Abstract
A new method for photoinduced dearomatization of arenes via an intramolecular cycloaddition with aza-o-xylylenes generated by the excited-state intramolecular proton transfer (ESIPT) in the readily available photoprecursors is developed. The topology of this cycloaddition, [2+4], is unprecedented for photo-dearomatizations of benzenoid aromatic carbocycles. It provides rapid access to novel heterocycles, cyclohexadieno-oxazolidino-quinolinols, as valuable synthons for a broad range of postphotochemical transformations.
Keywords: photochemistry, dearomatization, aza-o-xylylenes, polyheterocycles
Graphical Abstract
Synthesis of complex heterocycles via photoassisted dearomatization of benzenoid aromatics
Dearomatization of aryls provides an appealing preparative shortcut from the ubiquitous aromatic hydrocarbons to complex sp3-rich molecular topologies (see an excellent review by Porco1), offering access to the vast areas of underexplored chemical space.2 Dearomatization of electron-rich heterocycles such as indoles,3 furans,4 and pyrroles5 is precedented in the literature, including our own recent contributions.6,7
In contrast, the options for ground state dearomatization of carbocyclic benzenoid arenes are limited,8 with the predominance of phenolic oxidation9 or transition metal-assisted dearomatization.10 Photocycloadditions to arenes complement nicely these methods leading to diverse products topology, Scheme 1.11 Among these, the [3+2] reactions12 were often used as key steps in the synthesis of natural products,13 while the [2+2],14 [4+2],15 and [4+4]16 cycloadditions of arenes remain somewhat underutilized. Furthermore, [2+4] photocycloadditions – with arene acting as a 2π “dienophile” – are unknown.17 Instructively, such [2+4] reactions in the ground state are very rare.18
Scheme 1.
Molecular topologies accessible through photochemical dearomatization of benzenoid arenes.
We now report a new photo-dearomatization of arenes via the missing [2+4] topology, resulting in the formation of unique cyclohexadiene-fused heterocycles as primary photoproducts, amenable for further growth of framework complexity via straightforward postphotochemical transformations.
As we shown recently, cycloadditions of the ESIPT-generated azaxylylenes involve triplet species.6d The reaction is initiated by the electrophilic N-centered radical, with the overall process resembling a formal inverse electron demand Diels-Alder reaction. We hypothesized that a similar initial step should occur with donor-substituted benzenoid arenes. This indeed was the case: a readily available anilide of phenoxyacetic acid (1a) proved cycloaddition-competent, furnishing cyclohexadieno-quinolinol 4a upon irradiation with 365nm LEDs, Scheme 2.
Scheme 2.
A typical [2+4] cycloaddition of 1a.
The scope of this cycloaddition was assessed with a matrix of amides comprised of three photoactive cores, o-amino-benzaldehyde 1, aminoacetophenone 2, and aminotetralone 3, and twelve aromatic pendants: derivatives of phenoxyacetic- (a–h), phenylpropanoic- (i–k) acids, and biphenyl l, Figure 1.
Figure 1.
Substrate matrix.
Optimization of reaction conditions and solvent led to DMSO as a solvent of choice. The products resulting from irradiations of 1a–1c, 1h, 1j, 2d, 3d, 3e, 3j are summarized in Figure 2. For aldehyde-based precursors 1a–1c the sole syn-diastereomer was observed, where “syn” refers to the relative position of the benzylic OH and the newly formed cyclohexadiene ring. The stereochemical assignment was based on the analysis of proton spin-spin coupling constants (SSCC) and their comparison with the values calculated with our relativistic force field (rff), DU8c, method,19 Table S1.
Figure 2.
Primary photoproducts from anilides 1a–1c, 1h, 1j, 2d, 3d, 3e, 3j; isolated yields after chromatographic separation of diastereomers; a7% of anti-4j’ was additionally isolated
Phenylpropanoic derivatives, such as 1j, gave two regioisomers syn-4j and syn-4j’ in the ratio ca. 2:1.
The reaction scope is not limited to photoprecursors derived from benzaldehyde. Compounds 2d, 3d, 3e and 3j containing acetophenone and tetralone-based photoactive cores were also cycloaddition-competent. The stereo- and regiochemistry of cycloaddition for compounds anti-6d,e,j and j’ as well as syn- and anti-5d was unambiguously confirmed by x-ray analysis.
In several cases the methoxy-cyclohexadiene moiety in the primary photoproducts underwent hydrolysis into cyclohexenone. This reaction can be spontaneous as in the case of syn-4g, or happen during the chromatography as in the case of anti-6d, Scheme 3.
Scheme 3.
Spontaneous hydrolysis of primary photoproducts (isolated yields are over two steps).
A special case of interrupted postphotochemical hydrolysis is represented by syn-products 4, YR=OMe or NHAc, Scheme 4 and Figure 3. After protonation of the vinyl ether moiety, the methoxyallyl cation is trapped by the benzylic hydroxy-group yielding cyclic ketal 9.
Scheme 4.
Ketal Formation from the syn-Photoproduct.
Figure 3.
Formation of cyclic ketals. Conditions for ketal formation: (a) spontaneous; (b) HCl in ether, (c) p-TsOH•H2O
In this context, reaction of the aldehyde-based photoprecursor 1d presents a special case, Scheme 5. The initially formed syn- and anti-4d are unstable on the column. Upon addition of tosic acid anti-4d hydrolyzes to enone anti-10d. The syn-4d initially undergoes cyclic ketalization to form 9d, but its hydrolysis could be driven further to enone syn-10d, with subsequent acid-catalyzed nucleophilic capture of the benzylic hydroxyl group by the α,β-unsaturated ketone, producing stable ether 11d. The structure of ether 11d was initially assigned based on the analysis of its NMR, with the rff-calculated SSCCs matching experimental data with high accuracy (rmsd = 0.09 Hz), and later was also proved by x-ray analysis.
Scheme 5.
Acid-catalyzed postphotochemical transformations.
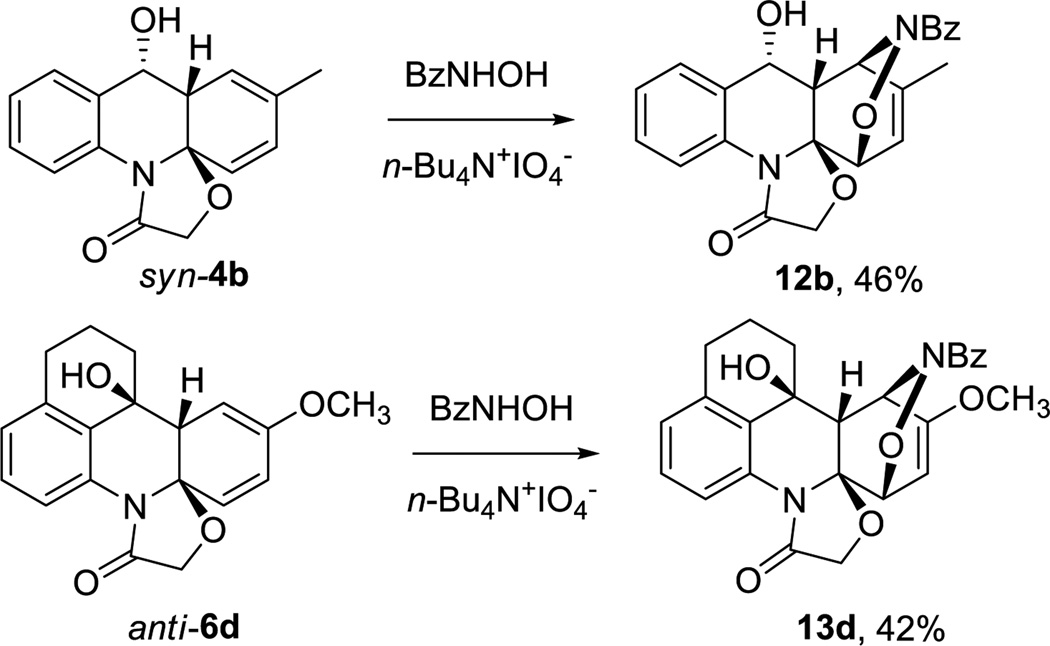
Synthetically appealing postphotochemical modifications to diversify and grow complexity of the resulting core scaffolds are not limited to ketalization. The primary photoproducts possess a reactive cyclohexadiene fragment that can be readily engaged in [4+2] cycloadditions, for example, hetero Diels-Alder reactions with in situ generated benzoylnitroso compounds, Scheme 6.
Scheme 6.
Hetero-Diels-Alder reaction of primary photoproducts
Another prominent postphotochemical transformation is a reaction with singlet oxygen yielding endoperoxides, such as 14 and 15, Scheme 6. Peroxides are ubiquitous in natural products,20 many possessing antitumor, antibacterial, and antimalarial activity. Additionally, these endoperoxides can be ring-opened with urea or bases, offering rapid access to complex pentasubstituted cyclohexenes fused to oxazolidino-quinolinol cores, for example ketal 16, triol 17, or enone 18, Scheme 7.
Scheme 7.
Reaction of primary photoproducts with singlet oxygen (isolated yields for compounds 16–18 were calculated over two steps).
In conclusion, we developed a new method for dearomatization of benzenoid arenes, with the arene reacting as the 2π component – an unprecedented topology for a photochemical reaction of benzenoid aromatics. The primary photoproducts are cyclohexadieno-quinolinol fused heterocycles which can be engaged in experimentally simple postphotochemical transformations to further grow scaffold diversity and complexity.
Supplementary Material
Acknowledgments
This work is supported by the NIH (GM GM093930)
References
- 1.Roche SP, Porco JA., Jr Angew. Chem. Int. Ed. 2011;50:4068. doi: 10.1002/anie.201006017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.On the importance of sp3-rich structures in drug discovery: Lovering F, Bikker J, Humblet C. J. Med. Chem. 2009;52:6752. doi: 10.1021/jm901241e. Young RJ, Green DVS, Luscombe CN, Hill AP. Drug Discovery Today. 2011;16:822. doi: 10.1016/j.drudis.2011.06.001. Ritchie TJ, Macdonald SJF. Drug Discovery Today. 2009;14:1011. doi: 10.1016/j.drudis.2009.07.014.
- 3.For reviews, see Denizot N, Tomakinian T, Beaud R, Kouklovsky C, Vincent G. Tetrahedron Lett. 2015;56:4413. Roche SP, Tendoung J-JY, Tréguier B. Tetrahedron. 2015;71:3549. Zi W, Zuo Z, Ma D. Acc. Chem. Res. 2015;48:702. doi: 10.1021/ar5004303. Ding Q, Zhou X, Fan R. Org. Biomol. Chem. 2014;12:4807. doi: 10.1039/c4ob00371c.
- 4. Padwa A, Flick AC. Adv. Heterocycl. Chem. 2013;110:1. Donohue TJ, Pullin RDC. Chem. Commun. 2012;48:11924. doi: 10.1039/c2cc36040c. For a recent example see Foster RW, Benhamou L, Porter MJ, Bučar D-K, Hailes H, Tame CJ, Sheppard TD. Chem. Eur. J. 2015;21:6107. doi: 10.1002/chem.201406286.
- 5. Antoline JE, Hsung RP, Huang J, Song Z, Li G. Org. Lett. 2007;9:127. doi: 10.1021/ol070103n. Howard JK, Rihak KJ, Bissember AC, Smith JA. Chem. Asian J. 2015 doi: 10.1002/asia.201500659. see also 4b
- 6.(a) Mukhina OA, Kumar NNB, Arisco TM, Valiulin RA, Metzel GA, Kutateladze AG. Angew. Chem., Int. Ed. 2011;50:9423. doi: 10.1002/anie.201103597. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nandurkar NS, Kumar NNB, Mukhina OA, Kutateladze AG. ACS Comb. Sci. 2013;15:73. doi: 10.1021/co3001296. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kumar NNB, Mukhina OA, Kutateladze AG. J. Am. Chem. Soc. 2013;135:9608. doi: 10.1021/ja4042109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mukhina OA, Cronk WC, Kumar NNB, Sekhar M, Samanta A, Kutateladze AG. J. Phys. Chem. A. 2014;118:10487. doi: 10.1021/jp504281y. [DOI] [PubMed] [Google Scholar]; (e) Cronk WC, Mukhina OA, Kutateladze AG. J. Org. Chem. 2014;79:1235. doi: 10.1021/jo4026447. [DOI] [PubMed] [Google Scholar]; (f) Kumar NNB, Kuznetsov DM, Kutateladze AG. Org. Lett. 2015;17:438. doi: 10.1021/ol5033909. [DOI] [PubMed] [Google Scholar]; (g) Mukhina OA, Kumar NNB, Cowger TM, Kutateladze AG. J. Org. Chem. 2014;79:10956. doi: 10.1021/jo5019848. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Umstead WJ, Mukhina OA, Kutateladze AG. Eur. J. Org. Chem. 2015:2205. doi: 10.1002/ejoc.201403620. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Umstead WJ, Mukhina OA, Kutateladze AG. Aust. J. Chem. 2015;68:1672. doi: 10.1071/CH15266. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Mukhina OA, Kuznetsov DM, Cowger TM, Kutateladze AG. Angew. Chem. Int. Ed. 2015;39:11516. doi: 10.1002/anie.201504455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alternative approaches to generate azaxylylenes: Wojciechowski K. Eur. J. Org. Chem. 2001:3587.
- 8.Pigge FC. In: Reaction Mechanisms and Methods for Aromatic Compounds. Morimer J, editor. John Wiley & Sons; 2016. p. 399. [Google Scholar]
- 9.(a) Harned AM. etrahedron Lett. 2014;55:4681. doi: 10.1016/j.tetlet.2014.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pouységu L, Deffieux D, Quideau S. Tetrahedron. 2010;66:2235. [Google Scholar]; (c) Ding Q, Ye Y, Fan R. Synthesis. 2013;45:1. [Google Scholar]; (d) Dohi T, Kita Y. Chem. Commun. 2009:2073. doi: 10.1039/b821747e. [DOI] [PubMed] [Google Scholar]
- 10.(a) Zhuo C-X, Zheng C, You S-Y. Acc. Chem. Res. 2014;47:2558. doi: 10.1021/ar500167f. [DOI] [PubMed] [Google Scholar]; (b) Keane JM, Harman WD. Organometallics. 2005;24:1786. [Google Scholar]; (c) Smith PL, Chordia MD, Harman WD. Tetrahedron. 2001;57:8203. [Google Scholar]
- 11.(a) Stret U, Bochet CG. Beilstein J. Org. Chem. 2011;7:525. doi: 10.3762/bjoc.7.61. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hoffmann N. Photochem. Photobiol. Sci. 2012;11:1613. doi: 10.1039/c2pp25074h. [DOI] [PubMed] [Google Scholar]; (c) Gaich T. In: Comprehensive Organic Synthesis. 2nd. Knochel P, Molander G, editors. Vol. 5. 2014. p. 703. [Google Scholar]
- 12.For reviews, see Cornelisse J. Chem. Rev. 1993;93:615. Chappell D, Russell AT. Org. Biomol. Chem. 2006;4:4409. doi: 10.1039/b614011b. For recent examples, see Shi H, De S, Wang Q, Gao S, Wang X, Chen C. Tetrahedron Lett. 2015;56:3225. doi: 10.1016/j.tetlet.2014.12.054. Wegmann M, Bach T. J. Org. Chem. 2015;80:2017. doi: 10.1021/jo5028613. Gaich T T, Mulzer J. J. Am. Chem. Soc. 2009;131:452. doi: 10.1021/ja8083048.
- 13.(a) Hoffmann N. Chem. Rev. 2008;108:1052. doi: 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]; (b) Bach T, Hehn JP. Angew. Chem. Int. Ed. 2011;50:1000. doi: 10.1002/anie.201002845. [DOI] [PubMed] [Google Scholar]
- 14.For reviews, see: Cornelisse J, de Haan R. In: Molecular and Supramolecular Photochemistry. Ramamurthy V, Schanze KS, editors. New York: Marcel Dekker; 2001. p. 1. Wagner P. Acc. Chem. Res. 2001;32:1. doi: 10.1021/ar000113n. For recent examples, see: Hoffmann N. Tetrahedron. 2002;58:7933. Kohmoto S, Hisamatsu S, Mitsuhashi H, Takahashi M, Masu H, Azumaya I, Yamaguchi K, Kishikawa K. Org. Biomol. Chem. 2010;8:2174. doi: 10.1039/c000179a.
- 15.arene as a diene: Streit U, Birbaum F, Quattropani A, Bochet CG. J. Org. Chem. 2013;78:6890. doi: 10.1021/jo4002307. Kalena GP, Pradhan P, Puranik VS, Banerji A. Tetrahedron Lett. 2003;44:2011. Kishikawa K, Akimoto S, Kohmoto S, Yamamoto M, Yamada K. J. Chem. Soc., Perkin Trans. 1. 1997:77.
- 16.(a) Yang C, Nakamura A, Wada T, Inoue Y Y. Org. Lett. 2006;8:3005. doi: 10.1021/ol061004x. [DOI] [PubMed] [Google Scholar]; (b) Yang C, Mori T, Inoue Y. J. Org. Chem. 2008;73:5786. doi: 10.1021/jo800533y. [DOI] [PubMed] [Google Scholar]; (c) Kohmoto S, Masu H, Tatsuno C, Kishikawa K, Yamamoto M, Yamaguchi K. J. Chem. Soc. Perkin Trans. 1. 2000:4464. [Google Scholar]; (e) Khatri BB, Vruliauskas D, Sieburth SMcN. Tetrahedron Lett. 2015;56:4520. [Google Scholar]; (f) Khatri BB, Kulyk S, Sieburth SMcN. Org. Chem. Front. 2014;1:961. [Google Scholar]
- 17.Photochemical reactions of arenes with dienes: Berridge JC, Forrester J, Foulger BE, Gilbert A. J. Chem. Soc., Perkin Trans. 1. 1980:2425. Kimura M, Sagara S, Morosawa S. J. Org. Chem. 1982;47:4344. Ellis-Davies GCR, Gilbert A, Warrington JV, Westover DL. Photochem. 1984;27:259. Albini A, Fasani E, Giavarini F. J. Org. Chem. 1988;53:5601. Noh T, Kim D. Tetrahedron Lett. 1996;52:9329. and computationally by van der Hart JA, Mulder JJC, Cornelisse J. J. Photochem Photobiol. A. 1995;86:141.
- 18.(a) Jarre W, Bienlek D, Korte F. Angew. Chem Int. Ed. 1975;14:181. [Google Scholar]; (b) Saito K, Omura Y, Maekawa E. Tetrahedron Lett. 1984;25:2573. [Google Scholar]; (c) Seitz G, Hoferichter R, Mohr R. Angew. Chem. Int. Ed. 1987;26:332. [Google Scholar]; (d) Hoferichter R, Seitz G. Lieb. Ann. Chem. 1992:1153. [Google Scholar]; (e) Inagaki Y, Nakamoto M, Sekiguchi A. Nature Commun. 2013;5 doi: 10.1038/ncomms4018. article number 3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Kutateladze AG, Mukhina OA. J. Org. Chem. 2015;80:5218. doi: 10.1021/acs.joc.5b00619. [DOI] [PubMed] [Google Scholar]; (b) Kutateladze AG, Mukhina OA. J. Org. Chem. 2014;79:8397. doi: 10.1021/jo501781b. [DOI] [PubMed] [Google Scholar]
- 20.(a) Castel DA. Nat. Prod. Rep. 1999;16:55. [Google Scholar]; (b) Dembitsky VM. Eur. J. Med. Chem. 2008;43:223. doi: 10.1016/j.ejmech.2007.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.