Abstract
Background
Cardiovascular diseases (CVD) are the leading cause of death in the world, and diet plays a major role in CVD incidence, especially through lipid oxidation mechanisms. This, in turn, leads to tissue inflammation and formation of atheromatous plaques.
Methods and Results
Our objective was to evaluate the association between the inflammatory potential of the diet and the incidence of overall CVD or its subclasses. We included 7743 participants from the Supplémentation en Vitamines et Minéraux AntioXydants (SU.VI.MAX) cohort. All cardiovascular events were recorded using self‐reported information or clinical visits, and were validated. The dietary inflammatory index (DII) was computed using repeated 24‐hour dietary records (mean=9.5±3.4 records/subject). Hazard ratio and 95% CI for outcomes (CVD and subclasses) were estimated across sex‐specific quartiles of the DII using Cox proportional hazard models. A total of 292 cardiovascular events were recorded and validated during an average of 11.4 years of follow‐up: 93 myocardial infarctions, 58 strokes, 128 angina pectoris and revascularization interventions, and 13 sudden deaths. When considering CVD subclasses, a diet with pro‐inflammatory properties, as expressed by higher DII scores, was significantly associated with a higher risk of myocardial infarction (hazard ratioQuartile 4 versus Quartile 1=2.24, 95% CI: 1.08–4.67). No significant association was observed between the DII score and stroke or both angina pectoris and revascularization intervention.
Conclusions
A pro‐inflammatory diet, as measured by a higher DII score, was prospectively associated with a higher risk of myocardial infarction. Promotion of a diet exhibiting anti‐inflammatory properties may help prevent myocardial infarctions.
Keywords: cardiovascular disease, diet, epidemiology, inflammation, nutrition
Subject Categories: Epidemiology, Diet and Nutrition, Cardiovascular Disease, Primary Prevention
Introduction
As a country develops, the types of diseases that affect its population shift from primarily infectious, such as diarrhea and pneumonia, to primarily noncommunicable diseases, such as cardiovascular disease (CVD), obesity, and cancer.1 The global burden of CVD has increased, mostly in wealthy countries. However, many low‐ and middle‐income countries now face a growing burden from the modern health risks (physical inactivity, tobacco), while still fighting an unfinished battle with traditional health risks (undernutrition, sanitation).1, 2, 3 CVDs are now the leading cause of mortality in the world, causing 17.5 million deaths in 2012, which represented 31% of total global mortality.4 In France, CVDs are the second major cause of mortality—the first among women—and represent nearly one third (28%) of total mortality.5
Optimistically, simple and cost‐effective measures such as dietary changes could reduce the obesity epidemic and the resultant CVD burden, as it has been estimated that lifestyle choices can account for up to 40% of premature CVD deaths.5 In the past few decades, associations between individual nutritional factors and chronic diseases, especially CVD, have been widely investigated. More recently, alternative approaches that consider the whole diet (for example, dietary indices reflecting diet quality) have been used, making it feasible to capture nutrient interactions in the food matrix.6, 7 In particular, the adherence to a traditional Mediterranean dietary pattern, including a high consumption of plant foods and olive oil, low intakes of saturated fat and sugar, and a low/moderate consumption of wine, has been associated with a lower risk of CVD and mortality in many epidemiological studies,8, 9 as well as with lower levels of inflammation.10, 11, 12 Indeed, the association between inflammatory mechanisms and the development of CVD has been largely brought to light.13, 14, 15 Inflammatory pathways promote thrombosis, a late complication of atherosclerosis responsible for myocardial infarctions (MI) and most strokes.14
To assess the adherence to a dietary pattern, several dietary scores or indices have been developed, such as the Dietary Inflammatory Index (DII). This index was developed in 200916 and then updated in 2014.17 The DII aims to measure the inflammatory potential of the diet based on the pro‐ and anti‐inflammatory properties of its various components, including macronutrients, vitamins, minerals, flavonoids, specific food items, and energy intake, according to the existing literature based on tissue culture, and animal and human studies.
To our knowledge, only 3 published studies have investigated the association between the DII and the risk of cardiovascular disease. All 3 studies reported a protective role of a more anti‐inflammatory diet.18, 19, 20 These recent studies have begun to fill an important gap in understanding the relationship between systemic inflammation—a phenomenon with multiple underlying causes—and cardiovascular disease, by focusing specifically on diet, which appears to be a key modifiable factor involved in inflammatory pathways.10, 11, 12 Additionally, to the best of our knowledge, no study has yet investigated the association of the DII with the incidence of specific CVD subtypes.
In order to contribute to filling this research gap, we assessed the prospective association between the inflammatory potential of the diet, as measured by the DII, and the incidence of overall CVD and subtypes of CVD—namely, MI; strokes; angina pectoris and revascularization interventions—in a large cohort of French adults with a long‐term follow‐up.
Subjects and Methods
Study Population
Subjects were selected from the “SUpplémentation en VItamines et Minéraux AntioXydants” (SU.VI.MAX) study. The SU.VI.MAX study was a randomized, double‐blind, placebo‐controlled primary prevention trial conducted between 1994 and 2002. It included 12 741 French adults (women aged 35–60 years and men aged 45–60 years). Its primary aim was to evaluate the potential efficacy of daily supplementation with antioxidant vitamins and minerals (ascorbic acid, vitamin E, β‐carotene, selenium, and zinc) delivered at nutritional (ie, nonpharmacologic) doses on the prevention of cancer, ischemic heart disease, and overall mortality.21 The trial phase ended in 2002 and health events monitoring was pursued until September 2007.
The SU.VI.MAX study was registered at clinicaltrials.gov (number NCT00272428) and was conducted according to the guidelines laid down in the Helsinki Declaration of 1975 as revised in 1983, and was approved by the Ethics Committee for Studies with Human Participants of Paris‐Cochin Hospital (CCPPRB no. 706) and the Commission Nationale Informatique et Liberté (CNIL no. 334641), which ensures that medical information be kept confidential. All subjects provided written informed consent.
Inclusion Criteria
In this analysis, we selected men and women who provided at least 3 valid 24‐hour dietary records during the first 2 years of follow‐up (n=8108) and who had no missing data for covariables (7743) to be included in the models (Figure).
Figure 1.
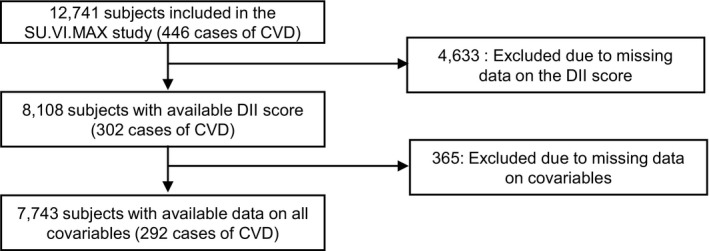
Selection of participants for our analyses, SU.VI.MAX study, France. CVD indicates cardiovascular diseases; DII, dietary inflammatory index; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants.
Dietary Data
Throughout the entire SU.VI.MAX study trial phase (1994–2002), participants were asked to provide a 24‐hour dietary record every 2 months, for a total of 6 records per year covering all days of the week and all seasons of the year. For these analyses, mean dietary intakes were calculated across all available, valid 24‐hour dietary records obtained during the first 2 years of follow‐up, in order to obtain a proxy for habitual dietary intakes. Participants with <3 valid records during this period were excluded from analyses. Dietary records were considered invalid if energy intake was <100 or >6000 kcal/day. Additionally, men reporting <800 kcal/day and women reporting <500 kcal/day across one third or more of records were excluded to account for energy underreporting.
An instruction manual including validated photographs of generic foods and portion sizes was provided to facilitate coding food portions.22 A food composition table was used to estimate nutrient intake.23 We used the DII to determine the “inflammatory potential” of the diet. This index relies on information contained in 1943 published studies reporting association between nutritional factors and inflammatory markers.17 Briefly, points were assigned to every food parameter on the basis of relevant articles found in the literature, according to whether each food parameter increased (+1), decreased (−1), or had no (0) effect on 6 inflammatory biomarkers: interleukin‐1b, interleukin‐4, interleukin‐6, interleukin‐10, tumor necrosis factor‐α, and C‐reactive protein.
The DII computed from the 24‐hour records in this study includes data on 36 of the 45 food parameters comprising the DII: carbohydrate, protein, total fat, energy, alcohol, fiber, cholesterol, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, omega 3, omega 6, niacin, thiamin, riboflavin, vitamin B6, vitamin B12, iron, magnesium, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β‐carotene, anthocyanidins, flavan‐3‐ol, flavonols, flavonones, flavones, isoflavones, garlic, ginger, pepper, onion, and tea. Dietary intakes for these parameters were first standardized using a “world” mean and SD, derived from nutritional databases from 11 populations around the world, then transformed to percentiles and multiplied by literature‐derived inflammatory points for each food parameter, then summed up. Thus, lower DII scores indicate a lower inflammatory potential of the diet. DII scores were computed on the basis of participant‐specific mean nutrient and food intakes, which were obtained by averaging intakes across all available dietary records, as explained in the first paragraph of the Dietary Data section (ie, DII scores were not first calculated per day and then averaged). More details on the score computation can be found elsewhere.17
Case Ascertainment
Information about health was self‐reported via a monthly questionnaire during the follow‐up. Other sources of information regarding outcome included yearly clinical examinations. In case of no contact with a participant for a long period, or nonattendance at the yearly visit, an investigation was launched to determine the reasons. In case of a suspected event, investigations were conducted to obtain relevant medical data (clinical, biochemical, histological, and radiological reports) from participants, physicians, and/or hospitals. All cardiovascular events were reviewed and validated by an independent expert committee, unaware of the supplementation group assignment, and then classified using the International Classification of Diseases, 10th Revision, Clinical Modification (codes I20–I24, I64).24 First, we considered as outcome the occurrence of all CVDs. Second, we studied the incidence of 3 subclasses of CVD—MI, stroke, and angina pectoris or revascularization intervention—separately.
Covariates
Sociodemographic data, including sex, date of birth, education (primary education only, up to secondary education, higher education), as well as information on marital status (couple or single), smoking status (never‐smoked, former or current smoker), and physical activity level were obtained by a (nonvalidated) baseline questionnaire. The items concerning physical activity inquired whether a participant had a regular physical activity, and if yes, whether they estimated this activity to be equivalent to ≥1 hour of walking per day. Body mass index was calculated using baseline weight and height measurements performed by trained personnel according to standardized procedures, using an electronic scale (Seca®, Hamburg, Germany) and a wall‐mounted stadiometer.
Statistical Analysis
Subjects contributed person‐time up to the date of the cardiovascular event (n=292), death (n=193), the last completed questionnaire or last contact (n=1704) or September 1, 2007 (n=5554), whichever occurred first. For analyses concerning subclasses of CVD, participants who reported another CVD event than the one studied during follow‐up were included as noncases for the studied CVD and censored at the date of that diagnosis. Participants’ baseline characteristics were compared across sex‐specific quartiles (Q) of DII scores. Nutritional intakes were energy‐adjusted using the residual method,25 and thus presented as adjusted (least squares) means with 95% CI. DII values were presented as means with interquartile ranges. Values for all other quantitative variables were presented as means (SD), and values for qualitative variables as percentages. Chi‐square tests and ANOVA were carried out for quantitative and qualitative variables, respectively.
We estimated hazard ratio (HR) of CVD risk and 95% CIs both for the continuous DII score and for quartiles of DII scores, using Cox proportional hazard models.26 Graphic methods (log–log [survival] versus log–time plots) were used to check for proportional hazards assumptions for this study. The lowest quartile of DII score (Q1, reflecting the most anti‐inflammatory diet) was used as the reference category. Tests for linear trend were performed by modeling DII quartiles as an ordinal variable.
The first model was crude (model 0), with only age as the time‐scale in the Cox model. A second model was adjusted for age (time‐scale in the Cox model), sex, and daily energy intake without alcohol (continuous, in kcal/day) (model 1). A multivariate model was further adjusted for number of available 24‐hour dietary records (continuous), smoking status, physical activity, education level, marital status, and treatment allocation group (placebo or active) (model 2—our main model). Two additional models (3a and 3b) were created in order to verify whether our results would remain stable even when adjusting for baseline body mass index (continuous, in kg/m2) and for alcohol intake (continuous, in g/day), respectively. The reason why model 3b was, in addition to body mass index, further adjusted for alcohol intake is that alcohol consumption is considered in a linear manner for the computation of the DII score while only moderate alcohol consumption is associated with a reduced CVD risk.27 The same models were used for both overall CVDs and analyses focused on CVD subtypes.
In order to verify the robustness of our findings, we performed a set of sensitivity analyses. First, we reanalyzed our data after removing participants who developed a cardiovascular event during the first 2 years of follow‐up. Second, we selected only participants with at least six 24‐hour records during the first 2 years of follow‐up in order to improve the accuracy of nutritional estimates. Finally, we repeated our analyses with a DII score in which dietary intakes for all parameters were first adjusted for overall energy intake, using the energy density method ([crude intakes/total energy intake]×1000), named “DII 2”.
All tests were 2‐sided and P<0.05 was considered statistically significant, except for interaction tests for which statistical power is often weak in observational studies.28 Thus, P<0.10 was applied for these models. Interactions between the DII score (modeled both as a continuous variable and as quartiles) and sex and intervention group were investigated. All analyses were performed using SAS® software (version 9.3; SAS Institute Inc, Cary, NC).
Results
These analyses included a total of 7743 participants (3197 men and 4546 women). Mean age at baseline (±SD) was 51.9±4.7 and 47.1±6.6 years for men and women, respectively. The DII score ranged from −5.31 to 6.26 with a mean of 0.69±1.88.
Baseline characteristics of the study population are presented by sex‐specific quartiles of the DII in Table 1. Given the relatively large size of our sample, the following comparisons of characteristics across quartiles should be interpreted with caution, as not every statistically significant result is biologically and clinically meaningful. Compared to those with a lower DII score (Q1), reflecting an anti‐inflammatory diet, participants with higher DII scores (Q4), (ie, more pro‐inflammatory) were slightly younger, less educated, less physically active, smokers, and less often postmenopausal women. A higher DII score also was associated with slightly higher glycemia, slightly higher total blood cholesterol concentration, and slightly higher blood pressure, both systolic and diastolic. In addition, a higher DII score was associated with lower total daily energy intake, as well as a lower fruit and vegetable consumption, a lower intake of mono‐ and polyunsaturated fatty acids—including n‐3 and n‐6—vitamins (C, E, B6, B9, and B12), β‐carotene, calcium, magnesium, sodium, iron, iodine, potassium, phosphorus, and fibers but a higher intake of saturated fatty acids and a higher alcohol consumption (Table 2).
Table 1.
Baseline Characteristics Across Sex‐Specific Quartiles of the DII Score, SU.VI.MAX Study, France (N=7743)
| Quartile 1DII | Quartile 2DII | Quartile 3DII | Quartile 4DII | P Valuea | |
|---|---|---|---|---|---|
| N | 1935 | 1936 | 1937 | 1935 | |
| DII, points | −1.7 (1.1) | 0.0 (0.8) | 1.3 (0.7) | 3.1 (1.3) | <0.0001b |
| Male (%) | 41.3 | 41.3 | 41.3 | 41.3 | 1.0 |
| Intervention group of the SU.VI.MAX trial (%) | 50.7 | 50.4 | 50.3 | 49.6 | 0.90 |
| Age, y | 49.6 (6.4) | 49.2 (6.4) | 49.2 (6.3) | 48.4 (6.2) | <0.0001b |
| Body mass index, kg/m2 | 19.9 (9.4) | 20.0 (9.6) | 19.9 (10.0) | 19.8 (10.0) | 0.65 |
| Education (%) | <0.0001b | ||||
| Primary | 15.4 | 18.0 | 21.5 | 26.4 | |
| Secondary | 36.9 | 38.1 | 39.8 | 39.0 | |
| University level or equivalent | 47.7 | 44.0 | 38.8 | 34.6 | |
| Marital status (%) | 0.40 | ||||
| Alone | 16.2 | 15.3 | 14.3 | 15.8 | |
| Cohabiting | 83.8 | 84.7 | 85.7 | 84.2 | |
| Physical activity (%) | <0.0001b | ||||
| Irregular | 21.3 | 23.7 | 27.2 | 28.9 | |
| <1 h/day | 32.6 | 31.3 | 31.0 | 28.5 | |
| ≥1 h/day | 46.1 | 45.0 | 41.8 | 42.6 | |
| Smoking status (%) | <0.0001b | ||||
| Nonsmoker | 49.0 | 48.3 | 47.4 | 45.7 | |
| Former smoker | 41.9 | 38.6 | 38.2 | 35.6 | |
| Smoker | 9.1 | 13.1 | 14.5 | 18.7 | |
| Oral contraceptive use (%)c | 15.2 | 19.4 | 13.2 | 15.1 | 0.003b |
| Postmenopausal women (%)c | 30.0 | 24.4 | 26.9 | 22.7 | 0.0009b |
| Among which with HTM medication (%)c | 71.4 | 69.0 | 73.6 | 64.7 | 0.15 |
| Glycemia, mmol/Lc | 5.67 (0.77) | 5.69 (0.92) | 5.71 (0.84) | 5.75 (0.86) | 0.04b |
| Cholesterol, mmol/Lc | 5.97 (1.01) | 5.97 (1.01) | 6.04 (1.02) | 6.09 (1.03) | 0.0004b |
| HDL‐cholesterol, mmol/Lc | 1.81 (0.33) | 1.80 (0.32) | 1.81 (0.32) | 1.82 (0.32) | 0.14 |
| LDL‐cholesterol, mmol/Lc | 3.71 (0.75) | 3.70 (0.75) | 3.74 (0.76) | 3.76 (0.77) | 0.06 |
| Blood pressure, mm Hg | |||||
| Systolicc | 122.6 (14.1) | 123.2 (14.2) | 124.6 (14.7) | 124.1 (15.0) | 0.0004b |
| Diastolicc | 78.9 (8.8) | 79.1 (9.0) | 80.1 (9.1) | 79.9 (9.4) | 0.0003b |
Values for quantitative variables are means (SD), except for the DII where mean (interquartile range) is presented. Values for qualitative variables are percentages. DII indicates dietary inflammatory index; HDL, high‐density lipoprotein; HTM, hormonal treatment for menopause; LDL, low‐density lipoprotein; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants.
P‐values are based on χ2 tests or ANOVA.
P<0.05.
Information was available for a reduced number of participants. Oral contraceptive use: 3621; postmenopausal women: 4197; HTM medication: 1092; glycemia: 7514; cholesterol: 7669; HDL‐ and LDL‐ cholesterol: 7618; systolic and diastolic blood pressure: 6422.
Percentages describe the respective proportion among female participants only.
Table 2.
Nutritional Data Across Sex‐Specific Quartiles of the DII Score, SU.VI.MAX Study, France (N=7743)
| Quartile 1DII | Quartile 2DII | Quartile 3DII | Quartile 4DII | P Valuea | |
|---|---|---|---|---|---|
| Number of 24‐h dietary recordsb | 9.4 (3.4) | 9.7 (3.3) | 9.5 (3.3) | 9.2 (3.5) | <0.0001c |
| Energy intake, kcal/dayb | 2403.2 (653.4) | 2200.4 (552.3) | 2017.7 (517.0) | 1759.0 (484.2) | <0.0001c |
| Energy intake without alcohol, kcal/dayb | 2267.5 (594.8) | 2063.9 (497.9) | 1888.6 (463.4) | 1649.6 (439.1) | <0.0001c |
| Carbohydrate, %b | 40.0 (6.6) | 39.3 (6.7) | 38.9 (6.8) | 38.5 (6.7) | <0.0001c |
| Fat, %b | 38.0 (5.2) | 38.2 (5.0) | 38.4 (5.2) | 38.6 (5.4) | 0.003c |
| Protein, %b | 16.7 (2.7) | 16.6 (2.7) | 16.7 (2.7) | 17.2 (3.0) | <0.0001c |
| Fruit and vegetable consumption, g/dayd | 499 (493, 505) | 393 (387, 399) | 326 (320, 332) | 244 (237, 250) | <0.0001c |
| Alcohold, g/day | 13.9 (13.0, 14.7) | 17.6 (16.8, 18.4) | 19.8 (19.0, 20.7) | 21.6 (20.8, 22.5) | <0.0001c |
| SFAd, g/day | 34.6 (34.3, 34.8) | 36.1 (35.8, 36.3) | 37.3 (37.0, 37.6) | 38.3 (38.0, 38.6) | <0.0001c |
| MUFAd, g/day | 34.0 (33.8, 34.3) | 33.8 (33.5, 34.0) | 33.6 (33.4, 33.8) | 33.3 (33.0, 33.5) | 0.0007c |
| PUFAd, g/day | 14.8 (14.6, 14.9) | 13.6 (13.4, 13.7) | 12.7 (12.6, 12.9) | 11.7 (11.5, 11.8) | <0.0001c |
| N‐3 PUFAd, g/day | 1.44 (1.43, 1.46) | 1.28 (1.26, 1.29) | 1.19 (1.17, 1.20) | 1.10 (1.08, 1.12) | <0.0001c |
| N‐6 PUFAd, g/day | 12.68 (12.54, 12.82) | 11.69 (11.55, 11.82) | 10.94 (10.81, 11.08) | 9.99 (9.84, 10.13) | <0.0001c |
| Vitamin Cd, mg/day | 128.6 (126.9, 130.3) | 101.2 (99.5, 102.8) | 83.9 (82.3, 85.6) | 62.2 (60.5, 64.0) | <0.0001c |
| Vitamin Ed, mg/day | 15.1 (15.0, 15.3) | 13.3 (13.1, 13.4) | 11.9 (11.8, 12.1) | 10.3 (10.2, 10.5) | <0.0001c |
| Vitamin B6d, mg/day | 1.95 (1.94, 1.97) | 1.74 (1.73, 1.76) | 1.62 (1.61, 1.64) | 1.52 (1.50, 1.53) | <0.0001c |
| Vitamin B9d, mg/day | 379.2 (376.3, 382.0) | 322.6 (319.9, 325.3) | 288.3 (285.5, 291.0) | 248.3 (245.4, 251.1) | <0.0001c |
| Vitamin B12d, mg/day | 7.9 (7.7, 8.1) | 7.2 (7.0, 7.4) | 6.8 (6.6, 7.0) | 6.6 (6.4, 6.8) | <0.0001c |
| β‐Carotened, μg/day | 5605 (5505, 5706) | 4309 (4211, 4407) | 3413 (3315, 3510) | 2493 (2392, 2595) | <0.0001c |
| Calciumd, mg/day | 1005 (993, 1018) | 949 (937, 961) | 921 (909, 933) | 894 (881, 906) | <0.0001c |
| Magnesiumd, mg/day | 323.5 (321.5, 325.4) | 296.8 (294.9, 298.7) | 284.7 (282.8, 286.6) | 271.5 (269.5, 273.4) | <0.0001c |
| Sodiumd, mg/day | 3440 (3401, 3478) | 3436 (3398, 3473) | 3400 (3362, 3437) | 3385 (3346, 3424) | 0.17 |
| Irond, mg/day | 13.5 (13.4, 13.6) | 12.8 (12.7, 12.9) | 12.3 (12.2, 12.4) | 11.9 (11.8, 12.0) | <0.0001c |
| Iodined, μg/day | 152.9 (151.1, 154.7) | 144.4 (142.6, 146.1) | 139.6 (137.8, 141.3) | 135.0 (133.2, 136.8) | <0.0001c |
| Potassiumd, mg/day | 3316 (3297, 3335) | 3037 (3018, 3055) | 2876 (2857, 2894) | 2682 (2663, 2702) | <0.0001c |
| Phosphorusd, mg/day | 1354 (1346, 1363) | 1283 (1275, 1292) | 1250 (1241, 1258) | 1230 (1221, 1239) | <0.0001c |
| Fibersd, g/day | 23.2 (23.0, 23.4) | 19.6 (19.4, 19.8) | 17.4 (17.2, 17.6) | 15.2 (15.0, 15.4) | <0.0001c |
Values are means (SDs) or least squares means (95% CI), as specified. DII indicates dietary inflammatory index; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants.
Values are based on analysis of variance (ANOVA).
Values are means (SDs). Carbohydrate, fat, and protein are presented as percentage of total daily energy intake.
P<0.05.
Data were energy‐adjusted using the residual method, and thus presented as least squares means (95% CIs).
During the 13‐year follow‐up (mean duration was 11.4 years, corresponding to 87 932 person‐years of observation), 292 CVD were reported: 238 in men and 54 in women. The distribution was as follows: 93 MI, 58 strokes, 128 angina pectoris and revascularization interventions, and 13 sudden deaths. In the crude model 0, we observed a positive association between the DII score, reflecting a pro‐inflammatory diet, and risk of CVD (HRQ4 versus Q1=1.73, 95% CI=1.25–2.39) (Table 3). However, this association was no longer observed after adjustment for confounding factors in any of the models (1, 2, 3a, or 3b). Interactions tested between the DII score and sex or intervention group were not significant, whether the DII was modeled as a continuous variable or as quartiles.
Table 3.
Association Between the DII and Incidence of Overall Cardiovascular Disease, SU.VI.MAX Study, France (N=7743)
| Q1DII | Q2DII | Q3DII | Q4DII | P trend a | Continuous DII | P continuous | |
|---|---|---|---|---|---|---|---|
| DII, mean (IQR) | −1.7 (1.1) | 0.0 (0.8) | 1.3 (0.7) | 3.1 (1.3) | |||
| N | 1935 | 1936 | 1937 | 1935 | |||
| Number of cases | 63 | 71 | 69 | 89 | |||
| Person‐time, y | 22 432 | 22 117 | 21 912 | 21 471 | |||
| Model 0 | 1 (ref) | 1.21 [0.86–1.70] | 1.18 [0.84–1.66] | 1.73 [1.25–2.39] | 0.002b | 1.05 [0.98–1.11] | 0.16 |
| Model 1 | 1 (ref) | 1.06 [0.75–1.50] | 0.99 [0.69–1.41] | 1.26 [0.87–1.82] | 0.30 | 1.05 [0.97–1.13] | 0.24 |
| Model 2 | 1 (ref) | 1.05 [0.74–1.48] | 0.94 [0.65–1.34] | 1.15 [0.79–1.69] | 0.59 | 1.03 [0.95–1.11] | 0.47 |
| Model 3a | 1 (ref) | 1.05 [0.74–1.48] | 0.94 [0.66–1.35] | 1.16 [0.79–1.69] | 0.59 | 1.03 [0.96–1.11] | 0.45 |
| Model 3b | 1 (ref) | 1.05 [0.74–1.48] | 0.93 [0.65–1.34] | 1.15 [0.79–1.68] | 0.61 | 1.03 [0.95–1.11] | 0.49 |
The values listed next to “Model 0”–“Model 3b” are hazard ratio (95% CIs), estimated through Cox proportional hazard models, corresponding to sex‐specific quartiles of the DII. Model 0 is crude. Model 1 is adjusted for sex and energy intake without alcohol. Model 2: Model 1+supplementation group, number of 24‐h records, education level, marital status, smoking status, and physical activity. Model 3a: Model 2+body mass index. Model 3b: Model 2+alcohol consumption. DII indicates dietary inflammatory index; IQR, interquartile range; Q, sex‐specific quartile; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants.
P for trend (obtained by modeling DII quartiles as an ordinal variable).
P<0.05.
When separating CVDs into subclasses (Table 4), being in the highest DII score quartile was associated with a higher incidence of MI, as compared to being in the lowest quartile (HRQuartile 4 versus Quartile 1=2.24, 95% CI: 1.08–4.67) in the fully adjusted model (model 2), although the HR for a one‐point‐increase in DII was not statistically significant (HR continuous DII=1.11, 95% CI=0.97–1.27). No significant relationship was found regarding incidence of cerebrovascular accident or angina pectoris and revascularization intervention.
Table 4.
Association Between the DII and Incidence of Subclasses of Cardiovascular Disease, SU.VI.MAX Study, France (N=7743)
| Q1DII | Q2DII | Q3DII | Q4DII | P trend a | Continuous DII | P continuous | |
|---|---|---|---|---|---|---|---|
| MI (N cases) | 13 | 21 | 24 | 35 | |||
| Model 0 | 1 (ref) | 1.74 [0.87–3.48] | 1.99 [1.01–3.91] | 3.29 [1.74–6.23] | 0.0002b | 1.13 [1.01–1.26] | 0.03b |
| Model 1 | 1 (ref) | 1.53 [0.76–3.09] | 1.69 [0.84–3.40] | 2.45 [1.19–5.04] | 0.02b | 1.13 [0.99–1.30] | 0.06 |
| Model 2 | 1 (ref) | 1.51 [0.75–3.06] | 1.62 [0.80–3.28] | 2.24 [1.08–4.67] | 0.03b | 1.11 [0.97–1.27] | 0.12 |
| Model 3a | 1 (ref) | 1.51 [0.75–3.07] | 1.64 [0.81–3.32] | 2.26 [1.08–4.71] | 0.03b | 1.12 [0.98–1.28] | 0.11 |
| Model 3b | 1 (ref) | 1.51 [0.74–3.05] | 1.63 [0.80–3.30] | 2.28 [1.09–4.75] | 0.03b | 1.12 [0.98–1.28] | 0.11 |
| Stroke (N cases) | 16 | 12 | 10 | 20 | |||
| Model 0 | 1 (ref) | 0.79 [0.38–1.68] | 0.66 [0.30–1.46] | 1.47 [0.76–2.85] | 0.32 | 1.07 [0.93–1.23] | 0.36 |
| Model 1 | 1 (ref) | 0.75 [0.35–1.60] | 0.61 [0.27–1.38] | 1.26 [0.59–2.72] | 0.64 | 1.06 [0.90–1.24] | 0.51 |
| Model 2 | 1 (ref) | 0.74 [0.35–1.59] | 0.59 [0.26–1.34] | 1.22 [0.56–2.66] | 0.71 | 1.05 [0.89–1.24] | 0.57 |
| Model 3a | 1 (ref) | 0.74 [0.35–1.59] | 0.58 [0.26–1.34] | 1.22 [0.56–2.65] | 0.72 | 1.05 [0.89–1.24] | 0.57 |
| Model 3b | 1 (ref) | 0.74 [0.35–1.59] | 0.57 [0.25–1.31] | 1.16 [0.53–2.51] | 0.82 | 1.03 [0.88–1.22] | 0.69 |
| AP/RI (N cases) | 32 | 34 | 30 | 32 | |||
| Model 0 | 1 (ref) | 1.15 [0.71–1.86] | 1.02 [0.62–1.68] | 1.24 [0.76–2.03] | 0.51 | 0.99 [0.90–1.09] | 0.85 |
| Model 1 | 1 (ref) | 0.97 [0.59–1.58] | 0.81 [0.48–1.36] | 0.82 [0.46–1.43] | 0.38 | 0.99 [0.88–1.11] | 0.83 |
| Model 2 | 1 (ref) | 0.95 [0.58–1.57] | 0.75 [0.45–1.28] | 0.73 [0.41–1.30] | 0.21 | 0.97 [0.87–1.09] | 0.59 |
| Model 3a | 1 (ref) | 0.95 [0.58–1.57] | 0.76 [0.45–1.28] | 0.73 [0.41–1.30] | 0.20 | 0.97 [0.87–1.09] | 0.61 |
| Model 3b | 1 (ref) | 0.96 [0.58–1.57] | 0.75 [0.45–1.27] | 0.73 [0.41–1.30] | 0.20 | 0.97 [0.86–1.08] | 0.57 |
Values are hazard ratio (95% CIs), estimated through Cox proportional hazard models, corresponding to sex‐specific quartiles of the DII. Person‐time per quartile (Q) in days: Q1, 22 432; Q2, 22 117; Q3, 21 912; Q4, 21 471. Model 0 is crude. Model 1 is adjusted for sex and energy intake without alcohol. Model 2: Model 1+supplementation group, number of 24‐h records, education level, marital status, smoking status, and physical activity. Model 3a: Model 2+body mass index. Model 3b: Model 2+alcohol consumption. AP indicates angina pectoris; DII, dietary inflammatory index; MI, myocardial infarction; Q, sex‐specific quartile; RI, revascularization intervention; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants.
P for trend (obtained by modeling DII quartiles as an ordinal variable).
P<0.05.
Three sets of supplemental analyses were conducted in order to investigate the stability of our results concerning MI. First, when removing participants with cardiovascular events declared during the first 2 years of follow‐up (N=7602), findings were very similar (Table 5). Next, when selecting participants with at least six 24‐hour records (N=6362), findings were strengthened (HRQ4 versus Q1 for MI risk=3.23, 95% CI=1.37–7.62), and the HR for a one‐point‐increase in DII was significantly different from 1.00 (HR continuous=1.18, 95% CI=1.01–1.38) (Table 6). Finally, in analyses using a DII score based on energy‐adjusted nutritional parameters, the results were broadly consistent with those of our main analysis, although the observed association was weaker (Table 7).
Table 5.
Association Between the DII and Incidence of Myocardial Infarction Among Participants Without Early Cardiovascular Events, SU.VI.MAX Study, France (N=7602)
| Q1DII | Q2DII | Q3DII | Q4DII | P trend a | Continuous DII | P continuous | |
|---|---|---|---|---|---|---|---|
| DII, mean (IQR) | −1.7 (1.1) | 0.0 (0.8) | 1.3 (0.7) | 3.1 (1.3) | |||
| N | 1900 | 1900 | 1902 | 1900 | |||
| Number of cases | 12 | 16 | 21 | 30 | |||
| Person‐time, y | 22 306 | 22 041 | 21 847 | 21 538 | |||
| Model 0 | 1 (ref) | 1.45 [0.68–3.06] | 1.90 [0.94–3.87] | 3.12 [1.59–6.10] | 0.0004b | 1.12 [1.00–1.27] | 0.06 |
| Model 1 | 1 (ref) | 1.32 [0.62–2.82] | 1.72 [0.82–3.61] | 2.61 [1.21–5.62] | 0.009b | 1.15 [0.99–1.32] | 0.06 |
| Model 2 | 1 (ref) | 1.28 [0.60–2.75] | 1.63 [0.77–3.43] | 2.38 [1.09–5.19] | 0.02b | 1.12 [0.97–1.29] | 0.12 |
| Model 3a | 1 (ref) | 1.29 [0.60–2.77] | 1.64 [0.78–3.47] | 2.39 [1.09–5.22] | 0.02b | 1.12 [0.97–1.30] | 0.11 |
| Model 3b | 1 (ref) | 1.28 [0.60–2.75] | 1.62 [0.77–3.43] | 2.36 [1.08–5.17] | 0.02b | 1.12 [0.97–1.29] | 0.13 |
Values are hazard ratio (95% CIs), estimated through Cox proportional hazard models, corresponding to sex‐specific quartiles of the DII. Model 0 is crude. Model 1 is adjusted for sex and energy intake without alcohol. Model 2: Model 1+supplementation group, number of 24‐h records, education level, marital status, smoking status, and physical activity. Model 3a: Model 2+body mass index. Model 3b: Model 2+alcohol consumption. DII indicates dietary inflammatory index; IQR, interquartile range; Q, sex‐specific quartile; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants.
P for trend (obtained by modeling DII quartiles as an ordinal variable).
P<0.05.
Table 6.
Association Between the DII and Incidence of Myocardial Infarction Among Participants With at Least Six 24‐H Dietary Records, SU.VI.MAX Study, France (N=6362)
| Q1DII | Q2DII | Q3DII | Q4DII | P trend a | Continuous DII | P continuous | |
|---|---|---|---|---|---|---|---|
| DII, mean (IQR) | −1.6 (1.1) | 0.0 (0.8) | 1.3 (0.8) | 3.0 (1.3) | |||
| N | 1590 | 1591 | 1591 | 1590 | |||
| Number of cases | 9 | 16 | 20 | 30 | |||
| Person‐time, y | 18 741 | 18 538 | 18 342 | 17 952 | |||
| Model 0 | 1 (ref) | 1.87 [0.83–4.24] | 2.35 [1.07–5.16] | 4.01 [1.90–8.47] | 0.0001b | 1.17 [1.03–1.32] | 0.02b |
| Model 1 | 1 (ref) | 1.78 [0.78–4.08] | 2.24 [0.99–5.10] | 3.65 [1.57–8.49] | 0.002b | 1.22 [1.05–1.42] | 0.01b |
| Model 2 | 1 (ref) | 1.69 [0.73–3.88] | 2.12 [0.93–4.83] | 3.23 [1.37–7.62] | 0.005b | 1.18 [1.01–1.38] | 0.03b |
| Model 3a | 1 (ref) | 1.69 [0.73–3.89] | 2.13 [0.93–4.87] | 3.22 [1.36–7.62] | 0.005b | 1.19 [1.02–1.38] | 0.03b |
| Model 3b | 1 (ref) | 1.69 [0.73–3.88] | 2.12 [0.93–4.83] | 3.23 [1.37–7.63] | 0.005b | 1.18 [1.01–1.38] | 0.03b |
Values are hazard ratio (95% CIs), estimated through Cox proportional hazard models, corresponding to sex‐specific quartiles of the DII. Model 0 is crude. Model 1 is adjusted for sex and energy intake without alcohol. Model 2: Model 1+supplementation group, number of 24‐h records, education level, marital status, smoking status, and physical activity. Model 3a: Model 2+body mass index. Model 3b: Model 2+alcohol consumption. DII indicates dietary inflammatory index; IQR, interquartile range; Q, sex‐specific quartile; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants.
P for trend (obtained by modeling DII quartiles as an ordinal variable).
P<0.05.
Table 7.
Association Between a DII Calculated on the Basis of Energy‐Adjusted Nutritional Parameters (DII 2) and Incidence of Myocardial Infarction, SU.VI.MAX Study, France (N=7743)
| Q1DII | Q2DII | Q3DII | Q4DII | P trend a | Continuous DII | P continuous | |
|---|---|---|---|---|---|---|---|
| DII, mean (IQR) | −1.86 (1.20) | −0.19 (0.92) | 0.91 (0.78) | 2.41 (1.00) | |||
| N | 1935 | 1936 | 1937 | 1935 | |||
| Number of cases | 19 | 19 | 29 | 26 | |||
| Person‐time, y | 22 050 | 22 064 | 21 905 | 21 913 | |||
| Model 0 | 1 (ref) | 1.05 [0.56–1.99] | 1.77 [0.99–3.16] | 1.71 [0.95–3.10] | 0.02b | 1.23 [1.09–1.39] | 0.001b |
| Model 1 | 1 (ref) | 1.11 [0.59–2.10] | 1.96 [1.09–3.52] | 1.83 [1.00–3.33] | 0.01b | 1.15 [1.01–1.31] | 0.04b |
| Model 2 | 1 (ref) | 1.08 [0.57–2.05] | 1.87 [1.03–3.37] | 1.59 [0.86–2.92] | 0.05 | 1.11 [0.98–1.27] | 0.11 |
| Model 3a | 1 (ref) | 1.09 [0.57–2.06] | 1.88 [1.04–3.39] | 1.62 [0.88–2.97] | 0.04b | 1.12 [0.98–1.27] | 0.10 |
| Model 3b | 1 (ref) | 1.07 [0.56–2.03] | 1.84 [1.01–3.33] | 1.56 [0.85–2.89] | 0.06 | 1.11 [0.97–1.26] | 0.13 |
Values are hazard ratio (95% CIs), estimated through Cox proportional hazard models, corresponding to sex‐specific quartiles of the DII. Model 0 is crude. Model 1 is adjusted for sex and energy intake without alcohol. Model 2: Model 1+supplementation group, number of 24‐h records, education level, marital status, smoking status, and physical activity. Model 3a: Model 2+body mass index. Model 3b: Model 2+alcohol consumption. DII indicates dietary inflammatory index; IQR, interquartile range; Q, sex‐specific quartile; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants.
P for trend (obtained by modeling DII quartiles as an ordinal variable).
P<0.05.
Discussion
In this large cohort study of middle‐aged French adults, a prospective association was found between a pro‐inflammatory diet, as reflected by higher DII scores, and the incidence of myocardial infarction over a 13‐year period, even after adjusting for a wide range of potential confounders including sociodemographic data and lifestyle habits. No statistically significant relationship was observed for overall CVD risk, stroke, or angina pectoris and revascularization intervention incidence.
To the best of our knowledge, no previous study had investigated the association between the DII score and different subtypes of CVD. As indicated by the distribution of nutrient intakes by DII quartiles in our study (Table 2), lower DII scores do not simply reflect diets with a higher anti‐inflammatory potential, but also diets that are generally healthier. Thus, our findings also should be interpreted in the light of studies that have found a preventive role of diets with an overall high quality with respect to MI incidence. For example, lower risks of MI were found among individuals exhibiting a healthy diet, as defined by a score above 27 points on a slightly modified version of the SmartDiet index in the Tromsø Study,29 and defined by being in the top quintile of Recommended Food Score in a prospective cohort of Swedish men.30 The Mediterranean diet also has been related to a lower MI incidence.31
The DII was associated with increased MI risk but not with other CVD outcomes in our study. A possible explanation could be that inflammation is more strongly linked to MI than other subtypes of CVD. Indeed, the infiltration and retention of low‐density lipoprotein cholesterol in the arterial intima initiates an inflammatory response in the artery wall, involving the binding of blood cells, especially monocytes, which then differentiate into macrophages and internalize a broad range of molecules including oxidized low‐density lipoprotein particles. Ultimately, the macrophages are transformed into foam cells, the prototypical cells in atherosclerosis. Several endogenous and microbial molecules can ligate pattern‐recognition receptors (Toll‐like receptors) on these cells, leading to the release of pro‐inflammatory cytokines and ultimately to tissue damage. Angina pectoris can occur at this stage. As activated macrophages produce inflammatory cytokines that can destabilize lesions and initiate thrombus formation, all of these inflammatory reactions can finally lead to the activation and rupture of plaques, and MI.13, 14, 15
Angina pectoris and revascularization interventions—which are generally conducted among high‐risk people before any cardiovascular event—might reflect the early stages of inflammatory mechanism,14 compared to MI, which reflects the cumulative effect of advanced‐stage inflammation in the artery. On the other hand, ischemic or hemorrhagic strokes may or may not be linked to mechanisms of inflammation32; nevertheless, it was hard to separate the 2 types in our study.
To the best of our knowledge, only 3 other studies have investigated the association between the DII score and overall CVD risk using a prospective design. Two of these studies that were based on the Spanish cohorts PREDIMED (Prevención con Dieta Mediterránea)18 and SUN (Seguimiento Universidad de Navarra)19 have investigated quartiles of the DII, and have found a positive association, showing a linear dose–response trend, with overall CVD risk. The respective HR, comparing the fourth with the first quartile, were 1.73 (95% CI=1.15–2.60),18 and 2.03 (1.06–3.88),19 respectively. Another study, conducted in a cohort of Australian men,20 has investigated the DII as a binary variable (negative versus positive scores); it also found positive DII scores to be associated with an elevated overall CVD risk: odds ratio=2.07 (1.20, 3.55).
Other studies have focused on other dietary indexes. For instance, a higher level of adherence to the French dietary guidelines, as reflected in Programme National Nutrition Santé‐Guideline Score (PNNS‐GS), was associated with a lower risk of CVD.33 This result is consistent with those obtained examining other diet quality indexes.34, 35 Nevertheless, these dietary indices do not focus on the inflammatory potential of the diet, and thus the measured association may reflect different mechanisms of action.
Some limitations of this study should be acknowledged. First, the subjects in our analysis, who were volunteers participating in a nutritional intervention study,21 generally had a higher educational level than the general population, and probably a higher general interest in nutrition. Furthermore, we included only participants who had completed at least 3 dietary records during the first 2 years of the study; thus, they may have been particularly compliant and health conscious. This quite rigorous selection may limit the external validity of our findings. Moreover, participants of the SU.VI.MAX cohort were aged between 35 (women) or 45 (men) and 60 years old at baseline. Thus, any generalization of these results to younger or older subjects should be done cautiously; further studies may be needed in other populations. Additionally, the limited number of cases may have impaired our ability to detect some of the hypothesized associations, especially for specific CVD subtypes. Finally, these findings are based on observational data; thus, residual confounding cannot be ruled out.
Strengths of the present study include its large sample size and its prospective design, allowing us to assess the association between baseline inflammatory potential of the diet and CVD risk through a long follow‐up period of 13 years. In addition, our clinical and dietary data exhibited a high level of accuracy, with a mean of nine 24‐hour dietary records per participant. Finally, we used an index that was specifically designed to measure the inflammatory potential of any diet: The DII was based on peer‐reviewed literature focusing specifically on inflammation; furthermore, this index can be adapted to virtually any dietary assessment method that provides estimates of nutrient intake; and it is standardized to dietary intakes from representative populations around the world, thus facilitating easy quantitative comparisons across studies.
Conclusions
The results of this study provide new arguments for dietary prevention of MI, suggesting that favoring anti‐inflammatory nutritional compounds and limiting pro‐inflammatory foods and nutrients may contribute to lower the risk of MI. This is in line with the major role of inflammation in the development of atherosclerosis, the most important contributor to the growing burden of CVD.
Author Contributions
The authors’ responsibilities were as follows: Galan, Hercberg, and Kesse‐Guyot were responsible for developing the concept, design, and protocol of the study and for coordinating data collection; Neufcourt performed the statistical analysis and wrote the article; Kesse‐Guyot provided methodological guidance and had primary responsibility for the final content; Neufcourt, Assmann, Fezeu, Touvier, Graffouillère, Shivappa, Hébert, Wirth, Hercberg, Galan, Julia, and Kesse‐Guyot were involved in interpreting results and editing the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Sources of Funding
This work was supported by grants from the Fondation Coeur et Artères, the French National Research Agency (Agence Nationale de Recherche, ANR) (grant no. ANR‐05‐PNRA‐010) and the French Ministry of Health (Direction Générale de Santé, DGS). Assmann was supported by a doctoral fellowship from the Ecole Doctorale Galilée, University of Paris 13, Sorbonne Paris Cité. Drs Shivappa, Hébert, and Wirth were supported by the United States National Institute for Diabetes, Digestive and Kidney Diseases (grant no. R44DK103377).
Disclosures
Dr James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smartphone applications for patient counseling and dietary intervention in clinical settings. Drs Nitin Shivappa and Michael Wirth are employees of CHI. None of the other authors declare any conflicts of interest.
Acknowledgments
We thank Younes Esseddik, Gwenaël Monot, Paul Flanzy, Mohand Aït Oufella, Yasmina Chelghoum, and Than Duong Van (computer scientists), Rachida Mehroug (logistic assistant), and Nathalie Arnault, Véronique Gourlet, Fabien Szabo, Laurent Bourhis, and Stephen Besseau (statisticians) for their technical contribution to the SU.VI.MAX study.
(J Am Heart Assoc. 2016;5:e002735 doi: 10.1161/JAHA.115.002735)
References
- 1. World Health Organization . Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: WHO; 2009. [Google Scholar]
- 2. Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health. 2005;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danaei G, Singh GM, Paciorek CJ, Lin JK, Cowan MJ, Finucane MM, Farzadfar F, Stevens GA, Riley LM, Lu Y, Rao M, Ezzati M. The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation. 2013;127:1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization—Media Centre . Cardiovascular diseases (CVDs)—fact sheet No. 317. 2015.
- 5. Schroeder SA. Shattuck Lecture. We can do better–improving the health of the American people. N Engl J Med. 2007;357:1221–1228. [DOI] [PubMed] [Google Scholar]
- 6. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 7. Jacobs DR Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr. 2009;89:1543S–1548S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez‐Lopez FR, Chedraui P, Haya J, Cuadros JL. Effects of the Mediterranean diet on longevity and age‐related morbid conditions. Maturitas. 2009;64:67–79. [DOI] [PubMed] [Google Scholar]
- 9. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta‐analysis. Am J Clin Nutr. 2010;92:1189–1196. [DOI] [PubMed] [Google Scholar]
- 10. Ahluwalia N, Andreeva VA, Kesse‐Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39:99–110. [DOI] [PubMed] [Google Scholar]
- 11. Casas R, Sacanella E, Urpi‐Sarda M, Chiva‐Blanch G, Ros E, Martinez‐Gonzalez MA, Covas MI, Salas‐Salvado J, Fiol M, Aros F, Estruch R. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS One. 2014;9:e100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta‐analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24:929–939. [DOI] [PubMed] [Google Scholar]
- 13. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 14. Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 15. Calder PC, Albers R, Antoine JM, Blum S, Bourdet‐Sicard R, Ferns GA, Folkerts G, Friedmann PS, Frost GS, Guarner F, Lovik M, Macfarlane S, Meyer PD, M'Rabet L, Serafini M, van Eden W, van Loo J, Vas Dias W, Vidry S, Winklhofer‐Roob BM, Zhao J. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009;101(suppl 1):S1–S45. [DOI] [PubMed] [Google Scholar]
- 16. Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hebert JR. A new dietary inflammatory index predicts interval changes in serum high‐sensitivity C‐reactive protein. J Nutr. 2009;139:2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature‐derived, population‐based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia‐Arellano A, Ramallal R, Ruiz‐Canela M, Salas‐Salvado J, Corella D, Shivappa N, Schroder H, Hebert JR, Ros E, Gomez‐Garcia E, Estruch R, Lapetra J, Aros F, Fiol M, Serra‐Majem L, Pinto X, Babio N, Gonzalez JI, Fito M, Martinez JA, Martinez‐Gonzalez MA; Investigators TP . Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED Study. Nutrients. 2015;7:4124–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramallal R, Toledo E, Martinez‐Gonzalez MA, Hernandez‐Hernandez A, Garcia‐Arellano A, Shivappa N, Hebert JR, Ruiz‐Canela M. Dietary inflammatory index and incidence of cardiovascular disease in the SUN cohort. PLoS One. 2015;10:e0135221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Neil A, Shivappa N, Jacka FN, Kotowicz MA, Kibbey K, Hebert JR, Pasco JA. Pro‐inflammatory dietary intake as a risk factor for CVD in men: a 5‐year longitudinal study. Br J Nutr. 2015;114:2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briancon S. The SU.VI.MAX study: a randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–2342. [DOI] [PubMed] [Google Scholar]
- 22. Le Moullec N, Deheeger M, Preziosi P, Montero P, Valeix P, Rolland‐Cachera M‐F, Potier de Courcy G, Christides J‐P, Galan P, Hercberg S. Validation du manuel photos utilisé pour l'enquête alimentaire de l’étude SU.VI.MAX. Cahier de Nutrition et de Diététique. 1996;31:158–164. [Google Scholar]
- 23. Hercberg S (coordinator). Table de composition SU.VI.MAX des aliments. Paris: Inserm. Economica Ed.; 2005. [Google Scholar]
- 24. World Health Organization (WHO) . ICD‐10, International classification of diseases and related health problems. 10th revision. 2010.
- 25. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 26. Cox DR. Regression models and life tables (with discussion). J R Stat Soc. 1972;B 34:187–202. [Google Scholar]
- 27. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta‐analysis. BMJ. 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansen‐Krone IJ, Enga KF, Njolstad I, Hansen JB, Braekkan SK. Heart healthy diet and risk of myocardial infarction and venous thromboembolism. The Tromso Study. Thromb Haemost. 2012;108:554–560. [DOI] [PubMed] [Google Scholar]
- 30. Akesson A, Larsson SC, Discacciati A, Wolk A. Low‐risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population‐based prospective cohort study. J Am Coll Cardiol. 2014;64:1299–1306. [DOI] [PubMed] [Google Scholar]
- 31. Martinez‐Gonzalez MA, Fernandez‐Jarne E, Serrano‐Martinez M, Marti A, Martinez JA, Martin‐Moreno JM. Mediterranean diet and reduction in the risk of a first acute myocardial infarction: an operational healthy dietary score. Eur J Nutr. 2002;41:153–160. [DOI] [PubMed] [Google Scholar]
- 32. Luna JM, Moon YP, Liu KM, Spitalnik S, Paik MC, Cheung K, Sacco RL, Elkind MS. High‐sensitivity C‐reactive protein and interleukin‐6‐dominant inflammation and ischemic stroke risk: the Northern Manhattan Study. Stroke. 2014;45:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kesse‐Guyot E, Touvier M, Henegar A, Czernichow S, Galan P, Hercberg S, Castetbon K. Higher adherence to French dietary guidelines and chronic diseases in the prospective SU.VI.MAX cohort. Eur J Clin Nutr. 2011;65:887–894. [DOI] [PubMed] [Google Scholar]
- 34. Kourlaba G, Panagiotakos DB. Dietary quality indices and human health: a review. Maturitas. 2009;62:1–8. [DOI] [PubMed] [Google Scholar]
- 35. Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123:2870–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]


