Abstract
Background
In heart failure (HF), weight loss (WL) has been associated with an adverse prognosis whereas obesity has been linked to lower mortality (the obesity paradox). The impact of WL in obese patients with HF is incompletely understood. Our objective was to explore the prevalence of WL and its impact on long‐term mortality, with an emphasis on obese patients, in a cohort of patients with chronic HF.
Methods and Results
Weight at first visit and the 1‐year follow‐up and vital status after 3 years were assessed in 1000 consecutive ambulatory, chronic HF patients (72.7% men; mean age 65.8±12.1 years). Significant WL was defined as a loss of ≥5% weight between baseline and 1 year. Obesity was defined as body mass index ≥30 kg/m2 (N=272). Of the 1000 patients included, 170 experienced significant WL during the first year of follow‐up. Mortality was significantly higher in patients with significant WL (27.6% versus 15.3%, P<0.001). In univariable Cox regression analysis, patients with significant WL had 2‐fold higher mortality (hazard ratio 1.95 [95% CI 1.39–2.72], P<0.001). In multivariable analysis, adjusting for age, sex, body mass index, New York Heart Association functional class, left ventricular ejection fraction, HF duration, ischemic etiology, diabetes, and treatment, significant WL remained independently associated with higher mortality (hazard ratio 1.89 [95% CI 1.32–2.68], P<0.001). Among obese patients with HF, significant WL was associated with an even more ominous prognosis (adjusted hazard ratio for death of 2.38 [95% CI 1.31–4.32], P=0.004) than that observed in nonobese patients (adjusted hazard ratio 1.83 [95% CI 1.16–2.89], P=0.01).
Conclusions
Weight loss ≥5% in patients with chronic HF was associated with high long‐term mortality, particularly among obese patients with HF.
Keywords: cachexia, heart failure, mortality, obesity, weight
Subject Categories: Heart Failure, Mortality/Survival, Metabolic Syndrome
Introduction
Obesity increases the risk of heart failure (HF)1 and cardiovascular death in the general population.2 However, a significant survival benefit of obesity in patients with established HF has been extensively described in different settings: short‐ and long‐term follow‐up, acute and chronic HF, and in reduced and preserved ejection fraction.3, 4, 5, 6, 7 The prevalence of overweight or obesity in patients with HF can reach about 35% to 45%8, 9 when defined using body mass index (BMI).
Among weight indices, BMI has been shown to properly correlate with the thickness of the subcutaneous fat layer10 and has been rapidly adopted as the primary obesity index in HF. Other parameters used for measuring body composition (waist circumference, skinfold estimates of percentage of body fat, DEXA, and bioelectrical impedance analysis) have also demonstrated an independent relationship with mortality in HF patients,11, 12, 13 although none has proved to be clearly superior to BMI; thus, the use of BMI in HF remains routine clinical practice.
Despite the potential benefits of weight loss (WL) in obese and overweight patients, this recommendation may not apply to patients with HF. Indeed, both the American College of Cardiology/American Heart Association and the European Society of Cardiology guidelines in HF do not provide conclusive recommendations about WL.14, 15 Moreover, the prognostic impact of WL in obese patients with HF is not completely known, despite possible beneficial effects of WL on hemodynamics, cardiac structure, diastolic function, and even systolic function.16 Robust evidence regarding the relation of WL and long‐term prognosis in patients with chronic HF is missing.17, 18, 19 Accordingly, our aim was to analyze the prevalence of WL over a 1‐year follow‐up period and its impact on mortality in a large, real‐life, outpatient population with HF, with special attention to obese patients.
Subjects and Methods
Design and Study Population
All consecutive, ambulatory patients referred between August 1, 2001 and September 30, 2011 to a structured HF clinic at a university hospital, irrespective of etiology, with available weight data at the first visit and at a 1‐year follow‐up visit were included in an outpatient setting. The criteria for clinical practice referral to the HF clinic have been reported elsewhere.20, 21 Briefly, the criteria were HF with at least one hospitalization and/or reduced left ventricular ejection fraction (LVEF) of <40%. Most patients were referred from cardiology and internal medicine departments, and fewer were from the emergency room/short‐stay unit or other hospital departments. Less than 10% of patients were admitted to the HF unit due to asymptomatic, reduced LVEF after acute myocardial infarction.
BMI was analyzed according to weight and height using the formula weight (kg)/(height [m])2. We then evaluated the relationship between BMI and survival throughout follow‐up. Patients were classified according to BMI into 4 strata following the criteria defined by the World Health Organization (WHO Technical Report Series, no 854, Geneva, 1999) as follows: low weight (BMI <20.5 kg/m2), normal weight (BMI 20.5 to <25.5 kg/m2), overweight (BMI 25.5 to <30 kg/m2), and obese (BMI ≥30 kg/m2). Significant WL was defined as a loss of ≥5% from the initial weight (ie, baseline) to end of first year of follow‐up.
All patients were seen regularly during follow‐up visits at the HF clinic according to their clinical needs. The follow‐up visit schedule (nurses and physicians) has been reported elsewhere.21, 22 Losing weight with dietary restrictions was not routinely advised for overweight, nor for the majority of obese patients, although a healthy diet and physical exercise is always recommended. Only some morbid obese patients were managed by the Endocrinology and Nutrition Service. The nutritionist at the HF Unit was primarily involved in undernourished patients with lack of appetite. Patients in NYHA class II to IV were evaluated by a rehabilitation physician and an exercise program was offered. Nevertheless, less than one third of the patients in the present study actually participated in such an established program. All patients provided written consent for analytical samples and the use of their clinical data for research purposes. The study was performed in compliance with laws protecting personal data in accordance with the international guidelines on clinical investigation of the World Medical Association's Declaration of Helsinki, it was approved by the local ethics committee, and all patients signed informed consent. At the first visit, we recorded the patient's demographic characteristics, baseline clinical status, physical examination data, and treatment following a standardized protocol.
Mortality Assessment
Death from all causes was the main outcome. The number and causes of death during follow‐up were obtained from clinical records at the HF clinic, other hospital departments, other hospital records, or by contacting the patient's relatives. Data were verified using the databases of the Catalan and Spanish Health System. One patient was lost during follow‐up and was adequately censored in the survival analysis. Follow‐up was closed September 30, 2014.
Statistical Analysis
Categorical variables were described by frequencies and percentages. Continuous variables were described by the mean±SD, or median and 25th to 75th percentiles (Q1–Q3) for cases with skewed distribution. Normal distribution was assessed with normal Q‐Q plots. Statistical differences between groups were assessed using the χ2 test for categorical variables, Student t test for continuous variables with normal distribution, or the Mann–Whitney U test for non‐normal distributions. Cox proportional hazards regression analyses were performed using all‐cause mortality and also cardiovascular mortality as the dependent variable and significant WL, as defined, as the independent variable. Afterwards, multivariable analyses were also performed, including as covariates age, sex, New York Heart Association (NYHA) functional class, HF duration, LVEF, etiology of HF, diabetes, baseline BMI, and treatment with β‐blockers, angiotensin‐converting enzyme inhibitors–angiotensin II receptor blockers, and mineralocorticoid receptor antagonists. These analyses were repeated after categorizing BMI in 2 groups: obese or nonobese, which included underweight, normal weight, and overweight. Also, adjusted survival curves for all‐cause and cardiovascular death were plotted according to the presence or absence of significant WL for both obese and nonobese patients. Finally, the Cox regression multivariable analyses were repeated using standardized WL as continuous variable (with 1 SD decrease). Statistical analyses were performed using SPSS 15 (SPSS Inc, Chicago, IL). A 2‐sided P<0.05 was considered statistically significant.
Results
Of 1322 patients admitted to the HF Unit, a total of 1000 patients (72.7% men; mean age 65.8±12.1 years) were included in the study. Causes for noninclusion were as follows: 139 patients died during the first year of follow‐up, 152 did not attend the 1‐year visit, and 31 had no weight available (wheelchair or impossibility to stand up). The demographic and clinical characteristics of the patients are summarized in Table 1. The patients were predominantly male, with a median duration of HF of 10.5 months (Q1–Q3 2–48 months), and a mean LVEF of 32.4±12.6%. One hundred seventy patients (17%) experienced significant WL during the first year of follow‐up. Figure 1 illustrates the percentage of patients with WL ≥5%, showing a significantly higher rate in overweight and obese patients.
Table 1.
Demographic and Clinical Characteristics
| Total Cohort | Significant WL | No Significant WL | P Value | |
|---|---|---|---|---|
| N=1000 | N=170 | N=830 | ||
| Agea, y | 65.8±12.1 | 67.3±11.0 | 65.5±12.3 | 0.08 |
| Female sex | 273 (27.3%) | 61 (35.9%) | 212 (25.5%) | 0.006 |
| Etiology | 0.31 | |||
| IHD | 557 (57.5%) | 82 (48.2%) | 475 (57.2%) | |
| DCM | 111 (11.1%) | 22 (12.9%) | 89 (10.7%) | |
| HYP | 88 (8.8%) | 21 (12.4%) | 67 (8.1%) | |
| Alcohol induced | 59 (5.9%) | 13 (7.6%) | 46 (5.5%) | |
| Toxic (MEDS) | 19 (1.9%) | 3 (1.8%) | 16 (1.9%) | |
| Valvular | 86 (8.6%) | 13 (7.6%) | 73 (8.8%) | |
| Other | 80 (8.0%) | 16 (9.4%) | 64 (7.7%) | |
| NHYA functional class | <0.001 | |||
| I | 56 (5.6%) | 6 (3.5%) | 50 (6.0%) | |
| II | 649 (64.9%) | 90 (52.9%) | 559 (67.3%) | |
| III | 283 (28.3%) | 70 (41.2%) | 213 (25.7%) | |
| IV | 12 (1.2%) | 4 (2.4%) | 8 (1.0%) | |
| HF duration, monthsb | 10.5 (2–48) | 14.5 (2–54) | 10 (1–48) | 0.16 |
| LVEFa, % | 32.4±12.6 | 33.9±14.2 | 32.2±12.3 | 0.10 |
| BMI, kg/m2 | 27.6±5.1 | 29.3±4.9 | 27.2±5.0 | <0.001 |
| Diabetes | 368 (36.8) | 65 (38.2) | 368 (36.8) | 0.67 |
| Smoking habit | ||||
| Current | 159 (15.9) | 28 (16.5) | 131 (15.8) | 0.82 |
| Past | 418 (41.8) | 70 (41.1) | 348 (41.9) | 0.86 |
| NTproBNP, ng/Lb, c | 1586 (576–3661) | 1766 (694–4792) | 1524 (554–3519) | 0.35 |
| ST2, ng/mLb, c | 39.7 (31.9–52.5) | 42.6 (31.7–62.4) | 39.2 (31.9–51.1) | 0.29 |
| Hs‐CRP, mg/Lb, c | 4.19 (1.69–9.91) | 4.37 (1.84–8.03) | 4.14 (1.62–10.46) | 0.94 |
| Hs‐TnT, ng/Lb, c | 26.5 (13.1–44.3) | 32.6 (19.0–55.8) | 26.2 (12.1–42.9) | 0.05 |
| STfR, mg/Lb, c | 3.7 (2.9–4.9) | 3.9 (3.1–4.8) | 3.7 (2.9–4.9) | 0.31 |
| Treatments (follow‐up) | ||||
| ACEI or ARB | 914 (91.4%) | 153 (90.0%) | 761 (91.7%) | 0.48 |
| β‐Blockers | 910 (91.0%) | 148 (87.1%) | 762 (91.8%) | <0.05 |
| MRA | 585 (58.5%) | 114 (67.1%) | 471 (56.7%) | 0.01 |
| Loop diuretics | 911 (91.1%) | 163 (95.9%) | 748 (90.1%) | 0.02 |
| Digoxin | 397 (39.7%) | 89 (52.4%) | 308 (37.1%) | <0.001 |
| ICD | 132 (13.2%) | 22 (12.9%) | 110 (13.3%) | 0.91 |
| CRT | 81 (8.1%) | 10 (5.9%) | 71 (8.6%) | 0.25 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CRT, cardiac resynchronization therapy; DCM, dilated cardiomyopathy; HF, heart failure; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐TnT, high sensitivity cardiac troponin T; HYP, hypertension; ICD, implantable cardiac defibrillator; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; MEDS, medications; MRA, mineralocorticoid receptor antagonist; NTproBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; ST2, high‐sensitivity soluble ST2; STfR, soluble transferrin receptor; WL, weight loss.
Mean±SD.
Median (Q1–Q3).
NTproBNP available in 422 patients; ST2 available in 340 patients; hs‐CRP, hs‐TnT, and STfR available in 332 patients.
Figure 1.
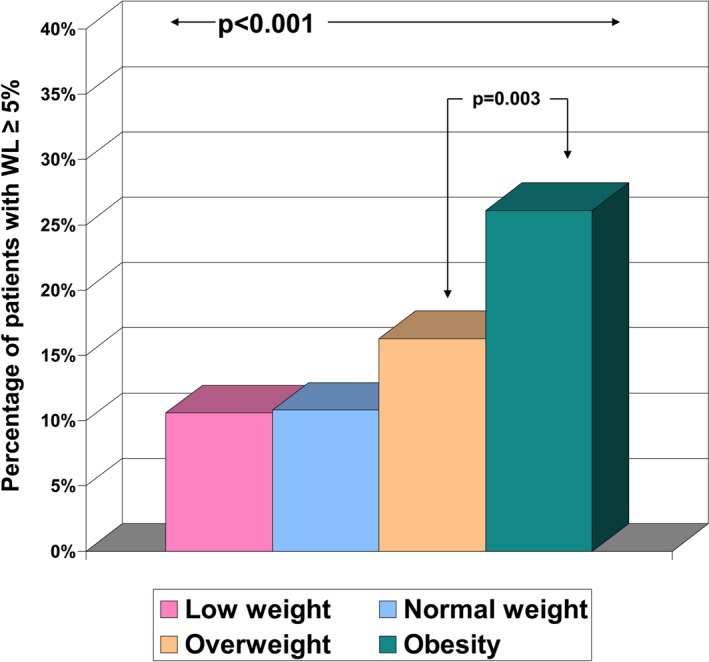
Prevalence of significant WL according to body mass index strata. Significant WL was considered the loss of ≥5% of the initial weight during the first year of follow‐up. WL indicates weight loss.
Table 1 shows the clinical differences between patients with and without significant WL. In addition to sex, most were related to HF severity and treatment. In a limited sample of patients, we have data on biomarkers, without differences between both groups; only high‐sensitivity troponin T tended to be higher in patients with significant WL (P=0.05, Table 1).
After 3 years of follow‐up from the first visit (ie, 2 years after the second weight assessment), 174 patients (17.4%) died—121 from cardiovascular causes (54 worsening HF, 30 sudden death, 11 acute myocardial infarction, 5 stroke, and 21 other), 40 from noncardiovascular causes, and 13 from unknown causes. Mortality in significant WL patients was significantly higher than in patients without significant WL (27.6% versus 15.3%, P<0.001). This was basically due to cardiovascular mortality (20.0% versus 10.9%, respectively, P=0.01), whereas differences in noncardiovascular mortality were nonsignificant (6.0% versus 3.7%, respectively, P=0.16). Of note, death due to worsening HF was 11.2% and 4.2%, respectively, P<0.001.
In univariable Cox regression analysis, patients with significant WL had 2‐fold all‐cause and cardiovascular higher mortality (hazard ratio [HR] 1.95 [95% CI 1.39–2.72], P<0.001 and HR 2.06 [95% CI 1.39–3.06], P<0.001, respectively). In the multivariable model adjusted by and age, sex, BMI, NYHA functional class, LVEF, HF duration, ischemic etiology, diabetes, and treatment with β‐blockers, angiotensin‐converting enzyme inhibitors–angiotensin II receptor blockers, and mineralocorticoid receptor antagonists, significant WL remained highly and independently associated with higher all‐cause mortality (HR 1.89 [95% CI 1.32–2.68], P<0.001) (Table 2). Among obese HF patients, significant WL was associated with an even higher risk of all‐cause death (adjusted HR 2.38 [95% CI 1.31–4.32], P=0.004) than that observed in nonobese patients (adjusted HR 1.83 [95% CI 1.16–2.89], P=0.01) (Table 2). Figure 2 shows adjusted survival curves for all‐cause death relative to the presence or absence of significant WL for nonobese and obese patients.
Table 2.
Multivariable Cox Regression Analysis for All‐Cause Death
| Total | Nonobese | Obese | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N=1000 | N=725 | N=275 | |||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Weight loss ≥5% | 1.88 | (1.32–2.68) | <0.001 | 1.83 | (1.16–2.89) | 0.01 | 2.38 | (1.31–4.32) | 0.004 |
| Age | 1.04 | (1.02–1.05) | <0.001 | 1.04 | (1.02–1.06) | <0.001 | 1.04 | (1.01–1.08) | 0.02 |
| Female sex | 0.70 | (0.48–1.02) | 0.06 | 0.70 | (0.44–1.12) | 0.14 | 0.45 | (0.22–0.95) | 0.04 |
| Ischemic etiology | 1.63 | (1.16–2.30) | 0.005 | 1.46 | (0.96–2.23) | 0.08 | 2.66 | (1.37–5.17) | 0.004 |
| HF duration | 1.00 | (1.00–1.01) | 0.001 | 1.00 | (1.00–1.01) | <0.001 | 1.00 | (1.00–1.01) | 0.66 |
| LVEF | 1.00 | (0.98–1.01) | 0.39 | 1.00 | (0.98–1.01) | 0.22 | 1.00 | (0.98–1.03) | 0.86 |
| NYHA functional class | 1.62 | (1.24–2.12) | <0.001 | 1.62 | (1.82–2.47) | <0.001 | 1.07 | (0.59–1.95) | 0.82 |
| Diabetes | 1.29 | (0.95–1.76) | 0.11 | 1.08 | (0.73–1.58) | 0.11 | 2.08 | (1.16–3.73) | 0.01 |
| BMI | 1.00 | (0.97–1.03) | 0.90 | 0.93 | (0.97–1.03) | 0.02 | 1.06 | (0.97–1.15) | 0.19 |
| β‐Blockers | 0.43 | (0.29–0.66) | <0.001 | 0.49 | (0.30–0.81) | 0.006 | 0.36 | (0.16–0.83) | 0.02 |
| ACEI or ARB | 0.41 | (0.28–0.61) | <0.001 | 0.38 | (0.24–0.60) | <0.001 | 0.50 | (0.21–1.21) | 0.12 |
| MRA | 0.77 | (0.56–1.05) | 0.09 | 0.80 | (0.56–1.16) | 0.24 | 0.73 | (0.40–1.33) | 0.30 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Figure 2.
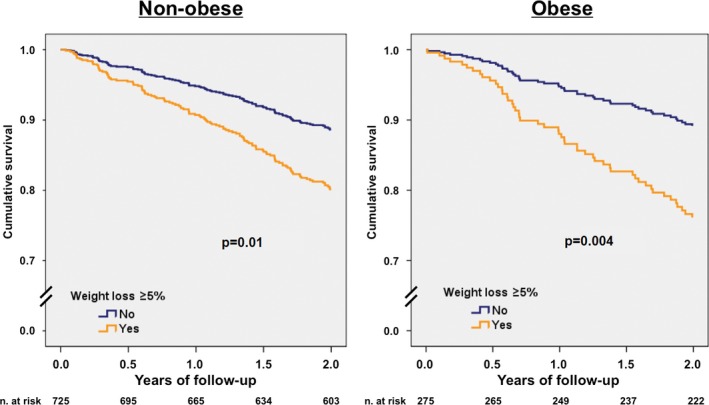
Adjusted survival curves for all‐cause death according to the presence of significant weight loss. A, Nonobese patients. B, Obese patients. Survival curves plotted from the multivariate analysis that included age, sex, New York Heart Association functional class, left ventricular ejection fraction, etiology of heart failure, diabetes, and treatment with β‐blockers, angiotensin‐converting enzyme inhibitors–angiotensin II receptor blockers, and mineralocorticoid receptor antagonists as covariates.
Focus in cardiovascular death provided similar results: Significant WL remained highly and independently associated with higher cardiovascular mortality (HR 1.89 [95% CI 1.29–2.90], P=0.003) (Table 3), and again among obese HF patients, significant WL was associated with higher risk of cardiovascular death (adjusted HR 2.51 [95% CI 1.23–5.14], P=0.01) than that observed in nonobese patients (adjusted HR 1.75 [95% CI 1.00–3.06], P<0.05) (Table 3). Figure 3 shows adjusted survival curves for cardiovascular death relative to the presence or absence of significant WL for nonobese and obese patients. Tables 4 and 5 show Cox regression multivariable analyses for all‐cause and cardiovascular death, respectively, using standardized WL as continuous variable (with 1 SD decrease).
Table 3.
Multivariable Cox Regression Analysis for Cardiovascular Death
| Total | Nonobese | Obese | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N=987a | N=716 | N=271 | |||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Weight loss ≥5% | 1.89 | (1.24–2.90) | 0.003 | 1.75 | (1.00–3.06) | <0.05 | 2.51 | (1.23–5.14) | 0.01 |
| Age | 1.04 | (1.02–1.06) | <0.001 | 1.05 | (1.02–1.07) | <0.001 | 1.05 | (1.01–1.09) | 0.01 |
| Female sex | 0.58 | (0.36–0.92) | 0.02 | 0.59 | (0.33–1.06) | 0.08 | 0.36 | (0.14–0.92) | 0.03 |
| Ischemic etiology | 2.10 | (1.37–3.23) | 0.001 | 1.84 | (1.08–3.15) | 0.03 | 3.58 | (1.59–8.08) | 0.002 |
| HF duration | 1.00 | (1.00–1.01) | <0.001 | 1.00 | (1.00–1.01) | <0.001 | 1.00 | (1.00–1.01) | 0.55 |
| LVEF | 1.00 | (0.98–1.01) | 0.53 | 1.00 | (0.98–1.01) | 0.64 | 0.99 | (0.96–1.02) | 0.63 |
| NYHA functional class | 1.62 | (1.17–2.25) | 0.004 | 1.84 | (1.26–2.68) | <0.001 | 1.00 | (0.50–2.02) | 0.99 |
| Diabetes | 1.59 | (1.10–2.31) | 0.01 | 1.41 | (0.90–2.23) | 0.14 | 2.37 | (1.19–4.71) | 0.01 |
| BMI | 1.02 | (0.99–1.07) | 0.23 | 0.97 | (0.97–1.05) | 0.47 | 1.10 | (0.99–1.07) | 0.04 |
| β‐Blockers | 0.37 | (0.23–0.60) | <0.001 | 0.40 | (0.22–0.73) | 0.003 | 0.34 | (0.13–0.91) | 0.03 |
| ACEI or ARB | 0.41 | (0.26–0.66) | <0.001 | 0.36 | (0.21–0.62) | <0.001 | 0.79 | (0.23–2.65) | 0.79 |
| MRA | 0.90 | (0.62–1.31) | 0.57 | 0.96 | (0.61–1.49) | 0.84 | 0.84 | (0.41–1.73) | 0.64 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Thirteen patients excluded because of unknown cause of death.
Figure 3.
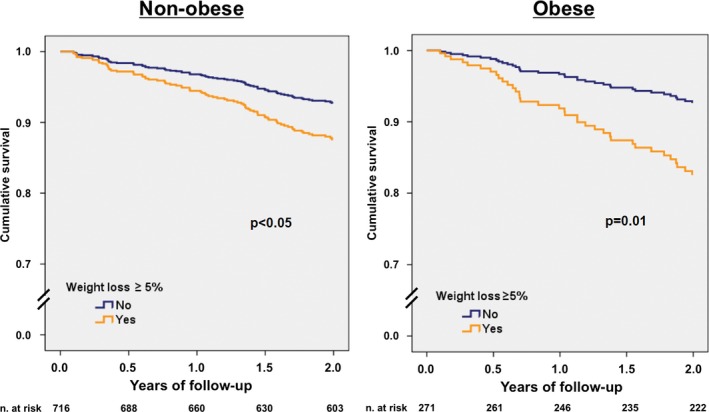
Adjusted survival curves for cardiovascular death according to the presence of significant weight loss. A, Nonobese patients. B, Obese patients. Survival curves plotted from the multivariate analysis that included age, sex, New York Heart Association functional class, left ventricular ejection fraction, etiology of heart failure, diabetes, and treatment with β‐blockers, angiotensin‐converting enzyme inhibitors‐angiotensin II receptor blockers, and mineralocorticoid receptor antagonists as covariates. Thirteen patients were excluded from the analysis because of unknown cause of death.
Table 4.
Multivariable Cox Regression Analysis for All‐Cause Death, Using Weight Loss as Continuous Variable
| Total | Nonobese | Obese | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N=1000 | N=725 | N=275 | |||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Weight lossa | 1.34 | (1.18–1.61) | 0.002 | 1.29 | (1.04–1.60) | 0.02 | 1.67 | (1.19–2.33) | 0.003 |
| Age | 1.03 | (1.02–1.05) | <0.001 | 1.04 | (1.02–1.05) | <0.001 | 1.04 | (1.01–1.08) | 0.02 |
| Female sex | 0.74 | (0.51–1.08) | 0.12 | 0.74 | (0.47–1.18) | 0.21 | 0.46 | (0.22–96) | 0.04 |
| Ischemic etiology | 1.60 | (1.14–2.26) | 0.007 | 1.43 | (0.94–2.17) | 0.09 | 2.62 | (1.35–5.10) | 0.004 |
| HF duration | 1.00 | (1.00–1.01) | 0.001 | 1.00 | (1.00–1.01) | <0.001 | 1.00 | (1.00–1.01) | 0.64 |
| LVEF | 1.00 | (0.98–1.01) | 0.43 | 0.99 | (0.98–1.01) | 0.26 | 1.00 | (0.98–1.03) | 0.92 |
| NYHA functional class | 1.69 | (1.29–2.20) | <0.001 | 1.89 | (1.39–2.56) | <0.001 | 1.12 | (0.62–2.03) | 0.70 |
| Diabetes | 1.33 | (0.97–1.81) | 0.07 | 1.14 | (0.94–2.17) | 0.51 | 2.00 | (1.35–5.10) | 0.02 |
| BMI | 0.99 | (0.96–1.61) | 0.60 | 0.92 | (0.86–0.98) | 0.007 | 1.04 | (0.96–1.13) | 0.32 |
| β‐Blockers | 0.45 | (0.30–0.68) | <0.001 | 0.51 | (0.31–0.85) | 0.009 | 0.37 | (0.16–0.83) | 0.02 |
| ACEI or ARB | 0.44 | (0.30–0.65) | <0.001 | 0.41 | (0.26–0.64) | <0.001 | 0.51 | (0.21–1.24) | 0.26 |
| MRA | 0.75 | (0.55–1.03) | 0.07 | 0.79 | (0.55–1.14) | 0.20 | 0.71 | (0.39–1.29) | 0.26 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Per 1 SD decrease in weight.
Table 5.
Multivariable Cox Regression Analysis for Cardiovascular Death, Using Weight Loss as Continuous Variable
| Total | Nonobese | Obese | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N=987a | N=716 | N=271 | |||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Weight lossb | 1.30 | (1.04–1.61) | 0.02 | 1.28 | (0.98–1.06) | 0.07 | 1.41 | (0.96–2.07) | 0.08 |
| Age | 1.04 | (1.02–1.06) | <0.001 | 1.04 | (1.02–1.07) | 0.001 | 1.05 | (1.01–1.09) | 0.02 |
| Female sex | 0.62 | (0.39–0.98) | 0.04 | 0.62 | (0.35–1.11) | 0.11 | 0.44 | (0.18–1.06) | 0.07 |
| Ischemic etiology | 2.07 | (1.35–3.19) | 0.001 | 1.81 | (1.06–3.08) | 0.03 | 3.36 | (1.51–7.48) | 0.003 |
| HF duration | 1.00 | (1.00–1.01) | <0.001 | 1.00 | (1.00–1.01) | <0.001 | 1.00 | (1.00–1.01) | 0.49 |
| LVEF | 1.00 | (0.98–1.01) | 0.54 | 1.00 | (0.98–1.01) | 0.71 | 0.99 | (0.96–1.02) | 0.49 |
| NYHA functional class | 1.70 | (1.23–2.35) | 0.001 | 1.90 | (1.31–2.76) | 0.001 | 1.07 | (0.55–2.11) | 0.84 |
| Diabetes | 1.62 | (1.12–2.35) | 0.01 | 1.48 | (0.94–2.33) | 0.09 | 2.17 | (1.10–4.27) | 0.03 |
| BMI | 1.02 | (0.98–1.06) | 0.34 | 0.96 | (0.89–1.04) | 0.33 | 1.08 | (0.99–1.19) | 0.08 |
| β‐Blockers | 0.38 | (0.23–0.62) | <0.001 | 0.42 | (0.23–0.75) | 0.004 | 0.34 | (0.13–0.86) | 0.02 |
| ACEI or ARB | 0.44 | (0.28–0.71) | 0.001 | 0.39 | (0.23–0.67) | 0.001 | 0.84 | (0.25–2.84) | 0.78 |
| MRA | 0.89 | (0.61–1.29) | 0.54 | 0.94 | (0.60–1.46) | 0.78 | 0.85 | (0.42–1.72) | 0.64 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Thirteen patients excluded because of unknown cause of death.
Per 1 SD decrease in weight.
Discussion
The main conclusion of this study may seem perplexing, which is that significant WL might be not beneficial even in obese patients with HF. This finding may, in part, provide a rationale for the obesity paradox in HF, which is extensively described,3, 4, 5, 6, 7 not universally observed,22, 23, 24, 25, 26 and incompletely understood. Obese patients with HF are thought to have metabolic reserves to better tolerate the catabolic stress of HF, thus explaining the better prognosis. However, the occurrence of unintentional WL may be a surrogate for the loss of metabolic reserves in obese patients and may be the trigger for adverse clinical outcomes. On the other hand, being obese while having HF seems incongruous with undernourishment, which is associated with a very poor prognosis in patients with chronic HF.27 Indeed, it is possible that WL secondary to malnutrition might exacerbate underlying undernourishment. Purposeful WL is usually recommended for patients with HF and morbid obesity. As suggested by Lavie et al, this seems particularly sound for those with a BMI ≥40 kg/m2 and reasonable for most HF patients with BMI of ≥35 kg/m2.16
A few reports with small sample populations exist on the effect of intentional weight reduction on quality of life and cardiac function in patients with HF. Mariotti et al evaluated the impact of a planned body weight reduction plan on quality of life and cardiac function in 34 obese and overweight chronic HF patients through a 6‐month nutritional and physical activity program. Those patients who achieved a loss of at least 3 kg (about 3.2% of WL) showed a significant improvement in LVEF and mean NYHA functional class and quality of life. The study did not evaluate mortality.28 Another small randomized clinical trial evaluated the benefit of losing weight via a lipase inhibitor–assisted diet in 21 severely obese patients. Significant WL (5% absolute reduction in body weight) and improvement in the 6‐minute walking test and functional class were observed in the treated group at 12 weeks.29 Again, no analysis of mortality could be performed due to the small sample size and short follow‐up.
Data on unintentional WL in obese patients are even scarcer. In a post‐hoc analysis of the SOLVD trial, Anker et al30 were the first to suggest that any WL independent of the patients' weight at baseline is related to poor survival, although no specific comment on obese patients was reported. More recently, Rossignol et al31 observed significant WL in 16.4% and 15.7% of patients from the GISSI‐HF and Val‐HeFT studies, respectively, with results quite similar to ours. As in Val‐Heft, we found that significant WL (using similar criteria) was associated with female sex, worse NYHA functional class, higher BMI, and less use of β‐blockers. It has been suggested that β‐blockers may exert anticachexic effects by inhibiting catecholamine‐induced lipolysis.32 We also found an association between significant WL and treatment with mineralocorticoid receptor antagonists and loop diuretics—the latter of which was also observed in GISSI‐HF—and with digoxin, treatments all associated with the severity of HF.
Whether biomarkers are useful to mirror or as surrogate markers of WL is unknown. Song et al33 showed an independent association between high‐sensitivity C‐reactive protein and unintentional WL in 243 patients with HF enrolled during an index hospitalization for HF exacerbation (OR, 1.49; 95% CI, 1.15–1.92). In the Val‐HeFT “biomarker” substudy, both N‐terminal pro‐brain natriuretic peptide and high‐sensitivity C‐reactive protein were associated with significant WL.31 In our study, we found no association between significant WL and any of these 2 biomarkers or with ST2 and soluble transferrin receptor. Only high‐sensitivity troponin T tended to be higher in patients with significant WL (P=0.05), which may eventually indicate myocyte damage caused by WL itself.
From a prognostic point of view, significant WL was independently associated with mortality, with an increased risk of death of 20% (GISSI‐HF) to 150% (Val‐HeFT).31 In our study, the risk of death for significant WL after adjustment in the multivariable model was 89% higher. Very remarkably, among obese patients (BMI ≥30 kg/m2), significant WL was associated with an even higher risk of death (138% increase) than in nonobese patients (83% increase). This finding was very unexpected, even more so considering that low‐weight patients were not excluded from the analysis. In an analysis of the CHARM study,34 those patients with 5% or greater WL in 6 months had a >50% increase in hazard both for cardiovascular and for other causes of death compared to those with stable weight. The impact of WL on mortality was not significantly related to BMI, although WL carried a particularly high risk in patients who were already lean at study entry. In our study, in contrast, cardiovascular mortality was almost doubled and the patients with highest adjusted hazard of death were the obese patients. Remarkably, in our study the higher mortality rate observed in patients with significant WL was mainly caused by worsening HF. On the other hand, although noncardiovascular death was non‐negligible in our series (23%), it was nonsignificantly higher in patients with significant WL.
Nevertheless, WL was not associated with an adverse prognosis in all HF settings. The RICA Registry35 analyzed weight changes in 731 patients admitted with acute HF during a 1‐year follow‐up. One hundred fifty‐two patients (20.8%) experienced WL. No differences were observed in terms of mortality and rehospitalization between the 2 groups. These results, observed in the acute setting in decompensated patients, are in disagreement with our data and those of others obtained in ambulatory, stable patients without overt congestion.
Finally, it has been suggested that incorporating physical activity and exercise training into a purposeful WL program might be of benefit in patients with HF.36 In the HF‐ACTION study, nonsignificant reductions in all‐cause mortality and hospitalization were observed across BMI categories, although no association with WL was identified.9 Further studies are needed to ascertain whether this strategy might improve outcomes in obese patients with HF.
Limitations
The present data are based on BMI‐based WL without further anthropometric characterization (muscle or fat mass wasting assessments), and we cannot fully ascertain whether WL was in part intentional or nonintentional. However, the result of intentional WL is difficult to address, and most of the analyses performed in HF trials included both intentional and nonintentional WL as we did. We defined significant WL as a loss of ≥5% of the initial weight during the first year of follow‐up, as previously described.31, 34, 35 This cut‐off can be considered arbitrary as were all the definitions used in previous studies; however, no definite cut‐off exists. Ideally, dry weight should be used to track WL. We only found statistically significant differences between patients with and without significant WL in NYHA functional class, sex, and baseline BMI; nevertheless, we cannot completely discard the possibility that they were sicker from the start, since significant WL is just a marker of such a situation without playing a causative role. Further studies are needed to fully clarify the putative causative role of significant WL in outcomes. We have no data on what happened to weight during follow‐up between year 1 and year 3 or the time of death, and we only analyzed weight loss during the first year of follow‐up. In the present study, we focused on stable outpatients, but whether subclinical congestion may have influenced our results remains a caveat. Although our population is a general population attending a HF unit, the unit is located at a tertiary university hospital and the cohort patients are mainly male and of ischemic etiology, with the great majority having had a hospital admission in the previous year or a depressed LVEF. Therefore, we cannot disregard the possibility of bias due to selection of patients who may not necessarily represent the general HF population.
Conclusions
Weight loss of ≥5% during 1 year in ambulatory HF patients was associated with an ominous prognosis in the subsequent 2 years. This association was even more apparent in obese patients. Significant WL might not be beneficial in obese HF patients, and indiscriminate advice to lose weight in this population might not be indicated. Further studies are needed to ascertain whether WL and the mode of WL achievement might ultimately be beneficial in obese or even very obese patients with HF.
Sources of Funding
This work was supported by Redes Temáticas de Investigación Cooperativa en Salud (RETICS), and Red Cardiovascular (RD12/0042/0047).
Disclosures
None.
Acknowledgments
We thank Beatriz González, Roser Cabanes, and Margarita Rodríguez, nurses in the HF Unit, for data collection and their invaluable work in the unit.
(J Am Heart Assoc. 2016;5:e002468 doi: 10.1161/JAHA.115.002468)
References
- 1. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 2. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body‐mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. [DOI] [PubMed] [Google Scholar]
- 3. Oreopoulos A, Padwal R, Kalantar‐Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta‐analysis. Am Heart J. 2008;156:13–22. [DOI] [PubMed] [Google Scholar]
- 4. Padwal R, McAlister FA, McMurray JJV, Cowie MR, Rich M, Pocock S, Swedberg K, Maggioni A, Gamble G, Ariti C, Earle N, Whalley G, Poppe KK, Doughty RN, Bayes‐Genis A; Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) . The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta‐analysis of individual patient data. Int J Obes. 2014;38:1110–1114. [DOI] [PubMed] [Google Scholar]
- 5. Zamora E, Lupón J, Urrutia A, González B, Mas D, Pascual T, Domingo M, Valle V. Does body mass index influence mortality in patients with heart failure? Rev Esp Cardiol. 2007;60:1127–1134. [PubMed] [Google Scholar]
- 6. Zamora E, Lupón J, Urrutia A, Bayes‐Genis A. Obesity and long‐term prognosis in heart failure: the paradox persists. Rev Esp Cardiol. 2010;63:1210–1212. [DOI] [PubMed] [Google Scholar]
- 7. Shah R, Gayat E, Januzzi JL Jr, Sato N, Cohen‐Solal A, diSomma S, Fairman E, Harjola VP, Ishihara S, Lassus J, Maggioni A, Metra M, Mueller C, Mueller T, Parenica J, Pascual‐Figal D, Peacock WF, Spinar J, van Kimmenade R, Mebazaa A; GREAT (Global Research on Acute Conditions Team) Network . Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–785. [DOI] [PubMed] [Google Scholar]
- 8. Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, Pfeffer MA, Yusuf S, Swedberg K, Michelson EL, Granger CB, McMurray JJ, Solomon SD; CHARM Investigators . Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart Failure: assessment of reduction in mortality and morbididty (CHARM) program. Circulation. 2007;116:627–636. [DOI] [PubMed] [Google Scholar]
- 9. Horwich TB, Broderick S, Chen L, McCullough PA, Strzelczyk T, Kitzman DW, Fletcher G, Safford RE, Ewald G, Fine LJ, Ellis SJ, Fonarow GC. Relation among body mass index, exercise training, and outcomes in chronic systolic heart failure. Am J Cardiol. 2011;108:1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–343 and Int J Epidemiol. 2014;43:655–665. [DOI] [PubMed] [Google Scholar]
- 11. Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17:374–380. [DOI] [PubMed] [Google Scholar]
- 12. Puig T, Ferrero‐Gregori A, Roig E, Vazquez R, Gonzalez‐Juanatey JR, Pascual‐Figal D, Delgado J, Alonso‐Pulpon L, Borras X, Mendez A, Cinca J; REDINSCOR Researchers . Prognostic value of body mass index and waist circumference in patients with chronic heart failure (Spanish REDINSCOR Registry). Rev Esp Cardiol. 2014;67:101–106. [DOI] [PubMed] [Google Scholar]
- 13. Futter JE, Cleland JG, Clarck AL. Body mass indices and outcomes in patients with chronic heart failure. Eur J Heart Fail. 2011;13:207–213. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 16. Lavie CJ, Alpert MA, Ventura HO. Risks and benefits of weight loss in heart failure. Heart Fail Clin. 2015;11:125–131. [DOI] [PubMed] [Google Scholar]
- 17. Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2014;56:409–414. [DOI] [PubMed] [Google Scholar]
- 18. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 19. Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56:391–400. [DOI] [PubMed] [Google Scholar]
- 20. Zamora E, Lupón J, Vila J, Urrutia A, de Antonio M, Sanz H, Grau M, Ara J, Bayés‐Genís A. Estimated glomerular filtration rate and prognosis in heart failure: value of the MDRD‐4, CDK‐EPI, and Cockroft‐Gault formulas. J Am Coll Cardiol. 2012;59:1709–1715. [DOI] [PubMed] [Google Scholar]
- 21. Gastelurrutia P, Lupón J, de Antonio M, Urrutia A, Díez C, Coll R, Altimir S, Bayes‐Genis A. Statins in heart failure: the paradox between large randomized clinical trials and real life. Mayo Clin Proc. 2012;87:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banack HR, Kaufman JS. The “obesity paradox” explained. Epidemiology. 2013;24:461–462. [DOI] [PubMed] [Google Scholar]
- 23. Zamora E, Lupón J, de Antonio M, Urrutia A, Coll R, Díez C, Altimir S, Bayés‐Genís A. The obesity paradox in heart failure: is etiology a key factor? Int J Cardiol. 2013;166:601–605. [DOI] [PubMed] [Google Scholar]
- 24. Aktas MK, Zareba W, Huang DT, McNitt S, Polonsky S, Chen L, Stockburger M, Merkely B, Moss AJ, Kutyifa V. The effect of weight loss on clinical outcomes in patients implanted with a cardiac resynchronization therapy device‐A MADIT‐CRT substudy. J Card Fail. 2014;20:183–189. [DOI] [PubMed] [Google Scholar]
- 25. Adamopoulos C, Meyer P, Desai RV, Karatzidou K, Ovalle F, White M, Aban I, Love TE, Deedwania P, Anker SD, Ahmed A. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity‐matched study. Eur J Heart Fail. 2011;13:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nochioka K, Shiba N, Kohno H, Miura M, Shimokawa H. Both high and low body mass indexes are prognostic risks in Japanese patients with chronic heart failure: implications from the CHART study. J Card Fail. 2010;16:880–887. [DOI] [PubMed] [Google Scholar]
- 27. Gastelurrutia P, Lupón J, Domingo M, Ribas N, Noguero M, Martinez C, Cortes M, Bayes‐Genis A. Usefulness of body mass index to characterize nutritional status in patients with heart failure. Am J Cardiol. 2011;108:1166–1170. [DOI] [PubMed] [Google Scholar]
- 28. Mariotti R, Castrogiovanni F, Canale ML, Borelli G, Rondinini L. Weight loss and quality of life in chronic heart failure patients. J Cardiovasc Med (Hagerstown). 2008;9:576–580. [DOI] [PubMed] [Google Scholar]
- 29. Beck‐da‐Silva L, Higginson L, Fraser M, Williams K, Haddad H. Effect of Orlistat in obese patients with heart failure: a pilot study. Congest Heart Fail. 2005;11:118–123. [DOI] [PubMed] [Google Scholar]
- 30. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. [DOI] [PubMed] [Google Scholar]
- 31. Rossignol P, Masson S, Barlera S, Girerd N, Castelnovo A, Zannad F, Clemenza F, Tognoni G, Anand IS, Cohn JN, Anker SD, Tavazzi L, Latini R; GISSI‐HF and Val‐HeFT Investigators . Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: insights from the GISSI‐HF and Val‐HeFT trials. Eur J Heart Fail. 2015;17:424–433. [DOI] [PubMed] [Google Scholar]
- 32. Pureza V, Florea VG. Mechanisms for cachexia in heart failure. Curr Heart Fail Rep. 2013;10:307–314. [DOI] [PubMed] [Google Scholar]
- 33. Song EK, Lee Y, Moser DK, Dekker RL, Kang SM, Lennie TA. The link of unintentional weight loss to cardiac event‐free survival in patients with heart failure. J Cardiovasc Nurs. 2014;29:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Anker SD, Swedberg KB. Weight loss and mortality risk in patients with chronic heart failure in the Candesartan in Heart Failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:2641–2650. [DOI] [PubMed] [Google Scholar]
- 35. Trullàs JC, Formiga F, Montero M, Carrera‐Izquierdo M, Grau‐Amorós J, Chivite‐Guillén D, Manzano L; RICA Investigators . Impact of weight loss on mortality in chronic heart failure: findings from the RICA Registry. Int J Cardiol. 2013;168:306–311. [DOI] [PubMed] [Google Scholar]
- 36. Lavie CJ, Berra K, Arena R. Formal cardiac rehabilitation and exercise training programs in heart failure: evidence for substantial clinical benefits. J Cardiopulm Rehabil Prev. 2013;33:209–211. [DOI] [PubMed] [Google Scholar]


