Abstract
Background
Carotid atherosclerosis is associated with subclinical ischemic cerebrovascular disease, but its role in hemorrhage‐prone small vessel disease—represented by cerebral microbleed (CMB)—is unclear, although vascular risk factors underlie both conditions. We hypothesized that persons with carotid atherosclerosis would have higher risk of CMB, particularly in deep regions.
Methods and Results
We studied 1243 participants in the Framingham Offspring Study (aged 56.9±8.8 years; 53% women) with carotid ultrasound available on 2 occasions (1995–1998 and 2005–2008) prior to brain magnetic resonance imaging. Using multivariable logistic regression, we related baseline carotid stenosis, baseline intima–media thickness, and site‐specific carotid intima–media thickness progression (at internal and common carotid locations) to the prevalence and location (lobar or deep plus mixed) of CMB. In addition, we assessed effect modification by lipid levels and use of statin and antithrombotic medications. Carotid stenosis ≥25% (a marker of cerebrovascular atherosclerosis) was associated with presence of CMB overall (Odds Ratio 2.20, 95% CI 1.10–4.40) and at deep and mixed locations (odds ratio 3.60, 95% CI 1.23–10.5). Baseline carotid intima–media thickness was not associated with CMB. Progression of common carotid artery intima–media thickness among persons on hypertension treatment was associated with lower risk of deep and mixed CMB (odds ratio per SD 0.41, 95% CI 0.18–0.96).
Conclusions
Cumulative vascular risk factor exposure may increase the risk of CMB, especially in deep regions. The apparent paradoxical association of carotid intima–media thickness progression with lower risk of CMB may reflect benefits of intensive vascular risk factor treatment among persons with higher cardiovascular risk and deserves further investigation. If replicated, the results may have potential implications for assessment of preventive and therapeutic interventions for subclinical cerebral hemorrhage.
Keywords: brain magnetic resonance imaging, carotid atherosclerosis, carotid intima–media thickness, cerebral microbleeds
Subject Categories: Epidemiology, Magnetic Resonance Imaging (MRI), Ultrasound, Cerebrovascular Disease/Stroke, Atherosclerosis
Introduction
Carotid artery stenosis and carotid intima–media thickness (CIMT) are predictors of myocardial infarction, stroke, and dementia.1, 2 Furthermore, a decrease in the rate of CIMT progression may serve as a surrogate measure of the effectiveness of cardiovascular prevention treatments.3 Higher CIMT and stenosis represent the cumulative effect of vascular risk factors and also have been related to subclinical ischemic brain injury on magnetic resonance imaging (MRI; white matter hyperintensities and covert infarcts).4, 5 Hemorrhage‐prone cerebral small vessel disease, represented by cerebral microbleeds (CMBs) detected on brain MRI, is increasingly recognized as a marker of stroke, dementia, and risk of death.6 Vascular risk factors have been related to CMB, but the relation of CIMT and CIMT progression—representing their cumulative effect—has not been elucidated. Carotid atherosclerosis results from the interplay of vascular risk factors affecting large vessels and involves disease mechanisms such as inflammation, vascular remodeling, and endothelial dysfunction, which are shared by other forms of vascular disease including cerebral small vessel disease. Although CIMT has been related to ischemic cerebral small vessel disease, its relation to hemorrhage‐prone vessel disease is not clear. If they are related, large artery markers of atherosclerosis, such as higher CIMT, CIMT progression, and stenosis, may serve as clinical markers of preclinical hemorrhage‐prone cerebral small vessel disease. In addition, they may help clarify the relative contribution of vascular risk factors to the main vascular pathologies causing intracerebral hemorrhage, cerebral amyloid angiopathy, and hypertensive vasculopathy, which are represented by CMB according to their topographic location. Although cerebral amyloid and hypertensive angiopathy represent distinct vascular entities, they coexist in most persons, and the role of vascular risk factors in persons with evidence of both (ie, mixed‐location CMB) is not entirely clear. Whether CIMT progression is predictive of hemorrhage‐prone cerebral small vessel disease has not been studied and may have potential screening and therapeutic implications.
We studied the associations of carotid stenosis, baseline CIMT, and site‐specific CIMT progression (ie, at common and internal carotid locations) with CMB presence overall and stratified by topography on brain MRI. We further explored the modification of any observed associations by treatments used for cardiovascular event prevention and by lipid levels.
Methods
Sample
Participants in the Framingham Offspring Study underwent carotid duplex ultrasound at the sixth examination cycle (1995–1998) and a repeat study at the eighth examination cycle (2005–2008). Among participants who underwent both carotid ultrasounds and also had information on levels of vascular risk factors at each examination (N=2377), 1332 participants had brain MRI measurements on or after their exam 8 study date. A total of 102 participants were excluded for having prevalent clinical stroke, dementia, or other neurological conditions that could affect MRI measures, and 23 participants were excluded for lack of CMB measurements on MRI, yielding a study sample of 1243 participants (Figure). The institutional review board of Boston University Medical Center approved the study protocol, and informed consent was obtained from all participants.
Figure 1.
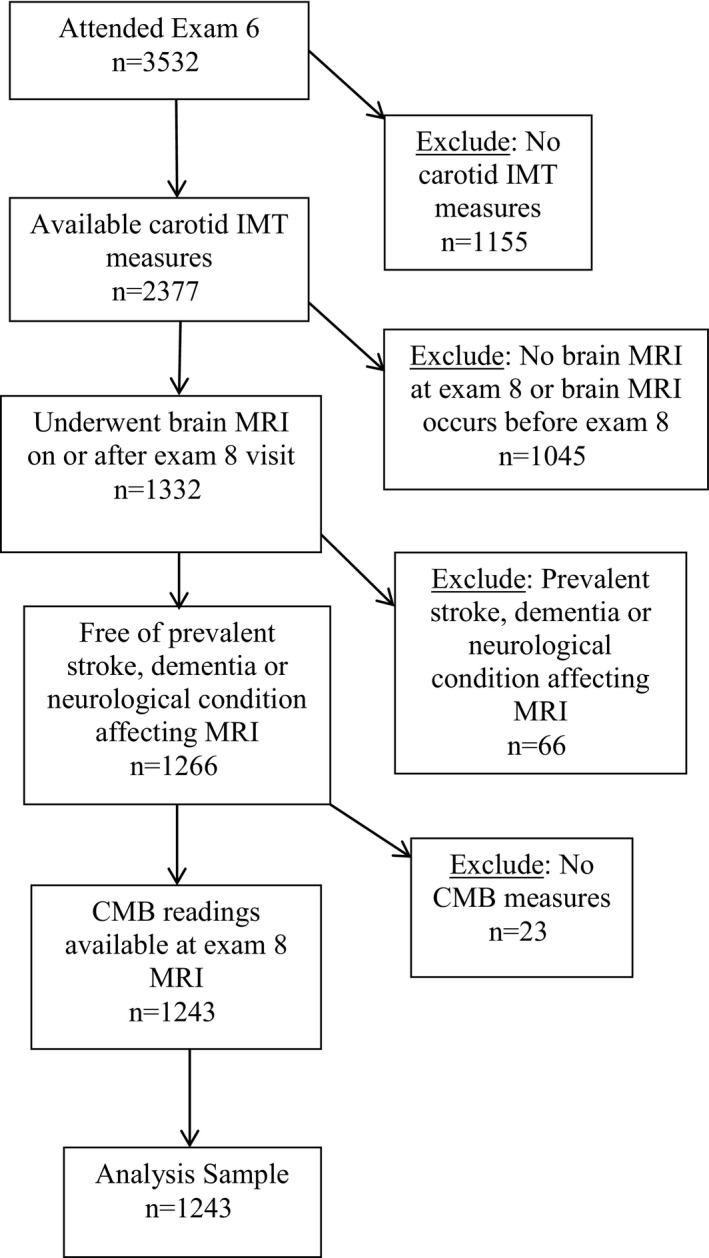
Flow chart of selection of the study sample. CMB indicates cerebral microbleed; IMT, intima‐media thickness; MRI, magnetic resonance imaging.
Carotid Ultrasound
Carotid ultrasound was acquired by a certified sonographer following a standard protocol and using an ultrasound device equipped with a high‐resolution linear‐array transducer with color Doppler and Doppler spectral analyzer (model SSH‐140A; Toshiba America Medical Systems). The common carotid arteries were imaged with a 7.5‐MHz transducer, and the carotid bulb and internal carotid arteries were imaged using a 5‐MHz transducer (−3‐dB point: 6.2 MHz). Images were gated to an electrocardiogram and taken at end‐diastole (peak of the R‐wave).
Carotid stenosis
An image of the distal common carotid artery (CCA), 2 of the carotid artery bulb and 2 of the proximal 2 cm of the internal carotid artery (ICA), were analyzed by 1 operator and overread by an experienced radiologist (J.F.P.). Hemodynamically significant stenosis (≥50%) was defined by peak‐systolic velocities ≥150 cm/s, and lower velocities were divided into 3 groups by the same operator: 0 (no stenosis), 1% to 24%, and 25% to 49%. The side with the more severe degree of ICA stenosis was used. Intrareader reproducibility of carotid stenosis ≥25% has been reported previously (κ=0.69).7
Carotid intima–media thickness
Intima–media thickness (IMT) was measured at the CCA, the carotid bulb, and the ICA bilaterally, and the mean of the maximal IMT measurements of the near and far walls was used (maximum 4 artery walls for the CCA). The internal carotid/bulb IMT was defined as the mean of the 4 maximal IMT measurements made in the carotid artery bulb and the ICA on both sides for a maximum of 16 wall segments. CIMT change was determined by an experienced investigator (J.F.P.). Reproducibility of IMT measurements has been reported previously. The Pearson correlation coefficient for replicated readings in 37 participants was 0.94 for the mean IMT of the CCA and 0.76 for the maximum IMT of the ICA.8
CIMT change
Carotid‐site IMT rate of change (mm/year) was defined as the difference between site‐specific IMT measured in the second carotid ultrasound minus the same site‐specific IMT measured in the first carotid ultrasound, divided by the time interval between the 2 studies.
Brain MRI
A 1.5‐T MRI machine (Siemens Magnetom) was used to obtain the following sequences: coronal T2‐weighted 2470/20 to 80 (repetition time/echo time), echo train length 8, field of view 22 cm, acquisition matrix 192×256 interpolated to 256×256 with 1 excitation, 4‐mm slice thickness from nasion to occiput, sagittal T1‐weighted 11.4/4.4, 3‐dimensional fast low‐angle shot, 192‐mm slab, 128 slices of 1.5‐mm thickness, and 12° flip angle and axial T2*gradient echo 656/26 (repetition time/echo time), field of view 22 cm, acquisition matrix 144×256, 30° flip angle, 19 slices of 5‐mm thickness, and 2‐mm gap.
MRI data were analyzed using a custom‐designed in‐house image analysis package, QUANTA 2, written for the Linux operating system. All analyses were done blind to each participant's demographic and clinical characteristics.
CMB definition
CMBs were assessed in the MRI scan following acquisition of the second carotid ultrasound during the time period specified above. CMBs were defined using standard criteria9 as rounded or ovoid hypointense lesions on T2*–gradient recalled echo weighted sequence measuring ≤10 mm in diameter and surrounded by brain parenchyma over at least half the circumference of the lesion and excluding CMB mimics. CMBs were grouped according to brain location into any CMB, lobar only, and deep plus mixed location (ie, CMB in deep location only plus any CMB in mixed deep and lobar location). Reliability measures for CMB readings have been published.10 The intrarater reliability based on blinded reading of 200 scans on 2 separate occasions was excellent (κ=0.78). Interrater reliability comparing 2 independent readers in a subset of 200 scans was excellent (κ=0.78).
Vascular Risk Factors
Systolic and diastolic blood pressures were taken as the average of the Framingham clinic physician's 2 measurements. Hypertension was defined by the classification of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg or use of antihypertensive medications). Current cigarette smoking was defined as self‐reported use in the year prior to the examination. Diabetes was defined as fasting glucose ≥126 mg/dL (≥7 mmol/L) or use of insulin or oral hypoglycemic medications. Prevalent cardiovascular disease included coronary heart disease, heart failure, and peripheral arterial disease.
Medication use was assessed by self‐report during interview at the corresponding examination cycle closest to baseline carotid duplex, including antiplatelet agents, anticoagulant therapies, and statin use.
Statistical Analysis
We used multivariable logistic regression analysis to relate carotid stenosis, baseline CIMT, and CIMT change (site specific) to CMB presence overall and CMB presence by brain location. The primary analysis was adjusted for age, sex, and time interval between the carotid study and brain MRI. A second model was additionally adjusted for levels of systolic blood pressure, diabetes, current smoking, hypertension, prevalent cardiovascular disease, and statin use. A third model was additionally adjusted for baseline CIMT.
We assessed for interactions with use of medications including statin and antithrombotic therapy, antihypertensive treatment, and lipid levels (dichotomized, <10th percentile versus higher), given their potential role in modifying the association between carotid atherosclerosis and CMB presence. All statistical analysis was done using SAS version 9.4 (SAS Institute). A P value of <0.05 was considered statistically significant; because these analyses were considered exploratory, we did not correct for multiple testing.
Results
The mean age of our sample was 56.9±8.8 years at the sixth examination cycle and 66.9±8.7 years at the time of MRI, and 53% were women (Table 1). We observed CMB in 8.3% of participants, with carotid stenosis ≥25% in 13.0% and ≥50% in only 1.5%. Baseline mean CIMT was 1.65 mm (SD 0.90 mm) and 0.62 mm (SD 0.12 mm) at the CCA and ICA, respectively. The mean rate of CIMT change was 0.008 mm/year (±0.010 mm/year) for the CCA and 0.065 mm/year (±0.087 mm/year) for the ICA. In multivariable analysis (Table 2), after adjusting for vascular risk factors and baseline IMT, we observed an association between baseline carotid stenosis ≥25% and presence of any CMB (odds ratio 2.20, P<0.05) and deep plus mixed CMB (odds ratio 3.60, P<0.05). Baseline CIMT was not related to presence of CMB overall or stratified by brain location. CIMT progression measured at the CCA was related to lower odds of the presence of deep plus mixed CMB after adjustment for vascular risk factors and baseline CIMT (odds ratio 0.41, P<0.01), whereas ICA IMT change was not associated with presence of CMB.
Table 1.
Characteristics of the Study Sample (n=1243)
| Clinical characteristics | |
| Women | 661 (53.2) |
| Age at exam 6, years | 56.9 (8.8) |
| Age at exam 8, years | 66.3 (8.7) |
| Age at MRI testing, years | 66.9 (8.7) |
| Time between exam 6 and MRI, years | 10.0 (1.1) |
| Time between exam 8 and MRI, years | 0.58 (0.71) |
| Exam 6 covariates | |
| Systolic blood pressure, mm Hg | 125 (18) |
| Diabetes mellitus | 94 (7.6) |
| Current smokers | 161 (13.0) |
| Prevalent cardiovascular disease | 78 (6.3) |
| Hypertension JNC 7 stage ≥1 | 405 (32.7) |
| Total cholesterol level, mg/dL | 204 (37) |
| Hypertension treatment | 262 (21.2) |
| Lipid lowering therapy use | 132 (10.6) |
| Statin use | 108 (8.7) |
| Antithrombotic use | 328 (26.4) |
| Carotid ultrasound measures | |
| ICA IMT duplex 1 | 1.65 (0.90) |
| ICA IMT duplex 2 | 2.26 (1.12) |
| ICA IMT change per year | 0.065 (0.087) |
| CCA IMT duplex 1 | 0.62 (0.12) |
| CCA IMT duplex 2 | 0.70 (0.17) |
| CCA IMT change per year | 0.0084 (0.010) |
| Stenosis ≥25% | 158 (12.8) |
| Stenosis ≥50% | 20 (1.6) |
| MRI measures | |
| Cerebral microbleeds | 101 (8.2) |
Values are mean (SD) for continuous variables and n (%) for categorical variables. Prevalent cardiovascular disease includes coronary heart disease, heart failure, and intermittent claudication. Participants were excluded if they were attending the baseline exam (exam 6) without MRI or with prevalent stroke, dementia, or other neurological condition affecting the MRI measures. CCA indicates common carotid artery; ICA, internal carotid artery; IMT, intima–media thickness; JNC 7, Seventh Report of the Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; MRI, magnetic resonance imaging.
Table 2.
Association Between Carotid Atherosclerosis Measures and CMB Presence
| Exposure | Model | CMB Location | |||||
|---|---|---|---|---|---|---|---|
| Any (n=101) | Lobar Only (n=67) | Deep Plus Mixed (n=34) | |||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Carotid stenosis (ICA)a | |||||||
| ≥25% | 1 | 1.62 (0.95–2.75) | 0.08 | 1.21 (0.62–2.38) | 0.66 | 2.64 (1.18–5.89)b | 0.02b |
| 2 | 1.44 (0.82–2.52) | 0.21 | 1.26 (0.62–2.54) | 0.53 | 1.90 (0.81–4.49) | 0.14 | |
| 3 | 2.20 (1.10–4.40)b | 0.03b | 1.69 (0.72–3.96) | 0.23 | 3.60 (1.23–10.5)b | 0.02b | |
| ≥50% | 1 | 1.38 (0.38–5.06) | 0.62 | 2.20 (0.60–8.12) | 0.24 | c | c |
| 2 | 1.16 (0.30–4.49) | 0.83 | 2.21 (0.58–8.46) | 0.25 | c | c | |
| 3 | 1.99 (0.48–8.27) | 0.35 | 3.66 (0.83–16.1) | 0.09 | c | c | |
| Baseline IMTa (per SD increment) | |||||||
| CCA | 1 | 0.93 (0.75–1.16) | 0.51 | 0.86 (0.65–1.13) | 0.27 | 1.06 (0.76–1.48) | 0.75 |
| 2 | 0.91 (0.73–1.13) | 0.39 | 0.85 (0.64–1.13) | 0.27 | 0.97 (0.68–1.39) | 0.88 | |
| ICA | 1 | 0.98 (0.79–1.20) | 0.81 | 0.94 (0.72–1.22) | 0.63 | 1.05 (0.76–1.45) | 0.76 |
| 2 | 0.88 (0.71–1.10) | 0.27 | 0.90 (0.69–1.18) | 0.46 | 0.86 (0.59–1.23) | 0.40 | |
| Change in IMT (per SD increment) | |||||||
| CCA | 1 | 0.88 (0.68–1.14) | 0.34 | 1.04 (0.85–1.26) | 0.72 | 0.46 (0.27–0.78)b | 0.004b |
| 2 | 0.79 (0.59–1.05) | 0.10 | 0.98 (0.77–1.26) | 0.89 | 0.41 (0.23–0.72)b | 0.002b | |
| 3 | 0.79 (0.59–1.05) | 0.11 | 1.01 (0.79–1.28) | 0.97 | 0.41 (0.23–0.72)b | 0.002b | |
| ICA | 1 | 1.13 (0.93–1.38) | 0.22 | 1.10 (0.87–1.40) | 0.42 | 1.18 (0.86–1.61) | 0.31 |
| 2 | 1.12 (0.92–1.36) | 0.27 | 1.10 (0.87–1.39) | 0.45 | 1.18 (0.85–1.63) | 0.32 | |
| 3 | 1.09 (0.89–1.35) | 0.41 | 1.08 (0.84–1.39) | 0.56 | 1.14 (0.81–1.62) | 0.45 | |
Standard deviation values for IMT are as follows: baseline IMT (CCA, 1 SD=0.12; ICA, 1 SD=0.90), change in IMT (CCA, 1 SD=0.010; ICA, 1 SD=0.087). Model 1 was adjusted for age, sex, time to MRI (years between exam 6 and MRI for carotid stenosis and baseline IMT; years between exam 8 and MRI for change in IMT). Model 2 was additionally adjusted for diabetes, smoking, hypertension, systolic blood pressure, prevalent cardiovascular disease, and statin use. Model 3 was additionally adjusted for baseline carotid IMT. CCA indicates common carotid artery; CMB, cerebral microbleed; ICA, internal carotid artery; IMT, intima–media thickness; MRI, magnetic resonance imaging; OR, odds ratio.
Carotid stenosis and baseline IMT measured at baseline ultrasound, exam 6.
P<0.05.
Insufficient sample size.
No statistically significant interactions (P<0.05) were observed with lipid levels or with antithrombotic or lipid‐lowering therapies (Table S1). In analysis stratified by hypertension treatment use, the relation of CCA IMT change and deep plus mixed CMB was attenuated and remained significant only in those using hypertension medications (Table 3). The direction of effect, however, was similar in those not taking hypertension medications, and the P value for interaction between change in CCA IMT and hypertension treatment was not statistically significant (P=0.57).
Table 3.
Association Between CCA IMT Progression and CMB Presence, Stratified by Hypertension Treatment
| Exposure | Outcome | Model | No Hypertension Treatment (n=963) | Hypertension Treatment (n=261) | P Value for Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Participants With CMBs | OR (95% CI) | P Value | Participants With CMBs | OR (95% CI) | P Value | ||||
| Change in CCA IMT (per SD increment) | Any CMB | 1 | 63 | 0.78 (0.53–1.15) | 0.22 | 36 | 0.89 (0.62–1.29) | 0.55 | 0.68 |
| 2 | 63 | 0.78 (0.53–1.14) | 0.20 | 36 | 0.84 (0.57–1.23) | 0.36 | 0.69 | ||
| 3 | 63 | 0.78 (0.53–1.16) | 0.22 | 36 | 0.83 (0.57–1.22) | 0.35 | 0.68 | ||
| Lobar only | 1 | 42 | 0.93 (0.62–1.39) | 0.72 | 23 | 1.04 (0.81–1.32) | 0.78 | 0.68 | |
| 2 | 42 | 0.95 (0.63–1.43) | 0.81 | 23 | 0.98 (0.75–1.28) | 0.90 | 0.60 | ||
| 3 | 42 | 0.97 (0.64–1.48) | 0.90 | 23 | 1.00 (0.76–1.30) | 0.97 | 0.60 | ||
| Deep plus mixed | 1 | 21 | 0.54 (0.27–1.09) | 0.08 | 13 | 0.38 (0.17–0.87) | 0.02 | 0.53 | |
| 2 | 21 | 0.51 (0.25–1.05) | 0.07 | 13 | 0.41 (0.18–0.94) | 0.03 | 0.57 | ||
| 3 | 21 | 0.51 (0.24–1.05) | 0.07 | 13 | 0.41 (0.18–0.96) | 0.04 | 0.57 | ||
Model 1 was adjusted for age, sex, and years between exam 8 and MRI. Model 2 was additionally adjusted for diabetes, smoking, hypertension, systolic blood pressure, prevalent cardiovascular disease, and statin use. Model 3 was additionally adjusted for baseline carotid IMT. CCA indicates common carotid artery; CMB, cerebral microbleed; IMT, intima media thickness; OR, odds ratio.
A 1‐SD increment is 0.010 for CCA IMT.
To better understand the apparently paradoxical association of higher CIMT progression with lower risk of CMB, we conducted additional exploratory analysis (Tables S1 through S5) and observed that among participants with higher CIMT progression, persons on hypertension treatment were older, were more likely to be men, and had higher blood pressure levels, higher cholesterol concentrations, and higher proportions of diabetes, smoking, and prevalent cardiovascular disease (Table S1). In analysis stratified by age, sex, diabetes, and smoking, we observed that the lower risk of CMB was present in participants aged <65 years, in women, in those with diabetes, and in nonsmokers (Table S2). Analysis stratified by blood pressure target (<140/90 versus higher) showed that the significantly lower risk of CMB was seen only among participants with blood pressure <140/90 mm Hg (Table S3). Similarly, among participants using statins, those on antihypertensive treatment and those with blood pressure <140/90 had significantly lower odds of CMB (Table S5). The formal test for interaction was statistically significant only for the group of statin users, not for any of the other abovementioned vascular risk factors.
We evaluated the associations among antihypertensive treatment class categorized into major groups (calcium channel blocker, beta blocker, diuretic, and angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker) and observed that lower CMB risk was seen only among those on an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker (Table S4).
Discussion
We previously reported an association of carotid stenosis and IMT with ischemic brain injury11 and now report an association with subclinical hemorrhage‐prone small vessel disease. Carotid atherosclerosis measured at the ICA was associated with increased odds of CMB, mainly in deep regions, and the association was stronger with increased severity of atherosclerosis; there was a trend suggesting an association of higher ICA IMT change with greater odds for deep plus mixed CMB and a significant association of ICA stenosis >25% with CMB presence. We also found lower odds of deep and mixed CMB presence among participants with greater CCA IMT progression. The findings suggest that there is a graded relationship of ICA carotid atherosclerosis and CMB risk and that the cumulative impact of vascular risk factors—represented by ICA IMT and stenosis—on the risk of subclinical hemorrhage‐prone brain injury predominantly affects deep cerebral regions.
To our knowledge, our study is the first relating progression of CIMT to presence of CMB. Others have related more advanced measures of carotid atherosclerosis, such as calcification measured using computed tomography, to presence of cerebral microbleeds, with findings suggesting an association between carotid calcification and CMB, mainly in deep regions.12 Our results concur with the predominant relation of carotid atherosclerosis measures with deep CMBs and expand previous studies by suggesting that carotid atherosclerosis measures at early stages of disease, before hemodynamically significant degrees of stenosis and severe atherosclerotic plaques develop, also relate to subclinical hemorrhage‐prone small vessel disease.
Exploratory analysis of the apparently paradoxical observation of lower odds of CMB presence among participants with greater CCA IMT progression showed several findings. The changes observed in stratified analysis by hypertension treatment suggest that, at least in part, the effect is related to use of hypertensive medications. We observed that this group of participants had a higher cardiovascular risk profile, suggesting bias by indication (ie, these persons likely had higher CIMT progression reflecting their higher vascular risk factor burden); therefore, more intensive management for their vascular risk factors may have led to lowering of CMB risk. The observed associations, however, were not attenuated by adjustment for lipid levels or use of antithrombotic or statin therapies. Among participants treated for hypertension, it appeared that lower blood pressure level (<140/90) was related to lower CMB risk, and the relation was seen only with antihypertensive‐class angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker. In this regard, a recent meta‐analysis suggested that this antihypertensive class may improve endothelial function,13 a potential mechanism leading to lower CMB risk. An alternative possibility is that progression of CCA atherosclerotic changes may result from release of prothrombotic inflammatory/endothelial cytokines with resulting lower risk of hemorrhages represented by CMB. The site of IMT measurement appears relevant, as reflected by a nonsignificant increase in odds of CMB in the same location when using ICA measurements versus those at the CCA. In fact, prior studies also suggested varying relations between CIMT measured at the CCA and prediction of ischemic events versus intracerebral hemorrhage.14, 15, 16 The site and features of the atherosclerotic lesions in the carotid artery may differ with regard to their relation to cerebrovascular disease. In a prior study including stroke patients, the characteristics of carotid lesions were relevant regarding the relation of carotid artery atherosclerosis to presence of CMBs: Fatty plaques increased the odds of CMB, but calcified plaques did not.17 The role of chance or residual confounding is not entirely excluded, and further studies are required to confirm our exploratory observations. A limitation of our study is the primarily European ancestry of the Framingham participants, preventing generalization of these results to other ethnic or racial groups. In addition, we did not have carotid plaque data to further evaluate the relation of carotid lesions and CMB. Advantages include the careful assessment of carotid and brain MRI measurements with reliable ratings, the former on 2 occasions, independent of each other, with raters blinded to clinical and demographic characteristics.
Conclusions
Our results suggest an association between carotid atherosclerosis measures considered preclinical markers of cumulative exposure to vascular risk factors and hemorrhage‐prone cerebral small vessel disease represented by CMB. This was particularly true for deep CMB, consistent with the hypothesis that CMBs in deep brain regions are due to hypertensive vasculopathy. Exploratory analysis in the group with higher CIMT progression and lower CMB presence generated important questions and observations that deserve further study. If confirmed in further studies, the findings in the present study have potential implications for detection and assessment of preventive and therapeutic interventions for cerebral hemorrhage in subclinical stages, before devastating outcomes occur.
Sources of Funding
This work (design and conduct of the study, collection and management of the data) was supported by the Framingham Heart Study's National Heart, Lung, and Blood Institute contract (N01‐HC‐25195, HHSN268201500001I) and by grants from the National Institute of Neurological Disorders and Stroke (R01 NS17950), the National Institute on Aging (R01 AG16495; AG08122; K23AG038444; 1 R03 AG048180‐01A1); NIH grant (1RO1 HL64753; R01 HL076784; 1 R01 AG028321, P30 AG010129), and NHLBI grants (HL67288, and 2K24HL04334).
Disclosures
None.
Supporting information
Table S1. Association Between Intima–Media Thickness Progression and Cerebral Microbleed, Stratified by Medication Use and Total Cholesterol Level
Table S2. Clinical Characteristics Among Participants With Carotid Intima–Media Thickness Progression by Hypertension Treatment (n=1238)
Table S3. Association Between Common Carotid Artery Intima–Media Thickness Progression and Cerebral Microbleed, Stratified by Vascular Risk Factors
Table S4. Association Between Common Carotid Artery Intima–Media Thickness* Progression and Cerebral Microbleed, Stratified by Antihypertensive Medication Use and Blood Pressure Level
Table S5. Association Between Common Carotid Artery Intima–Media Thickness Progression and Cerebral Microbleed, Stratified by Antihypertensive Medication Type.
(J Am Heart Assoc. 2016;5:e002377 doi: 10.1161/JAHA.115.002377)
Accompanying Tables S1 through S5 are available at http://jaha.ahajournals.org/content/5/3/e002377/suppl/DC1
References
- 1. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 2. Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bonaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the Tromso Study. Neurology. 2004;62:695–701. [DOI] [PubMed] [Google Scholar]
- 3. Peters SA, den Ruijter HM, Bots ML. Attenuation of rate of change in carotid intima‐media thickness by lipid‐modifying drugs: impact on clinical outcomes. Am J Cardiovasc Drugs. 2011;11:253–263. [DOI] [PubMed] [Google Scholar]
- 4. Ozaki K, Kubo T, Imaki R, Shinagawa H, Fukaya H, Ohtaki K, Ozaki S, Izumi T, Aizawa Y. The anti‐atherosclerotic effects of lipid lowering with atorvastatin in patients with hypercholesterolemia. J Atheroscler Thromb. 2006;13:216–219. [DOI] [PubMed] [Google Scholar]
- 5. Yu CM, Zhang Q, Lam L, Lin H, Kong SL, Chan W, Fung JW, Cheng KK, Chan IH, Lee SW, Sanderson JE, Lam CW. Comparison of intensive and low‐dose atorvastatin therapy in the reduction of carotid intimal‐medial thickness in patients with coronary heart disease. Heart. 2007;93:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–1360. [DOI] [PubMed] [Google Scholar]
- 7. Elosua R, Ordovas JM, Cupples LA, Fox CS, Polak JF, Wolf PA, D'Agostino RA Sr, O'Donnell CJ. Association of ApoE genotype with carotid atherosclerosis in men and women: the Framingham Heart Study. J Lipid Res. 2004;45:1868–1875. [DOI] [PubMed] [Google Scholar]
- 8. Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB Sr. Carotid‐wall intima‐media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al‐Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM; Microbleed Study G . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez‐Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45:1492–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, Au R, DeCarli C, Wolf PA. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung PW, Park KY, Kim JM, Shin DW, Ha SY. Carotid artery calcification is associated with deep cerebral microbleeds. Eur Neurol. 2014;72:60–63. [DOI] [PubMed] [Google Scholar]
- 13. Li S, Wu Y, Yu G, Xia Q, Xu Y. Angiotensin II receptor blockers improve peripheral endothelial function: a meta‐analysis of randomized controlled trials. PLoS One. 2014;9:e90217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsivgoulis G, Vemmos KN, Spengos K, Papamichael CM, Cimboneriu A, Zis V, Zakopoulos N, Mavrikakis M. Common carotid artery intima‐media thickness for the risk assessment of lacunar infarction versus intracerebral haemorrhage. J Neurol. 2005;252:1093–1100. [DOI] [PubMed] [Google Scholar]
- 15. Vemmos KN, Tsivgoulis G, Spengos K, Papamichael CM, Zakopoulos N, Daffertshofer M, Lekakis JP, Mavrikakis M. Common carotid artery intima‐media thickness in patients with brain infarction and intracerebral haemorrhage. Cerebrovasc Dis. 2004;17:280–286. [DOI] [PubMed] [Google Scholar]
- 16. Nagai Y, Kitagawa K, Yamagami H, Kondo K, Hougaku H, Hori M, Matsumoto M. Carotid artery intima‐media thickness and plaque score for the risk assessment of stroke subtypes. Ultrasound Med Biol. 2002;28:1239–1243. [DOI] [PubMed] [Google Scholar]
- 17. Saba L, Montisci R, Raz E, Sanfilippo R, Suri JS, Piga M. Association between carotid artery plaque type and cerebral microbleeds. AJNR Am J Neuroradiol. 2012;33:2144–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association Between Intima–Media Thickness Progression and Cerebral Microbleed, Stratified by Medication Use and Total Cholesterol Level
Table S2. Clinical Characteristics Among Participants With Carotid Intima–Media Thickness Progression by Hypertension Treatment (n=1238)
Table S3. Association Between Common Carotid Artery Intima–Media Thickness Progression and Cerebral Microbleed, Stratified by Vascular Risk Factors
Table S4. Association Between Common Carotid Artery Intima–Media Thickness* Progression and Cerebral Microbleed, Stratified by Antihypertensive Medication Use and Blood Pressure Level
Table S5. Association Between Common Carotid Artery Intima–Media Thickness Progression and Cerebral Microbleed, Stratified by Antihypertensive Medication Type.


