Abstract
Background
Atrial fibrillation is associated with higher mortality. Identification of causes of death and contemporary risk factors for all‐cause mortality may guide interventions.
Methods and Results
In the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) study, patients with nonvalvular atrial fibrillation were randomized to rivaroxaban or dose‐adjusted warfarin. Cox proportional hazards regression with backward elimination identified factors at randomization that were independently associated with all‐cause mortality in the 14 171 participants in the intention‐to‐treat population. The median age was 73 years, and the mean CHADS 2 score was 3.5. Over 1.9 years of median follow‐up, 1214 (8.6%) patients died. Kaplan–Meier mortality rates were 4.2% at 1 year and 8.9% at 2 years. The majority of classified deaths (1081) were cardiovascular (72%), whereas only 6% were nonhemorrhagic stroke or systemic embolism. No significant difference in all‐cause mortality was observed between the rivaroxaban and warfarin arms (P=0.15). Heart failure (hazard ratio 1.51, 95% CI 1.33–1.70, P<0.0001) and age ≥75 years (hazard ratio 1.69, 95% CI 1.51–1.90, P<0.0001) were associated with higher all‐cause mortality. Multiple additional characteristics were independently associated with higher mortality, with decreasing creatinine clearance, chronic obstructive pulmonary disease, male sex, peripheral vascular disease, and diabetes being among the most strongly associated (model C‐index 0.677).
Conclusions
In a large population of patients anticoagulated for nonvalvular atrial fibrillation, ≈7 in 10 deaths were cardiovascular, whereas <1 in 10 deaths were caused by nonhemorrhagic stroke or systemic embolism. Optimal prevention and treatment of heart failure, renal impairment, chronic obstructive pulmonary disease, and diabetes may improve survival.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT00403767.
Keywords: atrial fibrillation, mortality, rivaroxaban, stroke, warfarin
Subject Categories: Atrial Fibrillation, Sudden Cardiac Death, Heart Failure, Ischemic Stroke, Intracranial Hemorrhage
Introduction
Patients with atrial fibrillation (AF) are known to be at increased risk of stroke, heart failure, and death.1, 2, 3, 4 Oral anticoagulation has been shown to improve survival of patients with AF at risk of stroke.5, 6, 7 Although stroke or systemic embolism accounts for only ≈10% of deaths in AF patients,7, 8, 9 few if any therapies other than stroke prevention have been shown to improve survival in AF patients. Despite the large proportion of AF mortality related to cardiovascular events, rhythm control has not been shown to improve survival in AF patients.10
Given the increasing frequency of AF and its association with higher mortality, additional strategies to improve survival are needed.11 The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) study was designed to compare the efficacy and safety of rivaroxaban with warfarin for prevention of stroke or systemic embolism in patients with AF at moderate or high risk of stroke.12 The goal of this analysis was to identify factors associated with all‐cause mortality and to describe cause‐specific death to better inform potential strategies for improving survival in patients with nonvalvular AF.
Methods
The rationale and design of the ROCKET AF study have been reported previously (NCT00403767).13 In summary, ROCKET AF was an international, double‐blind, double‐dummy, randomized noninferiority trial of fixed‐dose rivaroxaban versus adjusted‐dose warfarin for the prevention of stroke or systemic embolism. The study was funded by Johnson & Johnson Pharmaceutical Research and Development and Bayer HealthCare AG. The Duke Clinical Research Institute coordinated the trial and performed the statistical analyses for this study independent of the sponsors. An international executive committee designed the study and took responsibility for the accuracy and completeness of the analyses. All appropriate national regulatory authorities and institutional review boards at all study centers approved the study.
Definitions, End Points, and Baseline Variables
Patients were evaluated at a minimum of every 4 weeks throughout the trial for study drug management, ascertainment of adverse events, and surveillance for the primary end points and other clinical events. The primary efficacy end point in the ROCKET AF clinical trial was the composite of stroke (ischemic or hemorrhagic) or systemic embolism.13 For the purpose of this analysis, intention‐to‐treat data were used, and the primary outcome of interest was all‐cause mortality. The secondary outcome of interest was cause‐specific death. All deaths were reviewed, and an independent clinical end point committee blinded to treatment assignment adjudicated causes of death.
Heart failure was defined as a clinical diagnosis of heart failure or a left ventricular ejection fraction ≤35%. Hypertension was defined as antihypertensive medications within 6 months of enrollment, systolic blood pressure >140 mm Hg, or diastolic blood pressure >90 mm Hg. The CHADS2 score was calculated as 1 point for heart failure, hypertension, age ≥75 years, or diabetes and 2 points for stroke or transient ischemic attack. Consistent with the method used in the conduct of ROCKET AF, creatinine clearance was calculated using a creatinine value measured at a site and the Cockcroft‐Gault formula.14
Vascular deaths included deaths from spontaneous bleeding, myocardial infarction, stroke, heart failure, sudden death, or arrhythmias. Patients who died within 30 days of the onset of a stroke were classified as having had a fatal stroke. Patients who had a stroke and then died >30 days after the onset of the stroke were classified based on the more immediate cause of death. Sudden death was defined as witnessed or unwitnessed death in a patient seen within 24 hours of death and known to be asymptomatic at that time (Data S1).
Statistical Analysis
Baseline characteristics, stratified by survival, were summarized numerically for categorical variables and as median values with 25th and 75th percentiles for continuous variables. Kaplan–Meier plots for all‐cause death were presented according to treatment allocation. Cox proportional hazards regression was used to identify the factors at randomization that were independently associated with the occurrence of all‐cause death in the 14 171 intention‐to‐treat patients. All baseline variables recorded at randomization were included in the model selection process, and covariates were selected via backward elimination: age, sex, race, ethnicity (Hispanic or non‐Hispanic), region, heart rate, body mass index, systolic blood pressure, diastolic blood pressure, years since AF diagnosis, type of AF (persistent, paroxysmal, recent onset), prior stroke or transient ischemic attack, heart failure, hypertension, diabetes mellitus, creatinine clearance, creatinine, peripheral arterial disease, chronic obstructive pulmonary disease (COPD), prior gastrointestinal bleeding, liver disease, alcohol use, obstructive sleep apnea, and left bundle branch block. Associations are reported as hazard ratios (HR) with 95% confidence intervals (CI). Following the development of this model, an additional model was constructed with the inclusion of baseline medications, including aspirin, prior vitamin K antagonist, and antiarrhythmic drug therapy, as covariates. Predictive models were also generated for cardiovascular death and sudden or unwitnessed death, using the Fine‐Gray method to account for competing risks. All analyses were performed using SAS version 9.2 (SAS Institute, Inc). The Duke institutional review board approved this project, and all patients with data included in this analysis signed informed consent.
Results
Patient Characteristics
There were 14 171 patients in the intention‐to‐treat population of ROCKET AF, with a median age of 73 years and a mean CHADS2 score of 3.5±0.9. There was a median follow‐up of 1.9 years (25th, 75th percentiles: 1.4, 2.4 years) during which 1214 (8.6%) patients died, with an event rate of 4.7 deaths per 100 patient‐years. The Kaplan–Meier mortality rate was 4.2% at 1 year and 8.9% at 2 years.
Among patients who died, the median age was 76 years (25th, 75th percentiles: 69, 80 years), the mean CHADS2 score was 3.6±1.0, and 47.9% had prior stroke or transient ischemic attack. Patients who died during follow‐up were older (aged 76 versus 72 years, P<0.0001), more frequently male (66.1% versus 59.9%, P<0.0001), and more likely to have a history of heart failure (70.3% versus 61.7%, P<0.0001) or vascular disease (34.9% versus 22.2%) (Table 1). Patients who died also were more likely to have lower creatinine clearance by the Cockcroft‐Gault formula (59 versus 68 mL/min, P<0.0001) relative to patients who survived.
Table 1.
Baseline Characteristics According to Survival
| Characteristic | Died During Follow‐Up (n=1214) | Alive (n=12 957) | P Valuea |
|---|---|---|---|
| Age, y | 76 (69, 80) | 72 (65, 78) | <0.0001 |
| Female sex | 411 (33.9) | 5194 (40.1) | <0.0001 |
| Region | |||
| Asia Pacific | 168 (13.8) | 1941 (15.0) | <0.0001 |
| East Europe | 407 (33.5) | 5000 (38.6) | |
| Latin America | 203 (16.7) | 1675 (12.9) | |
| North America | 285 (23.5) | 2396 (18.5) | |
| West Europe | 151 (12.4) | 1945 (15.0) | |
| Body mass index, kg/m2 | 27.5 (24.3, 31.2) | 28.3 (25.2, 32.0) | <0.0001 |
| Systolic pressure, mm Hg | 130 (120, 140) | 130 (120, 140) | 0.0001 |
| Diastolic pressure, mm Hg | 80 (70, 84) | 80 (70, 86) | <0.0001 |
| Heart rate, bpm | 77 (68, 86) | 76 (67, 86) | 0.09 |
| Creatinine clearance, Cockcroft/Gault, mL/min | 59 (45, 77) | 68 (53, 88) | <0.0001 |
| Type of atrial fibrillation | |||
| Persistent | 1029 (84.8) | 10 456 (80.7) | |
| Paroxysmal | 170 (14.0) | 2320 (17.9) | 0.001 |
| New | 15 (1.2) | 180 (1.4) | |
| CHADS2 score | |||
| Median (25th, 75th percentile) | 3 (3, 4) | 3 (3, 4) | <0.0001 |
| Mean±SD | 3.6±1.0 | 3.5±0.9 | |
| Aspirin | 516 (42.5) | 4668 (36.0) | <0.0001 |
| Vitamin K antagonists | 740 (61.0) | 8113 (62.6) | 0.003 |
| Thienopyridine | 36 (3.0) | 322 (2.5) | 0.25 |
| Hypertension | 1105 (91.0) | 11 719 (90.4) | 0.72 |
| Congestive heart failure | 854 (70.3) | 7997 (61.7) | <0.0001 |
| Diabetes | 540 (44.5) | 5107 (39.4) | <0.0001 |
| Stroke or TIA | 582 (47.9) | 6849 (52.9) | 0.12 |
| COPD | 221 (18.2) | 1260 (9.7) | <0.0001 |
| Vascular disease (PAD or MI) | 424 (34.9) | 2872 (22.2) | <0.0001 |
| Any alcohol use | 370 (30.5) | 4641 (35.8) | <0.0001 |
Data are shown as n(%), median (25th, 75th percentiles), or median±standard deviation. bpm indicates beats per minute; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; PAD, peripheral artery disease; SD, standard deviation; TIA, transient ischemic attack.
P values come from univariable Cox proportional hazards regression models.
Classification of Cause of Death
Adjudicated cause‐specific death rates are shown in Table 2 for the 1214 deaths, although 133 (11%) deaths were unable to be classified. The majority of the classified deaths (1081) were cardiovascular (72%), but only 6% of the classified deaths were due to nonhemorrhagic stroke or systemic embolism. Among the classified deaths in the trial, sudden or unwitnessed death was the most frequent classification (n=343, 32%), followed by death from heart failure (n=157, 15%). Nonvascular deaths accounted for 305 events (28%). Intracranial hemorrhage was determined to be the cause of death in 70 patients (0.3 deaths per 100 patient‐years).
Table 2.
Cause‐Specific Mortality Among Classified Deaths in the Overall Population and According to Heart Failure Status, Randomized Treatment, and Age
| Subclassified Cause of Death | Overall (n=1081) | HF (n=765) | No HF (n=316) | HF P Value | Age <75 (n=476) | Age ≥75 (n=605) | Age P Value | Rivaroxaban (n=523) | Warfarin (n=558) | Treatment P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Vascular | 776 (3.0) | 579 (3.7) | 197 (2.0) | <0.0001 | 363 (2.5) | 413 (3.7) | <0.0001 | 375 (2.9) | 401 (3.1) | 0.35 |
| Myocardial infarction | 38 (0.2) | 31 (0.2) | 7 (0.1) | 0.014 | 16 (0.1) | 22 (0.2) | 0.07 | 18 (0.1) | 20 (0.2) | 0.75 |
| Directly related to revascularization | 1 | 1 | 0 | — | 1 | 0 | — | 0 | 1 | — |
| Nonhemorrhagic stroke | 63 (0.2) | 40 (0.3) | 23 (0.2) | 0.73 | 23 (0.2) | 40 (0.4) | 0.001 | 27 (0.2) | 36 (0.3) | 0.26 |
| Intracranial hemorrhage | 70 (0.3) | 35 (0.2) | 35 (0.4) | 0.049 | 31 (0.2) | 39 (0.4) | 0.033 | 27 (0.2) | 43 (0.3) | 0.06 |
| Pulmonary embolism | 7 (0.03) | 6 (0.04) | 1 (0.01) | 0.22 | 2 (0.01) | 5 (0.1) | 0.15 | 4 (0.03) | 3 (0.02) | 0.71 |
| Noncoronary atherosclerotic disease | 7 (0.03) | 4 (0.03) | 3 (0.03) | 0.84 | 2 (0.01) | 5 (0.1) | 0.15 | 2 (0.02) | 5 (0.04) | 0.27 |
| Sudden/unwitnessed death | 343 (1.3) | 263 (1.7) | 80 (0.8) | <0.0001 | 176 (1.2) | 167 (1.5) | 0.030 | 169 (1.3) | 174 (1.4) | 0.79 |
| Dysrhythmia (other than sudden death) | 16 (0.1) | 13 (0.1) | 3 (0.03) | 0.12 | 11 (0.1) | 5 (0.1) | 0.35 | 9 (0.1) | 7 (0.1) | 0.62 |
| Heart failure/cardiogenic shock | 157 (0.6) | 131 (0.8) | 26 (0.3) | <0.0001 | 65 (0.4) | 92 (0.8) | <0.0001 | 88 (0.7) | 69 (0.5) | 0.13 |
| Extracranial hemorrhage | 22 (0.1) | 17 (0.1) | 5 (0.1) | 0.13 | 11 (0.1) | 11 (0.1) | 0.50 | 7 (0.1) | 15 (0.1) | 0.10 |
| Other vascular | 52 (0.2) | 38 (0.2) | 14 (0.1) | 0.08 | 25 (0.2) | 27 (0.2) | 0.19 | 24 (0.2) | 28 (0.2) | 0.58 |
| Nonvascular | 305 (1.2) | 186 (1.2) | 119 (1.2) | 0.95 | 113 (0.8) | 192 (1.7) | <0.0001 | 148 (1.2) | 157 (1.2) | 0.61 |
| Accidental/trauma | 15 (0.1) | 9 (0.1) | 6 (0.1) | 0.94 | 9 (0.1) | 6 (0.1) | 0.83 | 5 (0.04) | 10 (0.1) | 0.21 |
| Suicide | 4 (0.02) | 3 (0.02) | 1 (0.01) | 0.55 | 2 (0.01) | 2 (0.02) | 0.75 | 2 (0.02) | 2 (0.02) | 1.00 |
| Respiratory failure | 44 (0.2) | 28 (0.2) | 16 (0.2) | 0.69 | 14 (0.1) | 30 (0.3) | 0.001 | 19 (0.2) | 25 (0.2) | 0.37 |
| Liver failure | 1 | 1 | 0 | — | 0 | 1 | — | 1 | 0 | — |
| Infection/sepsis | 97 (0.4) | 62 (0.4) | 35 (0.4) | 0.54 | 35 (0.2) | 62 (0.6) | <0.0001 | 45 (0.4) | 52 (0.4) | 0.48 |
| Renal failure | 12 (0.1) | 9 (0.1) | 3 (0.03) | 0.31 | 3 (0.02) | 9 (0.1) | 0.042 | 7 (0.1) | 5 (0.04) | 0.55 |
| Malignancy | 118 (0.5) | 65 (0.4) | 53 (0.5) | 0.21 | 46 (0.3) | 72 (0.7) | <0.0001 | 63 (0.5) | 55 (0.4) | 0.46 |
| Other nonvascular | 14 (0.1) | 9 (0.1) | 5 (0.1) | 0.85 | 4 (0.03) | 10 (0.1) | 0.042 | 6 (0.1) | 8 (0.1) | 0.59 |
Data are summarized as number of events (event rate per 100 patient‐years of follow‐up). P values come from univariable Cox proportional hazards regression models in which different cause of death is censored. HF indicates heart failure.
Among the 22 deaths related to extracranial hemorrhage and 70 intracranial hemorrhage–related deaths, 6 (27%) and 8 (11%) of those patients, respectively, had a nonhemorrhagic stroke within 1 year of death. There were 92 hemorrhage‐related deaths, and 14 (15%) of those patients had a nonhemorrhagic stroke within 1 year of death.
Heart failure was associated with higher all‐cause mortality (HR 1.5, 95% CI 1.3–1.7, P<0.0001). Relative to patients without heart failure, those with heart failure were more likely to die from heart failure (0.8 versus 0.3 events per 100 patient‐years, P<0.001) and sudden or unwitnessed death (1.7 versus 0.8 events per 100 patient‐years, P<0.001). Patients without heart failure were more likely than those with heart failure to die of intracranial hemorrhage (0.4 versus 0.2 events per 100 patient‐years, P=0.049).
Given the concerns regarding safety of oral anticoagulants in elderly patients, the population was dichotomized based on age ≥75 years. Age ≥75 years was associated with higher all‐cause mortality relative to age <75 years (HR 1.7, 95% CI 1.5–1.9, P<0.0001). There were several significant differences in the causes of death according to age <75 versus ≥75 years, including a higher prevalence of nonhemorrhagic stroke (0.4 versus 0.2 events per 100 patient‐years, P=0.001) and sudden or unwitnessed death among patients <75 years (1.5 versus 1.2 events per 100 patient‐years, P=0.030). Noncardiovascular causes of death such as malignancy and infection were more common among patients aged ≥75 versus <75 years (0.7 versus 0.3 and 0.6 versus 0.2 events per 100 patient‐years, respectively, P<0.0001 for both).
Mortality by Treatment Strategy
The cumulative incidence of all‐cause mortality was not significantly different between the rivaroxaban and warfarin arms (P=0.15) (Figure 1). There were 582 deaths (48% of all deaths) on rivaroxaban and 632 deaths (52% of all deaths) on warfarin (4.5 versus 4.9 events per 100 patient‐years, respectively; HR 0.92, 95% CI 0.82–1.03; P=0.15). There was a trend toward lower incidence of death due to intracranial hemorrhage with rivaroxaban compared with warfarin (HR 0.63, 95% CI 0.39–1.02, P=0.06). There was no significant difference in the associations between treatment with rivaroxaban or warfarin and vascular or nonvascular death (Table 3).
Figure 1.
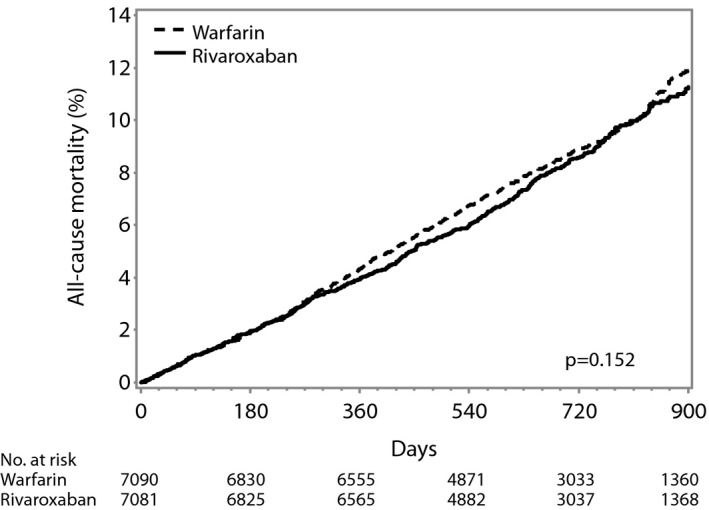
Cumulative incidence of all‐cause mortality in rivaroxaban vs warfarin arms in the intention‐to‐treat population.
Table 3.
Intention‐to‐Treat Treatment Effect of Rivaroxaban Versus Warfarin on Type of Death
| Rivaroxaban (n=582) | Warfarin (n=632) | HR (95% CI) | P Value | |
|---|---|---|---|---|
| All‐cause mortality | 582 (4.5) | 632 (4.9) | 0.92 (0.82–1.03) | 0.15 |
| Vascular death | 375 (2.9) | 401 (3.1) | 0.94 (0.81–1.08) | 0.35 |
| Nonvascular death | 148 (1.2) | 157 (1.2) | 0.94 (0.75–1.18) | 0.61 |
| Death unknown cause | 59 (0.5) | 74 (0.6) | 0.80 (0.57–1.12) | 0.20 |
| Sudden/unwitnessed death | 169 (1.3) | 174 (1.4) | 0.97 (0.79–1.20) | 0.79 |
| CHF/shock cause of death | 88 (0.7) | 69 (0.5) | 1.28 (0.93–1.75) | 0.13 |
| Malignancy cause of death | 63 (0.5) | 55 (0.4) | 1.14 (0.80–1.64) | 0.46 |
| Intracranial hemorrhage death | 27 (0.2) | 43 (0.3) | 0.63 (0.39–1.02) | 0.06 |
Data are summarized as number of events (event rate per 100 patient‐years of follow‐up), unless otherwise indicated. CHF indicates congestive heart failure; HR, hazard ratio.
Factors Associated With All‐Cause Mortality
The independent, significant predictors of increased mortality were lower creatinine clearance, COPD, male sex, peripheral vascular disease, older age, diabetes, heart failure, increased heart rate, residence in Latin America, and prior stroke or transient ischemic attack (Figure 2) (C‐index 0.677). For each 10‐mL/min decrease in creatinine clearance <60 mL/min, the hazard of death increased by 25%. The presence of COPD was associated with a 65% higher hazard of death. Alcohol use, paroxysmal (versus persistent) AF, higher diastolic blood pressure, and higher body mass index up to an index of 25 were associated with a lower risk of death. When baseline medications were included as candidate variables, prior vitamin K antagonist use was associated with lower mortality (HR 0.77, 95% CI 0.68–0.87), whereas there was no significant association between mortality and prior antiarrhythmic drug therapy or aspirin.
Figure 2.
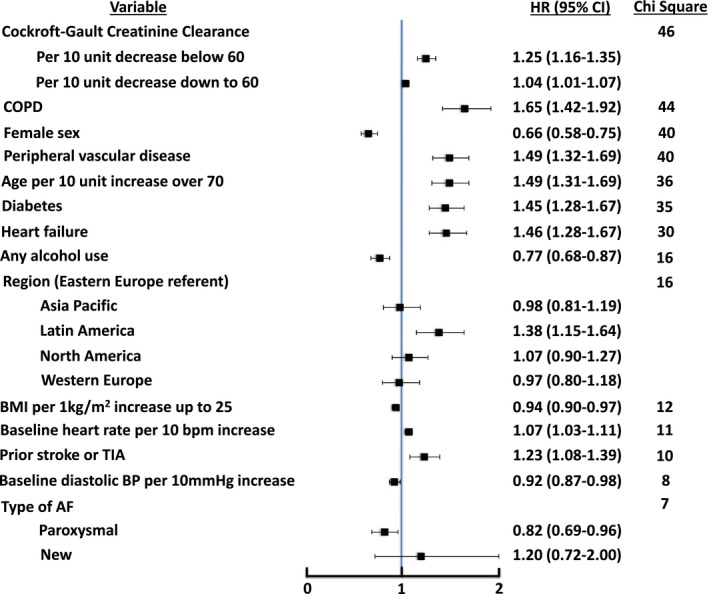
Factors associated with all‐cause mortality, with points to the left of unity being associated with lower likelihood of all‐cause mortality and points to the right of unity being associated with higher likelihood of all‐cause mortality. C‐index 0.677 (25th, 75th percentiles: 0.661, 0.693). AF indicates atrial fibrillation; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; TIA, transient ischemic attack.
Factors Associated With Cause of Death
Vascular disease, heart failure, and diabetes were among the factors most strongly associated with a higher likelihood of cardiovascular death, with a 78%, 72%, and 44% increased hazard of cardiovascular death, respectively (Figure 3) (C‐index 0.698). Female sex was associated with a lower likelihood of cardiovascular death (HR 0.68, 95% CI 0.58–0.80). Similarly for sudden or unwitnessed death, vascular disease and heart failure were again among the factors most strongly associated, with a 98% and 75% higher hazard of sudden or unwitnessed death, respectively (Figure 4) (C‐index 0.691). As with cardiovascular death, female sex was associated with a lower likelihood of sudden or unwitnessed death.
Figure 3.
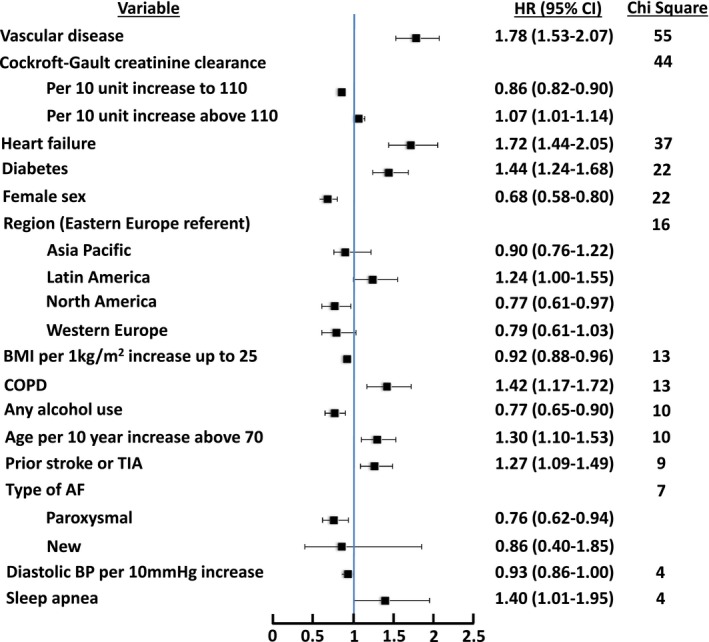
Factors associated with cardiovascular death, with points to the left of unity being associated with lower likelihood of cardiovascular death and points to the right of unity being associated with higher likelihood of cardiovascular death. C‐index 0.698. AF indicates atrial fibrillation; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; TIA, transient ischemic attack.
Figure 4.
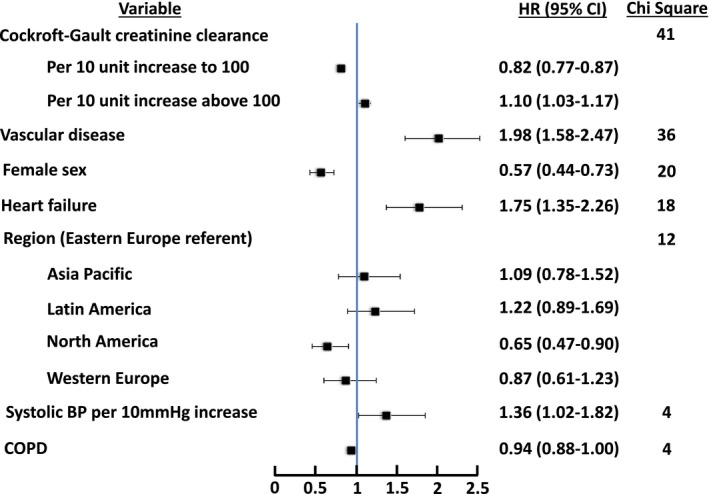
Factors associated with sudden or unwitnessed death, with points to the left of unity being associated with lower likelihood of sudden or unwitnessed death and points to the right of unity being associated with higher likelihood of sudden or unwitnessed death. C‐index 0.691. BP indicates blood pressure; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
Discussion
The use of oral anticoagulation reduces all‐cause mortality in AF patients,5 but a better understanding of risk factors for mortality among anticoagulated AF patients is necessary to identify additional interventions to improve care for these patients. This analysis of the ROCKET AF data demonstrates the high rate of aggregated cardiovascular death, which occurs 2.1 times more often than stroke, 4.5 times more often than myocardial infarction, and 1.4 times more often than major bleeding. The majority of deaths are not related to stroke or systemic embolism. There are no differences in event rates of any type of death among patients treated with rivaroxaban or warfarin. In addition to patients with pulmonary and renal disease, many of the patient characteristics associated with higher mortality rates are traditional cardiovascular risk factors.
The 1‐ and 2‐year mortality rates were 4% and 9%, respectively, with 4.7 deaths per 100 patient‐years. The primary end point in ROCKET AF was stroke or systemic embolism, and in the intention‐to‐treat analysis, the primary end point occurred 2.1 and 2.4 times per 100 patient‐years in the rivaroxaban and warfarin populations, respectively.12 Any major bleeding occurred 3.6 and 3.4 times per 100 patient‐years for rivaroxaban and warfarin, respectively. The mortality rates in ROCKET AF were lower than the previously published 1‐year mortality rate of 23% for patients (mean age 73 years) in Olmsted County, Minnesota, with new‐onset AF between 1980 and 2000.15 Mortality rates in the current study were also lower than the 1‐ and 2‐year mortality rates of 20% and 28%, respectively, for an older Medicare population (mean age 80 years) with newly diagnosed AF; however, similar to the findings in ROCKET AF, death was the most common event within 2 years of diagnosing AF.16 In the Medicare population, death was >3 times more common than heart failure and occurred 8 times more frequently than stroke or gastrointestinal bleed. The Danish Registry of AF patients treated with warfarin or dabigatran 150 mg is another example of death being more common than stroke, major bleeding, or myocardial infarction in general practice outside of clinical trials.17
Oral anticoagulants remain an important prevention strategy in AF patients, and nonadherence is associated with increased risk of stroke or death.18 In fact, oral anticoagulation is the only intervention shown in randomized trials to improve all‐cause survival in patients with nonvalvular AF. A meta‐analysis of randomized controlled trials showed that warfarin had 26% relative risk reduction (95% CI 3–43%) in all‐cause mortality compared with placebo.5 It may be that treatment with an oral anticoagulant matters more for mortality in AF patients than other baseline factors, and the fact that all patients were treated with an oral anticoagulant may partially explain the modest C‐index of 0.677 for the mortality model.
In our analysis of patients being prescribed oral anticoagulants in ROCKET AF, ≈1 in 20 patients died from stroke or systemic embolism, whereas more than half of the overall deaths were from other cardiovascular causes. These proportions of cause‐specific death were similar to those reported in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) trial, in which 11% of deaths were vascular and 39% of the deaths were nonvascular cardiac deaths.8 In AFFIRM, there were no statistically significant differences in rates of vascular or cardiac deaths between rate‐ and rhythm‐control strategies. Similar findings of 10% vascular death and 37% cardiac death were seen in the clinical trial comparison of dabigatran and warfarin; 7% of classified deaths in the dabigatran study were due to stroke or systemic embolism, and this was similar to the 6% seen in this analysis, despite the fact that the mean CHADS2 score of patients who died was higher in our study (3.6 versus 2.5).9 The most common cause of noncardiovascular death was malignancy in our study (11%) and in the dabigatran analysis (14%).9
Patients with AF tend to be older and have multiple comorbid cardiovascular diseases that could be lethal and require appropriate prevention and treatment. Renal disease and pulmonary disease were among the factors most strongly associated with all‐cause mortality. Patients with stage 3 chronic kidney disease in our analysis had a 25% increase in the hazard of death associated with a 10‐mL/min decrease in creatinine clearance. Cardiovascular disease factors, such as peripheral vascular disease, diabetes, and heart failure, were also associated with all‐cause mortality. This emphasizes the importance of treating all cardiovascular conditions in AF patients. Reductions in weight and blood pressure along with better glycemic and lipid management have been shown to improve arrhythmia‐free survival after AF ablation in the Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation (ARREST‐AF) study.19 Despite the evidence for risk factor management, it has been demonstrated previously that the minority of AF patients receive guideline‐recommended therapies for their comorbid cardiovascular conditions, with nearly 60% of AF patients with peripheral vascular disease, diabetes, and heart failure not receiving all guideline‐based treatment.20
Sudden or unwitnessed death was the most common cause of death, seen in ≈1 in 3 deaths. Heart failure was among the strongest predictors of sudden or unwitnessed death (HR 1.75, 95% CI 1.35–2.26). A similar finding was noted in the dabigatran analysis, in which sudden death accounted for 20% of deaths, and heart failure was the strongest predictor, with a HR of 2.24 (95% CI 1.75–2.87).9 An analysis of the Oregon Sudden Unexpected Death Study identified a high prevalence of AF in cases of sudden cardiac death relative to controls (27% versus 18%, P=0.0001), but further analyses demonstrated that heart failure at least partially explained the association between AF and sudden cardiac death.21
Clinical Implications
These data have several important clinical implications. Patients with AF are most commonly dying from cardiovascular conditions. This emphasizes the importance of following the general cardiology guidelines of care in AF patients. Patients with diabetes should be treated with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and heart failure patients should be on optimal medical therapy. Patients with AF should be monitored closely for the development of comorbidities, such as heart failure, peripheral vascular disease, COPD, diabetes, and hypertension. Early detection of these conditions and aggressively optimizing treatment regimens may improve mortality in AF patients on anticoagulation. Some data show that AF ablation may treat heart failure in patients with AF, and further research is needed on the effect of AF ablation on mortality in heart failure patients.22, 23 Future research should be focused on improving mortality in patients with AF and COPD, diabetes, peripheral vascular disease, and heart failure because these modifiable conditions are all associated with higher mortality with AF.
Limitations
Several important limitations must be kept in mind when considering our results. The generalizability of these results may be limited because this was a clinical trial population. Patients with the highest risk of mortality, such as those with active malignancy, were not included in ROCKET AF. Nevertheless, the ROCKET AF trial included patients with a greater number of comorbidities by CHADS2 score relative to the other trials comparing non–vitamin K oral anticoagulants with warfarin.24, 25, 26 The multivariable models were established based on baseline demographics and characteristics, and they may not have reflected patients’ comorbidities at the time of death. Spectrums of disease severity exist for peripheral vascular disease, COPD, diabetes, and heart failure; however, for the purposes of modeling, these diseases were treated as binary events. Data on treatment regimens for the comorbidities were not available and were not incorporated into the model. Although multivariable adjustment was performed, there may be significant, unmeasured confounding in the comparison between patients who died and those that lived. A large percentage of the classified deaths (32%) were sudden or unwitnessed, and autopsy data were not frequently available for event adjudication. It is possible that these cases resulted in underestimation of the incidence of death related to stroke.
Conclusions
Overall, >1 in 2 deaths in AF patients treated with oral anticoagulation were cardiovascular deaths unrelated to stroke or systemic embolism. In a large population of patients with nonvalvular AF, the strongest predictors of mortality were reduced renal function, COPD, male sex, peripheral vascular disease, and age. Cardiovascular disease risk factor modification needs to be emphasized for all AF patients, including optimal medical therapy to treat heart failure, smoking cessation to prevent COPD, and blood pressure control and angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers for diabetic patients to prevent renal dysfunction. Continued investigations of the mortality impact of management of nonvalvular AF should focus on patients with a high burden of comorbidities, including patients with concomitant renal and pulmonary disease.
Appendix
ROCKET AF Trial Investigators
United States: J. Anderson, N. Bedwell, M. Bilsker, G. Bruce, R. Agah, M. DeSantis, S. Eisenberg, A. Flores, W. Herzog, S. Klein, H. Snyder, S. Krueger, E. Almaguer, E. Lavie, C. Lee, G. Mallis, M. Modi, G. Woodworth, I. Niazi, B. Peart, S. Sundaram, B. Snoddy, R. Sotolongo, J. Moloney, K. Vijayaraghavan, F. Whittier, L. Yellen, S. Banerjee, D. Lustgarten, D. Suresh, M. Gelernt, L. Levinson, R. Ghanekar, I. Niazi, G. Kneller, C. Hall, Y. Fadl, D. Suresh, M. Pirwitz, W. French, N. Mayer, J. Pugeda, K. Steel, F. Mody, A. Malik, H. Chandna, A. Go, G. Emlein, W. Bowden, R. Moscoso, R. Hodson, M. Berk, D. Pan, J. Pappas, R. Orchard, G. Lynchard, N. Vijay, W. Khan, M. El Khadra, M. Antonishen, F. Cucher, M. Staab, J. Zebrack, S. Borromeo III, J. Heilman, S. Chaturvedi, S. Makam, S. Turk, T. Hyers, G. Williams, A. Labroo, S. Gill, D. Myears, J. Weinstein, J. Shanes, Y. Chandrashekhar, S. Shah, W. Reiter, T. Logemann, A. Almquist, R. Bhagwat, T. Tak, J. Shen‐Ling, P. Patel, A. Artis, A. Arouni, M. Lauer, K. Kinney, J. Elsen, P. Roan, R. Villafria, M. Sumpter, J. Ip, S. Welka, B. Schifferdecker, R. Sandoval, S. Speirs, A. Jones, T. Haldis, J. Kazmierski, J. Sutherland, D. Dietrich, E. Telfer, J. Berry, A. McElveen, J. Russell, M. Sackett, N. Antonios, D. Smith, K. Vora, A. Kirby, H. Lui, D. Mego, K. Ziada, J. Navas, A. Taussig, M. Koren, C. Vogel, F. Saba, C. Parrott, R. Schneider, A. Shirwany, M. Rubin, C. Treasure II, B. Bertolet, M. Chang, J. Langberg, R. Becker, Y. Cohen, F. McGrew, J. White, F. Arzola, J. Zelenka, A. Tannenbaum, V. Fernandes, P. Jamnadas, J. Agamasu, B. Collins, W. Jauch, B. Sasseen, D. Hotchkiss, R. Abadier, A. Osunkoya, A. Schlau, C. Chappel, M. Foster, E. Braun, E. Mostel, J. Capo, M. Ashchi, V. Howard, A. Albirini, A. Burger, D. Rolston, C. Staniloae, J. Bacon, A. Wiseman, J. McGarvey Jr, A. Sonel, G. Hamroff, D. Chang, N. Daboul, G. Broderick, A. Meholick, J. Corbelli, R. Silverman, J. Raffetto, R. Fishberg, S. Georgeson, J. Held, M. Seidner, H. Saint‐Jacques, J. Heitner, S. Kutalek, I. Friedlander, B. Hutchinson, J. Walia, N. Kondo, N. Smiley, L. Blitz, H. Dale, S. Sulman, I. Szulawski, F. Modares, R. Martin, A. Nahhas, M. Renzi, A. Akyea‐Djamson, A. Alfieri, J. Sandhu, S. Voyce, S. Amaram, G. Meyerrose, M. Shoukfeh, F. Lee, B. Villegas, O. Idowu, A. Khera, C. Sam, A. Vo, I. Lieber, T. Smith, N. Awan, C. Tsai, R. Ganim, G. Alzaghrini, W. Pitt, A. Shepherd, S. Tang, A. Go, S. Stoltz, W. Nelson, S. Cox, S. Meymandi, M. Melucci, G. Thomas, H. Gogia, C. Machell, S. Chandrasekaran, C. Brown, P. Jetty, G. Miller, G. Dykstra, N. Jaffrani, B. Zakhary, A. Caruso, R. Zolty, D. Fox, G. Jacobs, M. Lebenthal, S. Mukherjee, P. Zimetbaum, J. Kingsley, R. Jones, V. Robinson, D. Kenton, J. Usedom, S. Williams, C. Snipes, V. Wilson, R. Hasty, J. Shoemaker, V. Robinson, M. Donahue, Y. Al‐Saghir, E. Thomsen, S. Yarows, S. Chastain, P. McLaughlin, M. Wakham, D. Shrestha, J. Simmons, D. Fisher, Z. Seymour, B. Frandsen, B. First, C. Sharpe, L. Popeil, R. Guthrie, J. Hunter, O. Alvarado, J. Sandberg, N. Gutman, A. Belber; Russia: M. Arkhipov, M. Ballyzek, A. Baranov, O. Barbarash, V. Barbarich, D. Belenky, O. Berkovich, I. Bokarev, M. Boyarkin, S. Vaniev, E. Volkova, N. Gratsiansky, A. Demin, V. Zadionchenko, D. Zateyshchikov, K. Zrazhevsky, V. Mazaev, A. Martynov, S. Mikhailov, V. Mkrtchian, V. Novozhenov, T. Raskina, A. Rebrov, N. Sanina, V. Simanekov, M. Sitnikova, O. Smolenskaya, R. Stryuk, G. Storozhakov, B. Tankhilevich, S. Tereschenko, A. Khokhlov, O. Khrustalev, S. Chernov, Y. Shvarts, Y. Shubik, V. Shulman, S. Yakushin, O. Bugrova, A. Ivleva, R. Libis, N. Khozyainova, S. Maslov, E. Baranova, A. Sherenkov, I. Libov, V. Lusov, G. Chumakova, V. Kuznetsov, I. Ryamzina, O. Reshetko, S. Boldueva, N. Alekseeva, T. Novikova, V. Dvornikov, E. Idrisova, N. Shostak, N. Yarokhno, K. Tebloev, T. Treshkur, V. Mazurov, E. Loktin, I. Sedavnyh, O. Alexeeva, P. Yakhontova, A. Repin, N. Izmozherova, V. Kostenko, A. Fokin, G. Ketova; Canada: S. Kouz, R. Leader, F. Ayala‐Paredes, R. Luton, P. Ma, S. Pandey, Y. Pesant, R. Senior, G. Vertes, A. Bell, D. Crowley, S. Vizel, B. Lasko, D. Landry, L. Berger, J. Heath, R. Bessoudo, M. Ling, G. Tellier, J. Berlingieri, H. Kafka, L. Hill, G. Mazza, W. O'Mahony, M. Chilvers, M. O'Mahony, D. Newman, D. Newman, D. Newman, S. Silagy, M. Heffernan, M. Bennett, T. Bhesania, G. Rockman, K. Ng, B. Kalra, G. Meneses, W. Liang, M. Cheung, J. Kozak, G. Pugen, J. Vavougios, M. Kates, C. Nunes‐Vaz, S. Jaffer, J. Orfi, A. Faiers, C. Chung, S. Felsen, S. Bergman, I. Bernstein, L. Brownscombe, J. Stockdill, E. Silver, D. Ezekiel, N. Jagan, M. Khurana, H. Reisler, H. Goldman, T. Maung, F. Wong, G. Gillis, R. Vexler, B. Goldberg, M. Luterman, D. Gould, B. Coutu, A. Ouellet, P. MacDonald, M. Jones, R. Collette, P. Chong, T. Fargher, F. St‐Maurice, C. Fortin, R. Chehayeb, G. Proulx, R. Roy, J. Liutkus, G. Syan, D. Rupka, T. Lichtenstein, J. Kooy, D. Papastergiou, B. Lubelsky, W. Doyle, A. Rajakumar, J. Cha, A. Choudhry, H. Bhamjee, A. Mawji, M. Durfresne, C. Constance, J. Mutrie, A. Najarali, R. Warren, M. Mucha, D. Borts, P. Nord, S. Carrier, M. Dawood, G. Sabe‐Affaki, J. Archibald, N. Abram, E. Teitelbaum; South Africa: I. Ebrahim, R. Siebert, L. van Zyl, H. Theron, E. Lloyd, R. Sommers, G. Podgorski, L. Steingo, A. Dalby, J. Bayat, L. Herbst, F. Bester, C. Corbett, J. Bennett, A. Roodt, J. Roux, M. Abelson, Z. Mohamed, H. Nortje, A. Da Silva; Greece: K. Nikolaides, K. Liagkas, E. Papasteriadis, A. Achimastos, N. Koliopoulos, A. Trikas, A. Manolis; Netherlands: J. Ruiter, D. Basart, H. Crijns, A. Withagen, M. Janssen, R. Van Langeveld, I. van Gelder, B. Hamer, R. Van Der Heijden, D. Hertzeberger, M. Van Hessen, M. Pieterse, R. Groutars, A. Kuijper, G. De Ruiter, A. van Boven, P. Hoogslag, H. Kragten, H. Thijssen, R. Veldkamp; Belgium: C. Scavee, H. Heidbuchel, P. Debruyne, B. Deruyter, H. El Ali, M. Goethals, R. Cytryn, H. Striekwold, L. De Wolf, P. Goethals, F. Provenier, S. Hellemans; France: M. Galinier, D. Coisne, A. Koenig, D. Galley, S. Destrac, J. Leduc, A. Rifai, B. Citron, E. Ellie, P. Fournier, G. Steg, R. Landel, A. Robinson, F. Ziegler, J. Boulliat, M. Zuber; Spain: M. Vida, E. Galve Basilio, M. Lopez, C. Íñiguez, L. Iglesias Alonso, M. Cavero Gibanel, J. Olivan Martinez, F. Calvo Iglesias, P. Marco Vera, J. Bruguera Cortada, A. Jaber Houbani, J. Merino, F. Olaz Preciado, J. Balaguer, J. de la Hera Galarza, A. Martinez Rubio, J. Fontcuberta, J. Sotillo Marti, J. Gonzalez Juanatey, R. Del Campo, G. Vivanco, P. Alvarez Garcia, M. Pelayo; Hungary: J. Lippai, K. Zamolyi, T. Károly, A. Vertes, A. Nagy, I. Kosa, A. Janosi, G. Lupkovics, E. Kalo, T. Forster, E. Kis, J. Tenczer, D. Bereczki, S. Komoly, A. Csanyi, R. Kiss, A. Valikovics, P. Dioszeghy; Italy: F. Masini, P. Terrosu, V. Cirrincione, C. Marabotti, F. Cosmi, A. Salvioni, G. Binetti, G. Piovaccari, D. Nassiacos, G. Boriani, V. Calvi, R. De Caterina, V. Pengo, G. Parati, A. Carolei, A. D'Angelo, M. Di Biase, L. Fattore, G. Agnelli, P. Merlini, M. Furlan, M. Rasura, C. Gandolfo, W. Ageno, F. Piovella, G. Micieli; Romania: M. Cinteza, C. Fierbinteanu, D. Natase‐Melicovici, D. Ionescu, C. Macarie, I. Nanea, M. Radoi, G. Tatu‐Chitoiu, S. Dragulescu, A. Tudose, C. Militaru, C. Bengus, G. Ungureanu, A. Tau, V. Popa, O. Pirvu, M. Bojinca, D. Sipciu, M. Popescu, M. Chiru, D. Vinereanu, M. Tudoran, T. Cojocaru, M. Vintila, G. Aron, O. Petrascu, F. Bolohan; Switzerland: R. Baumgartner, L. Sekoranja; Czech Republic: J. Vojacek, B. Lacnak, I. Kellnerova, M. Dunaj, C. Cihalik, T. Janota, J. Janousek, P. Bouchal, R. Spacek, J. Choi Siruckova, P. Heinc, P. Vojtisek, M. Pirchala, J. Malecha, F. Padour, A. Linhart, E. Mandysova, J. Jandik, E. Zidkova, D. Sipula, P. Ostadal, R. Polasek, V. Stransky, G. Marcinek, D. Rysava, P. Osmancik; Austria: K. Huber, H. Drexel, M. Brainin, S. Eichinger‐Hasenauer, W. Lang, E. Pilger; United Kingdom: A. Moriarty, I. Hudson, K. Tang, J. Cleland, R. MacWalter, J. Cooke, G. McInnes, R. Durairaj, M. MacLeod, D. Murdoch, H. Kadr, G. Lip, R. Andrews, B. Hunt, P. Jackson, M. MacLeod, C. Roffe, H. Syed, P. Bath, J. Coyle, D. Kelly; Denmark: S. Stender, C. TorpPedersen, C. Tuxen, G. Jensen, T. Melchior, K. Klarlund, C. Dahlstrom, T. Nielsen, E. Nielsen, J. Bronnum‐Schou, R. Sykulski; Sweden: P. Blomstrom, C. Lindholm, T. Wallen, C. Nilsson, E. Bertholds, J. Carlsater; Norway: P. Sirnes, S. Elle, K. Risberg, K. Furuseth, A. Skag, H. Hoivik, N. Landmark, T. Kjaernli, J. Berg‐Johansen, G. Gradek; Poland: A. Drzewiecki, W. Pluta, H. Szwed, M. Trusz‐Gluza, M. Ogorek, K. Loboz‐Grudzien, P. Ruszkowski, R. Sciborski, J. Kopaczewski, K. Jaworska, J. Kubica, G. Opolski, A. Hoffman, M. Krzciuk, W. Sinkiewicz, W. Piotrowski, P. Kolodziej, M. Goszczynska, A. Rynkiewicz, L. Chojnowska, J. Lewczuk, M. Biedrzycka, M. Piepiorka, J. Kowal, A. Karczmarczyk, P. Pruszczyk, M. Tendera, Z. Gaciong, M. Krzeminska‐Pakula, Z. Kornacewicz‐Jach, G. Kania; Germany: J. Brachmann, H. Lawall, H. Guelker, S. Spitzer, S. MoebiusWinkler, C. Dempfle, C. Bode, H. Darius, S. Genth‐Zotz, S. Sommer, J. Roehnisch, R. Strasser, W. Daenschel, C. Schwencke, J. vom Dahl, M. Meuser, S. Behrens‐Spandau, S. Behrens‐Humbold, A. Muegge, N. Schoen, P. Grooterhorst, H. Ebert, A. Kraemer, B. Kohler, J. Taggeselle, G. Claus, H. Sarnighausen, A. Al‐Zoebi, T. Schroeder, M. Weissbrodt, R. Lange, M. Gabelmann, S. Kaeaeb, M. Doerr, D. Boscher, R. Bosch, F. Sonntag, C. Bauknecht, H. Omran, M. Leicht, R. Veltkamp, H. Hohensee, H. Dieckmann, B. Winkelmann, P. Bernhardt, A. Schnabel, C. Kadel, N. Proskynitopoulos, K. Seidl, S. Schellong; Peru: C. Rios, C. Guevara, R. Coloma, H. Torrejon, J. Parra Galvan, J. Drago Silva, J. Gallegos, A. Mendoza, S. Negron, L. Watanabe, F. Medina; Mexico: L. Virgen Carrilo, H. Alvarez Lopez, I. Rodriguez, J. Leiva‐Pons, A. Baños Velasco, J. Villarreal‐Careaga, M. De los Rios, M. Alcocer Gamba, G. Llamas Esperon, E. Villeda; Argentina: A. Ahuad Guerrero, A. Alvariqueta, M. Amuchastegui, J. Bluguermann, G. Caime, C. Cuneo, A. Gabito, D. Garcia Brasca, M. Hominal, H. Jure, H. Luquez, O. Montana, D. Piskorz, S. Listorti, J. Serra, H. Sessa, S. Varini, N. Vita, J. Aiub, I. MacKinnon, S. Chekherdemian, J. Castagnino, J. Cimbaro Canella, H. Sgammini, A. Escudero, G. Albina, C. Rapallo, C. Balparda, M. Chahin, V. Fuentealba, M. Riccitelli, J. Casabe, L. Lobo Marquez, R. Kevorkian, J. Cuadrado, R. Dran, J. Muntaner, M. Gonzalez, L. Cartasegna, E. Hasbani, A. Hrabar, A. Sanchez, D. Vogel, A. Hershson; Brazil: A. Avezum, J. Jaber, P. Ernesto Leaes, A. Bozza, A. Lorga Filho, P. Pimentel Filho, J. Moura Jorge, L. Maia, E. Manenti, R. D'Aurea Mora Jr, J. de Souza Neto, D. Precoma, A. Rabelo, J. Rocha, P. Rossi, J. Kerr Saraiva, L. Zimerman, L. Bodanese, E. Figueiredo, W. Sebba Barroso de Souza, J. Braga, S. Alessi, M. Gomes, R. Silva, M. Teixeira, F. Costa, M. Motta, D. Sobral Filho, G. Reis, B. Garbelini Jr, S. Zimmermann, A. Pereira Barretto, H. Dohmann, J. Barreto Filho, N. Ghorayeb, F. Borelli, F. Rossi dos Santos, M. Lopes Prudente; Chile: M. Vejar, F. Lanas, R. Del Pino, S. Potthoff, G. Charme, A. Aguirre, A. Saldana, E. Garces, L. Bunster, H. Figueroa, C. Olivares, C. Raffo, E. Vergara, P. Sepulveda, G. Jano, J. Morales Alvarado; Colombia: R. Suarez, M. Urina, G. Perez, A. Quintero, L. Pava, R. Botero Lopez, C. Luengas, E. Hernandez, D. Sanchez, C. Poveda, J. Coronel, R. Beltran, C. Jaramillo, J. Pardo; Venezuela: C. Ponte Negretti, J. Isea, G. Vergara, I. Morr; Malaysia: K. Sim, W. Wan Ahmad, Z. Yusof, A. Rosman, H. Basri; Australia: P. Thompson, I. Jeffery, P. Purnell, P. Roberts‐Thomson, W. Heddle, J. Waites, D. Walters, J. Amerena, P. Challa, J. Karrasch, A. Lowy, D. Fitzpatrick, M. Parsons, T. Phan, J. Karrasch, J. Karrasch, C. Bladin, G. Donnan, G. Aroney, R. Gerraty, C. Anderson, P. Blombery, P. Martin, K. Tissa Wijeratne, D. Cross, D. Crimmins, D. Packham, D. Jackson; Philippines: W. Chua, R. Merino, M. Magno, L. Tirador, E. Batalla, C. Manalo, N. Uy, G. Ebo, E. Reyes, A. Bernan; New Zealand: M. Richards, H. Hart, S. Mann, R. Fisher, R. Stewart, G. Wilkins, A. Barber; Singapore: R. Tan, H. Ong, R. Singh; Thailand: A. Sukonthasarn, S. Tanomsup, R. Krittayaphong, C. Piamsomboon, D. Piyayotai, B. Sunsaneewitayakul; Korea: S. Baek, H. Seo, S. Rim, C. Kim, K. Kim, K. Ryu, S. Jo, S. Tahk, H. Lee, C. Kim, Y. Kim, D. Shin, Y. Choi, N. Chung, J. Namgung, C. Kim, T. Hong, W. Shin, S. Jin; China: X. Yan, G. Fu, G. Lu, K. Yang, D. Xu, J. Chen, J. Liu, S. Wu, J. Song, Y. Liao, B. Xu, Z. Li, S. Ma, Y. Yin, Y. Zhao, D. Hu, C. Ma, J. Ma, J. Sun, H. Li, X. Hong, B. Yu, Q. Lu, J. Yang, Z. Wu, Y. Li, Y. Huang, Y. Wang, M. Liu, Y. Cheng, T. Yang, K. Chen, H. Wang, Z. Yuan, J. Wang, Z. Zeng, Y. Chen; Turkey: O. Yavuzgil, O. Kozan, M. Etemoglu, E. Diker, A. Belgi, C. Ceyhan, V. Cin, O. Yilmaz, N. Ata, B. Altunkeser, A. Agacdiken Agir, A. Karadede, R. Topsakal; India: R. Gulati, A. Madhavan, S. Jain, A. Oomman, S. Janorkar, P. Kumar, A. Madhukar Naik, H. Thacker, V. Rajasekhar, R. Reddy, C. Keshavamurthy, P. Jain, B. Gowdappa, M. Gadkari, A. Abhyankar, B. Ramesh Babu, P. Vydianathan, S. Sinha, N. Garg, S. Rao, P. Gautam, K. Chockalingam, M. Kumbla, R. Panwar, D. Banker; Finland: M. Kaste, P. Jäkälä, R. Roine; Bulgaria: A. Mihov, D. Raev, V. Yordanova, S. Dimitrova, H. Benov, V. Tsanova, M. Kyolean, S. Marchev, A. Stoikov, N. Zdravkov, K. Ramshev, A. Krastev, P. Stamenova, I. Angelova, G. Pencheva, V. Grigorova; Lithuania: B. Petrauskiene, I. Skripkauskiene, R. Raugaliene, S. Norkiene, R. Mazutavicius, A. Kavoliuniene, S. Aidietiene, J. Aganauskiene, A. Dailydkiene, J. Marcinkeviciene, L. Grigoniene, J. Anusauskiene, R. Kavaliauskiene; Ukraine: V. Lizogub, L. Rudenko, V. Tseluyko, L. Voronkov, O. Sychov, Y. Svyshchenko, Y. Sirenko, V. Serkova, N. Seredyuk, T. Pertseva, V. Netyazhenko, V. Lishnevska, O. Kupchynska, O. Koval, G. Koshukova, O. Karpenko, O. Grishyna, A. Faynyk, G. Dzyak, O. Dyadyk, L. Yena, V. Volkov, I. Rudyk, M. Kopytsya, L. Kononenko, K. Amosova, S. Zhurba, V. Kazimirko, I. Iuzkiv, O. Shershnyova, T. Khomazyuk, V. Batushkin, I. Vykhovanyuk, G. Popik, V. Skrebkov, A. Skurtov, T. Mishchenko, N. Lytvynenko, L. Sokolova, M. Vatutin, M. Shved, B. Rebrov, L. Kadina, M. Vajda, G. Ursol, V. Zheleznyy, T. Vysochanska, A. Gozhenko; Hong Kong: K. Fan, D. Ho, H. Tse, C. Yu, L. Wong; Taiwan: H. Yeh, P. Pai, I. Hsieh, C. Huang, Y. Hsieh, W. Yin, L. Tsai, T. Huang, C. Chen, F. Chiang, K. Ueng, M. Charng, H. Yeh; Israel: A. Marmor, A. Katz, A. Butnaru, B. Lewis, M. Eldar, S. Rosenhack, N. Elias, B. Koifman, M. Shochat, M. Swissa, R. Zimlichman, T. Bental, A. Weiss, R. Ganam, M. Elias, W. Nseir, A. Oliven, B. Brenner, M. Dayan
Sources of Funding
The ROCKET AF trial was sponsored by Johnson & Johnson Pharmaceutical Research & Development (Raritan, NJ) and Bayer HealthCare AG (Leverkusen, Germany).
Disclosures
Pokorney: Research grants from AstraZeneca, Boston Scientific, and Gilead. Consultant/advisory board from Janssen Pharmaceutical. Piccini: Research grants from ARCA biopharma, Boston Scientific, GE Healthcare, Janssen Scientific, Johnson & Johnson, and ResMed. Consultant/advisory boards for ChanRx, Johnson & Johnson, and Spectranetics. Stevens: None to report. Patel: Consultant/advisory board for Bayer, Janssen, AstraZeneca, and Genzyme; Institutional research grant for Johnson & Johnson, and AstraZeneca. Pieper: None to report. Halperin: Consulting fees from AstraZeneca, Bayer AG HealthCare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Janssen, Johnson & Johnson, Medtronic, Ortho‐McNeil‐Janssen Pharmaceuticals, Pfizer, and Sanofi Aventis. Breithardt: Honoraria from Bayer HealthCare, BMS/Pfizer; Consultant/advisory board for Bayer HealthCare, BMS/Pfizer, and Sanofi Aventis. Singer: Consulting/advisory board for Bayer HealthCare, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, and Pfizer; Research grants from Bristol‐Myers Squibb and Johnson & Johnson. Hankey: Honoraria/consulting fees from Bayer and Medscape (theheart.org). Hacke: Consulting fees from Boehringer Ingelheim, Bayer HealthCare, and Daiichi Sankyo; Research grants from Boehringer Ingelheim. Becker: Consulting fees/honoraria from Portola, Daiichi‐Sankyo, Bristol‐Myers Squibb, and Boehringer Ingelheim; Research grants from AstraZeneca and Johnson & Johnson. Berkowitz: Employee of Bayer HealthCare Pharmaceuticals. Nessel: Employee of Janssen Research & Development. Mahaffey: Research grants from Amgen, Daiichi‐Sankyo, Johnson & Johnson, Medtronic, St. Jude, and Tanax; consulting fees/honoraria from American College of Cardiology, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cubist, Eli Lilly, Elsevier, Epson, Forest, Glaxo Smith Kline, Johnson & Johnson, Medtronic, Merck, Mt. Sinai, Myokardia, Omthera, Portola, Purdue Pharma, Spring Publishing, The Medicines Company, Vindico, and WebMD; and equity stake in BioPrint Fitness. Fox: Consulting fees/honoraria from Boehringer Ingelheim, Sanofi‐Aventis, Astra Zeneca, Johnson & Johnson/Bayer, and Janssen; research grants from Eli Lilly. Califf: Consulting fees and research funding from Johnson & Johnson; all other industry interactions are listed at www.dcri.org.
Supporting information
Data S1. End point event definitions.
(J Am Heart Assoc. 2016;5:e002197 doi: 10.1161/JAHA.115.002197)
An accompanying Data S1 is available at http://jaha.ahajournals.org/content/5/3/e002197/suppl/DC1
References
- 1. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D; Framingham Heart Study . Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 5. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 6. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 7. Friberg L, Hammar N, Pettersson H, Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort‐Study of Atrial Fibrillation (SCAF). Eur Heart J. 2007;28:2346–2353. [DOI] [PubMed] [Google Scholar]
- 8. Steinberg JS, Sadaniantz A, Kron J, Krahn A, Denny DM, Daubert J, Campbell WB, Havranek E, Murray K, Olshansky B, O'Neill G, Sami M, Schmidt S, Storm R, Zabalgoitia M, Miller J, Chandler M, Nasco EM, Greene HL. Analysis of cause‐specific mortality in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109:1973–1980. [DOI] [PubMed] [Google Scholar]
- 9. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, Yusuf S; RE‐LY Investigators . Causes of death and influencing factors in patients with atrial fibrillation: a competing‐risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation. 2013;128:2192–2201. [DOI] [PubMed] [Google Scholar]
- 10. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD; Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) Investigators . A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 11. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 12. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 13. Rocket AF Study Investigators . Rivaroxaban‐once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;2159:340–347.e1. [DOI] [PubMed] [Google Scholar]
- 14. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 15. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21‐year community‐based study. J Am Coll Cardiol. 2007;49:986–992. [DOI] [PubMed] [Google Scholar]
- 16. Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larsen TB, Rasmussen LH, Skjoth F, Due KM, Callréus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in “real‐world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–2273. [DOI] [PubMed] [Google Scholar]
- 18. Shore S, Carey EP, Turakhia MP, Jackevicius CA, Cunningham F, Pilote L, Bradley SM, Maddox TM, Grunwald GK, Barón AE, Rumsfeld JS, Varosy PD, Schneider PM, Marzec LN, Ho PM. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J. 2014;167:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pathak RK, Middeldorp ME, Lau DH, Mehta A, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST‐AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 20. Hess PL, Kim S, Piccini JP, Allen LA, Ansell JE, Chang P, Freeman JV, Gersh BJ, Kowey PR, Mahaffey KW, Thomas L, Peterson ED, Fonarow GC. Use of evidence‐based cardiac prevention therapy among outpatients with atrial fibrillation. Am J Med. 2013;126:625–632.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reinier K, Marijon E, Uy‐Evabadi A, Teodorescu C, Narayanan K, Chugh H, Gunson K, Jui J, Chugh SS. The association between atrial fibrillation and sudden cardiac death: the relevance of heart failure. JACC Heart Fail. 2014;2:221–227. [DOI] [PubMed] [Google Scholar]
- 22. Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquie JL, Scavee C, Bordachar P, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. [DOI] [PubMed] [Google Scholar]
- 23. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, Goromonzi F, Sawhney V, Duncan E, Page SP, Ullah W, Unsworth B, Mayet J, Dhinoja M, Earley MJ, Sporton S, Schilling RJ. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7:31–38. [DOI] [PubMed] [Google Scholar]
- 24. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 25. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 26. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. End point event definitions.


