Abstract
Although surgical trauma activates the anterior pituitary gland and elicits an increase in prolactin (PRL) serum levels that can modulate nociceptive responses, the role of PRL and the PRL-receptor (PRL-R) in thermal and mechanical hyperalgesia in postoperative pain is unknown. Acute postoperative pain condition was generated with the use of the hindpaw plantar incision model. Results showed endogenous PRL levels were significantly increased in serum, operated hindpaw and spinal cords of male and female rats 24 hours after incision. These alterations were especially pronounced in females. We then examined the role of the PRL system in thermal and mechanical hyperalgesia in male and female mice 3-168 hours after plantar incision with the use of knock-out (KO) mice with PRL or PRL-R gene ablations and in wild-type (WT) mice. WT mice showed postoperative cold hyperalgesia in a sex-dependent manner (only in females), but with no effect on heat hyperalgesia or mechanical allodynia in either sex. Studies in KO mice showed no effect of PRL and PRL-R gene ablation on heat and cold hyperalgesia in male mice, while heat hyperlgesia were reduced 3-72 hours post-surgery in female PRL and PRL-R KO mice. In contrast, PRL and PRL-R ablations significantly attenuated mechanical allodynia 3-72 hours post-surgery in both male and female mice. Overall, we found elevated PRL levels in serum, hindpaws and spinal cords after incision, and identify a contributory role for the PRL system in postoperative pain responses to thermal stimuli in females and to mechanical stimuli in both males and females.
Keywords: Prolactin, Prolactin receptor, Post operative model, Pain, Sex-dependence
Introduction
The management of postoperative pain is an important healthcare issue since ineffective treatment of postoperative pain can prolong recovery, and increase both morbidity and mortality after surgery. Even though considerable progress has been made in understanding mechanisms contributing to postoperative pain (Pogatzki et al., 2002, Martin et al., 2005, Pogatzki-Zahn et al., 2007, Wu and Raja, 2011, Steyaert and De Kock, 2012, Deumens et al., 2013), a large percentage of patients still experience inadequate pain relief following surgical procedures (Phillips, 2000, Raghunathan and Pyati, 2013).
Findings from clinical studies indicate that women are at a substantially greater risk than men for certain aspects of postoperative and procedural pain (Fillingim et al., 2009). However, studies show male and female mice baseline responses to heat and mechanical nociception are not statistically different in a model of acute postoperative pain (Banik et al., 2006). Nevertheless, a number of physiological and pharmacological differences have been revealed regarding sex-dependency in responses to anesthesia and analgesic therapy in humans (Fillingim and Gear, 2004). Postoperative pain is conventionally managed with non-steroid anti-inflammatory drugs, acetaminophen, opioids and peripheral acting anesthetics (Kehlet et al., 2006, Butler et al., 2011, Wu and Raja, 2011). In this respect, women are more sensitive than men to opioid receptor agonists and to certain neuroblocking agents (Campesi et al., 2012) and these differences suggest that optimum analgesic regimes may be sex-dependent..
The sex-dependency in postoperative pain remains poorly understood, yet it appears that sexual hormones may play an important role (Fillingim et al., 2009, Campesi et al., 2012). The response of the hypothalamic-pituitary-adrenal axis in humans to surgery is well documented (Noel et al., 1972, Anand, 1986, Reiner et al., 1987). Stress, surgical trauma and postoperative conditions activate the anterior pituitary gland in humans and elicit an increase in serum levels of prolactin (PRL), an important sex hormone (Noreng et al., 1987, Yardeni et al., 2007). Plasma PRL concentrations in humans remain increased for up to 1-2 weeks postoperatively with a gradual return to normal levels (Chernow et al., 1987). Certain long-acting local anesthetics can decrease plasma levels of PRL in humans as well (Reiz et al., 1989). Inflammation is present postoperatively in both animals and humans (Moore et al., 1994, Wu and Raja, 2011), and can contribute to elevated systemic as well as local PRL levels (Berczi et al., 1984, Mateo et al., 1998, Scotland et al., 2011). Animal studies demonstrate that PRL can modulate the immune system (Bernton et al., 1988, Tseng et al., 1997) that plays a critical role in activation of nociceptors (Woolf et al., 1997). Importantly, PRL directly sensitizes nociceptors in rats (Diogenes et al., 2006). Moreover, pro-nociceptive actions of PRL could vary in males versus females, since PRL levels during inflammation are sex-dependent in humans and animals (Jimena et al., 1998, Mateo et al., 1998, Giraldo et al., 2008, Scotland et al., 2011). Altogether, despite the wealth of information on PRL elevation in serum of humans and animals during postoperative conditions, certain issues are still not clear. Questions remain on whether acute operative interventions trigger an increase in endogenous PRL locally at the site of surgical intervention or at distant sites such as the spinal cord that are important in processing pain signals, the specific contribution of the PRL system to modulating nociceptive responses during the acute postoperative pain period and if this contribution is sex-dependent? To investigate these questions, we measured endogenous PRL levels in serum, operated and non-operated hindpaws and spinal cords of male and female rats 24 hours after incision procedures on hindpaws. We also compared pain behaviors in wild-type (WT) versus PRL or PRL receptor (PRL-R) null-mutant (knockout; KO) male and female mice with the use of the hindpaw plantar incision postoperative pain model.
Experimental procedures
Animals
All animal experiments conformed to APS's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training, and to protocols approved by the University Texas Health Science Center at San Antonio (UTHSCSA) Animal Care and Use Committee (IACUC). We also followed guidelines issued by the National Institutes of Health and the Society for Neuroscience to minimize the number of animals used and their suffering.
Adult male and female Sprague-Dawley rats (200-250g, Charles River Laboratories, Wilmington, MA) were housed three per cage under a 12-h light/12-h dark cycle with food and water available ad libitum. Adult female and male PRL null-mutant (PRL KO), PRL-R null-mutant (PRL-R KO) and corresponding littermate wild-type (WT) mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). PRL KO and PRL-R KO mice are viable, normal in size and do not display any gross physical or behavioral abnormalities. The homozygous PRL KO females are infertile and have an irregular estrous cycle. Male and female homozygous PRL-R KO mice are completely sterile, and the serum PRL levels are increased 60 - 100 fold (Ormandy et al., 1997). The serum estradiol and progesterone levels are moderately decreased in estrus PRL-R KO females (estradiol: from 53 pg/ml for WT to 37 pg/ml for PRL-R KO; progesterone: from 17 ng/ml for WT to 7 ng/ml for PRL-R KO; (Clement-Lacroix et al., 1999). The serum total testosterone levels are at similar levels in WT and PRL-R KO male mice (Clement-Lacroix et al., 1999). PRL KO mice were generated via targeted disruption of coding exon 4, which results in a truncated 11 kDa-long PRL protein (normal PRL size is 24 kDa) that lacks any detectable bioactivity (Horseman et al., 1997). PRL-R KO mice were produced via creating an in-frame stop codon in exon 5 (Ormandy et al., 1997). The lack of functional PRL-R in homozygous mutant animals was confirmed using northern, western, and binding assays (Ormandy et al., 1997). PRL and PRL-R KO mice were produced in C57BL/6J line. Adult mice weighing 20-30 grams were used in the study. All animals were housed in a 12 hour light dark cycle.
Measurement of endogenous PRL
We have selected male and female rats for measurement of endogenous PRL, because reliable anti-mouse PRL are not available. Tissues and serum were collected 24 hours after sham and incision operative procedures on hind paws of animals (Pogatzki and Raja, 2003). Rat paw samples were collected with 6 mm biopsy punches (Healthlink®, Fray Corp., Buffalo, NY). The L4-L6 lumbar spinal cord was isolated. Serum samples were collected in blood collection tubes with sodium citrate (BD Biosciences, Franklin Lakes, NJ). Protein extracts were generated by adding 200-500 μl T-PER solution (Thermo Scientific, Rockford, IL) to samples, and disrupting them with TissueLyser LT (Millipore, Billerica, MA) at 50 oscillations per min for 5 min. Protein extracts were stored at −20° C until assayed using a commercially available rat PRL EIA kit (SPIbio, Montigny le Bretonneux, France, distributed by Cayman Chemical). Amount of protein in extracts was measured by Bradford assay (Scotland et al., 2011). Endogenous PRL levels in serum were presented as ng/ml of serum. Endogenous PRL levels in tissues were presented as ng/ml of protein extract. These values were normalized against total amount of protein in extracts.
Acute postoperative pain model
All behavioral experiments were conducted by a blinded observer. Since PRL KO and PRL-R KO female mice have irregular estrous cycles, surgical procedures on female mice were conducted in the morning on mice in estrus phase (Caligioni, 2009). The reproductive stage of cycling females was determined by vaginal lavage using methods previously described (Marcondes et al., 2002). Thermal and mechanical nociception were mainly measured in mice in estrus cycle, which have approximately 40-65 pg/ml of estradiol (Clement-Lacroix et al., 1999). However, 7 days post-incision, nociception in some female mice was measured in metestrus phase that is associated with low levels of E2. The plantar incision in mice was used as a model of acute postoperative pain as previously described (Pogatzki and Raja, 2003). Mice were anesthetized using 2% isoflurane. The right hindpaw was prepared for incision by application of antiseptic betadine solution. The incision model in mice was created by a 5 mm incision beginning 2 mm from the proximal edge of the right heel. Curved forceps were used to elevate the underlying muscle. A mattress suture of 8-0 nylon on a TG175-8 needle (ophthalmic, 1716G; Ethicon, Somerville, NJ) was used to close the incision. Antibiotic ointment (Bacitracin Zinc Ointment USP, Melville, NY) was applied to the wound and the sutures removed 2 days later. Sham treatment consisted of mice that received anesthesia, antiseptic preparation and topical antibiotic without an incision. Thermal, mechanical and cold hyperalgesia was measured in these animals over a period of 7 days.
Heat-induced nociception
Mice were habituated to the testing environment for at least 1 hour prior to testing. Heat nociception was assessed as previously described (Hargreaves et al., 1988). In brief, mice were placed on a glass surface with temperature held constant at ≈20°C. Following habituation, thermal withdrawal latencies to a radiant heat beam were recorded at each time point (3X measurements at each time point, averaged to obtain the data value used in analyses). In order to prevent tissue damage, the stimulus was terminated after ≈20 sec if the animal did not withdraw the hind paw.
Cold-induced nociception
Cold nociception in mice was measured using a modified IITC incremental hot/cold plate (IITC Life Sciences). Mice were placed on a metal plate maintained at room temperature. They were habituated to the apparatus for 45-60 min. The temperature was then decreased at a ramp of 10°C/min. To prevent any tissue damage due to extreme cold temperatures the cutoff temperature was −5°C. The threshold temperatures manifested by escape reflex (jump) of mice were recorded as a single response. One mouse at a time was used to measure cold nociception.
Mechanical stimulus-induced nociception
Mice were habituated for 45-60 minutes and then the baseline readings (three readings per animal) were taken on the right hind paw using the Dynamic Plantar Aesthesiometer (Ugo Basile) to record withdrawal thresholds for mechanical stimulation. The instrument applies constant ramp of increasing mechanical pressure to the paw (from 0 to 50 grams over 10 second intervals) and the withdrawal threshold was recorded in grams when the paw was withdrawn.
Data analyses
GraphPad Prism 5.0 (GraphPad, La Jolla, CA) was used for statistical analyses. The data in Figures were given as mean ± standard error of the mean (SEM), with the value of “n” referring to the number of analyzed animals for each group. All experiments were performed at least in duplicate with the use of a different set of mice. Differences between groups were assessed by two-way analysis of variance (ANOVA; no matching, regular 2-way ANOVA, not repeated measures) with Bonferroni's multiple comparison post-hoc tests (where each column was compared to all other columns). Differences between male and female post-operative hyperelgesia were conducted with 3-way ANOVA with sex, genotype and time as factors. A difference was accepted as statistically significant when p<0.05. Significance levels have p<0.05 (*), <0.01 (**) and <0.001 (***) values.
Results
Endogenous PRL levels are altered after plantar incision
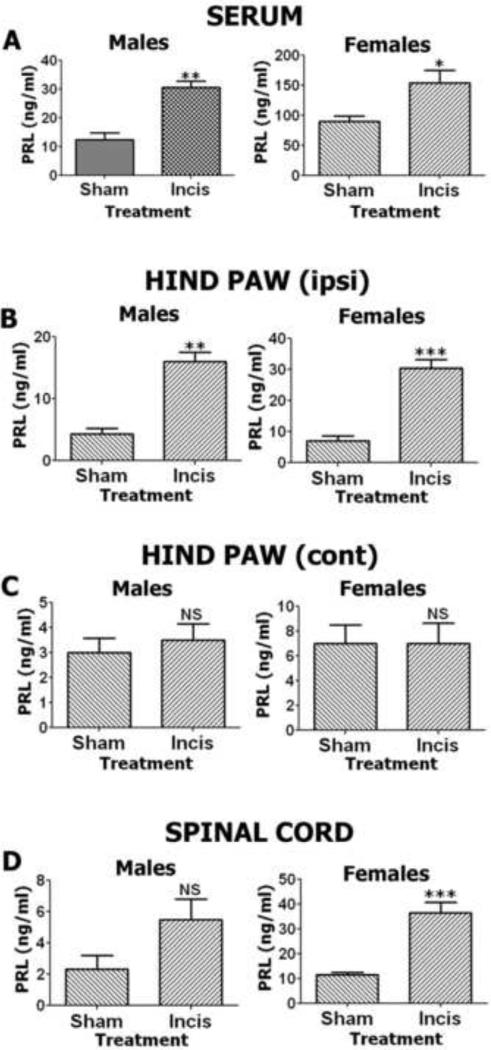
Previous studies have demonstrated that the serum level of PRL in humans increases after surgery (Noel et al., 1972, Anand, 1986, Reiner et al., 1987). Here, we evaluated if plantar incision alters endogenous PRL levels in hindpaw peripheral tissues, in the level of the spinal cord responsible for hindpaw innervations as well as in the systemic circulation. Systemic PRL levels were measured in serum of male and female rats 24 hours after sham (control) and incision interventions. There were significant changes in serum PRL levels in both male and female rats following incision compared to sham procedures (Fig 1A), but this increase was dramatically larger, ~8-fold greater in females when compared to males. Local PRL tissue levels were measured in punch biopsies collected 24 hours after incision surgery from non-operated (contralateral; Contra) and operated (ipsilateral; Ipsi) hindpaws of female and male rats. Elevations in local PRL levels were detected in the ipsilateral/operated paw, but not in the contralateral/non-operated hindpaw biopsies from both female and male rats (Fig 1B and 1C). Basal levels of local PRL were slightly, but not significantly higher (one-way ANOVA) in sham female rats. However, incision induced an elevation of PRL that was significantly higher in females when compared to males (one-way ANOVA; p<0.01; Fig 1B). Importantly, these incision-induced PRL levels identified in females, but not in male rat hindpaws can be high enough to activate PRL-R (Diogenes et al., 2006). We next examined whether PRL levels are increased in the L4-L6 spinal cord segment of male and female rats 24 hours after hindpaw incision surgery. Results showed sex-dependent differences in both PRL basal spinal cord expression levels and up-regulation of PRL spinal cord levels after hind paw incision. Specifically, higher basal PRL spinal cord levels were present in normal females when compared to males (one-way ANOVA; Fig 1D) while incision surgery caused up-regulation in PRL spinal cord levels in males, but with a significant increase in females spinal cords that again reaches levels that can activate PRL-R (Fig 1D). In summary, hindpaw incision surgery up-regulates serum, local and spinal cord PRL levels in female and male rats. However, this up-regulation in serum and tissue PRL levels is sex-dependent.
Figure 1. Endogenous PRL levels are regulated by hindpaw incision in intact female and male rats.
(A) Endogenous PRL levels in serum of male and female rats. Data were acquired 24 h after incision of hind paws (*p<0.05; **p<0.01; unpaired t-test; n=4). (B) Endogenous PRL levels in ipsi-lateral hind paw biopsies of male and female rats. Data were acquired 24 h after incision of hind paws (**p<0.01; ***p<0.001; unpaired t-test; n=4). (C) Endogenous PRL levels in contra-lateral hind paw biopsies of male and female rats. Data were acquired 24 h after incision of ipsi-lateral hind paws (NS – non-significant; unpaired t-test; n=4). (D) Endogenous PRL levels in L4-L6 level lumbar spinal cord of male and female rats. Data were acquired 24 h after incision of hind paws (NS – non-significant; ***p<0.001; unpaired t-test; n=4).
Postoperative heat hyperalgesia in male and female WT, PRL KO and PRL-R KO mice
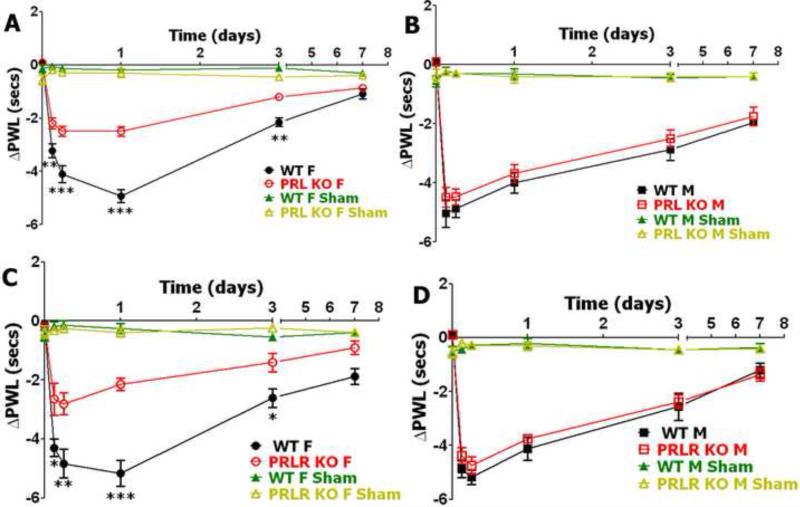
To investigate whether PRL and PRL-R is involved in the regulation of behavioral responses to heat stimuli after surgery, we used the plantar incision model in WT, PRL KO and PRL-R KO male and female mice and evaluated alterations in response to heat stimuli as a measure for heat hyperalgesia at different post-surgery time points. Basal levels of heat nociception have not been statistically changed in PRL KO and PRL-R KO versus WT male and female mice (Table 1). Data for heat hyperalgesia were presented as delta change in paw withdrawal latency (PWL). Postoperative heat hyperalgesia peaked at 24 hours post-surgery in WT female mice and at 6 hours post-surgery in WT male mice (Fig 2). WT male and female littermate mice for PRL KO and PRL-R KO produced similar incision-induced heat hyperalgesia at all post-surgery time points, except at 3 hours post-incision where male mice have higher heat hyperalgesia (**p<0.01; 3-way ANOVA with sex, genotype and time as factors). These results are in accordance with previously published data (Banik et al., 2006). Postoperative heat hyperalgesia was not affected at tested post-surgery time points (3, 6, 24, 72 and 168 hours) by the lack of PRL and PRL-R proteins in male mice (Fig 2B and 2D). However, postoperative-induced heat hyperalgesia was partially and significantly decreased at 3, 6, 24 and 72 hours post-surgery in PRL KO and PRL-R KO female mice when compared to WT female mice (Fig 2A and 2C). In summary, reduction of postoperative heat hyperalgesia in PRL and PRL-R KO mice is sex-dependent, and only present in female mice.
Table 1.
Baseline thermal and mechanical nociceptive thresholds in wild-type (WT), prolactin null-mutant (PRL KO) and prolactin receptor null-mutant (PRLR KO) female and male mice.
| WT vs PRL KO | WT vs PRLR KO | |||||
|---|---|---|---|---|---|---|
| Heat | Cold | Mechanical | Heat | Cold | Mechanical | |
| Female | 9.12±0.45 vs 8.21±0.24 (ns) | 4.11±0.30 vs 8.08±0.65(**) | 7.92±0.43 vs 7.41±0.54 (ns) | 8.42±0.37 vs 7.54±0.28 (ns) | 3.63±0.35 vs 6.88±0.26 (***) | 7.71±0.49 vs 7.16±0.61 (ns) |
| Male | 10.08±0.46 vs 9.45±0.52 (ns) | 7.28±0.59 vs 8.46±0.57 (ns) | 8.35±0.42 vs 8.01±0.37 (ns) | 9.22±0.43 vs 8.46±0.38 (ns) | 6.68±0.53 vs 7.39±0.72 (ns) | 8.02±0.33 vs 7.73±0.66 (ns) |
N=8-10. Significant changes (unpaired t-test) are in brackets: ns – non-significant
p<0.01
p<0.001.
Heat nociception is measured as paw withdrawal latency (PWL) in seconds.
Cold nociception is measured in threshold temperature in degrees (°C).
Mechanical nociception is measured in threshold force in grams (g).
Figure 2. Incision-induced heat hyperalgesia in WT, PRL KO and PRL-R KO female and male mice.
(A) Incision-induced heat hyperalgesia in WT and PRL KO female mice. Heat hyperalgesia is measured as changes in paw withdrawal latency from baseline measured before surgery (PWL; **p<0.01; ***p<0.001; 2-way ANOVA; n=8). (B) Incision-induced heat hyperalgesia in WT and PRL KO male mice (PWL; NS; 2-way ANOVA; n=8) (C) Incision-induced heat hyperalgesia in WT and PRL-R KO female mice ( PWL; *p<0.05; **p<0.01; ***p<0.001 2-way ANOVA; n=8). (D) Incision-induced heat hyperalgesia in WT and PRL-R KO male mice. ( PWL; NS; 2-way ANOVA; n=8). Post-incision time points are indicated above X-axis. Mouse lines and sex (i.e F and M) are noted. WT, PRL KO and PRL-R KO shams are for n=5 (each).
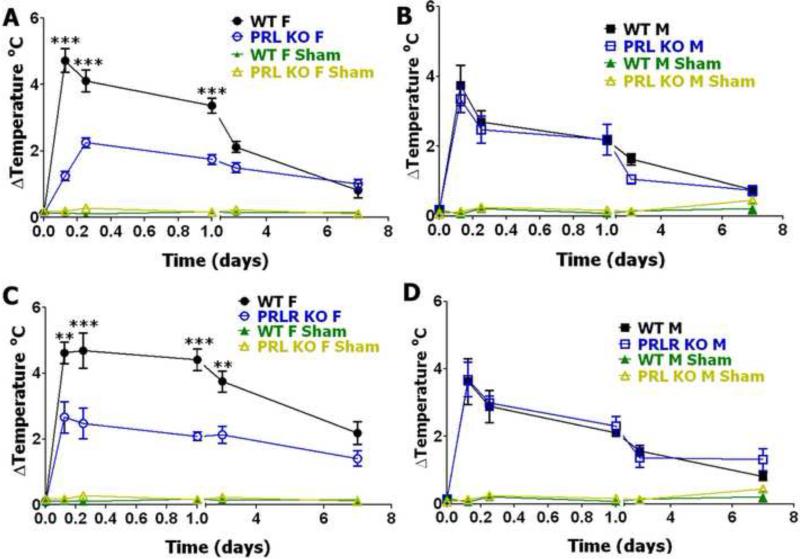
Postoperative cold hyperalgesia in male and female WT, PRL KO and PRL-R KO mice
Cold hyperalgesia in acute postoperative models has not been previously studied in detail. PRL KO and PRL-R KO female mice are dramatically sensitive to cold stimuli than WT animals, while in males cold nociception was not changed in PRL KO and PRL-R KO (Table 1). Postoperative cold hyperalgesia peaked at 3 hours in both WT male and female littermates and then gradually returned to baseline (Fig 3). Notably, WT female littermate mice for PRL KO and PRL-R KO produced significantly higher cold-induced postoperative hyperalgesia than WT male mice at all post-surgery time points, except 7 days post-incision (*p<0.05 3h post-surgery; ***p<0.001 6-24h post-surgery; **p<0.01 3 days post-surgery; 3-way ANOVA with sex, genotype and time as factors). Cold postoperative hyperalgesia behaved similar to heat postoperative hyperalgesia in PRL KO and PRL-R KO mice. Thus, cold postoperative hyperalgesia was not statistically changed in male mice with ablated PRL and PRL-R genes (Fig 3B and 3D). In contrast, the anti-hyperalgesic effects were even more pronounced for cold postoperative hyperalgesia at 3, 6, 24 and 72 h post-surgery in female mice with disrupted PRL and PRL-R genes (Fig 3A and 3C). However, since cold nociception is higher in female PRL KO and PRL-R KO mice, absolute values for cold hyperalgesia are not altered in both PRL KO and PRL-R KO females and males (Fig 3A, 3C and Table 1). Altogether, absolute values of cold hyperalgesia were not altered in both female and male PRL KO and PRL-R KO compare to WT littermate mice.
Figure 3. Incision-induced cold hyperalgesia in WT, PRL KO and PRL-R KO female and male mice.
(A) Incision-induced cold hyperalgesia in WT and PRL KO female mice. Cold hyperalgesia is measured as changes ( ) from baseline measured before surgery in threshold temperature producing animal responses (Temperature °C; ***p<0.001; 2-way ANOVA; n=9). (B) Incision-induced cold hyperalgesia in WT and PRL KO male mice (Temperature °C; NS; 2-way ANOVA; n=9). (C) Incision-induced cold hyperalgesia in WT and PRL-R KO female mice (Temperature °C; **p<0.01; ***p<0.001; 2-way ANOVA; n=8). (D) Incision-induced cold hyperalgesia in WT and PRL-R KO male mice (Temperature °C; NS; 2-way ANOVA; n=8). Post-incision time points are indicated below X-axis. Mouse lines and sex (i.e F and M) are noted. WT, PRL KO and PRL-R KO shams are for n=5 (each).
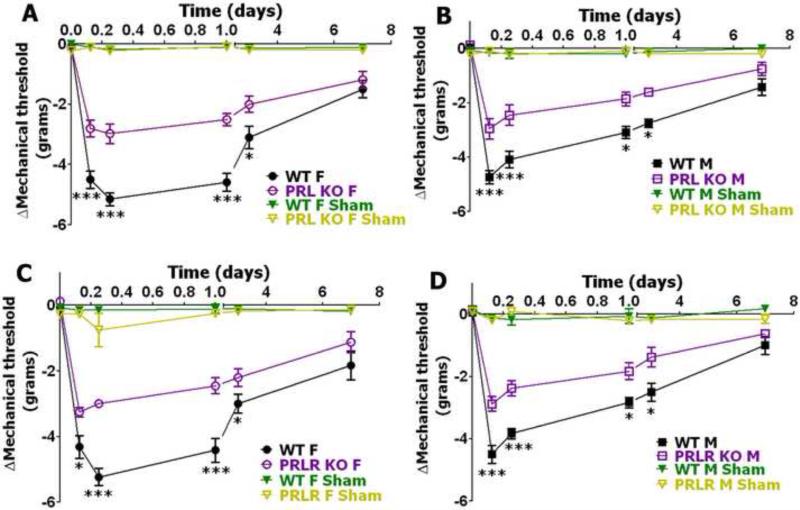
Postoperative mechanical allodynia in male and female WT, PRL KO and PRL-R KO mice
To further characterize the role of PRL and PRL-R hyperalgesia in an acute postoperative model, we evaluated whether postoperative-induced mechanical allodynia was altered in male and female mice lacking PRL or PRL-R proteins. Basal behavioral responses to mechanical stimuli as a measure for mechanical allodynia were approximately equal in PRL KO and PRL-R KO versus WT female as well as male mice (Table 1). Mechanical allodynia developed quickly after surgery in WT mice and reached a maximum at 6 hours post-surgery in females and 3 hours post-surgery in male mice (Fig 4). Statistical differences between WT male and female mice were noted only at the 24 hour post-surgery time period (Fig 4). At this time point, mechanical allodynia (mechanical threshold (g)) was higher in female versus male WT mice (−4.5±0.21 for females vs. −2.96±0.14 for male; 3-way ANOVA with sex, genotype and time as factors; **p<0.01, n=20). Disruption of PRL or PRL-R in male as well as female mice significantly altered postoperative mechanical allodynia at 3, 6, 24 and 72 hours post-surgery (Fig 4). Altogether, attenuation of postoperative-induced mechanical allodynia was equally affected in both male and female PRL KO and PRL-KO mice.
Figure 4. Incision-induced mechanical allodynia in WT, PRL KO and PRL-R KO female and male mice.
(A) Incision-induced mechanical allodynia in WT and PRL KO female mice. Mechanical allodynia is measured as changes from baseline measured before surgery ( ) in threshold force producing animal responses (***p<0.001; 2-way ANOVA; n=11). (B) Incision-induced mechanical allodynia in WT and PRL KO male mice (*p<0.05; ***p<0.001; 2-way ANOVA; n=11). (C) Incision-induced mechanical allodynia in WT and PRL-R KO female mice (*p<0.05; ***p<0.001; 2-way ANOVA; n=9). (D) Incision-induced mechanical allodynia in WT and PRL-R KO male mice (*p<0.05; ***p<0.001; 2-way ANOVA; n=9). Post- incision time points are indicated above X-axis. Mouse lines and sex (i.e. F and M) are noted. WT, PRL KO and PRL-R KO shams are for n=4 (each).
DISCUSSION
The current studies demonstrate that the hindpaw incision model for acute postoperative pain induces PRL levels systemically in serum as well as in the operated hind paw and in the spinal cord lumbar segment of both male and female rats. However, basal levels of both PRL and post-surgical PRL are higher in female animals (Fig 1). Our data also showed that ablations of PRL and PRL-R genes lead to significant reduction of thermal hyperalgesia in a sex-dependent manner following acute postoperative pain produced by the plantar incision model (Figs 2 and 3). Thus, eliminations of PRL and PRL-R genes resulted in attenuation of postoperative thermal (i.e. heat and cold) hyperalgesia in female mice (Fig 2 and 3), while postoperative mechanical allodynia was reduced in both male and female mice (Fig 4).
Surgical insults are associated with an activation of the anterior pituitary gland and increased serum levels of PRL in humans seen at 1-2 weeks and even longer (>2 weeks) after the insult (Noreng et al., 1987, Yardeni et al., 2007, Wu and Raja, 2011). Our results also show the systemic upregulation of endogenous PRL, but in addition show an upregulation locally at the site of the incision and in the lumbar spinal cord. Peripheral inflammation is also associated with a local increase in PRL levels in OVX-E rats as previously reported by our group (Scotland et al., 2011). Others have found that the magnitude of systemic up regulation seen during inflammatory insults is sex-dependent (Jimena et al., 1998, Mateo et al., 1998, Giraldo et al., 2008, Scotland et al., 2011) and it has been suggested that the PRL up regulation seen following surgical trauma could be dependent on the phase of estrous cycle and gender (Barni et al., 1991). Our data supports these possible influences since the incision-induced systemic and local PRL increases seen here were much higher in estrus female than males. The concentration values of systemic and especially local and spinal cord levels of PRL could have substantial effects on postoperative pain and hypersensitivity responses following surgical insults. PRL levels seen in estrus females 24 hours post-surgery were significantly higher than those needed to sensitize TRPV1 in nociceptors (20-25 ng/ml; (Diogenes et al., 2006). Since TRPV1 plays a key role in heat hyperalgesia (Caterina et al., 2000), the incision-induced elevation of PRL levels seen in females (but not in males) could contribute to the development of heat hyperalgesia by way of PRL modulation of TRPV1 channel activity. Other channels, such as TRPA1 and TRPM8, could also modulated by PRL in sensory neurons of females and males but this has yet to be studied. Thus, although PRL appears as a possible candidate to modulate heat hyperalgesia after surgical insults by way of a TRPV1 mechanism, PRL mechanisms contributing to postoperative cold hyperalgesia and mechanical hyperalgesia are less well known. It is not clear whether reduction of cold hyperalgesia and mechanical allodynia in PRL KO and PRL-R KO are mediated peripherally or centrally.
Local and systemic elevation of PRL could also have a stimulatory effect on the immune system (Matera, 1996, Tseng et al., 1997). Pro-inflammatory cytokine (IL-1 , IL-6 and TNF) production has been detected after treatment of immune cells with >15-20 ng/ml of PRL (Tseng et al., 1997). It is well documented that these pro-inflammatory cytokines are capable of sensitizing nociceptors and contribute to the development of inflammatory heat and cold hyperalgesia (Woolf et al., 1997, Khan et al., 2008). Even so, modulation of the immune system by increased PRL post-surgery may not explain the peripheral effect of the PRL system on mechanical hyperalgesia since it was present in both females and males, yet increased PRL levels were most notable in operated hind paws of females. Nevertheless, the elevation of local and systemic PRL levels seen in females, but not males, may be sufficient to both sensitize TRPV1 nociceptors and to stimulate the immune system and these combined effects could help explain the mechanism by which the PRL system is capable of producing sex-dependent thermal (heat and cold) hyperalgesia after hidpaw incision.
PRL is a member of the family of class I cytokines (Boutin et al., 1988). Its actions are mediated by the PRL-R, which has two forms, short and long (Kelly et al., 1991, Ginsburg et al., 2012). The PRL-R belongs to the tyrosine-kinase receptor family and can control long-term trophic effects of PRL via Jak/STAT and MAP kinase pathways (Brown et al., 2012). Transient and acute effects of PRL are mediated through PRL-R via PI3-kinase pathways (Bole-Feysot et al., 1998, Lyons et al., 2012). Detailed expression patterns for PRL-R, especially for its short and long forms, in the nociceptive pathway of males and females are not established as of yet. Also unknown is how PRL-R expression is regulated in nociceptors and the pain pathway following surgical trauma and during the postoperative period. Differential expression of PRL-R in pain conditions may be important since direct PRL effects on PRL-R may contribute to neuronal excitability. Such effects are possible since PRL is able to regulate tuberoinfundibular dopamine neuron discharge patterns, suggesting regulation of certain voltage-gated channels by PRL (Lyons et al., 2012). Thus, the short and long forms of the PRL-R represent possible candidates that may be responsible for sex-dependent contributions from the PRL system to pain responses during the postoperative period. This is an important observation, as postoperative conditions lead to sensitization of Aδ-fiber and C-fiber nociceptors and the conversion of silent Aδ nociceptors to mechanically sensitive fibers after incision (Pogatzki et al., 2002, Wu and Raja, 2011).
Surgery induces a significant elevation of PRL in spinal cords (Fig 1D). PRL was measured in whole spinal cords, but not dorsal horns. Besides, the source of the increased PRL is unknown. Possible candidates include non-neuronal cells, such as activated microglia that can release PRL (Moderscheim et al., 2007). Increased PRL levels in the dorsal horns could contribute to the development of hypersensitivity since elevated PRL could facilitate nociceptive transmission at pre- and post-synaptic sites in the spinal cord. For example, TRPV1 and TRPA1 play roles in mechanical allodynia via pre-synaptic mechanisms in the dorsal spinal cord (Patwardhan et al., 2009, Gregus et al., 2012). Hence, surgery elevated PRL in the spinal cord could directly affect mechanical allodynia by modulating these channels. Elevated PRL could also affect the functions of glia such as microglia (Ducret et al., 2002, Moderscheim et al., 2007, Yong, 2009). Thus alterations in the PRL system after surgical insults could contribute to central sensitization and postoperative pain by increasing the efficiency of nociceptive transmission and/or regulating the interaction of neurons and glia in dorsal spinal cord neurons.
In conclusion, the mechanisms that control the regulation of pain responses by PRL and PRL-R are important topics since changes in PRL levels could be present in many types of postoperative surgical conditions associated with increased pain. Further studies on hyperalgesia involving PRL and the PRL-R could uncover new mechanisms underlying sex-dependent pain via pathways that are independent or co-operating with other notable sex hormones such as testosterone, estrogen and progesterone.
Systemic and local up-regulations of incision-induced prolactin are sex-dependent.
Prolactin plays a role in postoperative thermal hyperalgesia in female mice.
Prolactin is involved in postoperative mechanical allodynia in females and males.
Acknowledgements
We thank Jackson Laboratory for providing PRL Ko and PRLR KO female and male mice for our studies and breeding. We thank Dr. Kenneth Hargreaves for advice on experimental procedures. We also thank Jei Li for technical assistance. This work was supported by the National Institute of Dental and Craniofacial Research (R01 DE017696 to A.N.A.). All work was carried out in facilities provided by the UTHSCSA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Anand KJ. The stress response to surgical trauma: from physiological basis to therapeutic implications. Prog Food Nutr Sci. 1986;10:67–132. [PubMed] [Google Scholar]
- Banik RK, Woo YC, Park SS, Brennan TJ. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006;105:1246–1253. doi: 10.1097/00000542-200612000-00025. [DOI] [PubMed] [Google Scholar]
- Barni S, Lissoni P, Mandelli D, Archili C, Real G, Sormani AL, Caprotti R, Tancini G. Relation between surgery-induced prolactin increase and the menstrual cycle phase at time of surgery in premenopausal breast cancer. Int J Biol Markers. 1991;6:103–106. doi: 10.1177/172460089100600204. [DOI] [PubMed] [Google Scholar]
- Berczi I, Nagy E, Asa SL, Kovacs K. The influence of pituitary hormones on adjuvant arthritis. Arthritis Rheum. 1984;27:682–688. doi: 10.1002/art.1780270612. [DOI] [PubMed] [Google Scholar]
- Bernton EW, Meltzer MS, Holaday JW. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988;239:401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- Boutin JM, Jolicoeur C, Okamura H, Gagnon J, Edery M, Shirota M, Banville D, Dusanter-Fourt I, Djiane J, Kelly PA. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988;53:69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Brown RS, Piet R, Herbison AE, Grattan DR. Differential actions of prolactin on electrical activity and intracellular signal transduction in hypothalamic neurons. Endocrinology. 2012;153:2375–2384. doi: 10.1210/en.2011-2005. [DOI] [PubMed] [Google Scholar]
- Butler M, Forte ML, Joglekar SB, Swiontkowski MF, Kane RL. Evidence summary: systematic review of surgical treatments for geriatric hip fractures. J Bone Joint Surg Am. 2011;93:1104–1115. doi: 10.2106/JBJS.J.00296. [DOI] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix. 2009;4 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesi I, Fois M, Franconi F. Sex and gender aspects in anesthetics and pain medication. Handb Exp Pharmacol. 2012:265–278. doi: 10.1007/978-3-642-30726-3_13. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chernow B, Alexander HR, Smallridge RC, Thompson WR, Cook D, Beardsley D, Fink MP, Lake CR, Fletcher JR. Hormonal responses to graded surgical stress. Arch Intern Med. 1987;147:1273–1278. [PubMed] [Google Scholar]
- Clement-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damotte D, Goffin V, Bouchard B, Amling M, Gaillard-Kelly M, Binart N, Baron R, Kelly PA. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology. 1999;140:96–105. doi: 10.1210/endo.140.1.6436. [DOI] [PubMed] [Google Scholar]
- Deumens R, Steyaert A, Forget P, Schubert M, Lavand'homme P, Hermans E, De Kock M. Prevention of chronic postoperative pain: Cellular, molecular, and clinical insights for mechanism-based treatment approaches. Prog Neurobiol. 2013 doi: 10.1016/j.pneurobio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26:8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret T, Boudina S, Sorin B, Vacher AM, Gourdou I, Liguoro D, Guerin J, Bresson-Bepoldin L, Vacher P. Effects of prolactin on intracellular calcium concentration and cell proliferation in human glioma cells. Glia. 2002;38:200–214. doi: 10.1002/glia.10056. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg E, Alexander S, Lieber S, Tarplin S, Jenkins L, Pang L, Heger CD, Goldsmith P, Vonderhaar BK. Characterization of ductal and lobular breast carcinomas using novel prolactin receptor isoform specific antibodies. BMC Cancer. 2012;10:678. doi: 10.1186/1471-2407-10-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo E, Hinchado MD, Garcia JJ, Ortega E. Influence of gender and oral contraceptives intake on innate and inflammatory response. Role of neuroendocrine factors. Mol Cell Biochem. 2008;313:147–153. doi: 10.1007/s11010-008-9752-2. [DOI] [PubMed] [Google Scholar]
- Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci U S A. 2012;109:6721–6726. doi: 10.1073/pnas.1110460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. The EMBO journal. 1997;16:6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimena P, Aguirre MA, Lopez-Curbelo A, de Andres M, Garcia-Courtay C, Cuadrado MJ. Prolactin levels in patients with systemic lupus erythematosus: a case controlled study. Lupus. 1998;7:383–386. doi: 10.1191/096120398678920361. [DOI] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Djiane J, Postel-Vinay MC, Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991;12:235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Khan AA, Diogenes A, Jeske NA, Henry MA, Akopian A, Hargreaves KM. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience. 2008;155:503–509. doi: 10.1016/j.neuroscience.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Lyons DJ, Hellysaz A, Broberger C. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: novel feedback control mechanisms in the lactotrophic axis. J Neurosci. 2012;32:8074–8083. doi: 10.1523/JNEUROSCI.0129-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kahn WR, Eisenach JC. Abdominal surgery decreases food-reinforced operant responding in rats: relevance of incisional pain. Anesthesiology. 2005;103:629–637. doi: 10.1097/00000542-200509000-00028. [DOI] [PubMed] [Google Scholar]
- Mateo L, Nolla JM, Bonnin MR, Navarro MA, Roig-Escofet D. High serum prolactin levels in men with rheumatoid arthritis. J Rheumatol. 1998;25:2077–2082. [PubMed] [Google Scholar]
- Matera L. Endocrine, paracrine and autocrine actions of prolactin on immune cells. Life Sci. 1996;59:599–614. doi: 10.1016/0024-3205(96)00225-1. [DOI] [PubMed] [Google Scholar]
- Moderscheim TA, Gorba T, Pathipati P, Kokay IC, Grattan DR, Williams CE, Scheepens A. Prolactin is involved in glial responses following a focal injury to the juvenile rat brain. Neuroscience. 2007;145:963–973. doi: 10.1016/j.neuroscience.2006.12.053. [DOI] [PubMed] [Google Scholar]
- Moore CM, Desborough JP, Powell H, Burrin JM, Hall GM. Effects of extradural anaesthesia on interleukin-6 and acute phase response to surgery. Br J Anaesth. 1994;72:272–279. doi: 10.1093/bja/72.3.272. [DOI] [PubMed] [Google Scholar]
- Noel GL, Suh HK, Stone JG, Frantz AG. Human prolactin and growth hormone release during surgery and other conditions of stress. J Clin Endocrinol Metab. 1972;35:840–851. doi: 10.1210/jcem-35-6-840. [DOI] [PubMed] [Google Scholar]
- Noreng MF, Jensen P, Tjellden NU. Per- and postoperative changes in the concentration of serum thyreotropin under general anaesthesia, compared to general anaesthesia with epidural analgesia. Acta Anaesthesiol Scand. 1987;31:292–294. doi: 10.1111/j.1399-6576.1987.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes & development. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DM. JCAHO pain management standards are unveiled. Joint Commission on Accreditation of Healthcare Organizations. JAMA. 2000;284:428–429. doi: 10.1001/jama.284.4.423b. [DOI] [PubMed] [Google Scholar]
- Pogatzki-Zahn EM, Zahn PK, Brennan TJ. Postoperative pain--clinical implications of basic research. Best Pract Res Clin Anaesthesiol. 2007;21:3–13. doi: 10.1016/j.bpa.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. Journal of neurophysiology. 2002;87:721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023–1027. doi: 10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- Raghunathan K, Pyati S. Persistent postsurgical pain--need for better methods. Reg Anesth Pain Med. 2013;38:73–74. doi: 10.1097/AAP.0b013e318276651c. [DOI] [PubMed] [Google Scholar]
- Reiner Z, Oreskovic M, Ribaric K. Endocrine responses to head and neck surgery in men. Acta Otolaryngol. 1987;103:665–668. [PubMed] [Google Scholar]
- Reiz S, Haggmark S, Johansson G, Nath S. Cardiotoxicity of ropivacaine--a new amide local anaesthetic agent. Acta Anaesthesiol Scand. 1989;33:93–98. doi: 10.1111/j.1399-6576.1989.tb02868.x. [DOI] [PubMed] [Google Scholar]
- Scotland PE, Patil M, Belugin S, Henry MA, Goffin V, Hargreaves KM, Akopian AN. Endogenous prolactin generated during peripheral inflammation contributes to thermal hyperalgesia. The European journal of neuroscience. 2011;34:745–754. doi: 10.1111/j.1460-9568.2011.07788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyaert A, De Kock M. Chronic postsurgical pain. Curr Opin Anaesthesiol. 2012;25:584–588. doi: 10.1097/ACO.0b013e32835743b7. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kessler MA, Schuler LA. Regulation of interleukin (IL)-1alpha, IL-1beta, and IL-6 expression by growth hormone and prolactin in bovine thymic stromal cells. Mol Cell Endocrinol. 1997;128:117–127. doi: 10.1016/s0303-7207(97)04028-8. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–2225. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- Yardeni IZ, Shavit Y, Bessler H, Mayburd E, Grinevich G, Beilin B. Comparison of postoperative pain management techniques on endocrine response to surgery: a randomised controlled trial. Int J Surg. 2007;5:239–243. doi: 10.1016/j.ijsu.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Yong VW. Prospects of repair in multiple sclerosis. J Neurol Sci 277 Suppl. 2009;1:S16–18. doi: 10.1016/S0022-510X(09)70006-1. [DOI] [PubMed] [Google Scholar]