Abstract
Biopolymer hydrogels are important materials for wound healing and cell culture applications. While current synthetic polymer hydrogels have excellent biocompatibility and are non-toxic, typically, they primarily function as a passive matrix that does not supply any additional bioactivity. Chitosan (CS) and pectin (Pec) are natural polymers with active properties that are desirable for wound healing. Unfortunately, previous CS:Pec materials have been limited by harsh acidic synthesis conditions. In this study, a thermoreversible hydrogel has been synthesized from a mixture of chitosan and pectin at biologically compatible conditions. We found that salt could be used to suppress long-range electrostatic interactions to generate a thermoreversible hydrogel that has temperature-sensitive gelation. Both the hydrogel and solution phases are highly elastic, with a power law index of close to −1. Dried hydrogels rapidly swelled to incorporate 2.7 times their weight in phosphate buffered saline solution. As a proof of concept, we removed the salt from our hydrogels, thus creating thick and easy to cast polyelectrolyte complex hydrogels, which proved to be compatible with human stem cells. We suggest that our development of an acid free CS:Pec hydrogel system that has excellent exudate uptake, holds potential for wound healing bandages.
Keywords: chitosan, hydrogel, pectin, rheology, stem cells, wound healing
Graphical abstract
INTRODUCTION
Biopolymer hydrogels are important materials for wound healing and cell culture applications.1,2 While modern synthetic polymer hydrogels are nontoxic and have excellent biocompatibility, their primary function is usually as a passive support matrix which does not supply any additional bioactivity.2 The biopolymer chitosan (CS) is the deacetylated derivative of chitin, the second most abundant polysaccharide. CS is commonly used in hydrogels due to its non-toxic, antibacterial, biodegradable, and biocompatible nature; these factors make CS hydrogels highly desirable products.2–8
CS is a polycation that requires being crosslinking in order to form structures with sufficient chemical stability and mechanical properties. Unfortunately, most commonly utilized crosslinking agents – glutaraldehyde,9 carbodiimide,10 and diphenylphosphoryl azide11 – are cytotoxic. Greener options like sodium tripolyphosphate12,13 and genipin3,14 are non-toxic, but do not contribute any active functionality. Polymer-polymer gelation featuring a polycation and a polyanion has also been utilized to effectively crosslink CS-based wound healing hydrogels.5,15 However, similar to the small molecules previously noted, most of these hydrogels have relied on passive polyanions. For example, alginate, a biocompatible polysaccharide, is the most common polyanion because it is hydrophilic and enables good exudate uptake.15,16 However, alginate has been reported to have a pro-inflammatory effect.17 This makes finding an alternative active polyanion desirable.
An underexplored polyanion that has promising anti-inflammatory18,19 properties is pectin (Pec). Pec is a hetro-polysaccharide derived from plant cell walls20 that is primarily comprised of partially esterified galacturonic acid residues, rhamnose residues, and a variety of sugar residues. The methyl-esterified and ethyl-esterified galacturonic acid residues are prone to hydrogen-bonding interactions and are unable to interact electrostatically. Due to this fact, the degree of esterification (DE) is an important physical characteristic of Pec varieties.20 Pec is a weak polyelectrolyte that deprotonates above a pH value of approximately 3.5.21 The high hydrophilicity of Pec is similar to that of other wound healing polyanions, but it has been reported to have an additional strong anti-inflammatory effect.19 This effect is very desirable for burns and chronic diabetic wounds. The strong anti-inflammatory activity is due to Pec’s high level of esterified galacturonic acid residues which suppress the expression of iNOS and COX-2, two of the most important enzymes in the inflammation process.18,19
Previously, the electrostatic interactions between CS and Pec have been used to generate a variety of structures, including millibeads,22 microbeads,23–25 nanoparticles,13 and thin films.5 Generating flat surfaces through CS:Pec electrostatic interactions requires long coalescence times and yields rough and irregular membranes.5 To-date, only one manuscript, by Norby et al.4, has demonstrated that thermoreversible CS:Pec hydrogels could be synthesized. They used 1 M hydrochloric acid (HCl) to protonate the Pec and enable gelation and hydrogen bonding between the two biopolymers.4 However, the high level of acid used is not conducive to cell culture and wound healing applications; a hydrogel synthesized under physiological conditions would be very desirable.
In this study, we characterized the rheological properties of a new CS:Pec hydrogel system, explored the rehydration of the hydrogels, and demonstrate the hydrogels’ compatibility with mammalian cells. By using salt to suppress long-range electrostatic interactions, we generated a thermoreversible hydrogel that has temperature-sensitive gelation. Changing from the previously demonstrated acid-based system4 to a salt-based system yields hydrogels that are much closer to physiological conditions immediately after synthesis. Using rheology, we analyzed the tan δ, the ratio of the dynamic moduli, and determined the gel point using the Winter-Chambon26 method. As a proof of concept, we removed the salt from our hydrogels, thus creating thick and easy to cast polyelectrolyte complex (PEC) hydrogels, which were previously limited by phase seperation.5,23–25 Our hydrogel system improves the biological compatibility of thermoreversible CS:Pec hydrogels and provides an easy to work with PEC hydrogel.
EXPERIMENTAL
Materials and Chemicals
Medium molecular weight chitosan (CS, poly(D-glucosamine)), pectin from citrus peel (Pec, galacturonic acid content ≥74%), ReagentPlus® grade acetic acid (AA, ≥99.0%), anhydrous sodium acetate (≥99.0%), sodium hexametaphosphate (65–70% P2O5), and bovine serum albumin (BSA, ≥98.0%) were obtained from Sigma-Aldrich (St. Louis, MO). Certified ACS grade sodium chloride (≥99.0%) and ACS plus grade hydrochloric acid (HCl, 12.1 N) were obtained from Fisher Scientific (Fair Lawn, NJ). Deionized (DI) water was obtained from a Barnstead Nanopure Infinity water purification system (Thermo Fisher Scientific, Waltham, WA).
Characterization of Chitosan and Pectin
The degree of acetylation of the CS was analyzed using a proton nuclear magnetic resonance (1H-NMR) method outlined in Fernandez-Megia et al.27, which is a modified version of the method originally outlined by Hirai et al.28 Briefly, CS was solvated in 2% DCl until the solution was clear and then allowed to cool. Spectra were recorded on an Avance 400 NMR spectrometer (Bruker, Billerica, MA) at 27 °C. The integrals of the acetyl groups and H2–H6 protons28,29 were compared to obtain a degree of acetylation.
The intrinsic viscosities of the polymers were determined using a capillary viscometry method. Dilute solutions of CS (0.2 M sodium chloride and 0.l M AA) and Pec (1 wt. % sodium hexametaphosphate) were measured at varying concentrations to obtain an intrinsic viscosity. The molecular weight was determined for CS using the Mark-Houwink parameters of K = 1.8 × 10−3 cm3 g−1 and a = 0.9330, whereas for Pec K = 9.55 × 10−2 cm3 g−1 and a = 0.73.4 The degree of esterification of the Pec was analyzed using a titration method outlined in the Food Chemicals Codex31. Pec is titrated to an end point using 0.1 M NaOH, saponified, and titrated to the end point again. The ratio of the two titers provides the ratio of esterified galacturonic acid to free galacturonic acid.
Preparation of CS:Pec Hydrogels
CS and Pec stock solutions were prepared at a polymer concentration of 1.5 wt. %. CS stock solutions were prepared by mixing in 0.1 M AA at 60 °C for 12 h. Pec stock solutions were prepared by mixing in DI water at room temperature (25 °C) for 12 h. Samples were stored at 4 °C until use in hydrogel preparation. Hydrogel samples were prepared by mixing 10 mL of CS stock solution with a measured amount of sodium chloride. A 2 mL volume of dilute HCl was added to bring the hydrogel to the desired acid concentration. The solution was then heated until it cleared. Pec solution (10 mL) was added, and the solution was heated to 97 °C. Samples were stored at 4 °C.
Characterization of Rheology and Swelling of Chitosan:Pectin Hydrogels
Small amplitude oscillatory shear measurements were performed in a Kinexus Pro rheometer (Malvern Instruments, UK) using a concentric cylinder geometry with a diameter of 25 mm and horizontal gap of 1 mm, run with a vertical gap of 1 mm. The sample was injected into the cell at 80 °C, the geometry was lowered into position, and the top of the geometry was sealed using mineral oil to prevent solvent evaporation. A strain amplitude sweep was performed to ensure that experiments were conducted within the linear viscoelastic region and a strain percent of 5% was selected. Oscillation frequency sweeps were conducted over an angular frequency domain. The measuring unit utilizes a temperature control unit (Peltier cylinder cartridge) to achieve rapid temperature changes and ±0.01 °C temperature control. The sample was allowed to gel for 4 h at 25 °C before measurement commenced, and was given 20 min to equilibrate at each temperature. Additional samples were run with 1 h thermal equilibration times over a narrower range to corroborate the results from the 20 min runs. There were no signs of degradation over the course of repeated oscillatory shear measurements at varying temperatures. No signs of hysteresis were found when the sample was heated or cooled, which demonstrated the reversibility of the gelation.
Samples used for swell testing were reheated until liquid and cast into 13 × 13 mm square petri dishes and allowed to gel. Discs with a diameter of 2.56 cm were then cut using a Spearhead 130 punch set (Zimmerman Packing and MFG., Cincinnati, Ohio) and placed on parafilm to dry in a fume hood for 6 days. Hydrogels were then removed from parafilm, weighed, and placed in 100 mL of phosphate buffered saline (pH 6.0 or 7.4) for a set period of time and removed, gently wiped dry, and weighed. The hydrogel degree of swelling was equal to (mf − mi)/mi × 100%, where degree of swelling in % is calculated from initial (mi) and final (mf) weights of the hydrogel.
Characterization of Cell Compatibility with Chitosan:Pectin Hydrogels
Telomerase-modified (hTERT), human marrow-derived stem cells (MSCs), a generous gift from Linda Griffith (Massachusetts Institute of Technology), were routinely cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 1% non-essential amino acids, 1% L-glutamine, and 1% sodium pyruvate at 37 °C and 5% CO2. All cell culture supplies were purchased from Life Technologies (Carlsbad, CA).
Sterile hydrogels were prepared by repeating the hydrogel synthesis protocol under sterile conditions. Each well of a 12-well plate was filled with 1.3 mL of sterile liquid hydrogel precursor by pipette. The plates were left to dry in a laminar flow hood for 2 days. Non-rinsed hydrogels were used immediately. Individual well plates containing rinsed hydrogels were rinsed twice in 500 ml sterile DI water before use. Rat-tail Collagen 1 (Life Technologies, Carlsbad, CA) was then passively adsorbed to hydrogels at 1 µg/cm2 at room temperature overnight. Hydrogels were rinsed four times in sterile phosphate-buffered saline, UV-sterilized for 6–12 hr, then incubated with complete cell culture medium overnight. MSCs were seeded onto the hydrogel surfaces at 10,000 cells/cm2 and the medium was changed every 2 days for 14 days. At days 1, 4, 7, 10, and 14, live cells were stained with 4 µM ethidium homodimer-1 (nuclear stain to indicate dead cells) and 2 µM calcein AM (cytosolic stain to stain all cells) (Life Technologies, Carlsbad, CA) in serum free medium for 30 min. Imaging was performed using a Zeiss Spinning Disc Cell Observer SD (Zeiss, Jenna, Germany) at 20× magnification. Area of cell spheroids was quantified via manual tracing in Image J 1.48p software (National Institutes of Health, Bethesda, MD). N = 2 independent biological replicates were performed and quantified.
Protein absorption was quantified by pipetting 100 µL of sterile hydrogel precursor into 96 well plates. These gels were dried and a subset were rinsed, as previously described. BSA concentrations ranging from 0–1250 µg/cm2 were passively absorbed to the hydrogel surfaces at room temperature for 24 h on a rotator. Hydrogels were rinsed four times with phosphate-buffered saline, 250 µL Bradford reagent (Pierce Biotechnology, Rockford, IL) was added per well, and absorption at 600 nm was read (Biotech ELx800, 783 Winooski, VT, USA) after 5 min incubation. N = 2 independent replicates were performed and quantified. Data was normalized to the blank for each hydrogel condition.
Statistical analysis was performed using Prism v6.0b. Data are reported as mean ± standard error. Statistical significance was evaluated using an unpaired, two-tailed t-test. < 0.05 was considered statistically significant. p < 0.05 is denoted with *, ≤ 0.01 with **, ≤ 0.001 with ***, and ≤ 0.0001 with ****; p ≥ 0.05 is considered not significant (‘ns’).
RESULTS AND DISCUSSION
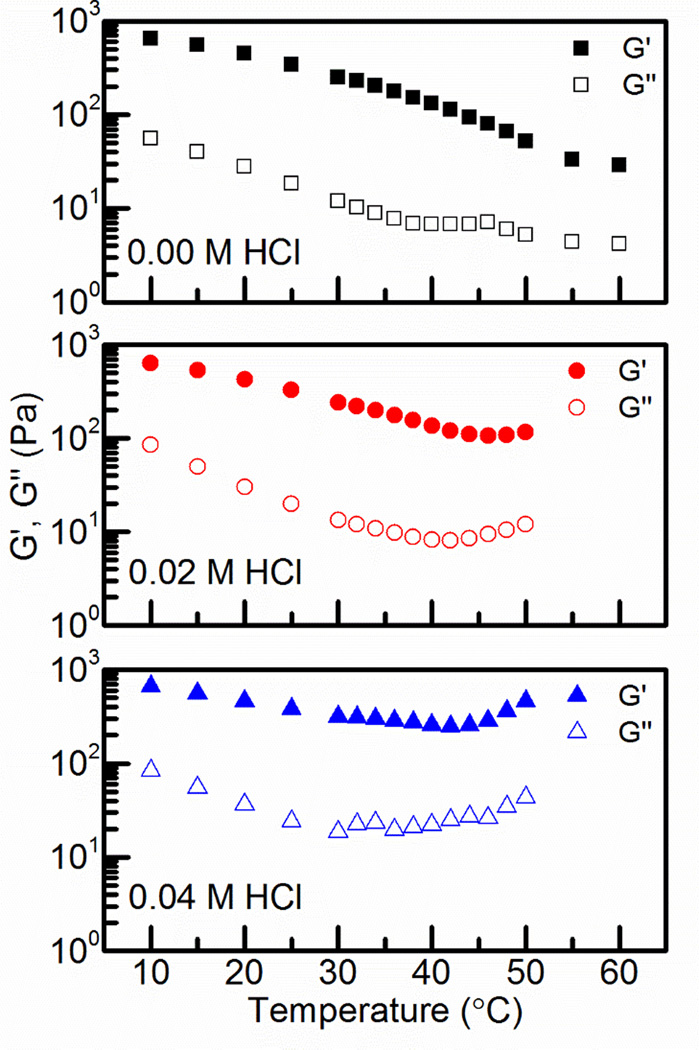
After successfully synthesizing CS:Pec hydrogels, samples were physically characterized using three methods. The first of these was bulk rheology to determine the strength and temperature response of the hydrogel systems. Temperature had a strong effect on the viscoelastic properties of the CS:Pec hydrogels at three HCl concentrations (0.00 M, 0.02 M, 0.04 M), a fixed ionic strength of 1.050 M, and a total polymer concentration of 1.5 wt. %, Figure 1. The elastic moduli dominates throughout, though the gap between the elastic and viscous moduli varies with temperatures. The elastically dominated behavior is common and implies that the material is a fairly elastic pseudo-plastic.4 The decreasing gap between the elastic and viscous moduli at high temperatures suggests that there is a phase transition that does not overcome the naturally elastic behavior of the heated CS:Pec solution.4,32
Figure 1.
Temperature dependence of the elastic and viscous moduli for aqueous mixtures of CS:Pec (top-to-bottom) in 0.00, 0.02, and 0.04 M HCl. A fixed frequency of 6.28 rad/s was used.
The rheological properties of hydrogels can also provide important insight into their potential end uses. In cases where a soft solid is involved, small amplitude oscillatory shear provides extensive rheological data without destroying the interior structures. The storage (G’) and loss moduli (G”) of the material help to illuminate the elastic and viscous natures of the hydrogel. The ratio of these moduli, tan δ, is the ratio of lost energy to stored energy, and is a valuable measure of material phase.4,33
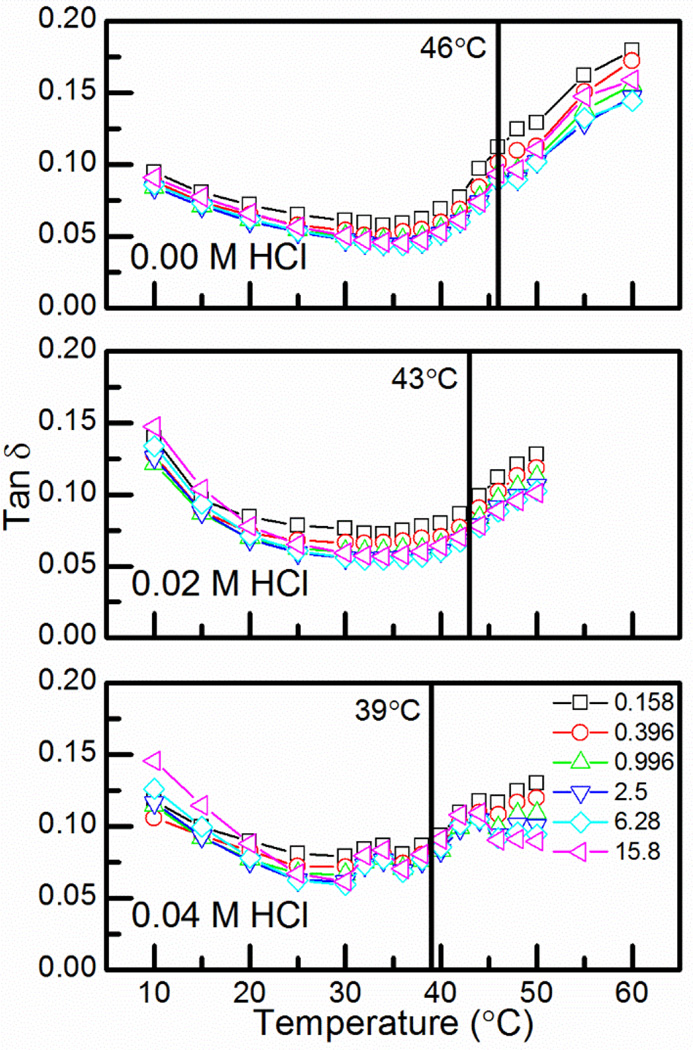
The Winter-Chambon method was utilized to quantify gelation temperatures for increasing acid concentrations.26 This method defines the gelation point as the point where the tan δ (the viscous modulus over the elastic modulus) values become frequency independent. This phenomenon is demonstrated in Figure 2, which shows the increase in the spread of tan δ with temperature. Tan δ does not collapse into total frequency independence, but does exhibit two distinct phases, a hydrogel phase with very low frequency dependence and a solution phase with higher frequency dependence. The gelation temperature has been found to be accurate (±1 °C) by close inspection of the data. The observed gelation temperature displays no sign of hysteresis: cooling and heating produces the same result. Results are independent of temperature change direction and magnitude.
Figure 2.
Temperature dependence of tan δ for CS:Pec (top-to-bottom) in 0.00, 0.02, and 0.04 M HCl. Gelation temperatures are shown as vertical black lines as calculated using the range of tan delta from the raw data from at least three runs.
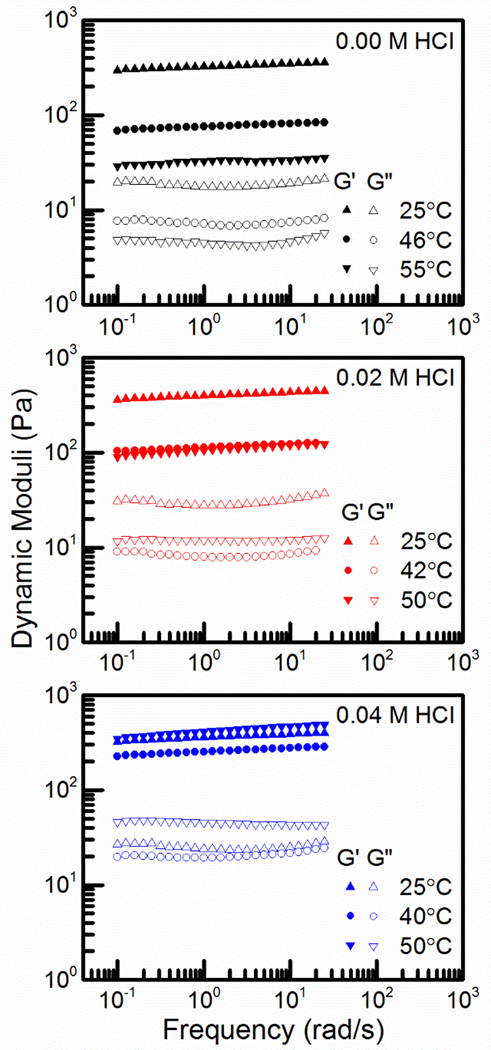
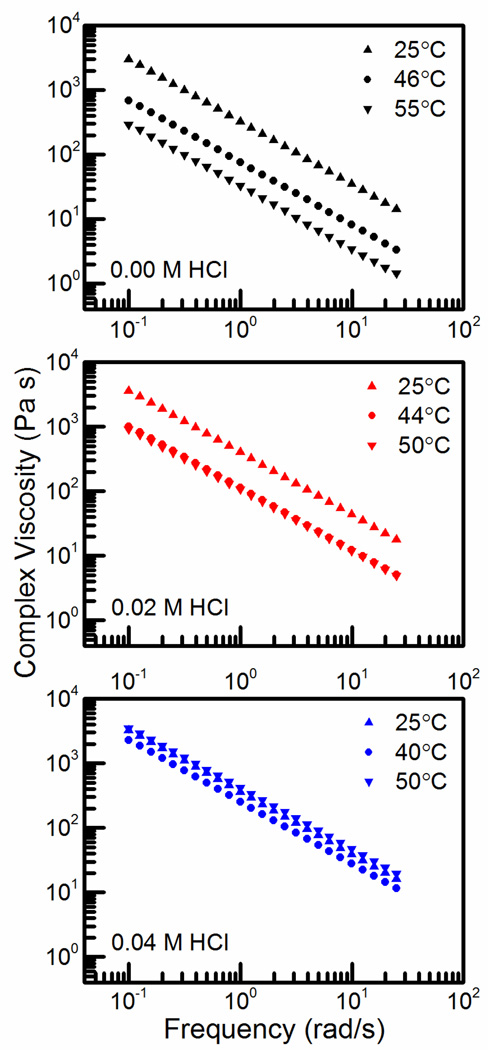
Figure 3 further demonstrates the frequency independence of the elastic moduli regardless of temperature or acid concentration. In all cases, the frequency dependence of the dynamic moduli increases as the temperature is near or above the gelation temperature. G’ remains above G” at all temperatures and the two lines remain parallel in most cases. When the complex viscosity is analyzed, Figure 4, the results are similar to the results from the dynamic moduli. The slopes of every line is very close to −1, meaning that even the solution phase is relatively elastic with a low level of viscous deformation. There is a strong temperature dependence in both the hydrogel and solution phases, however this effect is diminished by increasing the acid concentration. The similarity of behavior amongst the systems means that acid content can be fully minimized to 0.00 M HCl without losing thermoreversibility. Small amounts of acid can also be used to tune the gelation temperature very sensitively.
Figure 3.
Frequency dependence of the dynamic moduli for CS:Pec (top-to-bottom) in 0.00, 0.02, and 0.04 M HCl below the gelation temperature (25 °C), around the gelation temperature (46, 42, 40 °C), and above the gelation temperature (55, 50 °C).
Figure 4.
Frequency dependence of complex viscosity for CS:PEC in (top-to-bottom) 0.00, 0.02, and 0.04 M HCl below gelation temperature (25 °C), at gelation temperature (46, 42, 40 °C), and above gelation temperature (55, 50 °C).
The dynamic moduli and complex viscosity of an incipient hydrogel can be described by a simple power law where is described by equation 1, equation 2, and equation 3.4,26,32 The dynamic viscosity is defined by the Legendre gamma function (Γ(n)), the viscoelastic exponent (n), and the hydrogel strength parameter (s). The hydrogel strength parameter relates the molecular chain flexibility and the cross-linking density of the hydrogel.4 As we can see from equations 1 and 3, when the elastic modulus is frequency independent (n = 0), the complex viscosity should have a slope of −1.
| (1) |
| (2) |
| (3) |
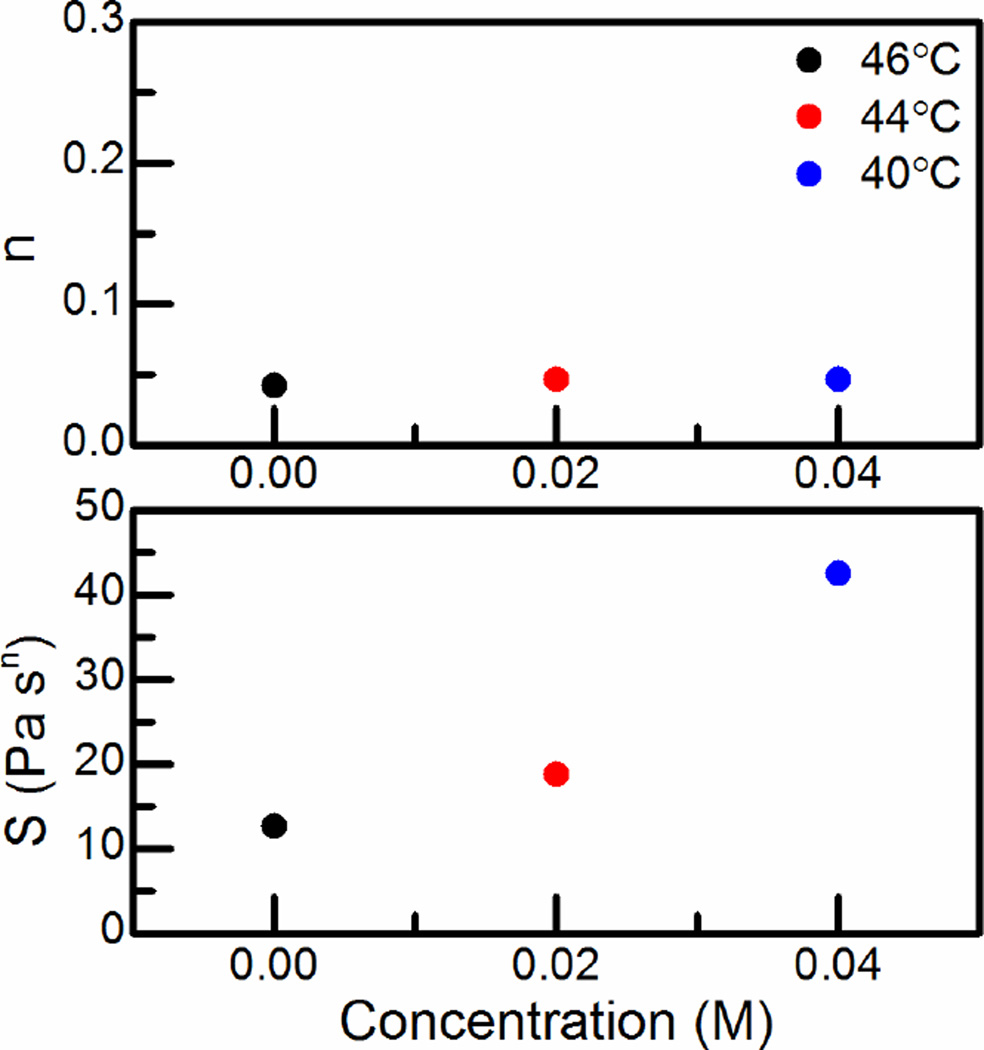
For each CS:Pec acid system’s gelation temperature, power law fits were obtained for the complex viscosities in Figure 4, and values for and were calculated, Figure 5. The incipient hydrogels remain almost entirely frequency independent (n ~0.05) regardless of acid concentration. However, the hydrogel strength changes strongly with acid concentration. This effect could be explained by a decrease in the density of hydrogen-bonding crosslinks as Pec protonates, or the result of the chain stiffness of CS and Pec changing as the pH increases.4
Figure 5.
The viscoelastic exponent, n, and the hydrogel strength parameter, S, for CS:PEC (top-to-bottom) in 0.00, 0.02, and 0.04 M HCl at gelation temperatures (46, 42, 40 °C).
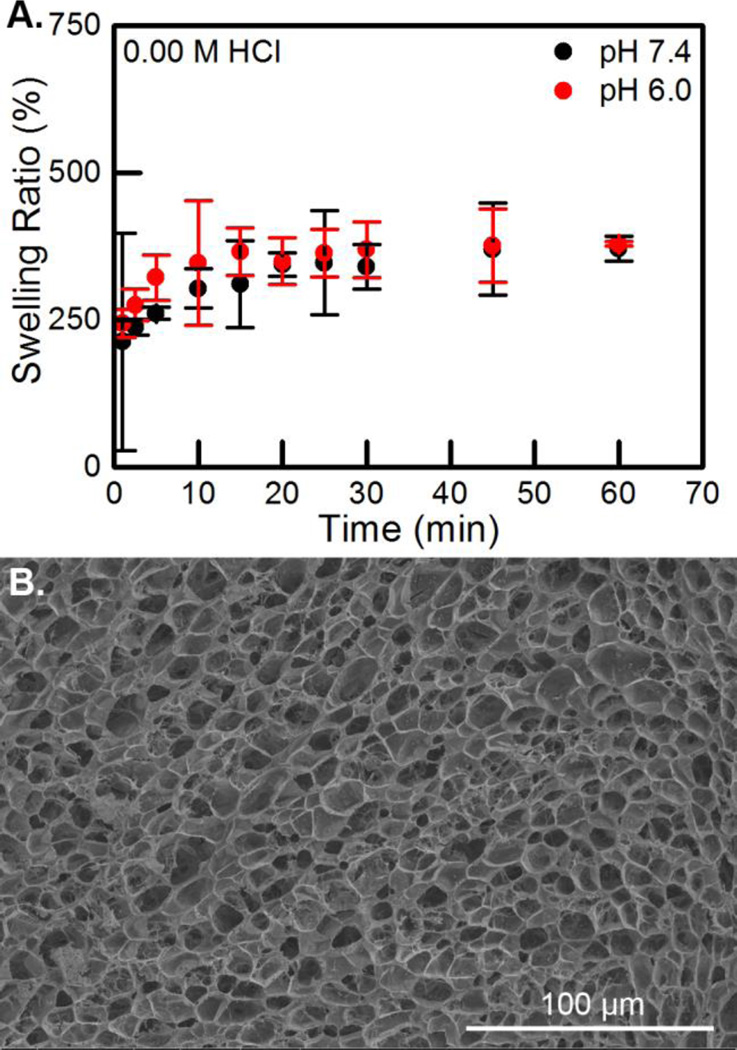
The other two characterization methods, swell testing and SEM, were used to gain insight into the ability of the hydrogel to uptake fluid and release loaded agents. The as-synthesized hydrogels had a theoretical swelling ratio of ~6,600%. However, when the hydrogels were systematically dried and then swollen, they were not able to take up anywhere near that amount of water. Figure 6 displays that dried hydrogels absorbed their maximum amount of phosphate buffered saline solution quickly, within 15 min. This is consistent with previously studied CS:Pec hydrogel systems.5 The swelling was not affected by the pH: after 1 hr, at pH values of 7.4 and 6.0 the CS:Pec hydrogels swelled to 370 ± 20% and 370 ± 4%, respectively, which is consistent with previous CS:Pec hydrogels.5 The two rehydration curves are statistically identical with the exception of the 5 min time points. SEM micrographs reveal that the hydrogel contains a wide range of pore sizes prior, some as large as 30 µm. Due to the high variability in pore size, swell testing data was extremely variable. It is likely that the statistical significance in the 5 min time point is not indicative of a real difference. It is likely that as swelling ratio decreased with drying and rehydration that pore size did as well.34 Even so, these large pores should allow the loading and release of even very large proteins. The rapid swelling, high equilibrium swelling percent, and large pore size suggest that our hydrogel system may be appropriate for a wound dressing since it can adsorb large volumes of exudate.
Figure 6.
(A) Swelling versus time for 0.00 M HCl CS:Pec hydrogels at pH values of 7.4 and 6.0. (B) SEM micrograph displays the cross-section of a lyophilized 0.00 M HCl CS:Pec hydrogel, the scale bar is 100 µm.
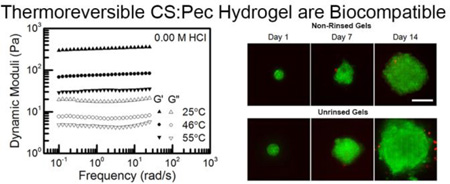
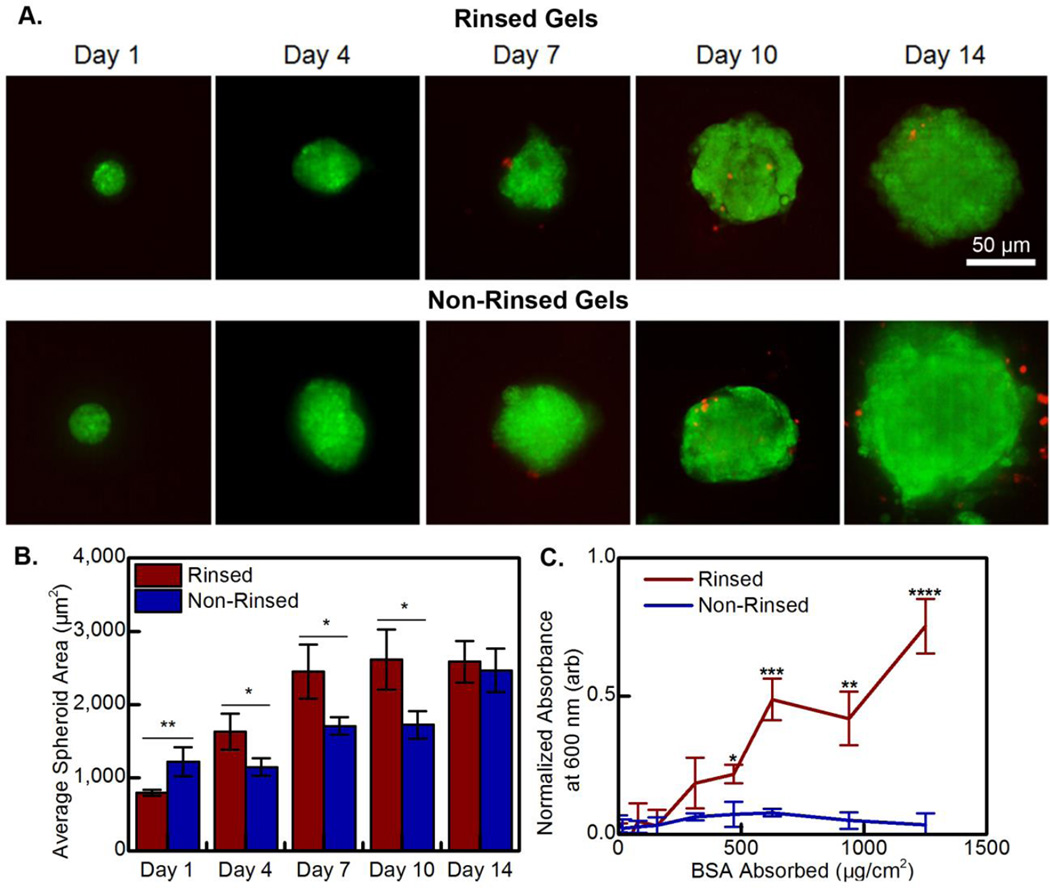
After fully characterizing the physical properties of the hydrogel, the system’s compatibility with cells in physiological conditions was explored. Human mesenchymal stem cells were used due to their extensive role in wound healing.35 We theorized that the high salt level in the dried hydrogels would result in cell death, so a rinsing procedure was implemented and both rinsed and non-rinsed hydrogels were tested for cell viability. Notably, Figure 7A displays that no significant cell death occurred as a result of cell contact with either hydrogel system over a 14 day period. Even with passive absorption of an adhesive ligand for cell adhesion, there was a low level of adhesion36 to the hydrogels. Thus, the cells did not adhere and spread. Instead, the cells adhered to other cells, and formed spheroids, which has been previously reported for chitosan-based hydrogels.37 At 1 day after seeding, spheroid size was greater on the non-rinsed gels, but there were fewer of these spheroids (data not shown). This led us to explore the possibility that the hydrogels differed in their ability to absorb proteins, resulting in the differential cell spheroid formation. Thus, BSA absorption was quantified, and the results are provided in Figure 7C. The non-rinsed gels absorbed less protein, likely resulting in the poor cell adhesion. The increased protein absorption we observe after rinsing is likely due to the removal of charge screening ions. However, these differences were only observed at protein concentrations significantly higher than the passively absorbed collagen or serum we introduced, explaining both the minimal differences in morphology and the lack of cell spreading that we observed in both systems.
Figure 7.
(A) Representative micrographs taken of hTERT MSC spheroids cultured on (top) rinsed and (bottom) non-rinsed 0.00 M CS:PEC hydrogels over a 14 day compatibility test. Cells are stained with live (green, Calcein AM) and dead (red, Ethidium homodimer-1) stains. (B) Average spheroid area is quantified as a function of time. (C) The passive absorption of BSA to the rinsed and non-rinsed 0.00 M CS:PEC hydrogels is provided.
We were interested in the ability of the hydrogels to promote long-term cell survival and proliferation. MSC spheroids were heterogeneous and increased in size over the 14 day culture period, but initially grew larger more quickly on the rinsed hydrogels, Figure 7B. The initial difference in spheroid growth rate tapered off and disappeared by day 14, likely due to salt leaching out from the non-rinsed hydrogels during media replacement. The low level of cell adhesion and lack of cell death over 14 days of culture shows that the hydrogels are both safe for physiological use and likely will not stick to wounds if used as a bandage, aiding the changing of dressings. The difference between the rinsed and non-rinsed gels can be partially explained by the differences in protein uptake, but it is likely that the removal of charge screening ions by rinsing also has some effect on gel stiffness and morphology. Neural stem cells have also been shown to form spheroids on chitosan films.38 However, keratinocytes, fibroblasts, and epithelial cells have shown good attachment and do not usually form spheroids.39,40 Even among very similar chitosan containing systems, physical properties can induce spheroid formation through a combination of factors.41 Further study is needed to determine if the spheroid behavior is due to the cell type used or the physical properties of the CS:Pec hydrogels.
CONCLUSION
In this paper, we have described the synthesis of a biologically compatible thermoreversible CS:PEC hydrogel and have fully characterized its physical properties. We examined the effects of three acid concentrations, 0.00, 0.02, and 0.04 M HCl, before determining that hydrogel strength and hydrogel temperature were both improved by fully replacing the acid content with salt. These hydrogels were found to continue to strengthen at lower temperatures. The dried hydrogels rapidly swelled to ~370% of their initial weight at pH values of 6.0 and 7.4. Our acid-free CS:PEC hydrogels were found to be biocompatible after drying, although protein absorption and cell proliferation were improved by rinsing. This system is simple, novel, and should prove a promising potential bandage for chronic wound healing.
Acknowledgments
Thanks to Dr. H. Henning Winters and Brian Momani for their rheological expertise. This work was partially funded by the James M. Douglas Career Development Faculty Award, start-up funds from the University of Massachusetts Amherst, and a grant from the NSF and NCI (DMR-1234852). SRP is a Pew Biomedical Scholar supported by the Pew Charitable Trusts. LEB was partially supported by National Research Service Award T32 GM008515 from the National Institutes of Health. We acknowledge the use of facilities at the W.M. Keck Center for Electron Microscopy.
REFERENCES
- 1.Rieger KA, Birch NP, Schiffman JD. J. Mater. Chem. B. 2013;1(36):4531–4541. doi: 10.1039/c3tb20795a. [DOI] [PubMed] [Google Scholar]
- 2.Varghese S, Elisseeff J. Polym. Regen. Med. 2006 Jan;203:95–144. [Google Scholar]
- 3.Moura MJ, Figueiredo MM, Gil MH. Biomacromolecules. 2007;8(12):3823–3829. doi: 10.1021/bm700762w. [DOI] [PubMed] [Google Scholar]
- 4.Nordby MH, Kjoniksen AL, Nystrom B, Roots J. Biomacromolecules. 2003;4(2):337–343. doi: 10.1021/bm020107+. [DOI] [PubMed] [Google Scholar]
- 5.Bernabé P, Peniche C, Argüelles-Monal W, Bernabe P, Arguelles-Monal W. Polym. Bull. 2005;55(5):367–375. [Google Scholar]
- 6.Zhang H, Oh M, Allen C, Kumacheva E. Biomacromolecules. 2004;5(6):2461–2468. doi: 10.1021/bm0496211. [DOI] [PubMed] [Google Scholar]
- 7.Morris GA, Kok MS, Harding SE, Adams GG. Biotechnol. Genet. Eng. Rev. 2010;27:257–283. doi: 10.1080/02648725.2010.10648153. [DOI] [PubMed] [Google Scholar]
- 8.Kong M, Chen XG, Xing K, Park HJ. Int. J. Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Xing K, Chen XG, Kong M, Liu CS, Cha DS, Park HJ. Carbohydr. Polym. 2009;76(1):17–22. [Google Scholar]
- 10.Ulubayram K, Aksu E, Gurhan SID, Serbetci K, Hasirci N. J. Biomater. Sci. Polym. Ed. 2002;13(11):1203–1219. doi: 10.1163/156856202320892966. [DOI] [PubMed] [Google Scholar]
- 11.Marinucci L, Lilli C, Guerra M, Belcastro S, Becchetti E, Stabellini G, Calvi EM, Locci P. J. Biomed. Mater. Res. A. 2003;67(2):504–509. doi: 10.1002/jbm.a.10082. [DOI] [PubMed] [Google Scholar]
- 12.Jonassen H, Kjøniksen A-L, Hiorth M. Biomacromolecules. 2012;13(11):3747–3756. doi: 10.1021/bm301207a. [DOI] [PubMed] [Google Scholar]
- 13.Birch NP, Schiffman JD. Langmuir. 2014;30(12):3441–3447. doi: 10.1021/la500491c. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Y, Chesnutt BM, Utturkar G, Haggard WO, Yang Y, Ong JL, Bumgardner JD. Carbohydr. Polym. 2007;68(3):561–567. [Google Scholar]
- 15.Lee Y-H, Chang J-J, Yang M-C, Chien C-T, Lai W-F. Carbohydr. Polym. 2012;88:809–819. [Google Scholar]
- 16.Wang L, Khor E, Wee A, Lim LY. J. Biomed. Mater. Res. 2002;63(5):610–618. doi: 10.1002/jbm.10382. [DOI] [PubMed] [Google Scholar]
- 17.Thomas a, Harding KG, Moore K. Biomaterials. 2000;21(17):1797–1802. doi: 10.1016/s0142-9612(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 18.Markov PA, Popov SV, Nikitina IR, Ovodova RG, Ovodov YS. Russ. J. Bioorganic Chem. 2011;37(7):817–821. [Google Scholar]
- 19.Chen C-H, Sheu M-T, Chen T-F, Wang Y-C, Hou W-C, Liu D-Z, Chung T-C, Liang Y-C. Biochem. Pharmacol. 2006;72(8):1001–1009. doi: 10.1016/j.bcp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Ovodov YS. Russ. J. Bioorganic Chem. 2009;35(3):269–284. [Google Scholar]
- 21.Opanasopit P, Apirakaramwong A, Ngawhirunpat T, Rojanarata T, Ruktanonchai U. AAPS PharmSciTech. 2008;9(1):67–74. doi: 10.1208/s12249-007-9007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barck K, Butler MF. J. Appl. Polym. Sci. 2005;98(4):1581–1593. [Google Scholar]
- 23.Yu C-Y, Yin B-C, Zhang W, Cheng S-X, Zhang X-Z, Zhuo R-X. Colloids Surf. B. Biointerfaces. 2009;68(2):245–249. doi: 10.1016/j.colsurfb.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira GF, Ferrari PC, Carvalho LQ, Evangelista RC. Carbohydr. Polym. 2010;82(3):1004–1009. [Google Scholar]
- 25.Kim TH, Park YH, Kim KJ, Cho CS. Int. J. Pharm. 2003;250(2):371–383. doi: 10.1016/s0378-5173(02)00553-7. [DOI] [PubMed] [Google Scholar]
- 26.Winter HH, Chambon F. J. Rheol. (N. Y. N. Y) 1986;30:367. [Google Scholar]
- 27.Fernandez-Megia E, Novoa-Carballal R, Quiñoá E, Riguera R. Carbohydr. Polym. 2005;61(2):155–161. [Google Scholar]
- 28.Hirai A, Odani H, Nakajima A. Polym. Bull. 1991;26:87–94. [Google Scholar]
- 29.Vårum K, Antohonsen M, Grasdalen H, Smidsrød O. Carbohydr. Res. 1991;211:17–23. doi: 10.1016/0008-6215(91)84142-2. [DOI] [PubMed] [Google Scholar]
- 30.Qurashi MT, Blair HS, Allen SJ. J. Appl. Polym. Sci. 1992;46(2):255–261. [Google Scholar]
- 31.Institute of Medicine (U.S.) Food Chemicals Codex. 5th. Washington, DC: National Academy Press; 2003. Committee on Food Chemicals Codex; pp. 322–323. [Google Scholar]
- 32.Chambon F, Winter H. J. Rheol. (N. Y. N. Y) 1987;31(8):683–697. [Google Scholar]
- 33.Morrison FA. In: Understanding Rheology. Gubbins KF, Barteau MA, Lauffenburger DA, Morari M, Ray WH, Russel WB, Tirrell MV, editors. New York: Oxford University Press; 2001. pp. 191–193. [Google Scholar]
- 34.Bryant SJ, Anseth KS. J. Biomed. Mater. Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 35.Maxson S, Lopez Ea, Yoo D, Danilkovitch-Miagkova a, LeRoux Ma. Stem Cells Transl. Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, De Boer J. Trends Biotechnol. 2013;31(2):108–115. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, Blumling J, Wang CF, Kohane DS, Langer R. Biomaterials. 2006;27:5259–5267. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 38.Hsu S, Lin Y, Lin T, Tseng T, Lee H, Liao Y, Chiu I. J. Med. Biol. 2012;32(2):85–90. [Google Scholar]
- 39.Gómez-Mascaraque LG, Méndez JA, Fernández-Gutiérrez M, Vázquez B, San Román J. Acta Biomater. 2014;10(2):798–811. doi: 10.1016/j.actbio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Guan L, Tian P, Ge H, Tang X, Zhang H, Du L, Liu P. J. Mol. Histol. 2013;44(5):609–618. doi: 10.1007/s10735-013-9508-5. [DOI] [PubMed] [Google Scholar]
- 41.Peschel G, Dahse H-M, Konrad A, Wieland GD, Mueller P-J, Martin DP, Roth M. J. Biomed. Mater. Res. A. 2008;85(4):1072–1081. doi: 10.1002/jbm.a.31666. [DOI] [PubMed] [Google Scholar]