Abstract
Background
Evidence shows that healthy diet, exercise, smoking interventions, and stress reduction reduce cardiovascular disease risk. We aimed to compare the effectiveness of these lifestyle interventions for individual risk profiles and determine their rank order in reducing 10‐year cardiovascular disease risk.
Methods and Results
We computed risks using the American College of Cardiology/American Heart Association Pooled Cohort Equations for a variety of individual profiles. Using published literature on risk factor reductions through diverse lifestyle interventions—group therapy for stopping smoking, Mediterranean diet, aerobic exercise (walking), and yoga—we calculated the risk reduction through each of these interventions to determine the strategy associated with the maximum benefit for each profile. Sensitivity analyses were conducted to test the robustness of the results. In the base‐case analysis, yoga was associated with the largest 10‐year cardiovascular disease risk reductions (maximum absolute reduction 16.7% for the highest‐risk individuals). Walking generally ranked second (max 11.4%), followed by Mediterranean diet (max 9.2%), and group therapy for smoking (max 1.6%). If the individual was a current smoker and successfully quit smoking (ie, achieved complete smoking cessation), then stopping smoking yielded the largest reduction. Probabilistic and 1‐way sensitivity analysis confirmed the demonstrated trend.
Conclusions
This study reports the comparative effectiveness of several forms of lifestyle modifications and found smoking cessation and yoga to be the most effective forms of cardiovascular disease prevention. Future research should focus on patient adherence to personalized therapies, cost‐effectiveness of these strategies, and the potential for enhanced benefit when interventions are performed simultaneously rather than as single measures.
Keywords: cardiovascular risk reduction, comparative effectiveness, lifestyle modification
Subject Categories: Lifestyle, Cardiovascular Disease, Primary Prevention, Exercise, Risk Factors
Introduction
Consensus exists that lifestyle changes such as stopping smoking, higher levels of physical activity, and certain dietary patterns can lead to lower rates of cardiovascular disease (CVD) and other chronic diseases.1, 2, 3, 4, 5 Current clinical guidelines in both the United States and Europe include such changes in their recommendations for reducing the risk of CVD.1, 6, 7 For example, the 2013 American College of Cardiology (ACC) and American Heart Association (AHA) Guidelines to Reduce Cardiovascular Risk give a strong, Grade A recommendation for adults who need low‐density lipoprotein and blood pressure (BP) lowering to follow a dietary pattern that emphasizes intake of vegetables, fruits, and whole grains and limit intake of sweets, sugar‐sweetened beverages, and red meats.1
Clinical guidelines for providers and patients rely on evidence that includes data analysis of individual studies, data synthesis that pools results from many studies, and models that use data to project future risk. An attractive feature of models and tools that predict risk is the ability to estimate the effectiveness of alternative strategies for different populations and subpopulations, and for individuals with specific characteristics. In fact, the ACC and AHA recently published a new set of equations that can be used to estimate 10‐year and lifetime risk of a first hard atherosclerotic CVD event, or first occurrence of nonfatal myocardial infarction or death from coronary heart disease or fatal or nonfatal stroke.8 These equations, known as the Pooled Cohort Equations, are based on data from diverse, community‐based cohorts that represent both black and white men and women in the United States. Full descriptions of the CVD risk equations and their development have been published elsewhere.8, 9
With the introduction of the ACC/AHA guidelines and the accompanying debates on use of statin therapy, calls for more focus on personal lifestyle modification and behavior change have been made.10, 11, 12 There has additionally been growing interest in using data and analytic tools to develop personalized recommendations that consider an individual's clinical risk profile.13, 14, 15 Personalized recommendations utilizing comparative effectiveness research have been done previously using life expectancy gains based on US Preventive Services Task Force guidelines,16, 17, 18 with substantial gains in life expectancy shown with greater use of preventive services.19
The objective of this study is to estimate the risk reductions that can be expected from diverse lifestyle modifications for patients with specified clinical characteristics and establish the comparative effectiveness of these strategies, depending on individual risk profiles. We calculated risk by inputting individual levels of key risk factors including age, BP, lipid levels, hypertension treatment, and smoking status into the Pooled Cohort Equations and then using the best available evidence on the effects of different interventions on each of these risk factors to establish the comparative effectiveness of interventions for individuals depending on their profiles. We considered the following lifestyle interventions: group therapy for stopping smoking, Mediterranean diet (a diet that emphasizes high intake of fruits, vegetables, whole grains, fatty fish, nuts, and olive oil),1 aerobic exercise (specifically walking), and yoga (a popular form of exercise focusing on the mind–body connection).
These interventions were chosen to encompass a range of strategies that may be part of a lifestyle modification regimen. For stopping smoking, already well‐established as a main driver of cardiovascular risk, we chose group therapy counseling sessions rather than nicotine replacement therapy or medication to more closely align with the nonpharmacological nature of the other strategies. A healthy diet and aerobic exercise are regarded as mainstays in prevention of CVD, with accumulating evidence on the impact of the Mediterranean diet on cardiovascular risk.5, 20, 21 Yoga, a form of physical activity that involves physical postures, breath work, and meditation or relaxation, is becoming more prevalent in the United States; a 2012 National Center for Health Statistics survey showing that 9.5% of adults (21 million) used yoga in the previous 12 months.22 Yoga is thought to reduce response to stress and affect the autonomic nervous system through relaxation techniques and breath control.23, 24 Three recent systematic reviews and meta‐analyses suggest that yoga may significantly improve risk factors for CVD such as body weight, lipid profile, and BP.25, 26, 27
Methods
This study uses the Pooled Cohort Equations published by Goff et al to calculate 10‐year CVD risk separately for white and black individuals.8 We focused on the nondiabetic white and black populations.
Risk factors in the equations are age, systolic BP, whether the patient is treated for hypertension or not, total cholesterol (TC) and high‐density lipoprotein cholesterol (HDL‐C), current smoking status, and history of diabetes. Levels of risk factors were entered in the Pooled Cohort Equations to calculate an individual's absolute 10‐year atherosclerotic CVD risk. The potential improvement in each risk factor that could be achieved through the 4 lifestyle management strategies—group therapy for smoking (only for current smokers), the Mediterranean dietary pattern, walking, and yoga—were obtained from published literature (Table 1). The risk factor effect sizes were then translated into reductions in 10‐year CVD risk for each intervention.
Table 1.
Effectiveness of Lifestyle Interventions on Reducing CVD Risk Through Changes in Risk Factors
| Risk Factor | Impact of Lifestyle Changes on Risk Factor Due to Interventions | |||
|---|---|---|---|---|
| Group Therapy for Smoking | Mediterranean Diet | Walking | Yoga | |
| Smoking (% quitting) | 9.90* (8.00, 12.30)28 | — | — | — |
| Systolic blood pressure, mm Hg | — | −1.70 (−3.35, −0.05)29 | −3.80 (−5.90, −1.70)30 | −4.45 (−6.99, −1.90)27 |
| Total cholesterol, mg/dL | — | −7.35 (−10.32, −4.39)29 | −3.48 (−12.37, 5.80)30 | −17.00 (−27.29, −6.71)27 |
| HDL cholesterol, mg/dL | — | 0.94 (−1.93, 3.82)29 | 2.32 (0.46, 5.41)30 | 2.87 (1.47, 4.26)27 |
CVD indicates cardiovascular disease; HDL, high‐density lipoprotein.
*Probability of quitting smoking with group therapy compared to 5% probability of quitting without intervention. The relative proportion of quitting (relative risk) is 1.98 for group therapy vs control (95% CI: 1.60–2.46).28
Interventions
Intervention effect sizes were obtained from meta‐analyses of randomized controlled trials (RCTs). Published literature was searched and meta‐analyses were selected if they included RCTs using intention‐to‐treat analysis, contained summary estimates of all relevant risk factors within a single report, and compared the treatment to a control group of no/minimal intervention or usual care. We used an alternate set of estimates in a sensitivity analysis.
For current smokers, group therapy for smoking cessation consisted of about 6 to 8 scheduled meetings led by professional facilitators for at least 6 months that included information, advice, and encouragement for quitting.28 For Mediterranean diet, data were taken from a meta‐analysis of RCTs in which patients followed a diet rich in fruits and vegetables, low in red meat, and moderate in fat from nuts and oils for at least 6 months with a follow‐up of 2 years.29 Evidence on change in risk factors from walking was obtained from a meta‐analysis on pedometer use as a means to increase physical activity.30 Specifically, participants were given a pedometer and encouraged to increase their daily steps (either with or without a daily step goal). This intervention resulted in an additional 2491 steps per day, roughly equivalent to 1 mile of walking. Walking was chosen to represent aerobic exercise as a low‐impact form of aerobic activity that is accessible and suitable for most individuals. Mean duration of the intervention and follow‐up was 18 weeks. Data on yoga came from the most recent meta‐analysis of RCTs evaluating a variety of movement‐based yoga styles including Hatha, Vinyasa, and Ashtanga and excluding breathing‐ or meditation‐only styles.27 Yoga was practiced 3 to 4 times per week on average, and the mean duration of the yoga interventions and follow‐up was 18 weeks.
Statistical Analysis
Calculations for risk factor changes as a result of lifestyle interventions were based on an intention‐to‐treat approach. The intervention effect estimates from Table 1 were added to the initial risk factor level for an individual with specific clinical characteristics to generate a new risk factor level. These new levels were then entered in the Pooled Cohort Equations to obtain a new CVD risk.
We calculated CVD risk with group therapy for smoking in 2 steps. First we calculated the CVD risk difference between a smoker and nonsmoker, with all other risk factors unchanged to get the pure effect of group therapy on CVD risk. Then we multiplied this number by the probability a smoker would successfully quit with group therapy versus no group therapy to get the intention‐to‐treat estimate (see Data S1 for details on calculations).
We calculated the absolute risk reduction of 10‐year CVD risk across the 4 different interventions for the average patient in each risk subcategory. Base‐case results were displayed using heatmaps, using color shading to represent gradation of risk across subgroups and strategies. Results were stratified by sex, smoking status, and treatment for hypertension.
We included 6 risk factor profiles of hypothetical patients with varying levels of systolic BP, TC, and HDL‐C with different combinations of smoking and hypertension treatment status to represent individuals with a wide range of CVD risk, extending from 1% to nearly 30%. The 6 profiles were created for both white and black patients for a total of 12 different profiles. For each profile, we estimated the 10‐year CVD risk in the absence of a lifestyle management intervention and with the addition of each of the 4 interventions.
Sensitivity Analysis
We conducted several analyses to explore the impact of parameter uncertainty and the impact of alternative assumptions.
First, we conducted a deterministic sensitivity analysis using the published 95% CI for the effect measure for each intervention. We conducted a “worse case” scenario analysis using the smallest favorable change for all risk factors and a “best case” scenario analysis using the largest favorable change for all risk factors.
Second, we conducted an analysis that explored the impact on CVD risk for an individual smoker who successfully quits. In contrast to the primary “intention‐to‐treat” analysis explained above, this secondary analysis is more akin to a per protocol type of analysis. For example, a provider who is considering options to recommend to a patient will discuss the relative benefits of those options based on evidence from study results analyzed by intention‐to‐treat. However, a patient might be interested in acquiring information for the “benefit” they would obtain if they successfully stopped smoking. Therefore, we conducted an analysis in which we assumed a patient successfully quits and refer to this second analysis as “successful smoking cessation.”
Third, we conducted a probabilistic sensitivity analysis to determine how often a strategy would be considered optimal for the 12 hypothetical patients. This analysis was done for both the base‐case analysis and the analysis with successful smoking cessation. In each analysis, 1000 random draws were performed in which we simultaneously varied the parameters in Table 1—the effect size of the interventions for each risk factor and the probability of successfully quitting smoking with group therapy—according to its probability density function. Intervention parameters were drawn from a normal distribution and the probability of quitting smoking drawn from a beta distribution. Probability of successfully quitting was designated as 100% for the individual patient who successfully quits smoking. The base‐case intervention effect estimates were used as means and the 95% CIs that were derived from published literature were used to inform the spread of the distribution.
Fourth, we used an alternative set of intervention effect estimates from published literature for the 12 hypothetical patient profiles to see if the ranking was sensitive to our choice of meta‐analysis. We have presented the table of alternative estimates in Table S1. For smoking cessation, we used another nonpharmaceutical intervention, physician advice for smoking cessation, to mimic the behavior change necessary to quit smoking.31 Another meta‐analysis on the Mediterranean dietary pattern for RCTs longer than 3 months from the Cochrane Database was selected for this sensitivity analysis32; however, only TC was pooled and reported and thus we varied only this parameter. To more broadly represent exercise as an intervention, we widened this intervention category to include more kinds of aerobic exercise including running, jogging, and cycling. For this, we used a recent meta‐analysis of RCTs of lipid measurements (TC and HDL)33 supplemented with a meta‐analysis of the effect of aerobic exercise on systolic BP.34 Both analyses used similar definitions of aerobic exercise, had similar patient populations, and both had median durations of 12 weeks (range 2–3 weeks to 2 years). An alternate meta‐analysis with similar inclusion criteria was employed for the yoga estimates.25
Lastly, we conducted a sensitivity analysis with the general Framingham risk score to examine if and how the relative rankings of the interventions would change with this commonly used risk algorithm.35 This Framingham risk score closely matches the Pooled Cohort Equations used in the base case, assessing all the same cardiovascular disease events plus cardiac failure.
This study does not constitute human subjects research and thus did not require Institutional Review Board approval.
Results
Figures 1A through 1H and 2A through 2H display heatmaps of risk reductions from the 4 interventions for all hypothetical groups of patients, dichotomized by race, sex, smoking status, and treatment for hypertension. Axes represent the covariates—age, systolic BP, TC, and HDL—in the Pooled Cohort Equations. In the heatmaps, the colors vary from red (smallest risk reduction) to green (moderate risk reduction) to blue (largest risk reduction). Differences among the strategies for an individual patient can be observed by comparing the same cell across the 4 different interventions.
Figure 1.
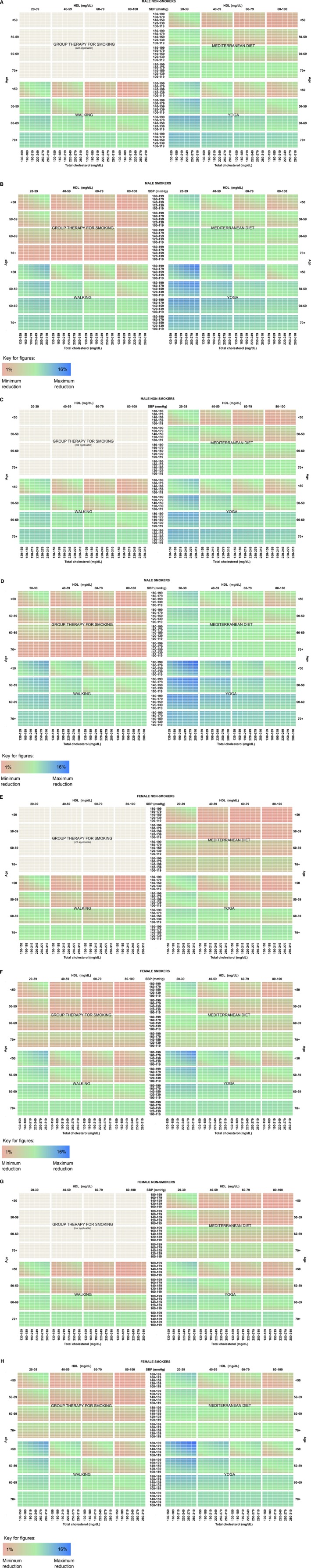
Risk reduction by intervention for (A) white males, not treated for hypertension, nonsmoker; (B) white males, not treated for hypertension, smoker; (C) white males, treated for hypertension, nonsmoker; (D) white males, treated for hypertension, smoker; (E) white females, not treated for hypertension, nonsmoker; (F) white females, not treated for hypertension, smoker; (G) white females, treated for hypertension, nonsmoker; (H) white females, treated for hypertension, smoker. Reductions range from smallest (red) to moderate (green) to largest (blue). HDL indicates high‐density lipoprotein cholesterol; SBP indicates systolic blood pressure.
Figure 2.
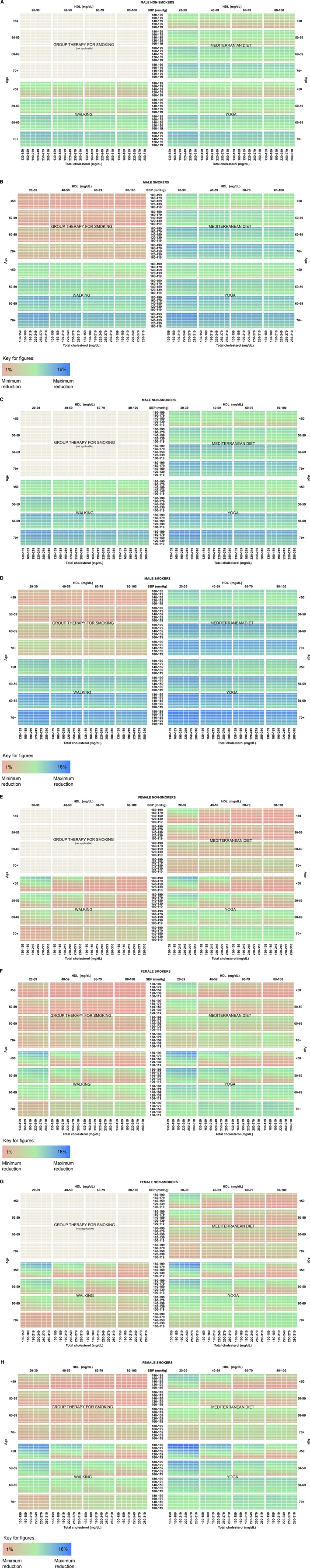
A, Risk reduction by intervention for black males, not treated for hypertension, nonsmoker; (B) black males, not treated for hypertension, smoker; (C) black males, treated for hypertension, nonsmoker; (D) black males, treated for hypertension, smoker; (E) black females, not treated for hypertension, nonsmoker; (F) black females, not treated for hypertension, smoker; (G) black females, treated for hypertension, nonsmoker; (H) black females, treated for hypertension, smoker. Reductions range from smallest (red) to moderate (green) to largest (blue). HDL indicates high‐density lipoprotein cholesterol; SBP indicates systolic blood pressure.
Base Case
In general across all profiles displayed in the heatmaps, the lowest risk reductions were with group therapy for smoking (red) and the highest reductions with yoga and walking exercise (blue). Although the results are heterogeneous across individual patients, the largest reductions on average were seen with yoga. The average risk reduction for all patients was 2.6% for yoga, compared to 1.9% for walking, 1.5% for Mediterranean diet, and 0.4% for smoking cessation counseling.
For the highest‐risk individuals who may experience the largest absolute reductions, the maximum reduction achieved with yoga was 16.7%. Walking typically fared second (max reduction 11.4%), followed by Mediterranean diet (max reduction 9.2%) and then group therapy for smoking if an individual was a current smoker (max reduction 1.6%). For some—white individuals with moderately low TC and high levels of HDL‐cholesterol and elderly black women—Mediterranean diet outranked walking. Black subjects generally achieved larger absolute risk reductions than white subjects.
Table 2 shows the risk reduction achieved through the various lifestyle modifications for the risk factor profiles representing 12 hypothetical individual patients.
Table 2.
10‐Year CVD Risk (%) for Different Risk Factor Profiles With and Without Lifestyle Interventions With 95% Credible Intervals
| Risk Profile | 10‐Year CVD Risk (%) With Intervention | ||||||
|---|---|---|---|---|---|---|---|
| None | Group Therapy for Smoking | MED Diet | Walking | Yoga | |||
| BC | SC | ||||||
| 1. 50‐yr‐old nonsmoking woman, SBP 120 mm Hg, not treated for hypertension, TC 160 mg/dL, HDL‐C 50 mg/dL | White | 0.9 | N/A | 0.8 (0.8, 0.9) | 0.8 (0.7, 0.8) | 0.7* (0.6, 0.8) | |
| Black | 1.4 | 1.2 (1.1, 1.3) | 1.1 (1.0, 1.2) | 1.0* (0.9, 1.1) | |||
| 2. 55‐yr‐old nonsmoking man, SBP 140 mm Hg, treated for hypertension, TC 170 mg/dL, HDL‐C 45 mg/dL | White | 7.0 | N/A | 6.4 (6.0, 6.8) | 6.2 (5.8, 6.6) | 5.5* (5.1, 6.0) | |
| Black | 12.7 | 10.0 (9.7, 10.3) | 9.7 (9.5, 9.9) | 9.3* (9.0, 9.7) | |||
| 3. 45‐yr‐old smoking man, SBP 160 mm Hg, treated for hypertension, TC 200 mg/dL, HDL‐C 40 mg/dL | White | 12.2 | 11.8 (11.6, 12.0) | 5.0† | 11.0 (10.1, 12.0) | 10.6 (9.8, 11.5) | 9.3* (8.4, 10.2) |
| Black | 18.2 | 17.8 (17.6, 17.9) | 9.4† | 14.5 (14.1, 14.9) | 14.1 (13.8, 14.4) | 13.7* (13.2, 13.7) | |
| 4. 70‐yr‐old smoking woman, SBP 155 mm Hg, not treated for hypertension, TC 145 mg/dL, HDL‐C 70 mg/dL | White | 18.6 | 18.3 (18.1, 18.4) | 12.1† | 18.0 (18.4, 17.6) | 17.6 (17.2, 18.0) | 17.0* (16.3, 17.6) |
| Black | 23.9 | 23.3 (23.1, 23.5) | 13.3† | 22.5 (21.9, 23.1) | 22.5 (21.3, 23.6) | 20.5* (19.1, 22.0) | |
| 5. 65‐yr‐old nonsmoking man, SBP 180 mm Hg, treated for hypertension, TC 200 mg/dL, HDL‐C 60 mg/dL | White | 23.2 | N/A | 22.1 (21.5, 22.8) | 21.7 (21.0, 22.4) | 20.5* (19.6, 21.5) | |
| Black | 27.3 | 22.1 (21.6, 22.6) | 21.6 (21.2, 22.0) | 21.0* (20.4, 21.7) | |||
| 6. 50‐yr‐old smoking man, SBP 170 mm Hg, treated for hypertension, TC 260 mg/dL, HDL‐C 35 mg/dL | White | 28.2 | 27.5 (27.2, 27.8) | 14.4† | 26.2 (24.4, 28.4) | 25.3 (24.1, 26.6) | 23.2* (21.7, 24.8) |
| Black | 28.1 | 27.5 (27.2, 27.7) | 15.0† | 22.8 (22.2, 23.4) | 22.1 (21.8, 22.5) | 21.6* (20.9, 22.3) | |
BC indicates base‐case; CVD, cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; MED, Mediterranean; N/A, not applicable; SBP, systolic blood pressure; SC, successful cessation; TC, total cholesterol.
Numbers with * represent the most effective CVD risk reduction strategy in the base‐case intention‐to‐treat analysis.
Numbers with † represent the most effective strategy assuming successful cessation of smoking.
Sensitivity Analysis
For both the best‐case and worse‐case scenarios, trends were similar to those found in the base case, with the largest risk reductions achieved with yoga for all 12 profiles.
Results of our secondary sensitivity analyses assuming successful smoking cessation are shown in column “SC” in Table 2 with optimal strategies indicated with †. If the patient is a smoker and successfully quits, then this lifestyle modification achieves the largest reductions in risk.
Probabilistic Sensitivity Analysis
Tables 3 and 4 show the results from the probabilistic sensitivity analysis based on 1000 random draws for the base case and successful smoking cessation analyses. The highest‐ranking strategy was yoga in over 88% of the simulations in the base case, with walking being the second most frequent optimal strategy. With successful cessation of smoking (ie, complete smoking cessation), group therapy becomes the dominant strategy.
Table 3.
Percentage of Simulations That the Strategy Was Ranked First (%) in the Base Case, by Race‐Specific Risk Profile
| Highest‐Ranked Strategy | Risk Profile | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| White | ||||||
| Group therapy for smoking | N/A | N/A | 0.0 | 0.0 | N/A | 0.0 |
| Mediterranean diet | 0.3 | 0.3 | 0.3 | 0.0 | 0.1 | 0.6 |
| Walking | 2.5 | 2.2 | 2.5 | 6.6 | 3.6 | 3.7 |
| Yoga | 97.2 | 97.5 | 97.2 | 93.4 | 96.3 | 95.7 |
| Black | ||||||
| Group therapy for smoking | N/A | N/A | 0.0 | 0.0 | N/A | 0.0 |
| Mediterranean diet | 0.1 | 0.0 | 0.3 | 0.6 | 0.0 | 0.5 |
| Walking | 5.9 | 8.1 | 8.5 | 3.1 | 7.6 | 10.6 |
| Yoga | 93.9 | 91.9 | 91.2 | 96.3 | 92.4 | 88.9 |
N/A indicates not applicable.
Table 4.
Percentage of Simulations That the Strategy Was Ranked First (%) in the Secondary Analysis Assuming Successful Cessation of Smoking, by Race‐Specific Risk Profile
| Highest‐Ranked Strategy | Risk Profile | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| White | ||||||
| Group therapy for smoking | N/A | N/A | 100.0 | 100.0 | N/A | 100.0 |
| Mediterranean diet | 0.4 | 0.4 | 0.0 | 0.0 | 0.2 | 0.0 |
| Walking | 4.9 | 4.9 | 0.0 | 0.0 | 4.9 | 0.0 |
| Yoga | 94.7 | 94.7 | 0.0 | 0.0 | 94.9 | 0.0 |
| Black | ||||||
| Group therapy for smoking | N/A | N/A | 100.0 | 100.0 | N/A | 100.0 |
| Mediterranean diet | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Walking | 7.8 | 10.3 | 0.0 | 0.0 | 9.6 | 0.0 |
| Yoga | 92.0 | 89.7 | 0.0 | 0.0 | 90.4 | 0.0 |
N/A, not applicable.
Alternative Estimates
Figure S1 shows the comparison of the base‐case estimates and alternative estimates for each hypothetical profile. As shown in the figure, the optimal strategy for each profile does not change, nor do the rankings among the interventions. In fact, some absolute risk reductions are lower with the alternate estimates compared to the base case.
When the Framingham algorithm is used (see Figure S2), the relative rankings among the interventions also remain the same. The absolute 10‐year risk using the Framingham risk equations is higher in some cases, as the Framingham risk score includes both populations in a single score and also predicts risk of heart failure in addition to the outcomes predicted in the Pooled Cohort Equations.
Discussion
We assessed the comparative effectiveness of 4 lifestyle management strategies on 10‐year CVD risk. In the base‐case intention‐to‐treat analysis, yoga resulted in the largest risk reduction out of the 4 interventions for nonsmokers and smokers alike. Walking as a form of aerobic exercise generally placed after yoga, followed by Mediterranean diet. In both the deterministic and probabilistic sensitivity analyses, the same trend in the base case held for our 12 hypothetical patients (yoga, walking, diet, and group therapy for smoking).
In the base‐case analysis, the impact of group therapy for stopping smoking on cardiac health was based on an intention‐to‐treat calculation, in order to be consistent across the 4 interventions; thus, only those who successfully quit realize reduced CVD risk. With the probability of successfully quitting being low, the benefits were correspondingly minimal. However, in a secondary analysis we demonstrated that for a current smoker, successful smoking cessation is the most effective of all lifestyle changes.
Our results are consistent with others who assessed the effect of CVD prevention. A simulation model by Kahn et al that focuses specifically on CVD interventions showed that the greatest benefits in life expectancy would be achieved through the treatment of hypertension and elevated low‐density lipoprotein‐C.36 On a population level, the large, multinational INTERHEART case–control study found that abnormal lipids was the most important risk factor with respect to population attributable risk for acute myocardial infarction across all age groups.37 Accordingly, our study shows that yoga, which provides the largest improvements in systolic BP and lipid levels, also provides the largest reduction in CVD risk.
In the analysis by Taksler et al, among US Preventive Services Task Force recommendations for preventive care, tobacco cessation, diabetes control, weight loss, and BP reduction were consistently ranked among the top guidelines for increases in life expectancy across patients.18 Tobacco cessation ranked high in their study as well as the INTERHEART study, which demonstrated that the odds of an acute myocardial infarction almost tripled for a current smoker versus a nonsmoker.37 Our finding that successful smoking cessation is the most effective of all lifestyle changes for current smokers is consistent with these studies.
Limitations
While risk assessment was based on recently published pooled cohort equations that allowed up‐to‐date, accurate, race‐specific estimation, there are several limitations of this study. First, we focused changes in risk factors translated into CVD risk in the nondiabetic population. Although we evaluate diverse sets of patient characteristics, results may not generalize to different populations. The ACC/AHA report did not include any measures of adiposity in their algorithm, although the lifestyle interventions studied could impact those measures, further altering CVD risk.
Second, insufficient data precluded subgroup‐specific pooled estimates of interventions on risk factors as well as any projections of future changes in disease outcomes. Future long‐term studies could determine the effects of these interventions on CVD outcomes rather than on risk factors and model additional changes in risk factors and other disease processes using more complex microsimulation models.
Third, we chose to use the new ACC/AHA pooled cohort equations to calculate 10‐year atherosclerotic CVD risk defined as a composite end point of first occurrence of nonfatal myocardial infarction, death from coronary heart disease, or fatal or nonfatal stroke. Other risk calculators—the Framingham Heart Study Coronary Heart Disease calculator,38 the EURO‐SCORE,39 and the Adult Treatment Panel (ATP) III40 to name a few—are also used to calculate risk scores for cardiovascular outcomes. While criticism over use of the ACC/AHA risk equations exists,41, 42, 43 our model focuses on a change in risk among strategies, so it would not be affected by choice of risk algorithm.
Fourth, results may be sensitive to model inputs. Our parameters are based on the best available published data, namely, meta‐analyses of controlled clinical trials. For example, data on the effectiveness of yoga on risk factors were obtained from a meta‐analysis of RCTs of moderate quality and generally small sample size.27 Other studies have, however, corroborated the results and found improvements of similar magnitude.25, 26 Additionally, estimates for Mediterranean diet were obtained from a meta‐analysis of RCTs involving overweight and obese patients, who in some cases have access to additional advice and counseling29; the actual benefit may differ based on individual characteristics and participation. As with all models, estimation is subject to the data available and results should be interpreted recognizing the limitations of the meta‐analyses used.
Next, we recognize that patients have preferences for certain approaches and doctors may have prescribing preferences, including taking and prescribing medication, respectively. We have presented a rank order of strategies that do not include taking any pills or medication. As such, nonadherence with lifestyle change and other health behaviors, including pill‐taking, is of concern and may dilute intervention effects. Our calculations are based on intention‐to‐treat rates from the clinical trials, which incorporate nonadherence. Personalized medicine may, in fact, aid adherence to lifestyle changes.44 Nonetheless, real‐world adherence and associated risk improvement may differ from what we have presented.
Lastly, we report risk reductions based on a single intervention. It is possible that patients may try multiple interventions simultaneously (eg, smoking cessation therapy and walking) and that these interventions would reduce CVD risk in a synergistic fashion. Furthermore, walking was chosen as a comparator intervention in this study as it is considered low‐impact and widely accessible. More vigorous exercise such as jogging may confer even greater cardiovascular and fitness benefits and may change the rank order of strategies.
Clinical Implications and Policy Relevance
Studies on personalized medicine for preventive care have found that some of the greatest gains in life expectancy come from services targeting CVD.18, 19, 36 Accurate, personalized recommendations regarding cardiovascular risk reduction can lead to better health and economic outcomes, reducing healthcare and patient time costs, and increasing quality of life and productivity. Use of risk estimators has been shown to have significant, modest effects on prescribing preventive therapies and on risk factor levels.9, 35, 45, 46
Based on this model and available data, yoga to reduce CVD risk can be considered by physicians and patients as a preventive‐care strategy alongside smoking cessation, diet, and exercise. The potential benefit of yoga in lowering lipids, BP, and body weight warrant further exploration of its health effects and dose–response relationship in future clinical trials.
The information presented in this study has the potential to help inform clinicians and patients on which activities could be more effective at reducing long‐term CVD risk based on their personal risk and preferences for interventions. These findings can be included in a decision aid used in the context of shared decision‐making between clinician and patient. Additionally, the lifestyle management strategies analyzed in this study require little to no equipment, no expensive medication, and can be performed at home or at the convenience of patients. Further research can examine whether these low‐cost strategies are cost‐effective compared to other CVD management strategies and demonstrate whether tailored therapies do improve patient adherence and health outcomes.
Conclusions
This study reports the comparative effectiveness of several forms of lifestyle modifications, from the commonly advised smoking cessation, diet, and exercise strategies to a more contemporary form of exercise, yoga, on 10‐year CVD risk. For a current smoker, successfully quitting smoking is the most effective lifestyle change. Smoking cessation is, however, difficult to achieve and group therapy for stopping smoking has only a small probability of success. From an intention‐to‐treat perspective, if yoga is as effective as reported in currently published meta‐analyses, then yoga could be considered among the strongest lifestyle interventions for reducing CVD risk. With more individualized estimates and recommendations, providers can better predict CVD risk and manage prevention more effectively, particularly among different ethnic groups who may be at higher risk of CVD and can benefit from early CVD risk discussion.47 Patients themselves can become more informed and involved in their own health and lifestyle choices.
Tailored recommendations for managing CVD risk have the potential to inform practice, improve care, and reduce healthcare expenditures. Our analysis supports the findings of several large‐scale studies that CVD risk can be modified by lifestyle changes. Additional research can take into account adherence to management strategies as well as investigate the effects of multiple interventions. As the interventions we have included are relatively low cost, a priority for future research is to analyze the effect of these management strategies on quality of life, costs (ie, affordability), and cost‐effectiveness (ie, value for money).
Sources of Funding
Support for this research was provided by a grant from the Harvard Global Health Institute, Cambridge, MA.
Disclosures
None.
Supporting information
Data S1. Risk equation calculation.
Table S1. Alternative Estimates for Effectiveness of Lifestyle Interventions on Reducing CVD Risk Through Changes in Risk Factors
Figure S1. Sensitivity analysis with alternate meta‐analyses, shown by hypothetical profile (y‐axes represent 10‐year CVD risk, x‐axes represent interventions). Note that the optimal intervention with the lowest 10‐year risk with the alternate estimates (red and purple bars) remains the same as that in the base case (blue and green bars) for each profile and race.
Figure S2. Sensitivity analysis with Framingham risk equations, shown by hypothetical profile (y‐axes represent 10‐year CVD risk, x‐axes represent interventions). Note that the optimal intervention with the lowest 10‐year risk with the Framingham equations (red bars) remains the same as that in the base case (blue and green bars) for each profile.
(J Am Heart Assoc. 2016;5:e002737 doi: 10.1161/JAHA.115.002737)
Accompanying Data S1, Table S1, and Figures S1, S2 are available at http://jaha.ahajournals.org/content/5/3/e002737/suppl/DC1
References
- 1. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, de Jesus JM, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Houston Miller N, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TW, Yanovski SZ. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960–2984. [DOI] [PubMed] [Google Scholar]
- 2. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109–3116. [DOI] [PubMed] [Google Scholar]
- 3. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non‐communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berlin JA, Colditz GA. A meta‐analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132:612–628. [DOI] [PubMed] [Google Scholar]
- 5. Estruch R, Ros E, Salas‐Salvado J, Covas MI, Corella D, Aros F, Gomex‐Gracia E, Ruiz‐Gutierrez V, Fiol M, Lapetra J, Lamuela‐Raventos RM, Serra‐Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez‐Gonzalez MA. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 6. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 7. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) . European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 8. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 9. National Heart, Lung, and Blood Institute . Assessing Cardiovascular Risk: Systematic Evidence Review From the Risk Assessment Work Group. Bethesda, MD: National Institutes of Health; 2013. [Google Scholar]
- 10. Kraus WE, Bittner V, Appel L, Blair SN, Church T, Despres JP, Franklin BA, Miller TD, Pate RR, Taylor‐Piliae RE, Vafiadis DK, Whitsel L; on behalf of the American Heart Association Physical Activity Committee of the Council on Lifestyle and Metabolic Health, Council on Clinical Cardiology, Council on Hypertension, and Council on Cardiovascular and Stroke Nursing . The National Physical Activity Plan: a call to action from the American Heart Association: a science advisory from the American Heart Association. Circulation. 2015;131:1932–1940. [DOI] [PubMed] [Google Scholar]
- 11. Lloyd‐Jones DM, Goff D, Stone NJ. Statins, risk assessment, and the new American prevention guidelines. Lancet. 2014;383:600–602. [DOI] [PubMed] [Google Scholar]
- 12. Mozaffarian D. The promise of lifestyle for cardiovascular health: time for implementation. J Am Coll Cardiol. 2014;64:1307–1309. [DOI] [PubMed] [Google Scholar]
- 13. Burke W, Psaty BM. Personalized medicine in the era of genomics. JAMA. 2007;298:1682–1684. [DOI] [PubMed] [Google Scholar]
- 14. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301–304. [DOI] [PubMed] [Google Scholar]
- 16. Maciosek MV, Edwards NM, Coffield AB, Flottemesch TJ, Nelson WW, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: methods. Am J Prev Med. 2006;31:90–96. [DOI] [PubMed] [Google Scholar]
- 17. Taksler GB, Braithwaite RS. Personalized estimates of benefit from preventive care guidelines. Ann Intern Med. 2014;160:140. [DOI] [PubMed] [Google Scholar]
- 18. Taksler GB, Keshner M, Fagerlin A, Hajizadeh N, Braithwaite RS. Personalized estimates of benefit from preventive care guidelines: a proof of concept. Ann Intern Med. 2013;159:161–168. [DOI] [PubMed] [Google Scholar]
- 19. Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38:600–609. [DOI] [PubMed] [Google Scholar]
- 20. Domenech M, Roman P, Lapetra J, Garcia de la Corte FJ, Sala‐Vila A, de la Torre R, Corella D, Salas‐Slavado J, Ruiz‐Gutierrez V, Lamuela‐Raventos RM, Toledo E, Estruch R, Coca A, Ros E. Mediterranean diet reduces 24‐hour ambulatory blood pressure, blood glucose, and lipids: one‐year randomized, clinical trial. Hypertension. 2014;64:69–76. [DOI] [PubMed] [Google Scholar]
- 21. Ruiz‐Canela M, Estruch R, Corella D, Salas‐Salvado J, Martinez‐Gonzalez MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415–417. [DOI] [PubMed] [Google Scholar]
- 22. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the Use of Complementary Health Approaches Among Adults: United States. 2002–2012. Hyattsville, MD: National Center for Health Statistics; 2015. [PMC free article] [PubMed] [Google Scholar]
- 23. Kiecolt‐Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF, Glaser R. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raub JA. Psychophysiologic effects of Hatha Yoga on musculoskeletal and cardiopulmonary function: a literature review. J Altern Complement Med. 2002;8:797–812. [DOI] [PubMed] [Google Scholar]
- 25. Cramer H, Lauche R, Haller H, Steckhan N, Michalsen A, Dobos G. Effects of yoga on cardiovascular disease risk factors: a systematic review and meta‐analysis. Int J Cardiol. 2014;173:170–183. [DOI] [PubMed] [Google Scholar]
- 26. Hartley L, Dyakova M, Holmes J, Clarke A, Lee MS, Ernst E, Rees K. Yoga for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;5:CD010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chu P, Gotink RA, Yeh GY, Goldie SJ, Hunink MM. The effectiveness of yoga in modifying risk factors for cardiovascular disease and metabolic syndrome: a systematic review and meta‐analysis of randomized controlled trials. Eur J Prev Cardiol. 2016;23:291–307. [DOI] [PubMed] [Google Scholar]
- 28. Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2005;2:CD001007. [DOI] [PubMed] [Google Scholar]
- 29. Nordmann AJ, Suter‐Zimmermann K, Bucher HC, Shai I, Tuttle KR, Estruch R, Briel M. Meta‐analysis comparing Mediterranean to low‐fat diets for modification of cardiovascular risk factors. Am J Med. 2011;124:841–851. [DOI] [PubMed] [Google Scholar]
- 30. Bravata DM, Smith‐Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. [DOI] [PubMed] [Google Scholar]
- 31. Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;5:CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M, Stranges S. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;8:CD009825. [DOI] [PubMed] [Google Scholar]
- 33. Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2015;4:e002014 doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. [DOI] [PubMed] [Google Scholar]
- 35. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 36. Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;118:576–585. [DOI] [PubMed] [Google Scholar]
- 37. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Liseng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 38. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 39. Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall‐Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 40. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 41. Amin NP, Martin SS, Blaha MJ, Nasir K, Blumenthal RS, Michos ED. Headed in the right direction but at risk for miscalculation: a critical appraisal of the 2013 ACC/AHA risk assessment guidelines. J Am Coll Cardiol. 2014;63:2789–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. [DOI] [PubMed] [Google Scholar]
- 44. van der Leeuw J, Ridker PM, van der Graaf Y, Visseren FL. Personalized cardiovascular disease prevention by applying individualized prediction of treatment effects. Eur Heart J. 2014;35:837–843. [DOI] [PubMed] [Google Scholar]
- 45. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 46. Harle CA, Downs JS, Padman R. Effectiveness of personalized and interactive health risk calculators: a randomized trial. Med Decis Making. 2012;32:594–605. [DOI] [PubMed] [Google Scholar]
- 47. Stuart‐Shor EM, Berra KA, Kamau MW, Kumanyika SK. Behavioral strategies for cardiovascular risk reduction in diverse and underserved racial/ethnic groups. Circulation. 2012;125:171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Risk equation calculation.
Table S1. Alternative Estimates for Effectiveness of Lifestyle Interventions on Reducing CVD Risk Through Changes in Risk Factors
Figure S1. Sensitivity analysis with alternate meta‐analyses, shown by hypothetical profile (y‐axes represent 10‐year CVD risk, x‐axes represent interventions). Note that the optimal intervention with the lowest 10‐year risk with the alternate estimates (red and purple bars) remains the same as that in the base case (blue and green bars) for each profile and race.
Figure S2. Sensitivity analysis with Framingham risk equations, shown by hypothetical profile (y‐axes represent 10‐year CVD risk, x‐axes represent interventions). Note that the optimal intervention with the lowest 10‐year risk with the Framingham equations (red bars) remains the same as that in the base case (blue and green bars) for each profile.


