Abstract
Background
The differing relations of steady and pulsatile components of central hemodynamics and aortic stiffness with cardiac dimensions and function have not been fully elucidated.
Methods and Results
Central hemodynamics and carotid‐femoral pulse wave velocity (CFPWV, a measure of aortic stiffness) were measured by arterial tonometry in 5799 participants of the Framingham Heart Study (mean age 51 years, 54% women) and related to echocardiographic left ventricular (LV) dimensions and systolic and diastolic function using multivariable‐adjusted partial Pearson correlations. Mean arterial pressure (MAP, steady component of central blood pressure) was associated positively with LV wall thickness (r=0.168; P<0.0001) but showed only a weak direct association with LV diastolic dimension (r=0.035, P=0.006). Central pulse pressure (pulsatile component of central blood pressure) showed a direct correlation with both LV diastolic dimension and LV wall thickness (r=0.08 and 0.044, both P<0.0001 in multivariable models that included MAP). CFPWV was not associated with LV structure (all P≥0.27) in MAP‐adjusted models). Both MAP and CFPWV were associated inversely with LV diastolic function (E′; r=−0.140 and −0.153, respectively; both P<0.0001), and these associations persisted after additional adjustment for LV mass and central pulse pressure (r=−0.142 and −0.108, both P<0.0001). MAP and CFPWV were not associated with LV fractional shortening (P≥0.10), whereas central pulse pressure was positively related (r=0.064, P<0.0001).
Conclusions
Pulsatile and steady components of central pressure are conjointly yet variably related to LV structure. CFPWV is related to LV diastolic function but not to systolic function. Additional studies are warranted to confirm these observations.
Keywords: aortic stiffness, diastolic dysfunction, left ventricle geometry, pulse wave velocity, systolic dysfunction
Subject Categories: Remodeling, Hypertrophy, Vascular Disease, Pathophysiology, Hemodynamics
Introduction
Congestive heart failure is a leading cause of morbidity and mortality in developed countries, with an estimated prevalence of 1% to 2% in the general population, reaching 7% to 8% in individuals aged >75 years.1 Elevated blood pressure appears to play an important role in the pathogenesis of both left ventricular (LV) systolic and diastolic dysfunction, at least partly by increasing cardiac afterload.2 This has led to the therapeutic concept of reducing cardiac afterload, which has proven highly beneficial in heart failure with reduced ejection fraction but not in heart failure with a preserved ejection fraction.3 Thus, broadening the understanding of the role of central hemodynamics in the pathogenesis of systolic and diastolic LV dysfunction is critical to inform the development of novel therapeutic approaches.
Blood pressure is routinely measured at the brachial artery, whereas the actual cardiac pressure load is determined by the hemodynamics in the proximal aorta. This is a relevant distinction, as central pulse pressure may differ from brachial pulse pressure, particularly in younger adults, due to age‐related differences in central pressure augmentation. The extent to which central pressure augmentation is explained by peripheral wave reflection or by the windkessel function of the proximal aorta is the subject of ongoing debate.4, 5
Limited data suggest that central pressure may be more closely related to cardiovascular disease than peripheral blood pressure.6 Furthermore, variable relations between aortic stiffness (which increases early systolic load on the heart) and wave reflection (which increases late systolic load) may have differing implications for systolic and diastolic LV structure and function.7 In contrast, the steady component of blood pressure, ie, the mean arterial pressure, is fairly constant throughout large arteries.8
In this context, the relations of central hemodynamics with cardiac structure and function have not been fully delineated. We hypothesized that the pulsatile component (and its determinants aortic stiffness and pressure augmentation) and the steady component of central blood pressure may have different relations with LV structure and function. Thus, we investigated the cross‐sectional relations of central hemodynamics and aortic stiffness to echocardiographic measures of LV structure and systolic and diastolic function in a large community‐based sample.
Methods
Study Sample
Individuals were derived from the Framingham Offspring and the Framingham Third Generation cohorts, which have been described.9, 10 The Framingham Offspring cohort was recruited in 1971–1974 and includes individuals who are children of the Framingham original cohort or the children's spouses. The Framingham Third Generation cohort, recruited in 2002–2005, comprises children of the Framingham Offspring cohort. Participants of both cohorts are evaluated approximately every 4 to 8 years in our study clinic during a visit that includes a medical history, physical examination, and phlebotomy.
The present investigation is based on examination cycle 8 of the Framingham Offspring cohort (2005–2008) and examination cycle 1 of the Third Generation cohort (2002–2005). Of 3021 participants who attended Offspring examination cycle 8 and 4095 participants recruited into the Third Generation cohort, we excluded 252 individuals with atrial fibrillation, 703 for missing echocardiographic measurements, 334 for missing tonometry, and 28 for missing covariates; that left 5799 (2184 Offspring, 3615 Third Generation) individuals for this investigation. Excluded individuals were generally older and had a higher morbidity (higher prevalence of diabetes, hypertension, and prevalent cardiovascular disease) than those included in the analyses. The study protocols were approved by the Boston University Medical Center Institutional Review Board, and participants signed informed consent. The study complies with the principles outlined in the Declaration of Helsinki.
Blood Pressure and Arterial Tonometry
Supine brachial systolic and diastolic blood pressures were obtained using an auscultatory device.11 Arterial tonometry measurements were performed as previously described.11, 12, 13 Briefly, arterial tonometry (using a standard applanation tonometry device) with simultaneous ECG was performed on the brachial, femoral, and carotid arteries. All recordings were performed on the right side of the body. Transit distances were assessed by body surface measurements from the suprasternal notch to the pulse‐recording site. Mean arterial pressure (MAP) was derived from integration of the brachial waveform calibrated with BP at the time of tonometry. Diastolic blood pressure and integrated MAP were used to calibrate carotid pressure tracings. Calibrated carotid pressure was used as a surrogate for central pressure. Direct measurement of carotid pressure, as compared to transfer function–based estimates, is associated with a smaller difference between central and peripheral pulse pressure.14 Details of signal analyses and data processing have been published elsewhere.11, 12, 13 We primarily assessed 4 measures of arterial stiffness and central hemodynamics: (1) carotid‐femoral pulse wave velocity (CFPWV), the current reference standard for aortic stiffness, (2) central pulse pressure, ie, the blood pressure amplitude in the proximal aorta, (3) augmentation index, ie, the fraction of central pulse pressure attributable to late systolic pressure augmentation (expressed as percentage), and (4) mean arterial pressure. Secondary analyses assessed additional measures of central pulse wave form and peripheral reflection, in particular forward wave amplitude, reflected wave amplitude, and the global reflection coefficient.
Echocardiography
Echocardiography was performed at both examinations using a Philips Sonos 5500 ultrasound machine. Two‐dimensional guided M‐Mode tracings were recorded with a minimum of 3 frames. All echocardiograms were evaluated by an experienced sonographer or cardiologist based on a standardized reading protocol. Cardiac dimensions were quantified using the leading‐edge technique as recommended by the American Society of Echocardiography (ASE). LV mass was calculated according to ASE guidelines, applying the method of Devereux et al.15 The sum of the diastolic thicknesses of the septum and posterior wall was used as an estimate of LV wall thickness. Early systolic mitral annulus velocity (E′) was measured at the lateral mitral annulus using tissue Doppler imaging and transmitral Doppler flow velocities recorded using a standardized protocol. Repeated analysis of diastolic function measures (mitral E and A peak velocity, tissue Doppler E′ and A′ peak velocity) yielded interobserver correlation coefficients of >0.97.
Statistical Analyses
CFPWV was inverse transformed to reduce heteroscedasticity and multiplied by −1000 to restore directionality. LV dimension, LV mass, and left atrial (LA) diameter distributions were skewed and therefore natural logarithmically transformed for all analyses. We estimated mutivariable‐adjusted partial Pearson correlations of tonometry measures with echocardiographic traits. Our primary analyses focused on CFPWV, central pulse pressure, mean arterial pressure, and augmentation index. Analyses of LV dimensions (LV mass, LV wall thickness, and diastolic dimension) were performed in 3 stages: (1) adjusting only for age, age², sex, height, and study cohort (Offspring vs Third Generation); (2) additionally adjusting for clinical risk factors (excluding blood pressure) and antihypertensive medication, ie, weight, heart rate, diabetes, serum total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), triglycerides, fasting glucose, prevalent cardiovascular disease, current smoking, intake of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, diuretics, calcium channel blockers (binary variable for each class); and (3) additionally adjusting for brachial MAP. Analyses evaluating LV systolic and diastolic function (fractional shortening, E′, E/E′) were constructed similarly in a staged design: (1) adjusting only for age, age², sex, height, and study cohort; (2) additionally adjusting for clinical risk factors (excluding blood pressure) and antihypertensive medication, ie, weight, heart rate, diabetes, total cholesterol, HDL‐C, triglycerides, fasting glucose, prevalent cardiovascular disease, current smoking, intake of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, diuretics, calcium channel blockers; (3) additionally adjusting for LV mass; and (4) further adjusting for either MAP (in models investigating pulsatile blood pressure traits) or central pulse pressure (in models investigating MAP). For figure construction we also estimated least‐squares means based on regression models with LV structure or function traits as dependent variable and tertiles of CFPWV as predictor variable, adjusting for the covariates of stage 2. Logarithmically transformed LV diastolic diameter was then backtransformed to original scale; thus, means represent geometric means.
Given that our primary analyses assessed the relation of 4 tonometry traits (central pulse pressure, MAP, CFPWV, augmentation index) to 3 LV structure traits (LV mass, LV diastolic diameter, LV wall thickness) in 3 statistical models, as well as the relation of the same 4 tonometry traits to 3 LV function traits (LV fractional shortening, E′, E/E′) in 4 statistical models, we introduced a Bonferroni correction for (4×3×3)+(4×3×4)=84 statistical tests and regarded a P‐value of 0.0006 (0.05/84) as statistically significant. Statistical analyses were carried out using SAS Version 9.1.
Results
Study Sample
Characteristics of the entire Framingham Offspring and Third Generation cohort are given in the left part of Table 1; the characteristics of the final study sample after exclusions (see Methods for details) are shown in the right part of Table 1. LV hypertrophy was present in 1574 individuals (27%) of the study sample; impaired systolic LV function was present in 46 individuals (1%). The unadjusted pairwise correlations between our primary tonometry and blood pressure traits are provided in Table 2. The correlation between forward and reflected wave amplitude was 0.79.
Table 1.
Clinical, Echocardiographic, and Hemodynamic Characteristics
| Characteristic | Entire Cohort (n=7116) | Study Sample (n=5799) |
|---|---|---|
| Age, y | 51±16 | 51±16 |
| Women, n (%) | 3846 (54) | 3132 (54) |
| Body mass index, kg/m² | 27.4±5.61 | 26.9±5.0 |
| Diabetes, n (%) | 545 (8)2 | 331 (6) |
| Hypertension, n (%) | 2467 (35)3 | 1730 (30) |
| Prevalent CVD, n (%) | 552 (8) | 260 (4) |
| Current smoking, n (%) | 979 (14)4 | 825 (14) |
| Fasting glucose, mg/dL | 100±225 | 99±20 |
| Total cholesterol, mg/dL | 187±366 | 189±36 |
| High density lipoprotein cholesterol, mg/dL | 55±177 | 56±17 |
| Triglycerides, mg/dL | 116±826 | 114±81 |
| Medication | ||
| Antihypertensive medication | 1909 (26)3 | 1242 (21) |
| ACEI or ARB, n (%) | 945 (13)8 | 624 (11) |
| Beta blockers, n (%) | 1051 (15)8 | 614 (11) |
| Calcium channel blockers, n (%) | 499 (7)8 | 308 (5) |
| Diuretics, n (%) | 884 (13)8 | 560 (10) |
| Lipid‐lowering medication, n (%) | 1596 (22)9 | 1313 (23) |
| Antidiabetic medication, n (%) | 360 (5)10 | 219 (4) |
| Echocardiography | ||
| LV mass, g | 163±4611 | 161±44 |
| LV diastolic diameter, cm | 4.9±0.4412 | 4.9±0.4 |
| LV wall thickness, mm | 1.86±0.2713 | 1.82±0.25 |
| LV fractional shortening, % | 36±4.614 | 36±4 |
| E′, cm/s | 11.2±2.915 | 11.4±2.9 |
| E/E′ | 6.4±2.016 | 6.3±1.9 |
| Tonometry | ||
| CFPWV, m/s | 8.46±3.1717 | 8.2±2.8 |
| Inverse CFPWV, ms/m | 130±3517 | 133±34 |
| Brachial diastolic blood pressure, mm Hg | 68±918 | 68±9 |
| Mean arterial pressure, mm Hg | 93±1219 | 92±12 |
| Brachial systolic blood pressure, mm Hg | 129±1918 | 128±19 |
| Brachial pulse pressure, mm Hg | 61±1720 | 60±16 |
| Central pulse pressure, mm Hg | 58±1920 | 57±19 |
| Augmentation index, % | 10.4±13.420 | 10.3±13.1 |
| Forward wave amplitude, mm Hg | 50±1520 | 49±14 |
| Reflected wave amplitude, mm Hg | 17±5.720 | 17±6 |
| Reflection factor | 0.35±0.0720 | 0.34±0.06 |
Available n values were as follows: 1n=7032, 2n= 6995, 3n=7096, 4n=7115, 5n=6986, 6n=6987, 7n=6984, 8n=7116, 9n=7094, 10n=6976, 11n=6497, 12n=6500, 13n=6751, 14n=6494, 15n=6823, 16n=6761, 17n=6587, 18n= 6918, 19n=6878, 20n=6797. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; E, carotid‐femoral pulse wave velocity; E, peak early diastolic mitral inflow velocity; E′, peak early diastolic mitral annulus velocity; LV, left ventricular.
Table 2.
Pairwise Correlation between Tonometry and Blood Pressure Measures
| SBP | Central SBP | PP | Central PP | MAP | CFPWV | AI | |
|---|---|---|---|---|---|---|---|
| DBP | 0.49 | 0.50 | 0.01a | 0.08 | 0.80 | 0.37 | 0.28 |
| SBP | 0.95 | 0.87 | 0.85 | 0.87 | 0.68 | 0.21 | |
| Central SBP | 0.82 | 0.90 | 0.88 | 0.66 | 0.31 | ||
| PP | 0.93 | 0.53 | 0.58 | 0.12 | |||
| Central PP | 0.61 | 0.58 | 0.25 | ||||
| MAP | 0.61 | 0.30 | |||||
| CFPWV | 0.21 |
Data are Pearson correlation coefficients. Correlations were highly significant (P<0.0001) unless indicated otherwise. AI indicates augmentation index; CFPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
P>0.05 (n.s.).
Central Hemodynamics, Aortic Stiffness, and LV Mass, Dimensions, and Wall Thickness
Unadjusted correlations between the tonometry measures and echocardiographic traits reflecting cardiac dimensions are presented in Table 3. Adjusted relations of central hemodynamics and aortic stiffness with LV dimensions are displayed in Table 4. Mean arterial pressure was positively associated with LV mass, even after multivariable adjustment (P<0.0001). Results for LV mass index and LV hypertrophy were essentially similar (data not shown). When we separately investigated the 2 components of LV mass, ie, LV wall thickness and LV diastolic diameter, we observed that the association of MAP with LV wall thickness was statistically robust and persisted after additional adjustment for central pulse pressure (P<0.0001 for all models), whereas the association of MAP with LV diastolic diameter was weaker and no longer statistically significant (P=0.15) after adjustment for central pulse pressure.
Table 3.
Pairwise Correlation between Vascular Measures and Cardiac Structure
| LV Mass | LV Diastolic Diameter | LV Wall Thickness | |
|---|---|---|---|
| Mean arterial pressure | 0.25a | 0.04 | 0.34a |
| CFPWV | 0.27a | 0.01 | 0.39a |
| Central pulse pressure | 0.17a | −0.001 | 0.26a |
| Augmentation index | −0.10a | −0.10a | −0.06a |
Data are Pearson correlation coefficients. CFPWV indicates carotid‐femoral pulse wave velocity; LV, left ventricular.
P<0.003. Correlations without asterisk were nonsignificant (P>0.003).
Table 4.
Central Hemodynamics, Aortic Stiffness, and Cardiac Geometry (n=5799)
| LV Massa | LV Diastolic Diametera | LV Wall Thickness | ||||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| Mean arterial pressure | ||||||
| Base model | 0.199 | <0.0001 | 0.035 | 0.008 | 0.237 | <0.0001 |
| Multivariable | 0.156 | <0.0001 | 0.035 | 0.006 | 0.168 | 0.0001 |
| Multivariable+CPP | 0.076 | <0.0001 | −0.018 | 0.15 | 0.112 | <0.0001 |
| Central pulse pressure | ||||||
| Base model | 0.195 | <0.0001 | 0.118 | <0.0001 | 0.162 | <0.0001 |
| Multivariable | 0.162 | <0.0001 | 0.086 | <0.0001 | 0.134 | <0.0001 |
| Multivariable+MAP | 0.089 | <0.0001 | 0.080 | <0.0001 | 0.044 | <0.0001 |
| Carotid‐femoral pulse wave velocityb | ||||||
| Base model | 0.112 | <0.0001 | −0.010 | 0.46 | 0.158 | <0.0001 |
| Multivariable | 0.061 | <0.0001 | 0.001 | 0.96 | 0.076 | <0.0001 |
| Multivariable+MAP | <0.001 | 0.99 | −0.014 | 0.27 | 0.011 | 0.40 |
| Augmentation index | ||||||
| Base model | 0.10 | <0.0001 | 0.110 | <0.0001 | 0.042 | 0.002 |
| Multivariable | 0.057 | <0.0001 | 0.039 | 0.003 | 0.041 | 0.002 |
| Multivariable+MAP | 0.007 | 0.61 | 0.029 | 0.03 | −0.015 | 0.24 |
Data are partial Pearson correlation coefficients and the respective P‐values. Base model adjusted for age, age2, sex, height, study cohort. Multivariable model additionally adjusted for weight, heart rate, diabetes, total cholesterol, HDL cholesterol, triglycerides, fasting glucose, prevalent cardiovascular disease, current smoking, and intake of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, β‐blockers, diuretics, or calcium channel blockers. CPP indicates central pulse pressure; LV, left ventricular; MAP, mean arterial pressure.
Natural log‐transformed for normality.
Inverse‐transformed for normality and multiplied by −1 to restore directionality.
Central pulse pressure was positively correlated with higher LV mass (P<0.0001), which was attributable to comparable relations with LV diastolic diameter and LV wall thickness (both P<0.0001). Relations remained statistically significant after adjustment for multiple clinical covariates and also persisted on additional adjustment for MAP (all P<0.0001). Figure 1 depicts multivariable‐adjusted means of LV diastolic diameter (Figure 1A) and LV wall thickness (Figure 1B), stratified by tertiles of mean arterial pressure or central pulse pressure.
Figure 1.
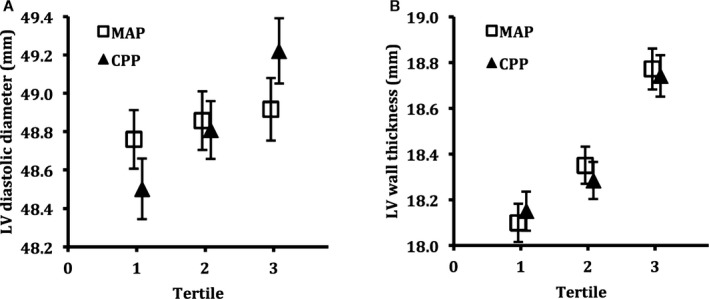
Multivariable adjusted means of left ventricular (LV) diastolic diameter (A) and LV anterior+posterior wall thickness (B), plotted by tertiles of mean arterial pressure (MAP; squares) and central pulse pressure (CPP; triangles). Error bars represent 95% confidence intervals.
Higher aortic stiffness, as assessed by CFPWV, was associated with greater LV mass and greater LV wall thickness, but the associations were attenuated on multivariable adjustment and rendered statistically nonsignificant on additional adjustment for mean arterial pressure. CFPWV was not associated with LV chamber size. Higher augmentation index correlated with higher LV mass and higher LV diastolic diameter (all P<0.0001); however, the associations were no longer significant in multivariable (including MAP)‐adjusted analyses (all P>0.003).
In secondary analyses, we related additional subphenotypes of the central pressure waveform, ie, forward wave, reflected wave, and reflection factor, to LV structure (Table 5). In multivariable (including MAP)‐adjusted analyses, forward and reflected waves generally showed similar associations with LV mass, LV diastolic diameter, and LV wall thickness (r=0.053–0.089, P<0.0001), except for a nonsignificant association of the reflected wave with LV wall thickness (r=0.034, P=0.01). In mutually adjusted analyses, forward wave relations with LV mass persisted after adjusting for reflected wave (Table 5, P<0.0001), whereas reflected wave was not related to LV mass after adjusting for forward wave (P=0.63). The reflection factor was not associated with LV structure after accounting for multiple statistical testing (all P≥0.003).
Table 5.
Secondary Analyses: Relations of Central Pressure Waveform Components With Left Ventricular Structure
| LV Mass | LV Diastolic Diameter | LV Wall Thickness | ||||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| Forward wave | ||||||
| Model 1 | 0.089 | <0.0001 | 0.069 | <0.0001 | 0.054 | <0.0001 |
| Model 2a | 0.059 | <0.0001 | 0.039 | 0.003 | 0.042 | <0.0014 |
| Reflected wave | ||||||
| Model 1 | 0.066 | <0.0001 | 0.059 | <0.0001 | 0.034 | 0.01 |
| Model 2b | 0.006 | 0.63 | 0.015 | 0.24 | −0.005 | 0.71 |
| Reflection factor | ||||||
| Model 1 | −0.029 | 0.03 | −0.017 | 0.18 | −0.022 | 0.09 |
Data are partial Pearson correlation coefficients. Model 1 adjusted for age, age2, sex, height, study cohort, weight, heart rate, diabetes, total cholesterol, HDL‐C, triglycerides, fasting glucose, prevalent cardiovascular disease, current smoking, intake of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, diuretics, or calcium channel blockers, and mean arterial pressure. LV indicates left ventricular.
Additionally adjusted for reflected wave.
Additionally adjusted for forward wave.
Central Hemodynamics, Aortic Stiffness, and LV Systolic and Diastolic Function
Unadjusted correlations between the assessed tonometry measures and echocardiographic measures of LV systolic and diastolic function are presented in Table 6. The adjusted relations of central hemodynamics and aortic stiffness with systolic and diastolic LV function are given in Table 7. Mean arterial pressure was not associated with LV fractional shortening (P>0.05 in all models). In contrast, higher MAP was associated with lower E′ and higher E/E′ (all P<0.0001). Correlations between MAP and LV filling measures persisted after adjustment for clinical covariates and also after additional adjustment for LV mass (all P<0.0001).
Table 6.
Pairwise Correlation between Vascular Measures and Cardiac Function
| Fractional Shortening | E′ | E/E′ | |
|---|---|---|---|
| Mean arterial pressure | 0.11 | −0.46 | 0.30 |
| CFPWV | 0.15 | −0.62 | 0.40 |
| Central pulse pressure | 0.21 | −0.39 | 0.39 |
| Augmentation index | 0.11 | −0.20 | 0.22 |
Data are Pearson correlation coefficients. All P‐values (for Pearson correlation) were P<0.0001. CFPWV indicates carotid‐femoral pulse wave velocity; E, maximum early diastolic mitral inflow velocity; E′, maximum early diastolic mitral annulus velocity.
Table 7.
Central Hemodynamics, Aortic Stiffness, and Cardiac Function (n=5799)
| LV Fractional Shortening | E′ | E/E′ | ||||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| Mean arterial pressure | ||||||
| Base model | 0.020 | 0.13 | −0.245 | <0.0001 | 0.164 | <0.0001 |
| Multivariable | 0.020 | 0.13 | −0.140 | <0.0001 | 0.125 | <0.0001 |
| Multivariable+LVM | 0.021 | 0.10 | −0.127 | <0.0001 | 0.118 | <0.0001 |
| Multivariable+LVM+CPP | −0.020 | 0.14 | −0.142 | <0.0001 | 0.050 | <0.0001 |
| Central pulse pressure | ||||||
| Base model | 0.073 | <0.0001 | −0.041 | 0.002 | 0.167 | <0.0001 |
| Multivariable | 0.063 | <0.0001 | −0.035 | 0.001 | 0.141 | <0.0001 |
| Multivariable+LVM | 0.065 | <0.0001 | −0.019 | 0.14 | 0.135 | <0.0001 |
| Multivariable+LVM+MAP | 0.064 | <0.0001 | 0.066 | <0.0001 | 0.082 | <0.0001 |
| Carotid‐femoral pulse wave velocitya | ||||||
| Base model | −0.019 | 0.16 | −0.260 | <0.0001 | 0.160 | <0.0001 |
| Multivariable | −0.007 | 0.61 | −0.153 | <0.0001 | 0.121 | <0.0001 |
| Multivariable+LVM | −0.006 | 0.63 | −0.148 | <0.0001 | 0.119 | <0.0001 |
| Multivariable+LVM+MAP | −0.016 | 0.23 | −0.108 | <0.0001 | 0.080 | <0.0001 |
| Augmentation index | ||||||
| Base model | 0.027 | 0.04 | 0.034 | 0.01 | 0.082 | <0.0001 |
| Multivariable | −0.047 | 0.0004 | −0.037 | 0.005 | 0.071 | <0.0001 |
| Multivariable+LVM | −0.046 | 0.0006 | −0.031 | 0.02 | 0.068 | <0.0001 |
| Multivariable+LVM+MAP | −0.056 | <0.0001 | 0.010 | 0.44 | 0.031 | 0.02 |
Data are partial Pearson correlation coefficients and the respective P‐values. Base model: adjusted for age, age2, sex, height, and study cohort. Multivariable model: additionally adjusted for weight, heart rate, diabetes, total cholesterol, HDL‐cholesterol, triglycerides, fasting glucose, prevalent cardiovascular disease, current smoking, intake of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, diuretics, calcium channel blockers. CPP indicates central pulse pressure; E, maximum early diastolic mitral inflow velocity; E′, maximum early diastolic mitral annulus velocity; LVM, left ventricular mass; MAP, mean arterial pressure.
Inverse‐transformed for normality and multiplied by −1 to restore directionality.
Central pulse pressure was moderately and positively correlated with fractional shortening (P<0.0001), even after multivariable adjustment (P<0.0001, Table 7). Central pulse pressure also correlated very modestly and inversely with E′ (a measure of diastolic relaxation), whereas we observed a stronger direct correlation of central pulse pressure with E/E′ (P<0.0001), a surrogate for LV filling pressure. Correlations between central pulse pressure and LV filling measures were only slightly attenuated by adjustment for multiple potential clinical confounders including LV mass. After additional adjustment for mean arterial pressure, the association of central pulse pressure with E/E′ was maintained, whereas the directionality of the modest negative association with E′ was reversed.
Higher CFPWV was not related to fractional shortening but correlated with worse diastolic function (as assessed by E′ and E/E′, all P<0.0001, Table 3). The correlations of CFPWV with measures of slowed relaxation and higher LV filling pressure persisted after multivariable adjustment, including adjustment for MAP and LV mass (all P<0.0001). Figure 2 depicts multivariable adjusted means of LV fractional shortening (Figure 2A) and E′ (Figure 2B), stratified by tertiles of CFPWV.
Figure 2.

Multivariable adjusted means of left ventricular (LV) fractional shortening (A) and early mitral valve annulus diastolic velocity (E′, B), plotted by tertiles of carotid‐femoral pulse wave velocity (CFPWV). Error bars represent 95% confidence intervals.
In a base model, higher augmentation index correlated modestly with greater fractional shortening. However, in multivariable‐adjusted analyses, higher augmentation index correlated modestly and inversely with LV systolic function, even after adjustment for LV mass and mean arterial pressure (P<0.0001, Table 7). We found similar reversal of directionality of very modest associations between augmentation index and E′ following multivariable adjustment. In contrast, augmentation index was associated positively with E/E′ in our base model (P>0.0001), and the association and directionality persisted with multivariable adjustment.
In secondary analyses (see Table 8), forward wave amplitude correlated with fractional shortening (r=0.086, P<0.0001), whereas reflected wave amplitude did not (P=0.06). Forward and reflected waves similarly correlated with measures of diastolic LV function (r=0.053–0.066, all P<0.0001). Reflection factor correlated inversely with fractional shortening (r=−0.077, P<0.0001) but was not related to diastolic function traits (all P>0.05).
Table 8.
Secondary Analyses: Relations of Central Pressure Waveform Components with Left Ventricular Function
| Fractional Shortening | E′ | E/E′ | ||||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| Forward wave | ||||||
| Model 1 | 0.086 | <0.0001 | 0.066 | <0.0001 | 0.057 | <0.0001 |
| Model 2a | 0.094 | <0.0001 | 0.036 | 0.008 | 0.027 | 0.03 |
| Reflected wave | ||||||
| Model 1 | 0.025 | 0.06 | 0.053 | <0.0001 | 0.055 | <0.0001 |
| Model 2b | −0.048 | 0.0002 | 0.010 | 0.46 | 0.022 | 0.09 |
| Reflection factor | ||||||
| Model 1 | −0.077 | <0.0001 | −0.02 | 0.18 | 0.009 | 0.51 |
Data are partial Pearson correlation coefficients, adjusted for age, age2, sex, height, study cohort, weight, heart rate, diabetes, total cholesterol, HDL‐C, triglycerides, fasting glucose, prevalent cardiovascular disease, current smoking, intake of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, diuretics, calcium channel blockers, mean arterial pressure and left ventricular mass. E′, maximum early diastolic mitral annulus velocity; E, maximum early diastolic mitral inflow velocity.
Additionally adjusted for reflected wave.
Additionally adjusted for forward wave.
Discussion
In the present investigation we examined differing associations of pulsatile and steady components of central blood pressure and aortic stiffness with left ventricular structure and function. Our major findings are 3‐fold. First, both steady (ie, MAP) and pulsatile (ie, central pulse pressure) components of central blood pressure were correlated positively with LV mass. However, whereas higher MAP was primarily associated with higher LV wall thickness, central pulse pressure was positively associated with both LV diameter and LV wall thickness. Second, higher MAP and greater aortic stiffness were correlated inversely with LV diastolic function, and these correlations at least partly persisted in models adjusting of LV mass. Third, aortic stiffness, as assessed by CFPWV, was associated inversely with LV diastolic function but showed no independent correlation with LV dimensions or circumferential LV systolic function.
Central Hemodynamics, Aortic Stiffness, and Cardiac Structure
Whereas the relation between peripheral blood pressure and cardiac structure has been well documented,16 few studies have assessed relations among cardiac structure, central hemodynamics, and vascular stiffness using detailed tonometry measures. These prior studies were limited by small sample size17 or an indirect assessment of central hemodynamics,18 restricted to non‐European ancestry individuals18, 19, 20 or relied on electrocardiographic LV hypertrophy.21 In a sample of 1272 Chinese indiviudals, Wang et al reported that central pulse pressure was associated with LV mass.19 However, their study did not separately assess associations with LV wall thickness versus LV dimensions. An independent association of proximal (ascending) aortic stiffness with LV mass was recently reported in 347 elderly participants of the Age, Gene/Environment Susceptibility (AGES)‐Reykjavik Study cohort.22 That study investigated the hypothesis of a direct coupling between LV and a stiffened proximal aorta as a novel type of mechanical, rather than hemodynamic, load on the left ventricle and thus did not evaluate relations with CFPWV. To our knowledge, the present analysis is the largest investigation of the relation of central hemodynamics and CFPWV (the reference measure of aortic stiffness) with cardiac structure and function in a sample of European ancestry. We observed that higher central pulse pressure is associated with both higher LV diameter and greater wall thickness, whereas higher MAP (steady component of central pressure) is primarily associated with higher LV wall thickness (rather than with LV diameter). Thus, the steady and pulsatile components of central blood pressure may conjointly yet variably contribute to differences in LV mass. Of note, however, the relation between LV mass and pulse pressure is likely bidirectional. The fact that neither aortic stiffness nor wave reflection is independently associated with LV mass in our analyses suggests that the relation between LV mass and pulse pressure may be largely attributable to mismatch between ventricular outflow and the ability of the aorta to accommodate that flow, as recently described by Torjesen et al.23
Central Hemodynamics, Aortic Stiffness, and LV Systolic and Diastolic Function
Existing literature on the relations among central hemodynamics, vascular stiffness, and cardiac function is sparse, and most studies were of limited size,24, 25, 26, 27, 28 restricted to certain patient populations,27 or did not include tissue Doppler assessment of LV diastolic function.20 Abhayaratna et al investigated the relation of arterial stiffness to LV diastolic dysfunction in a sample of 188 elderly individuals and observed a significant correlation between central pulse pressure and severity of diastolic dysfunction.29 However, CFPWV was not associated with diastolic dysfunction in their study. Russo et al reported in 983 individuals that several tonometry‐derived measures of central hemodynamics, greater arterial stiffness, and more wave reflection were all associated with worse LV diastolic function.30 However, after multivariable adjustment, only the ratio of central pulse pressure to stroke volume index (a measure of global arterial stiffness) remained associated with LV diastolic dysfunction. Kang et al measured brachial‐ankle pulse wave velocity in 1929 individuals in Shanghai and reported an association with diastolic heart failure.31 Notably, CFPWV was not measured in the latter studies.
Our considerably larger analysis demonstrates that higher mean arterial pressure and aortic stiffness are associated inversely with measures of LV diastolic function. The fact that these associations persisted after adjustment for LV mass is consistent with the notion that diastolic dysfunction may be only partly dependent on LV hypertrophy.32 Aortic and cardiac stiffness (hence LV diastolic dysfunction) may share etiologic mechanisms such as excessive tissue fibrosis. The extent to which aortic stiffness may contribute to LV diastolic dysfunction or that common pathophysiological mechanisms may contribute to parallel increases in both aortic and cardiac stiffness cannot be assessed in our cross‐sectional analyses and therefore remains to be elucidated. If greater aortic stiffness is indeed shown to contribute to LV diastolic dysfunction in additional studies, therapeutic interventions aimed at decreasing macrovascular stiffness may be a promising tool for the prevention and mitigation of diastolic dysfunction, a premise that warrants further study.
The relations of central pulse pressure and augmentation index to LV diastolic dysfunction were not straightforward in our analyses. Whereas we observed a consistent association of higher central pulse pressure with higher E/E′ (a surrogate measure of elevated LV filling pressure), the association of central pulse pressure with the filling‐phase measure of diastolic relaxation (ie, E′) was weak and changed directionality after adjustment for potential confounding by MAP. Hence, central pulse pressure and augmentation index appear to be primarily associated with LV filling pressures rather than with LV diastolic relaxation. Interestingly, we observed a positive correlation between higher central pulse pressure and LV systolic function, which may seem surprising. The most likely explanation for this finding is that a greater fractional shortening corresponds to higher stroke volume and peak flow rate in the proximal aorta and thus may be a cause rather than a consequence of higher central pulse pressure. Similarly, the lack of an association between MAP and LV systolic function in our cross‐sectional analysis may possibly be explained by various opposing effects of MAP on LV function. An acute increase in MAP reduces LV systolic function (inverse relation) but may promote LV hypertrophy and remodeling, which restore LV wall stress and systolic function to normal levels (thereby resulting in a null relation).
Limitations
Several limitations of our study should be addressed. First, our study sample is community based and predominantly comprised of middle‐aged adults of European ancestry. The applicability of our findings to younger individuals, to other ethnicities or certain patient groups, remains to be explored in future studies.
Second, our study is cross‐sectional and observational; thus, causal inferences cannot be drawn. This is particularly relevant as some of the observed correlations are likely bidirectional. In addition, the potential for residual confounding cannot be eliminated. Third, our echocardiographic measures are based on 2‐dimensional guided M‐mode echocardiographic tracings. Unfortunately, 2‐dimensional measures of ejection fraction were not available.
Also, we would like to emphasize that our study was focused on the physiological relations of central hemodynamics with cardiac structure and function. We did not investigate whether central blood pressure may be more strongly related to certain echocardiography traits than to arm blood pressure. In fact, brachial and central pulse pressure were highly correlated (R=0.93) in our data. Consequently, the findings for brachial pulse pressure were similar to those observed for central pulse pressure (see Table 9).14 Similarly, we did not study aggregate measures of pulsatile and steady pressure components, eg, central systolic blood pressure.
Table 9.
Brachial Versus Central Pressure in Relation to Echocardiographic Traits
| Brachial Pulse Pressure | Central Pulse Pressure | P for Difference | |
|---|---|---|---|
| LV structure | |||
| LV mass | 0.186 | 0.195 | 0.63 |
| LV diastolic diameter | 0.108 | 0.118 | 0.61 |
| LV wall thickness | 0.159 | 0.162 | 0.86 |
| LV function | |||
| LV fractional shortening | 0.081 | 0.073 | 0.66 |
| E′ | 0.037 | 0.041 | 0.83 |
| E/E′ | 0.154 | 0.167 | 0.49 |
E indicates maximum early diastolic mitral inflow velocity; E', maximum early diastolic mitral annulus velocity; LV, left ventricular.Data are partial Pearson correlations, adjusted for age, age2, sex, height and study cohort.
Next, most of the observed correlations were of modest strength. However, we would like to underscore that our main analyses were adjusted for multiple potential confounders. In unadjusted analyses, several correlations were markedly stronger. Last, we have performed multiple statistical tests, potentially inflating the type 1 error rate. However, we accounted for multiple testing using a Bonferroni correction. Of note, most of our findings were highly statistically significant (P<0.0001) and hence likely to be true associations.
Conclusions
In the present investigation, we assessed relations of steady and pulsatile components of central hemodynamics and aortic stiffness to cardiac structure and LV systolic and diastolic function in a large community‐based sample with a broad age range. We observed a distinct pattern of associations. Steady and pulsatile components of central blood pressure were jointly and variably related to components of LV structure. Aortic stiffness and MAP were associated inversely with measures of LV diastolic function but not with LV systolic function. Additional studies are warranted to confirm our findings.
Sources of Funding
This work was supported by the NHLBI, Framingham Heart Study (NHLBI/NIH Contracts N01‐HC‐25195 and HHSN268201500001I), the Boston University School of Medicine, and by HL076784, G028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, 2‐K24‐HL04334, and R01HL126136.
Disclosures
Dr Mitchell is owner of Cardiovascular Engineering Inc (a company that develops and manufactures devices to measure vascular stiffness) and serves as a consultant to Novartis, Merck, and Servier. The other authors report no potential conflicts to disclose.
(J Am Heart Assoc. 2016;5:e002693 doi: 10.1161/JAHA.115.002693)
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure. A clinical mechanistic overview. Arch Intern Med. 1996;156:1789–1796. [PubMed] [Google Scholar]
- 3. Liu Y, Haddad T, Dwivedi G. Heart failure with preserved ejection fraction: current understanding and emerging concepts. Curr Opin Cardiol. 2013;28:187–196. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell GF. Arterial stiffness and wave reflection in hypertension: pathophysiologic and therapeutic implications. Curr Hypertens Rep. 2004;6:436–441. [DOI] [PubMed] [Google Scholar]
- 5. Schultz MG, Davies JE, Hardikar A, Pitt S, Moraldo M, Dhutia N, Hughes AD, Sharman JE. Aortic reservoir pressure corresponds to cyclic changes in aortic volume: physiological validation in humans. Arterioscler Thromb Vasc Biol. 2014;34:1597–1603. [DOI] [PubMed] [Google Scholar]
- 6. Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. [DOI] [PubMed] [Google Scholar]
- 7. Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St. John Sutton M, Gillebert TC; Asklepios I . Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle‐aged adults: the Asklepios Study. Hypertension. 2013;61:296–303. [DOI] [PubMed] [Google Scholar]
- 8. Hamilton WF, Dow P. An experimental study of the standing waves in the pulse propagated through the aorta. Am J Physiol. 1938;125:48–59. [Google Scholar]
- 9. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 10. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 13. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Narayan O, Casan J, Szarski M, Dart AM, Meredith IT, Cameron JD. Estimation of central aortic blood pressure: a systematic meta‐analysis of available techniques. J Hypertens. 2014;32:1727–1740. [DOI] [PubMed] [Google Scholar]
- 15. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 16. Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saba PS, Roman MJ, Pini R, Spitzer M, Ganau A, Devereux RB. Relation of arterial pressure waveform to left ventricular and carotid anatomy in normotensive subjects. J Am Coll Cardiol. 1993;22:1873–1880. [DOI] [PubMed] [Google Scholar]
- 18. Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens. 2010;28:384–388. [DOI] [PubMed] [Google Scholar]
- 19. Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Li Y, Ding FH, Sheng CS, Huang QF, Wang JG. Cardiac structure and function in relation to central blood pressure components in Chinese. J Hypertens. 2011;29:2462–2468. [DOI] [PubMed] [Google Scholar]
- 21. Wohlfahrt P, Wichterle D, Seidlerova J, Filipovsky J, Bruthans J, Adamkova V, Cifkova R. Relation of central and brachial blood pressure to left ventricular hypertrophy. The Czech Post‐MONICA Study. J Hum Hypertens. 2011;26:14–19. [DOI] [PubMed] [Google Scholar]
- 22. Bell V, Sigurdsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, Harris TB, Gudnason V, de Roos A, Mitchell GF. Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility—Reykjavik Study. Circ Cardiovasc Imaging. 2015;8:e003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torjesen AA, Sigurethsson S, Westenberg JJ, Gotal JD, Bell V, Aspelund T, Launer LJ, de Roos A, Gudnason V, Harris TB, Mitchell GF. Pulse pressure relation to aortic and left ventricular structure in the Age, Gene/Environment Susceptibility (AGES)—Reykjavik Study. Hypertension. 2014;64:756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. [DOI] [PubMed] [Google Scholar]
- 26. Ikonomidis I, Tzortzis S, Papaioannou T, Protogerou A, Stamatelopoulos K, Papamichael C, Zakopoulos N, Lekakis J. Incremental value of arterial wave reflections in the determination of left ventricular diastolic dysfunction in untreated patients with essential hypertension. J Hum Hypertens. 2008;22:687–698. [DOI] [PubMed] [Google Scholar]
- 27. Sharman JE, Haluska BA, Fang ZY, Prins JB, Marwick TH. Association of arterial wave properties and diastolic dysfunction in patients with type 2 diabetes mellitus. Am J Cardiol. 2007;99:844–848. [DOI] [PubMed] [Google Scholar]
- 28. Subherwal S, de las Fuentes L, Waggoner AD, Heuerman S, Spence KE, Davila‐Roman VG. Central aortic pressure is independently associated with diastolic function. Am Heart J. 2010;159:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abhayaratna WP, Barnes ME, O'Rourke MF, Gersh BJ, Seward JB, Miyasaka Y, Bailey KR, Tsang TS. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients > or =65 years of age. Am J Cardiol. 2006;98:1387–1392. [DOI] [PubMed] [Google Scholar]
- 30. Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang S, Fan HM, Li J, Fan LY, Miao AY, Bao Y, Wu LZ, Zhu Y, Zhang DF, Liu ZM. Relationship of arterial stiffness and early mild diastolic heart failure in general middle and aged population. Eur Heart J. 2010;31:2799–2807. [DOI] [PubMed] [Google Scholar]
- 32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]


