalyst, impurities, NIR spectra, and Raman signature). Additional work is needed to develop techniques for quantification of unknown CNTs in an environmental or biological matrix. Along these lines, the impact of CNT heterogeneities (e.g., different lengths) on their quantification could also be helpful.
Supplementary Material
Acknowledgments
The contributions of AG and TDB were provided as part of the project “Effects of NANOparticles on beneficial soil MIcrobes and CROPS (NANOMICROPS)”, within the Swiss National Research Programme NRP 64 “Opportunities and Risks of Nanomaterials”. AG and TDB thank the Swiss National Science Foundation (SNSF) for financial support. DLP acknowledges support from NSF CBET # 1336794. D.X. Flores-Cervantes is very grateful to Hans-Peter Kohler for his continuous support and advice, and thanks the SNSF through the NRP 64 (Project Biocarb) for the financial support. We thank Dr. P. Lee Ferguson (Duke University) for helpful discussions.
Footnotes
Tables describing potential matrix interferences and interferences from changes to the CNTs on selected CNT quantification techniques, standard reference material carbon nanotubes, and standards and references materials related to standardization of carbon nanotube quantification techniques. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339(6119):535–9. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 2.Piccinno F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nano Res. 2012;14(9) Article number 1109. [Google Scholar]
- 3.Helland A, Wick P, Koehler A, Schmid K, Som C. Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ Health Perspect. 2007;115(8):1125–1131. doi: 10.1289/ehp.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen EJ, Zhang LW, Mattison NT, O’Carroll DM, Whelton AJ, Uddin N, Nguyen T, Huang QG, Henry TB, Holbrook RD, Chen KL. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ Sci Technol. 2011;45(23):9837–9856. doi: 10.1021/es201579y. [DOI] [PubMed] [Google Scholar]
- 5.Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ Sci Technol. 2008;42(16):5843–5859. doi: 10.1021/es8006904. [DOI] [PubMed] [Google Scholar]
- 6.Shen MW, Wang SH, Shi XY, Chen XS, Huang QG, Petersen EJ, Pinto RA, Baker JR, Weber WJ., Jr Polyethyleneimine-mediated functionalization of multiwalled carbon nanotubes: Synthesis characterization and in vitro toxicity assay. J Phys Chem C. 2009;113(8):3150–3156. [Google Scholar]
- 7.Shi XY, Wang SH, Shen MW, Antwerp ME, Chen XS, Li C, Petersen EJ, Huang QG, Weber WJ, Jr, Baker JR. Multifunctional dendrimer-modified multiwalled carbon nanotubes: Synthesis characterization and in vitro cancer cell targeting and imaging. Biomacromolecules. 2009;10(7):1744–1750. doi: 10.1021/bm9001624. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Mahendra S, Alvarez PJJ. Nanomaterials in the construction industry: A review of their applications and environmental health and safety considerations. ACS Nano. 2010;4(7):3580–3590. doi: 10.1021/nn100866w. [DOI] [PubMed] [Google Scholar]
- 9.Plata DL, Ferguson PL, Westerhoff P. Express It in Numbers: Efforts to Quantify Engineered Nanoparticles in Environmental Matrices Advance. Environ Sci Technol. 2012;46(22):12243–12245. doi: 10.1021/es302789n. [DOI] [PubMed] [Google Scholar]
- 10.Selck H, Handy RD, Fernandes TF, Klaine SJ, Petersen EJ. Nanomaterials in the aquatic environment: A European Union-United States perspective on the status of ecotoxicity testing, research priorities, and challenges ahead. Environmental Toxicology & Chemistry. 2016 doi: 10.1002/etc.3385. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahr JL, Mickelson ET, Bronikowski MJ, Smalley RE, Tour JM. Dissolution of small diameter single-wall carbon nanotubes in organic solvents? Chem Commun. 2001;(2):193–194. [Google Scholar]
- 12.Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J Am Chem Soc. 2004;126(48):15638–15639. doi: 10.1021/ja0466311. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Lorente AI, Simonet BM, Valcarcel M. Determination of carboxylic SWCNTs in river water by microextraction in ionic liquid and determination by Raman spectroscopy. Talanta. 2013;105:75–79. doi: 10.1016/j.talanta.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 14.Plata DL, Reddy CM, Gschwend PM. Thermogravimetry-Mass Spectrometry for Carbon Nanotube Detection in Complex Mixtures. Environ Sci Technol. 2012;46(22):12254–12261. doi: 10.1021/es203198x. [DOI] [PubMed] [Google Scholar]
- 15.Schierz A, Parks AN, Washburn KM, Chandler GT, Ferguson PL. Characterization and Quantitative Analysis of Single-Walled Carbon Nanotubes in the Aquatic Environment Using Near-Infrared Fluorescence Spectroscopy. Environ Sci Technol. 2012;46(22):12262–12271. doi: 10.1021/es301856a. [DOI] [PubMed] [Google Scholar]
- 16.Doudrick K, Herckes P, Westerhoff P. Detection of Carbon Nanotubes in Environmental Matrices Using Programmed Thermal Analysis. Environ Sci Technol. 2012;46(22):12246–12253. doi: 10.1021/es300804f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyung H, Fortner JD, Hughes JB, Kim JH. Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ Sci Technol. 2007;41(1):179–184. doi: 10.1021/es061817g. [DOI] [PubMed] [Google Scholar]
- 18.Sobek A, Bucheli TD. Testing the resistance of single- and multi-walled carbon nanotubes to chemothermal oxidation used to isolate soots from environmental samples. Environ Pollut. 2009;157(4):1065–1071. doi: 10.1016/j.envpol.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Koelmans AA, Nowack B, Wiesner MR. Comparison of manufactured and black carbon nanoparticle concentrations in aquatic sediments. Environ Pollution. 2009;157(4):1110–6. doi: 10.1016/j.envpol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Mansfield E, Kar A, Hooker SA. Applications of TGA in quality control of SWCNTs. Anal Bioanal Chem. 2010;396(3):1071–1077. doi: 10.1007/s00216-009-3319-2. [DOI] [PubMed] [Google Scholar]
- 21.Plata DL, Gschwend PM, Reddy CM. Industrially synthesized single-walled carbon nanotubes: compositional data for users, environmental risk assessments, and source apportionment. Nanotechnol. 2008;19(18) doi: 10.1088/0957-4484/19/18/185706. Article Number: 185706. [DOI] [PubMed] [Google Scholar]
- 22.Reed RB, Goodwin DG, Marsh KL, Capracotta SS, Higgins CP, Fairbrother DH, Ranville JF. Detection of single walled carbon nanotubes by monitoring embedded metals. Environ Sci Proc Imp. 2013;15(1):204–213. doi: 10.1039/c2em30717k. [DOI] [PubMed] [Google Scholar]
- 23.Doudrick K, Corson N, Oberdörster G, Eder AC, Herckes P, Halden RU, Westerhoff P. Extraction and Quantification of Carbon Nanotubes in Biological Matrices with Application to Rat Lung Tissue. ACS Nano. 2013;7(10):8849–8856. doi: 10.1021/nn403302s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khodakovskaya MV, de Silva K, Nedosekin DA, Dervishi E, Biris AS, Shashkov EV, Galanzha EI, Zharov VP. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc Natl Acad Sci US A. 2011;108(3):1028–1033. doi: 10.1073/pnas.1008856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen EJ, Pinto RA, Zhang L, Huang QG, Landrum PF, Weber WJ. Effects of polyethyleneimine-mediated functionalization of multi-walled carbon nanotubes on earthworm bioaccumulation and sorption by soils. Environ Sci Technol. 2011;45(8):3718–3724. doi: 10.1021/es103004r. [DOI] [PubMed] [Google Scholar]
- 26.Petersen EJ, Pinto RA, Mai DJ, Landrum PF, Weber WJ., Jr Influence of Polyethyleneimine Graftings of Multi-Walled Carbon Nanotubes on their Accumulation and Elimination by Toxicity to Daphnia magna. Environ Sci Technol. 2011;45(3):1133–1138. doi: 10.1021/es1030239. [DOI] [PubMed] [Google Scholar]
- 27.Edgington AJ, Petersen EJ, Herzing AA, Podila R, Rao A, Klaine SJ. Microscopic investigation of single-wall carbon nanotube uptake by Daphnia magna. Nanotoxicology. 2014;8(S1):2–10. doi: 10.3109/17435390.2013.847504. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Petersen EJ, Huang QG. Phase distribution of 14C-labeled multiwalled carbon nanotubes in aqueous systems containing model solids: Peat. Environ Sci Technol. 2011;45(4):1356–1362. doi: 10.1021/es1026097. [DOI] [PubMed] [Google Scholar]
- 29.Schierz A, Espinasse B, Wiesner MR, Bisesi JH, Sabo-Attwood T, Ferguson PL. Fate of single walled carbon nanotubes in wetland ecosystems. Environmental Science-Nano. 2014;1(6):574–583. [Google Scholar]
- 30.Ferguson PL, Chandler GT, Templeton RC, Demarco A, Scrivens WA, Englehart BA. Influence of sediment-amendment with single-walled carbon nanotubes and diesel soot on bioaccumulation of hydrophobic organic contaminants by benthic invertebrates. Environ Sci Technol. 2008;42(10):3879–3885. doi: 10.1021/es702830b. [DOI] [PubMed] [Google Scholar]
- 31.Bisesi JH, Merten J, Liu K, Parks AN, Afrooz A, Glenn JB, Klaine SJ, Kane AS, Saleh NB, Ferguson PL, Sabo-Attwood T. Tracking and Quantification of Single-Walled Carbon Nanotubes in Fish Using Near Infrared Fluorescence. Environ Sci Technol. 2014;48(3):1973–1983. doi: 10.1021/es4046023. [DOI] [PubMed] [Google Scholar]
- 32.Hanna SK, Miller RJ, Lenihan HS. Deposition of carbon nanotubes by a marine suspension feeder revealed by chemical and isotopic tracers. J Hazard Mater. 2014;279:32–37. doi: 10.1016/j.jhazmat.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 33.Jeong J, Lee Y-j, Hwang Ys, Hong IS. Selective detection and quantification of carbon nanotubes in soil. Environ Toxicol Chem. 2015;34(9):1969–1974. doi: 10.1002/etc.3035. [DOI] [PubMed] [Google Scholar]
- 34.Petersen EJ, Henry TB. Methodological considerations for testing the ecotoxicity of carbon nanotubes and fullerenes: Review. Environ Toxicol Chem. 2012;31(1):60–72. doi: 10.1002/etc.710. [DOI] [PubMed] [Google Scholar]
- 35.Su Y, Yan XM, Pu YB, Xiao F, Wang DS, Yang M. Risks of Single-Walled Carbon Nanotubes Acting as Contaminants-Carriers: Potential Release of Phenanthrene in Japanese Medaka (Oryzias latipes) Environ Sci Technol. 2013;47(9):4704–4710. doi: 10.1021/es304479w. [DOI] [PubMed] [Google Scholar]
- 36.Chilek JL, Wang RH, Draper RK, Pantano P. Use of Gel Electrophoresis and Raman Spectroscopy to Characterize the Effect of the Electronic Structure of Single-Walled Carbon Nanotubes on Cellular Uptake. Anal Chem. 2014;86(6):2882–2887. doi: 10.1021/ac403827m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang RH, Mikoryak C, Chen E, Li S, Pantano P, Draper RK. Gel Electrophoresis Method to Measure the Concentration of Single-Walled Carbon Nanotubes Extracted from Biological Tissue. Anal Chem. 2009;81(8):2944–2952. doi: 10.1021/ac802485n. [DOI] [PubMed] [Google Scholar]
- 38.Tong L, Liu YX, Dolash BD, Jung Y, Slipchenko MN, Bergstrom DE, Cheng JX. Label-free imaging of semiconducting and metallic carbon nanotubes in cells and mice using transient absorption microscopy. Nat Nanotech. 2012;7(1):56–61. doi: 10.1038/nnano.2011.210. [DOI] [PubMed] [Google Scholar]
- 39.von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, Koelmans AA, Horne N, Unrine JM. Analysis of engineered nanomaterials in complex matrices (environment and biota): General considerations and conceptual case studies. Environ Toxicol Chem. 2012;31(1):32–49. doi: 10.1002/etc.723. [DOI] [PubMed] [Google Scholar]
- 40.Herrero-Latorre C, Alvarez-Mendez J, Barciela-Garcia J, Garcia-Martin S, Pena-Crecente RM. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal Chem Acta. 2015;853:77–94. doi: 10.1016/j.aca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Fagan JA, Bauer BJ, Hobbie EK, Becker ML, Walker ARH, Simpson JR, Chun J, Obrzut J, Bajpai V, Phelan FR, Simien D, Huh JY, Migler KB. Carbon Nanotubes: Measuring Dispersion and Length. Adv Mat. 2011;23(3):338–348. doi: 10.1002/adma.201001756. [DOI] [PubMed] [Google Scholar]
- 42.Decker JE, Walker ARH, Bosnick K, Clifford CA, Dai L, Fagan J, Hooker S, Jakubek ZJ, Kingston C, Makar J, Mansfield E, Postek MT, Simard B, Sturgeon R, Wise S, Vladar AE, Yang L, Zeisler R. Sample preparation protocols for realization of reproducible characterization of single-wall carbon nanotubes. Metrologia. 2009;46(6):682–692. [Google Scholar]
- 43.Petersen EJ, Henry TB, Zhao J, MacCuspie RI, Kirschling TL, Dobrovolskaia MA, Hackley V, Xing B, White JC. Identification and Avoidance of Potential Artifacts and Misinterpretations in Nanomaterial Ecotoxicity Measurements. Environ Sci Technol. 2014;48(8):4226–4246. doi: 10.1021/es4052999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Worle-Knirsch JM, Pulskamp K, Krug HF. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006;6(6):1261–1268. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 45.Jakubek LM, Marangoudakis S, Raingo J, Liu XY, Lipscombe D, Hurt RH. The inhibition of neuronal calcium ion channels by trace levels of yttrium released from carbon nanotubes. Biomaterials. 2009;30(31):6351–6357. doi: 10.1016/j.biomaterials.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gogos A, Kaegi R, Zenobi R, Bucheli TD. Capabilities of asymmetric flow field-flow fractionation coupled to multi-angle light scattering to detect carbon nanotubes in soot and soil. Environmental Science-Nano. 2014;1(6):584–594. [Google Scholar]
- 47.Tagmatarchis N, Zattoni A, Reschiglian P, Prato M. Separation and purification of functionalised water-soluble multi-walled carbon nanotubes by flow field-flow fractionation. Carbon. 2005;43(9):1984–1989. [Google Scholar]
- 48.Niyogi S, Hu H, Hamon MA, Bhowmik P, Zhao B, Rozenzhak SM, Chen J, Itkis ME, Meier MS, Haddon RC. Chromatographic Purification of Soluble Single-Walled Carbon Nanotubes (s-SWNTs) J Am Chem Soc. 2001;123(4):733–734. doi: 10.1021/ja0024439. [DOI] [PubMed] [Google Scholar]
- 49.Fagan JA, Khripin CY, Batista CAS, Simpson JR, Haroz EH, Walker ARH, Zheng M. Isolation of Specific Small-Diameter Single-Wall Carbon Nanotube Species via Aqueous Two-Phase Extraction. Adv Mat. 2014;26(18):2800–2804. doi: 10.1002/adma.201304873. [DOI] [PubMed] [Google Scholar]
- 50.Pycke BFG, Benn TM, Herckes P, Westerhoff P, Halden RU. Strategies for quantifying C-60 fullerenes in environmental and biological samples and implications for studies in environmental health and ecotoxicology. Trends Anal Chem. 2011;30(1):44–57. doi: 10.1016/j.trac.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pycke BFG, Chao TC, Herckes P, Westerhoff P, Halden RU. Beyond nC(60): strategies for identification of transformation products of fullerene oxidation in aquatic and biological samples. Anal Bioanal Chem. 2012;404(9):2583–2595. doi: 10.1007/s00216-012-6090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isaacson CW, Kleber M, Field JA. Quantitative analysis of fullerene nanomaterials in environmental systems: A critical review. Environ Sci Technol. 2009;43(17):6463–6474. doi: 10.1021/es900692e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang JF, Wages M, Yu SY, Maul JD, Mayer G, Hope-Weeks L, Cobb GP. Bioaccumulation of fullerene (C60) and corresponding catalase elevation in Lumbriculus variegatus. Environ Toxicol Chem. 2014;33(5):1135–1141. doi: 10.1002/etc.2540. [DOI] [PubMed] [Google Scholar]
- 54.Pakarinen K, Petersen EJ, Leppanen MT, Akkanen J, Kukkonen JVK. Adverse effects of fullerenes (nC(60)) spiked to sediments on Lumbriculus variegatus (Oligochaeta) Environ Pollut. 2011;159(12):3750–3756. doi: 10.1016/j.envpol.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 55.Tervonen K, Waissi G, Petersen EJ, Akkanen J, Kukkonen JVK. Analysis of fullerene-C60 and kinetic measurements for its accumulation and depuration in Daphnia magna. Environ Toxicol Chem. 2010;29(5):1072–1078. doi: 10.1002/etc.124. [DOI] [PubMed] [Google Scholar]
- 56.Pakarinen K, Petersen EJ, Alvila L, Waissi-Leinonen GC, Akkanen J, Leppänen MT, Kukkonen JVK. A screening study on the fate of fullerenes (nC60) and their toxic implications in natural freshwaters. Environ Toxicol Chem. 2013;32(6):1224–1232. doi: 10.1002/etc.2175. [DOI] [PubMed] [Google Scholar]
- 57.Petersen EJ, Lam T, Gorham JM, Scott KC, Long CJ, Stanley D, Sharma R, Liddle JA, Pellegrin B, Nguyen T. Methods to assess the impact of UV irradiation on the surface chemistry and structure of multiwall carbon nanotube epoxy nanocomposites. Carbon. 2014;69:194–205. [Google Scholar]
- 58.Nowack B, David RM, Fissan H, Morris H, Shatkin JA, Stintz M, Zepp R, Brouwer D. Potential release scenarios for carbon nanotubes used in composites. Environ Intl. 2013;59:1–11. doi: 10.1016/j.envint.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Kingston C, Zepp R, Andrady A, Boverhof D, Fehir R, Hawkins D, Roberts J, Sayre P, Shelton B, Sultan Y, Vejins V, Wohlleben W. Release characteristics of selected carbon nanotube polymer composites. Carbon. 2014;68:33–57. [Google Scholar]
- 60.Wohlleben W, Meier MW, Vogel S, Landsiedel R, Cox G, Hirth S, Tomovic Z. Elastic CNT–polyurethane nanocomposite: synthesis, performance and assessment of fragments released during use. Nanoscale. 2013;5(1):369–380. doi: 10.1039/c2nr32711b. [DOI] [PubMed] [Google Scholar]
- 61.Green JM, Irin F, Canas JE, Saed MA. Detection of Carbon Nanotubes by Microwave-Induced Heating. 2012 [Google Scholar]
- 62.Irin F, Shrestha B, Canas JE, Saed MA, Green MJ. Detection of carbon nanotubes in biological samples through microwave-induced heating. Carbon. 2012;50(12):4441–4449. [Google Scholar]
- 63.Petersen EJ, Diamond SA, Kennedy AJ, Goss GG, Ho K, Lead J, Hanna SK, Hartmann NB, Hund-Rinke K, Mader B, Manier N, Pandard P, Salinas ER, Sayre P. Adapting OECD Aquatic Toxicity Tests for Use with Manufactured Nanomaterials: Key Issues and Consensus Recommendations. Environ Sci Technol. 2015;49(16):9532–9547. doi: 10.1021/acs.est.5b00997. [DOI] [PubMed] [Google Scholar]
- 64.Li ZF, Luo GH, Zhou WP, Wei F, Xiang R, Liu YP. The quantitative characterization of the concentration and dispersion of multi-walled carbon nanotubes in suspension by spectrophotometry. Nanotechnol. 2006;17(15):3692–3698. [Google Scholar]
- 65.Cerrillo C, Barandika G, Igartua A, Areitioaurtena O, Marcaide A, Mendoza G. Ecotoxicity of multiwalled carbon nanotubes: Standardization of the dispersion methods and concentration measurements. Environ Toxicol Chem. 2015;34(8):1854–1862. doi: 10.1002/etc.2999. [DOI] [PubMed] [Google Scholar]
- 66.Porter AE, Gass M, Muller K, Skepper JN, Midgley PA, Welland M. Direct imaging of single-walled carbon nanotubes in cells. Nat Nanotech. 2007;2(11):713–717. doi: 10.1038/nnano.2007.347. [DOI] [PubMed] [Google Scholar]
- 67.Mavrocordatos D, Perret D. Non-artifacted specimen preparation for transmission electron microscopy of submicron soil particles. Comm Soil Sci Plant Anal. 1995;26(15–16):2593–2602. [Google Scholar]
- 68.Gogos A. Ph D Thesis No 22589. Swiss Federal Insitute of Technology (ETH); 2015. Engineered nanomaterials in the agricultural environment: current state of applications and development of analytical methods. [Google Scholar]
- 69.Prasad A, Lead JR, Baalousha M. An electron microscopy based method for the detection and quantification of nanomaterial number concentration in environmentally relevant media. Sci Tot Environ. 2015;537:479–486. doi: 10.1016/j.scitotenv.2015.07.117. [DOI] [PubMed] [Google Scholar]
- 70.Gottschalk F, Sun TY, Nowack B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ Pollut. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Schwab F, Bucheli TD, Lukhele LP, Magrez A, Nowack B, Sigg L, Knauer K. Are Carbon Nanotube Effects on Green Algae Caused by Shading and Agglomeration? Environ Sci Technol. 2011;45(14):6136–6144. doi: 10.1021/es200506b. [DOI] [PubMed] [Google Scholar]
- 72.Pohl K, Cantwell M, Herckes P, Lohmann R. Black carbon concentrations and sources in the marine boundary layer of the tropical Atlantic Ocean using four methodologies. Atmos Chem Phys. 2014;14(14):7431–7443. [Google Scholar]
- 73.Dahm MM, Evans DE, Schubauer-Berigan MK, Birch ME, Deddens JA. Occupational Exposure Assessment in Carbon Nanotube and Nanofiber Primary and Secondary Manufacturers: Mobile Direct-Reading Sampling. Ann Occup Hyg. 2013;57(3):328–344. doi: 10.1093/annhyg/mes079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Fernback JE, Deddens JA. Carbon Nanotube and Nanofiber Exposure Assessments: An Analysis of 14 Site Visits. Ann Occup Hyg. 2015;59(6):705–723. doi: 10.1093/annhyg/mev020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vo E, Zhuang ZQ, Birch E, Zhao Q, Horvatin M, Liu YW. Measurement of Mass-Based Carbon Nanotube Penetration through Filtering Facepiece Respirator Filtering Media. Ann Occup Hyg. 2014;58(5):646–656. doi: 10.1093/annhyg/meu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hashimoto N, Ogura I, Kotake M, Kishimoto A, Honda K. Evaluating the capabilities of portable black carbon monitors and photometers for measuring airborne carbon nanotubes. J Nano Res. 2013;15(11) Article Number: UNSP 2033. [Google Scholar]
- 77.Zhang LW, Petersen EJ, Habteselassie MY, Mao L, Huang QG. Degradation of multiwall carbon nanotubes by bacteria. Environ Pollut. 2013;181:335–339. doi: 10.1016/j.envpol.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 78.Flores-Cervantes DX, Maes HM, Schaffer A, Hollender J, Kohler HP. Slow biotransformation of carbon nanotubes by horseradish peroxidase. Environ Sci Technol. 2014;48(9):4826–34. doi: 10.1021/es4053279. [DOI] [PubMed] [Google Scholar]
- 79.Andon FT, Kapralov AA, Yanamala N, Feng WH, Baygan A, Chambers BJ, Hultenby K, Ye F, Toprak MS, Brandner BD, Fornara A, Klein-Seetharaman J, Kotchey GP, Star A, Shvedova AA, Fadeel B, Kagan VE. Biodegradation of Single-Walled Carbon Nanotubes by Eosinophil Peroxidase. Small. 2013;9(16):2721–2729. doi: 10.1002/smll.201202508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farrera C, Bhattacharya K, Lazzaretto B, Andon FT, Hultenby K, Kotchey GP, Star A, Fadeel B. Extracellular entrapment and degradation of single-walled carbon nanotubes. Nanoscale. 2014;6(12):6974–6983. doi: 10.1039/c3nr06047k. [DOI] [PubMed] [Google Scholar]
- 81.Kagan VE, Kapralov AA, St Croix CM, Watkins SC, Kisin ER, Kotchey GP, Balasubramanian K, Vlasova, Yu J, Kim K, Seo W, MallampaIli RK, Star A, Shvedova AA. Lung Macrophages “Digest” Carbon Nanotubes Using a Superoxide/Peroxynitrite Oxidative Pathway. ACS Nano. 2014;8(6):5610–5621. doi: 10.1021/nn406484b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kotchey GP, Hasan SA, Kapralov AA, Ha SH, Kim K, Shvedova AA, Kagan VE, Star A. A Natural Vanishing Act: The Enzyme-Catalyzed Degradation of Carbon Nanomaterials. Acc Chem Res. 2012;45(10):1770–1781. doi: 10.1021/ar300106h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kotchey GP, Zhao Y, Kagan VE, Star A. Peroxidase-mediated biodegradation of carbon nanotubes in vitro and in vivo. Adv Drug Deliv Rev. 2013;65(15):1921–1932. doi: 10.1016/j.addr.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ Sci Technol. 2009;43(24):9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- 85.Mueller NC, Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol. 2008;42(12):4447–4453. doi: 10.1021/es7029637. [DOI] [PubMed] [Google Scholar]
- 86.Sun TY, Conroy G, Donner E, Hungerbuhler K, Lombi E, Nowack B. Probabilistic modelling of engineered nanomaterial emissions to the environment: a spatio-temporal approach. Environmental Science-Nano. 2015;2(4):340–351. [Google Scholar]
- 87.Montano MD, Badiei HR, Bazargan S, Ranville JF. Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times. Environ Sci: Nano. 2014;1(4):338–346. [Google Scholar]
- 88.Borovinskaya O, Hattendorf B, Tanner M, Gschwind S, Gunther D. A prototype of a new inductively coupled plasma time-of-flight mass spectrometer providing temporally resolved, multi-element detection of short signals generated by single particles and droplets. J Anal At Spectrom. 2013;28(2):226–233. [Google Scholar]
- 89.Borovinskaya O, Gschwind S, Hattendorf B, Tanner M, Gunther D. Simultaneous Mass Quantification of Nanoparticles of Different Composition in a Mixture by Microdroplet Generator-ICPTOFMS. Anal Chem. 2014;86(16):8142–8148. doi: 10.1021/ac501150c. [DOI] [PubMed] [Google Scholar]
- 90.Zhu Y, Ran TC, Li YG, Guo JX, Li WX. Dependence of the cytotoxicity of multi-walled carbon nanotubes on the culture medium. Nanotechnol. 2006;17(18):4668–4674. doi: 10.1088/0957-4484/17/18/024. [DOI] [PubMed] [Google Scholar]
- 91.Guo X, Dong S, Petersen EJ, Gao S, Huang Q, Mao L. Biological Uptake and Depuration of Radio-labeled Graphene by Daphnia magna. Environ Sci Technol. 2013;47(21):12524–12531. doi: 10.1021/es403230u. [DOI] [PubMed] [Google Scholar]
- 92.Li D, Fortner JD, Johnson DR, Chen C, Li QL, Alvarez PJJ. Bioaccumulation of 14C60 by the Earthworm Eisenia fetida. Environ Sci Technol. 2010;44(23):9170–9175. doi: 10.1021/es1024405. [DOI] [PubMed] [Google Scholar]
- 93.Liu JH, Yang ST, Wang X, Wang HF, Liu YM, Luo PJG, Liu YF, Sun YP. Carbon Nanoparticles Trapped in Vivo-Similar to Carbon Nanotubes in Time-Dependent Biodistribution. ACS Appl Mater Interf. 2014;6(16):14672–14678. doi: 10.1021/am504022s. [DOI] [PubMed] [Google Scholar]
- 94.Feng YP, Lu K, Mao L, Guo XK, Gao SX, Petersen EJ. Degradation of C-14-labeled few layer graphene via Fenton reaction: Reaction rates, characterization of reaction products, and potential ecological effects. Water Res. 2015;84:49–57. doi: 10.1016/j.watres.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 95.Mao L, Hu M, Pan B, Xie Y, Petersen EJ. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part Fibre Toxicol. 2016;13(1):1–12. doi: 10.1186/s12989-016-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang HF, Wang J, Deng XY, Sun HF, Shi ZJ, Gu ZN, Liu YF, Zhao YL. Biodistribution of carbon single-wall carbon nanotubes in mice. J Nanosci Nanotechnol. 2004;4(8):1019–1024. doi: 10.1166/jnn.2004.146. [DOI] [PubMed] [Google Scholar]
- 97.Wang HF, Yang ST, Cao AN, Liu YF. Quantification of Carbon Nanomaterials in Vivo. Acc Chem Res. 2013;46(3):750–760. doi: 10.1021/ar200335j. [DOI] [PubMed] [Google Scholar]
- 98.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci US A. 2006;103(9):3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang LW, Petersen EJ, Zhang W, Chen YS, Cabrera M, Huang QG. Interactions of C-14-labeled multi-walled carbon nanotubes with soil minerals in water. Environ Pollut. 2012;166:75–81. doi: 10.1016/j.envpol.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Zhao Q, Petersen EJ, Cornelis G, Wang X, Guo X, Tao S, Xing B. Retention of 14C-labeled multiwall carbon nanotubes by humic acid and polymers: Roles of macromolecule properties. Carbon. 2016;99:229–237. doi: 10.1016/j.carbon.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang D, Pan B, Cook RL, Xing BS. Multi-walled carbon nanotube dispersion by the adsorbed humic acids with different chemical structures. Environ Pollut. 2015;196:292–299. doi: 10.1016/j.envpol.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 102.Hyung H, Kim JH. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: Effect of NOM characteristics and water quality parameters. Environ Sci Technol. 2008;42(12):4416–4421. doi: 10.1021/es702916h. [DOI] [PubMed] [Google Scholar]
- 103.Parks AN, Chandler GT, Ho KT, Burgess RM, Ferguson PL. Environmental biodegradability of [C14] single-walled carbon nanotubes by Trametes versicolor and natural microbial cultures found in New Bedford Harbor sediment and aerated wastewater treatment plant sludge. Environ Toxicol Chem. 2015;34(2):247–251. doi: 10.1002/etc.2791. [DOI] [PubMed] [Google Scholar]
- 104.Bitter JL, Yang J, Beigzadeh Milani S, Jafvert CT, Fairbrother DH. Transformations of oxidized multiwalled carbon nanotubes exposed to UVC (254 nm) irradiation. Environmental Science: Nano. 2014;1(4):324–337. [Google Scholar]
- 105.Hou WC, BeigzadehMilani S, Jafvert CT, Zepp RG. Photoreactivity of Unfunctionalized Single-Wall Carbon Nanotubes Involving Hydroxyl Radical: Chiral Dependency and Surface Coating Effect. Environmental Science & Technology. 2014;48(7):3875–3882. doi: 10.1021/es500013j. [DOI] [PubMed] [Google Scholar]
- 106.Hou WC, BeigzadehMilani S, Jafvert CT, Zepp RG. Photoreactivity of Unfunctionalized Single-Wall Carbon Nanotubes Involving Hydroxyl Radical: Chiral Dependency and Surface Coating Effect. Environ Sci Technol. 2014;48(7):3875–3882. doi: 10.1021/es500013j. [DOI] [PubMed] [Google Scholar]
- 107.Bitter JL, Yang J, Milani SB, Jafvert CT, Fairbrother DH. Transformations of oxidized multiwalled carbon nanotubes exposed to UVC (254 nm) irradiation. Environmental Science-Nano. 2014;1(4):324–337. [Google Scholar]
- 108.Chen CY, Jafvert CT. Photoreactivity of Carboxylated Single-Walled Carbon Nanotubes in Sunlight: Reactive Oxygen Species Production in Water. Environ Sci Technol. 2010;44(17):6674–6679. doi: 10.1021/es101073p. [DOI] [PubMed] [Google Scholar]
- 109.Khripin CY, Tu X, Heddleston JM, Silvera-Batista C, Hight Walker AR, Fagan J, Zheng M. High-Resolution Length Fractionation of Surfactant-Dispersed Carbon Nanotubes. Anal Chem. 2013;85(3):1382–1388. doi: 10.1021/ac303349q. [DOI] [PubMed] [Google Scholar]
- 110.Hull M, Kennedy AJ, Detzel C, Vikesland P, Chappell MA. Moving beyond Mass: The Unmet Need to Consider Dose Metrics in Environmental Nanotoxicology Studies. Environ Sci Technol. 2012;46(20):10881–10882. doi: 10.1021/es3035285. [DOI] [PubMed] [Google Scholar]
- 111.Roebben G, Rasmussen K, Kestens V, Linsinger TPJ, Rauscher H, Emons H, Stamm H. Reference materials and representative test materials: the nanotechnology case. J Nano Res. 2013;15(3) Article Number: 1455. [Google Scholar]
- 112.ISO (International Organization for Standardization) TS 10798: Nanotechnologies -- Charaterization of single-wall carbon nanotubes using scanning electron microscopy and energy dispersive X-ray spectrometry analysis. Geneva, Switzerland: 2011. [Google Scholar]
- 113.ISO (International Organization for Standardization) TS 10867: Nanotechnologies -- Characterization of single-wall carbon nanotubes using near infrared photoluminescence spectroscopy. Geneva, Switzerland: 2010. [Google Scholar]
- 114.ISO (International Organization for Standardization) TS 10868: Nanotechnologies -- Characterization of single-wall carbon nanotubes using ultraviolet-visible-near infrared (UV-Vis-NIR) absorption spectroscopy. Geneva, Switzerland: 2011. [Google Scholar]
- 115.ISO (International Organization for Standardization) TS 10929: Nanotechnologies -- Characterization of multiwall carbon nanotube (MWCNT) samples. Geneva, Switzerland: 2012. [Google Scholar]
- 116.ISO (International Organization for Standardization) TS 11251: Characterization of volatile components in single-wall carbon nanotube samples using evolved gas analysis/gas chromatograph-mass spectrometry. Geneva, Switzerland: 2010. [Google Scholar]
- 117.ISO (International Organization for Standardization) TS 11888: Nanotechnologies -- Characterization of multiwall carbon nanotubes -- Mesoscopic shape factors. Geneva, Switzerland: 2011. [Google Scholar]
- 118.ISO (International Organization for Standardization) TS 13278: Nanotechnologies -- Determination of elemental impurities in samples of carbon nanotubes using inductively coupled plasma mass spectrometry. Geneva, Switzerland: 2011. [Google Scholar]
- 119.Organization for Economic Cooperation and Development. OECD Guideline 305. Paris, F: 2012. Bioaccumulation in fish: Aqueous and Dietary Exposure. [Google Scholar]
- 120.Edgington AJ, Roberts AP, Taylor LM, Alloy MM, Reppert J, Rao AM, Ma JD, Klaine SJ. The influence of natural organic matter on the toxicity of multiwalled carbon nanotubes. Environ Toxicol Chem. 2010;29(11):2511–2518. doi: 10.1002/etc.309. [DOI] [PubMed] [Google Scholar]
- 121.Leeuw TK, Reith RM, Simonette RA, Harden ME, Cherukuri P, Tsyboulski DA, Beckingham KM, Weisman RB. Single-walled carbon nanotubes in the intact organism: Near-IR imaging and biocompatibility studies in Drosophila. Nano Lett. 2007;7(9):2650–2654. doi: 10.1021/nl0710452. [DOI] [PubMed] [Google Scholar]
- 122.Mouchet F, Landois P, Puech P, Pinelli E, Flahaut E, Gauthier L. Carbon nanotube ecotoxicity in amphibians: assessment of multiwalled carbon nanotubes and comparison with double-walled carbon nanotubes. Nanomedicine. 2010;5(6):963–974. doi: 10.2217/nnm.10.60. [DOI] [PubMed] [Google Scholar]
- 123.Mouchet F, Landois P, Sarremejean E, Bernard G, Puech P, Pinelli E, Flahaut E, Gauthier L. Characterisation and in vivo ecotoxicity evaluation of double-wall carbon nanotubes in larvae of the amphibian Xenopus laevis. Aquat Toxicol. 2008;87(2):127–137. doi: 10.1016/j.aquatox.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 124.Maes HM, Stibany F, Giefers S, Daniels B, Deutschmann B, Baumgartner W, Schaffer A. Accumulation and Distribution of Multiwalled Carbon Nanotubes in Zebrafish (Danio rerio) Environ Sci Technol. 2014;48(20):12256–12264. doi: 10.1021/es503006v. [DOI] [PubMed] [Google Scholar]
- 125.Li SB, Irin F, Atore FO, Green MJ, Canas-Carrell JE. Determination of multi-walled carbon nanotube bioaccumulation in earthworms measured by a microwave-based detection technique. Sci Tot Environ. 2013;445:9–13. doi: 10.1016/j.scitotenv.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 126.Rösslein M, Elliott JT, Salit ML, Petersen EJ, Hirsch C, Krug HF, Wick P. The use of cause-and-effect analysis to design a high quality nano-cytotoxicology assay. Chem Res Toxicol. 2014;27(10):1877–1884. doi: 10.1021/tx500327y. [DOI] [PubMed] [Google Scholar]
- 127.Hammes K, Schmidt MWI, Smernik RJ, Currie LA, Ball WP, Nguyen TH, Louchouarn P, Houel S, Gustafsson Ö, Elmquist M, Cornelissen G, Skjemstad JO, Masiello CA, Song J, Peng Pa, Mitra S, Dunn JC, Hatcher PG, Hockaday WC, Smith DM, Hartkopf-Fröder C, Böhmer A, Lüer B, Huebert BJ, Amelung W, Brodowski S, Huang L, Zhang W, Gschwend PM, Flores-Cervantes DX, Largeau C, Rouzaud J-N, Rumpel C, Guggenberger G, Kaiser K, Rodionov A, Gonzalez-Vila FJ, Gonzalez-Perez JA, de la Rosa JM, Manning DAC, López-Capél E, Ding L. Comparison of quantification methods to measure fire-derived (black/elemental) carbon in soils and sediments using reference materials from soil, water, sediment and the atmosphere. Global Biogeochem Cycles. 2007;21(3) Article Number: GB3016. [Google Scholar]
- 128.Cleveland D, Long SE, Pennington PL, Cooper E, Fulton MH, Scott GI, Brewer T, Davis J, Petersen EJ, Wood L. Pilot estuarine mesocosm study on the environmental fate of silver nanomaterials leached from consumer products. Sci Tot Environ. 2012;421:267–272. doi: 10.1016/j.scitotenv.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 129.Bour A, Mouchet F, Silvestre J, Gauthier L, Pinelli E. Environmentally relevant approaches to assess nanoparticles ecotoxicity: A review. J Hazard Mater. 2015;283:764–777. doi: 10.1016/j.jhazmat.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 130.Chen BL, Selegue JP. Separation and characterization of single-walled and multiwalled carbon nanotubes by using flow field-flow fractionation. Anal Chem. 2002;74(18):4774–4780. doi: 10.1021/ac020111b. [DOI] [PubMed] [Google Scholar]
- 131.Moon MH, Kang DJ, Jung JH, Kim JM. Separation of carbon nanotubes by frit inlet asymmetrical flow field-flow fractionation. J Sep Sci. 2004;27(9):710–717. doi: 10.1002/jssc.200401743. [DOI] [PubMed] [Google Scholar]
- 132.Chun J, Fagan JA, Hobbie EK, Bauer BJ. Size separation of single-wall carbon nanotubes by flow-field flow fractionation. Anal Chem. 2008;80(7):2514–2523. doi: 10.1021/ac7023624. [DOI] [PubMed] [Google Scholar]
- 133.Gigault J, Grassl B, Lespes G. Size characterization of the associations between carbon nanotubes and humic acids in aqueous media by asymmetrical flow field-flow fractionation combined with multi-angle light scattering. Chemosphere. 2012;86(2):177–182. doi: 10.1016/j.chemosphere.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 134.Doorn SK, Fields RE, Hu H, Hamon MA, Haddon RC, Selegue JP, Majidi V. High resolution capillary electrophoresis of carbon nanotubes. JACS. 2002;124(12):3169–3174. doi: 10.1021/ja012364c. [DOI] [PubMed] [Google Scholar]
- 135.Doorn SK, Strano MS, O’Connell MJ, Haroz EH, Rialon KL, Hauge RH, Smalley RE. Capillary electrophoresis separations of bundled and individual carbon nanotubes. J Phys Chem B. 2003;107(25):6063–6069. [Google Scholar]
- 136.Suarez B, Simonet BM, Cardenas S, Valcarcel M. Separation of carbon nanotubes in aqueous medium by capillary electrophoresis. J Chromat A. 2006;1128(1–2):282–289. doi: 10.1016/j.chroma.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 137.Lopez-Pastor M, Dominguez-Vidal A, Ayora-Canada MJ, Simonet BM, Lendl B, Valcarcel M. Separation of single-walled carbon nanotubes by use of ionic liquid-aided capillary electrophoresis. Anal Chem. 2008;80(8):2672–2679. doi: 10.1021/ac701788r. [DOI] [PubMed] [Google Scholar]
- 138.Fagan JA, Becker ML, Chun JH, Nie PT, Bauer BJ, Simpson JR, Hight-Walker A, Hobbie EK. Centrifugal length separation of carbon nanotubes. Langmuir. 2008;24(24):13880–13889. doi: 10.1021/la801388a. [DOI] [PubMed] [Google Scholar]
- 139.Komatsu N, Wang F. A Comprehensive Review on Separation Methods and Techniques for Single-Walled Carbon Nanotubes. Materials. 2010;3(7):3818–3844. doi: 10.3390/ma3073818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fagan JA, Becker ML, Chun J, Hobbie EK. Length fractionation of carbon nanotubes using centrifugation. Adv Mat. 2008;20(9):1609–1613. [Google Scholar]
- 141.Duesberg GS, Blau W, Byrne HJ, Muster J, Burghard M, Roth S. Chromatography of carbon nanotubes. Synthetic Metals. 1999;103(1–3):2484–2485. [Google Scholar]
- 142.Duesberg GS, Burghard M, Muster J, Philipp G, Roth S. Separation of carbon nanotubes by size exclusion chromatography. Chem Commun. 1998;(3):435–436. [Google Scholar]
- 143.Duesberg GS, Muster J, Krstic V, Burghard M, Roth S. Chromatographic size separation of single-wall carbon nanotubes. Appl Phys A: Mater Sci Proces. 1998;67(1):117–119. [Google Scholar]
- 144.Farkas E, Anderson ME, Chen ZH, Rinzler AG. Length sorting cut single wall carbon nanotubes by high performance liquid chromatography. Chem Phys Let. 2002;363(1–2):111–116. [Google Scholar]
- 145.Zhao B, Hu H, Niyogi S, Itkis ME, Hamon MA, Bhowmik P, Meier MS, Haddon RC. Chromatographic Purification and Properties of Soluble Single-Walled Carbon Nanotubes. J Am Chem Soc. 2001;123(47):11673–11677. doi: 10.1021/ja010488j. [DOI] [PubMed] [Google Scholar]
- 146.Ziegler KJ, Schmidt DJ, Rauwald U, Shah KN, Flor EL, Hauge RH, Smalley RE. Length-dependent extraction of single-walled carbon nanotubes. Nano Lett. 2005;5(12):2355–2359. doi: 10.1021/nl0510208. [DOI] [PubMed] [Google Scholar]
- 147.Flavel BS, Moore KE, Pfohl M, Kappes MM, Hennrich F. Separation of Single-Walled Carbon Nanotubes with a Gel Permeation Chromatography System. ACS Nano. 2014;8(2):1817–1826. doi: 10.1021/nn4062116. [DOI] [PubMed] [Google Scholar]
- 148.Suarez B, Moliner-Martinez Y, Cardenas S, Simonet BM, Valcarcel M. Monitoring of carboxylic carbon nanotubes in surface water by using multiwalled carbon nanotube-modified filter as preconcentration unit. Environ Sci Technol. 2008;42(16):6100–6104. doi: 10.1021/es800896c. [DOI] [PubMed] [Google Scholar]
- 149.Ziolkowski LA, Druffel ERM. The feasibility of isolation and detection of fullerenes and carbon nanotubes using the benzene polycarboxylic acid method. Mar Poll Bull. 2009;59(4–7):213–218. doi: 10.1016/j.marpolbul.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 150.Mattison NT, O’Carroll DM, Rowe RK, Petersen EJ. Impact of Porous Media Grain Size on the Transport of Multi-walled Carbon Nanotubes. Environ Sci Technol. 2011;45(22):9765–9775. doi: 10.1021/es2017076. [DOI] [PubMed] [Google Scholar]
- 151.O’Carroll DM, Liu X, Mattison NT, Petersen EJ. Impact of diameter on carbon nanotube transport in sand. J Coll Interf Sci. 2013;390:96–104. doi: 10.1016/j.jcis.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 152.Parks AN, Portis LM, Schierz PA, Washburn KM, Perron MM, Burgess RM, Ho KT, Chandler GT, Ferguson PL. Bioaccumulation and toxicity of single-walled carbon nanotubes to benthic organisms at the base of the marine food chain. Environ Toxicol Chem. 2013;32(6):1270–1277. doi: 10.1002/etc.2174. [DOI] [PubMed] [Google Scholar]
- 153.Hennrich F, Krupke R, Lebedkin S, Arnold K, Fischer R, Resasco DE, Kappes M. Raman spectroscopy of individual single-walled carbon nanotubes from various sources. J Phys Chem B. 2005;109(21):10567–10573. doi: 10.1021/jp0441745. [DOI] [PubMed] [Google Scholar]
- 154.Heeg S, Malic E, Casiraghi C, Reich S. Quantitative composition of a single-walled carbon nanotube sample: Raman scattering versus photoluminescence. Phys Status Sol B: Basic Solid State Phys. 2009;246(11–12):2740–2743. [Google Scholar]
- 155.Dresselhaus MS, Jorio A, Hofmann M, Dresselhaus G, Saito R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010;10(3):751–758. doi: 10.1021/nl904286r. [DOI] [PubMed] [Google Scholar]
- 156.Caballero-Diaz E, Guzman-Ruiz R, Malagon MM, Simonet BM, Valcarcel M. Effects of the interaction of single-walled carbon nanotubes with 4-nonylphenol on their in vitro toxicity. J Hazard Mater. 2014;275:107–115. doi: 10.1016/j.jhazmat.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 157.Lopez-Lorente AI, Simonet BM, Valcarcel M. Raman spectroscopic characterization of single walled carbon nanotubes: influence of the sample aggregation state. Analyst. 2014;139(1):290–298. doi: 10.1039/c3an00642e. [DOI] [PubMed] [Google Scholar]
- 158.Lopez-Lorente AI, Simonet BM, Valcarcel M, Mizaikoff B. Bare gold nanoparticles mediated surface-enhanced Raman spectroscopic determination and quantification of carboxylated single-walled carbon nanotubes. Anal Chem Acta. 2013;788:122–128. doi: 10.1016/j.aca.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 159.Lopez-Lorente AI, Simonet BM, Valcarcel M. Qualitative detection and quantitative determination of single-walled carbon nanotubes in mixtures of carbon nanotubes with a portable Raman spectrometer. Analyst. 2013;138(8):2378–2385. doi: 10.1039/c3an36566b. [DOI] [PubMed] [Google Scholar]
- 160.Marches R, Mikoryak C, Wang RH, Pantano P, Draper RK, Vitetta ES. The importance of cellular internalization of antibody-targeted carbon nanotubes in the photothermal ablation of breast cancer cells. Nanotechnol. 2011;22(9) doi: 10.1088/0957-4484/22/9/095101. Article Number: 095101. [DOI] [PubMed] [Google Scholar]
- 161.Liu Z, Davis C, Cai WB, He L, Chen XY, Dai HJ. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci US A. 2008;105(5):1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salzmann CG, Chu BTT, Tobias G, Llewellyn SA, Green MLH. Quantitative assessment of carbon nanotube dispersions by Raman spectroscopy. Carbon. 2007;45(5):907–912. [Google Scholar]
- 163.Athalin H, Lefrant SL. A correlated method for quantifying mixed and dispersed carbon nanotubes: analysis of the Raman band intensities and evidence of wavenumber shift. J Raman Spectr. 2005;36(5):400–408. [Google Scholar]
- 164.Nunes A, Bussy C, Gherardini L, Meneghetti M, Herrero MA, Bianco A, Prato M, Pizzorusso T, Al-Jamal KT, Kostarelos K. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine. 2012;7(10):1485–1494. doi: 10.2217/nnm.12.33. [DOI] [PubMed] [Google Scholar]
- 165.Batista CAS, Zheng M, Khripin CY, Tu XM, Fagan JA. Rod Hydrodynamics and Length Distributions of Single-Wall Carbon Nanotubes Using Analytical Ultracentrifugation. Langmuir. 2014;30(17):4895–4904. doi: 10.1021/la404892k. [DOI] [PubMed] [Google Scholar]
- 166.Badireddy AR, Wiesner MR, Liu J. Detection, Characterization, and Abundance of Engineered Nanoparticles in Complex Waters by Hyperspectral Imagery with Enhanced Darkfield Microscopy. Environ Sci Technol. 2012;46(18):10081–10088. doi: 10.1021/es204140s. [DOI] [PubMed] [Google Scholar]
- 167.Mortimer M, Gogos A, Bartolome N, Kahru A, Bucheli TD, Slaveykova VI. Potential of Hyperspectral Imaging Microscopy for Semi-quantitative Analysis of Nanoparticle Uptake by Protozoa. Environ Sci Technol. 2014;48(15):8760–8767. doi: 10.1021/es500898j. [DOI] [PubMed] [Google Scholar]
- 168.Berciaud S, Cognet L, Poulin P, Weisman RB, Lounis B. Absorption spectroscopy of individual single-walled carbon nanotubes. Nano Lett. 2007;7(5):1203–1207. doi: 10.1021/nl062933k. [DOI] [PubMed] [Google Scholar]
- 169.Nedosekin DA, Shashkov EV, Galanzha EI, Hennings L, Zharov VP. Photothermal Multispectral Image Cytometry for Quantitative Histology of Nanoparticles and Micrometastasis in Intact, Stained and Selectively Burned Tissues. Cytometry Part A. 2010;77A(11):1049–1058. doi: 10.1002/cyto.a.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hong H, Chen F, Cai WB. Pharmacokinetic Issues of Imaging with Nanoparticles: Focusing on Carbon Nanotubes and Quantum Dots. Mol Imag Biol. 2013;15(5):507–520. doi: 10.1007/s11307-013-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lehman JH, Terrones M, Mansfield E, Hurst KE, Meunier V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon. 2011;49(8):2581–2602. [Google Scholar]
- 172.Mansfield E, Kar A, Wang CM, Chiaramonti AN. Statistical sampling of carbon nanotube populations by thermogravimetric analysis. Anal Bioanal Chem. 2013;405(25):8207–8213. doi: 10.1007/s00216-013-7221-6. [DOI] [PubMed] [Google Scholar]
- 173.Petersen EJ, Huang Q, Weber WJ., Jr Bioaccumulation of radio-labeled carbon nanotubes by Eisenia foetida. Environ Sci Technol. 2008;42(8):3090–3095. doi: 10.1021/es071366f. [DOI] [PubMed] [Google Scholar]
- 174.Petersen EJ, Huang Q, Weber WJ., Jr Ecological uptake and depuration of carbon nanotubes by Lumbriculus variegatus. Environ Health Perspect. 2008;116(4):496–500. doi: 10.1289/ehp.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Petersen EJ, Pinto RA, Zhang L, Huang QG, Landrum PF, Weber WJ. Effects of polyethyleneimine-mediated functionalization of multi-walled carbon nanotubes on earthworm bioaccumulation and sorption by soils. Environ Sci Technol. 2011;45(8):3718–3724. doi: 10.1021/es103004r. [DOI] [PubMed] [Google Scholar]
- 176.Zhang L, Petersen EJ, Qingguo H. Phase Distribution of 14C-Labeled Multiwalled Carbon Nanotubes in Aqueous Systems Containing Model Solids: Peat. Environ Sci Technol. 2011;45(4):1356–1362. doi: 10.1021/es1026097. [DOI] [PubMed] [Google Scholar]
- 177.Petersen EJ, Akkanen J, Kukkonen JVK, Weber WJ., Jr Biological Uptake and Depuration of Carbon Nano-tubes by Daphnia magna. Environ Sci Technol. 2009;43(8):2969–2975. doi: 10.1021/es8029363. [DOI] [PubMed] [Google Scholar]
- 178.Petersen EJ, Huang QG, Weber WJ., Jr Relevance of octanol-water distribution measurements to the potential ecological uptake of multi-walled carbon nanotubes. Environ Toxicol Chem. 2010;29(5):1106–1112. doi: 10.1002/etc.149. [DOI] [PubMed] [Google Scholar]
- 179.Larue C, Pinault M, Czarny B, Georgin D, Jaillard D, Bendiab N, Mayne-L’Hermite M, Taran F, Dive V, Carriere M. Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed. J Hazard Mater. 2012;227:155–163. doi: 10.1016/j.jhazmat.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 180.Zhang L, Petersen EJ, Habteselassie MY, Mao L, Huang Q. Degradation of multiwall carbon nanotubes by bacteria. Environ Pollut. 2013;181:335–339. doi: 10.1016/j.envpol.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 181.Rhiem S, Riding MJ, Baumgartner W, Martin FL, Semple KT, Jones KC, Schaffer A, Maes HM. Interactions of multiwalled carbon nanotubes with algal cells: Quantification of association, visualization of uptake, and measurement of alterations in the composition of cells. Environ Pollut. 2015;196:431–439. doi: 10.1016/j.envpol.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 182.Fagan JA, Zheng M, Rastogi V, Simpson JR, Khripin CY, Batista CAS, Walker ARH. Analyzing Surfactant Structures on Length and Chirality Resolved (6,5) Single-Wall Carbon Nanotubes by Analytical Ultracentrifugation. ACS Nano. 2013;7(4):3373–3387. doi: 10.1021/nn4002165. [DOI] [PubMed] [Google Scholar]
- 183.Arnold MS, Suntivich J, Stupp SI, Hersam MC. Hydrodynamic Characterization of Surfactant Encapsulated Carbon Nanotubes Using an Analytical Ultracentrifuge. ACS Nano. 2008;2(11):2291–2300. doi: 10.1021/nn800512t. [DOI] [PubMed] [Google Scholar]
- 184.Backes C, Karabudak E, Schmidt CD, Hauke F, Hirsch A, Wohlleben W. Determination of the Surfactant Density on SWCNTs by Analytical Ultracentrifugation. Chem- Europ J. 2010;16(44):13176–13184. doi: 10.1002/chem.200903461. [DOI] [PubMed] [Google Scholar]
- 185.Kim KT, Edgington AJ, Klaine SJ, Cho JW, Kim SD. Influence of multiwalled carbon nanotubes dispersed in natural organic matter on speciation and bioavailability of copper. Environ Sci Technol. 2009;43(23):8979–8984. doi: 10.1021/es900647f. [DOI] [PubMed] [Google Scholar]
- 186.Bourdiol F, Dubuc D, Grenier K, Mouchet F, Gauthier L, Flahaut E. Quantitative detection of carbon nanotubes in biological samples by an original method based on microwave permittivity measurements. Carbon. 2015;81:535–545. [Google Scholar]
- 187.Cano A, Kohl K, Deleon S, Payton P, Irin F, Saed M, Shah SA, Green MJ, Canas-Carrell JE. Determination of uptake, accumulation, and stress effects in corn (Zea mays L.) grown in single-wall carbon nanotube contaminated soil. Chemosphere. 2016 doi: 10.1016/j.chemosphere.2016.02.093. in press. [DOI] [PubMed] [Google Scholar]
- 188.Menendez JA, Arenillas A, Fidalgo B, Fernandez Y, Zubizarreta L, Calvo EG, Bermudez JM. Microwave heating processes involving carbon materials. Fuel Process Technol. 2010;91(1):1–8. [Google Scholar]
- 189.Garner KL, Suh S, Lenihan HS, Keller AA. Species Sensitivity Distributions for Engineered Nanomaterials. Environ Sci Technol. 2015;49(9):5753–5759. doi: 10.1021/acs.est.5b00081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.