Abstract
Background
The purpose of this study is to describe key elements, clinical outcomes, and potential uses of the Kaiser Permanente–Cardiac Device Registry.
Methods and Results
This is a cohort study of implantable cardioverter defibrillators (ICD), pacemakers (PM), and cardiac resynchronization therapy (CRT) devices implanted between January 1, 2007 and December 31, 2013 by ≈400 physicians in 6 US geographical regions. Registry data variables, including patient characteristics, comorbidities, indication for procedures, complications, and revisions, were captured using the healthcare system's electronic medical record. Outcomes were identified using electronic screening algorithms and adjudicated via chart review. There were 11 924 ICDs, 33 519 PMs, 4472 CRTs, and 66 067 leads registered. A higher proportion of devices were implanted in males: 75.1% (ICD), 55.0% (PM), and 66.7% (CRT), with mean patient age 63.2 years (ICD), 75.2 (PM), and 67.2 (CRT). The 30‐day postoperative incidence of tamponade, hematoma, and pneumothorax were ≤0.3% (ICD), ≤0.6% (PM), and ≤0.4% (CRT). Device failures requiring revision occurred at a rate of 2.17% for ICDs, 0.85% for PMs, and 4.93% for CRTs, per 100 patient observation years. Superficial infection rates were <0.03% for all devices; deep infection rates were 0.6% (ICD), 0.5% (PM), and 1.0% (CRT). Results were used to monitor vendor‐specific variations and were systematically shared with individual regions to address potential variations in outcomes, utilization, and to assist with the management of device recalls.
Conclusions
The Kaiser Permanente–Cardiac Device Registry is a robust tool to monitor postprocedural patient outcomes and postmarket surveillance of implants and potentially change practice patterns.
Keywords: electrophysiology, pacemakers, registries
Subject Categories: Quality and Outcomes
Introduction
As the indications for and utilization of cardiac implantable electronic devices (CIED) increase annually, so do concerns regarding procedural outcomes and safety risks associated with the implantation of these devices. The United States is the largest implanter of CIEDs, adding nearly 33 000 new implantable cardioverter defibrillators (ICDs) and 60 000 new pacemakers (PM) each year.1 Over the last decade, the use of these devices and procedures has increased substantially, a change commensurate with ever‐changing guidelines for device‐based therapy for cardiac rhythm abnormalities2 and the aging of the US population.3 For example, ICD implantation has increased 145% between 1997 and 2004,4 and PM implantation has increased by 56% between 1993 and 2009,5 despite smaller growth rates in the overall population (7.4% and 18% increase over the same time periods, respectively). Challenged by increases in utilization and cost, healthcare systems have sought to identify the most effective implants, reduce complication and readmission rates, and improve patient quality outcomes.
Over the last 10 years, the US Food and Drug Administration (FDA) has approved significantly more high‐risk (FDA class III) devices than previous decades, many of which require little premarket testing.5, 6, 7 Approximately 700 device recalls occur per year, many carrying moderate health risks.8 Healthcare delivery systems that monitor implant performance have an advantage in ensuring the safety of their patients and lessening the financial impact in the event of a recall. The FDA requires device vendors to maintain surveillance databases of all implanted ICDs and pacemakers. In addition, the Centers for Medicare and Medicaid Services mandates detailed documentation of patient and implant characteristics for reimbursement of qualifying procedures. Clinicians can satisfy the Centers for Medicare and Medicaid Services requirement by submitting data to the American College of Cardiology National Cardiovascular Data Registry (ACC‐NCDR).8, 9, 10 While the intent of the ACC‐NCDR is a national quality program, it is inherently limited as submission is dependent on Centers for Medicare and Medicaid Services requirements (eg, required only for recipients 65 years or older, eligible for reimbursement) and postprocedural follow‐up is restricted to the hospital stay. Nevertheless, the ACC‐NCDR and other registries have been shown to be valuable tools for clinical research, and have unique advantages over clinical trials and administrative claims databases.
In response to increasing device implant volume, costs, and safety risks as well as the need for robust, long‐term patient and device surveillance, the Kaiser Permanente–Cardiac Device Registry (KP‐CDR) was established to identify patients accurately and efficiently in the event of a device recall, measure comparative safety, effectiveness, and longevity of implants, and evaluate longitudinal patient outcomes. The purpose of this study is to describe the characteristics and outcomes of a cohort of registered patients from a large, geographically and demographically diverse US integrated healthcare system.
Methods
Registry Cohort and Setting
The registry includes 6 US geographical regions (Northern California, Southern California, Hawaii, Pacific Northwest, Colorado, and Mid‐Atlantic) of a large integrated healthcare system with over 9 million members (Figure 1). The study includes data from 385 medical facilities and ≈400 implanting cardiologists, electrophysiologists, and surgeons. Patients with an ICD, PM, or cardiac resynchronization therapy device (CRT) implanted between January 1, 2007 and December 31, 2013 were prospectively followed until March 31, 2014.
Figure 1.

The Kaiser Permanente healthcare system comprises 7 geographical regions spanning 8 states and nearly 9 million members. Data from Georgia not included in KP‐CDR.
Data Collection Procedures
Device data for initial and replacement ICD and PM procedures were imported on a quarterly schedule from multiple sources: device manufacturers, Paceart,10 and Apollo Data Repository.11 Complete implant data were unavailable from an individual source. Device manufacturers provided device data obtained at the time of implant from Kaiser Permanente medical centers. Data from the manufacturers did not include devices implanted in facilities outside the Kaiser Permanente network. Paceart is an additional source to ensure comprehensive data capture, regardless of whether the implant occurred within or outside of our network. Paceart is a software application that stores device data entered at the time of follow‐up care at a Kaiser Permanente facility. Finally, the Apollo Data Repository provided data for ICDs only and was generally limited to patients 65 years and older whose implants were subject to mandated reporting by the ACC‐NCDR.
All data were recorded and transferred to a centralized data repository for data management, validation, and reporting (Figure 2). Due to the electronic capture mechanism, 100% of device implants across all medical centers were captured.
Figure 2.
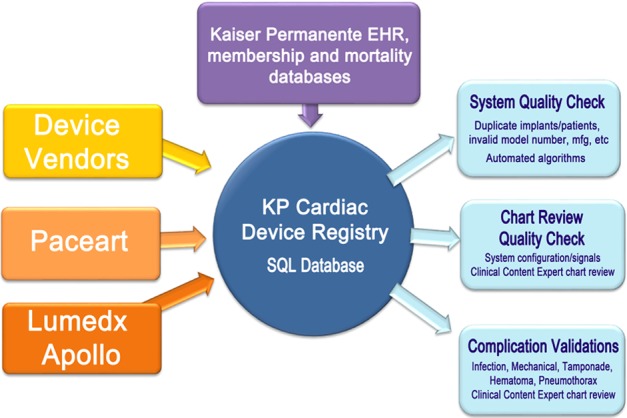
The Kaiser Permanente Cardiac Device Registry (KP‐CDR) retrieves device and patient data from device vendors, clinic‐based registry software such as Apollo and Paceart, as well as the program‐wide electronic medical record (EMR) system. Quality‐control queries are performed routinely to ensure high data quality and integrity.
The electronic medical record (EMR) was used to obtain patient characteristics (age, sex, race, and body mass index), comorbidities, mortality, and other diagnoses. Data from the EMR were integrated with device data to create a comprehensive patient registry, which includes patient and implant information (device and lead type and model). Specific functional parameters (ie, thresholds, impedances, episodes, etc) were not proactively stored in the registry but could easily be obtained from chart review of the EMR. For example, this was done when evaluating and confirming causes of device or lead malfunction (such as threshold or impedance changes suggesting dislodgments, fractures, insulation breaches, etc).
Quality Control
Key data elements in the KP‐CDR include device characteristics, patient demographics, clinical indications for implant, procedural details, and postoperative outcomes. Automated, ongoing quality control procedures were carried out to flag patient and device data anomalies that were adjudicated using the EMR by clinical content experts. The data were subjected to systematic review through these rigorous processes that included checks for illogical device configurations (eg, a single‐chamber device implanted with 2 active leads), implant/explant date anomalies, and missing data fields.
Procedural Outcomes Surveillance
Using electronic screening algorithms (see Appendix S1) based on International Classification of Disease, 9th Revision (ICD‐9) diagnostic and procedure codes, the EMR was queried for postoperative complications and re‐interventions every 3 months via an automated search. After initial identification of these end points, clinical content experts manually reviewed the EMR to adjudicate each suspected complication and/or re‐intervention. If confirmed, outcomes were included in the registry database and reports.
The cardiac device registry specifically monitored 4 main outcomes associated with device and/or lead procedures: early procedural complications, long‐term mechanical complications, premature explants, and procedural site infections (see Appendix S1 for definitions).
Continuous data are reported as mean and SD. Categorical data are reported as number and proportion. This is a retrospective data only study. The study was approved by each region's Institutional Review Board and was granted a waiver of informed consent.
Results
Study Cohort
Demographic characteristics of the 34 238 patients with primary implants registered between 2007 and 2013 are presented in Table 1. The mean age (years±SD) of each cohort was 63.2±13.5 for ICD, 75.2±12.4 for PM, and 67.2±11.9 for CRT. Patients receiving ICD or CRT implants were younger than those with PM implants (Table 1). A higher proportion of devices were implanted in males: 75.1% (ICD), 55.0% (PM), and 66.7% (CRT).
Table 1.
Patient Demographics at Initial Implant, 2007–2013
| Description | ICD | PM | CRT |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total N primary cases | 7929 | 23 772 | 2537 |
| Sex | |||
| Male | 5957 (75.1%) | 13 063 (55.0%) | 1692 (66.7%) |
| Female | 1960 (24.7%) | 10 615 (44.7%) | 833 (32.8%) |
| Unknown | 12 (0.2%) | 94 (0.4%) | 12 (0.5%) |
| Age category, y | |||
| <18 | 37 (0.5%) | 74 (0.3%) | 5 (0.2%) |
| 18 to 64 | 4019 (50.7%) | 3996 (16.8%) | 990 (39.0%) |
| ≥ 65 | 3872 (48.8%) | 19 684 (82.8%) | 1541 (60.7%) |
| Age, ±SD | 63.2 (13.5) | 75.2 (12.4) | 67.2 (11.9) |
| BMI, ±SD | 28.8 (6.5) | 27.8 (6.3) | 29 (6.4) |
| BMI category | |||
| <30 | 5054 (63.7%) | 16 661 (70.1%) | 1545 (60.9%) |
| 30 and ≤35 | 1662 (21.0%) | 4286 (18.0%) | 553 (21.8%) |
| >35 | 1185 (15.0%) | 2609 (11.0%) | 415 (16.4%) |
| Unknown | 28 (0.4%) | 216 (0.9%) | 24 (1.0%) |
| Race | |||
| White | 4401 (55.5%) | 16 437 (69.1%) | 1486 (58.6%) |
| Black | 1264 (15.9%) | 1666 (7.0%) | 382 (15.1%) |
| Asian | 758 (9.6%) | 2283 (9.6%) | 166 (6.5%) |
| Hispanic | 1113 (14.0%) | 2344 (9.9%) | 324 (12.8%) |
| Native American | 14 (0.2%) | 40 (0.2%) | 8 (0.3%) |
| Multiracial | 87 (1.1%) | 174 (0.7%) | 37 (1.5%) |
| Other | 29 (0.4%) | 99 (0.4%) | 12 (0.5%) |
| Unknown | 263 (3.3%) | 729 (3.1%) | 122 (4.8%) |
BMI indicates body mass index; CRT, cardiac resynchronization therapy device; ICD, implantable cardioverter defibrillator; PM, pacemaker.
The cohort was ethnically and racially diverse with 9.6% black, 9.4% Asian, and 11.0% Hispanic. There were 11 924 ICD implants (7929 initial and 3995 replacement); 33 519 PM implants (23 772 initial and 9747 replacement); and 4472 CRT devices (2537 initial and 1935 replacement) registered during the study period (2007–2013) in 47 312 patients. There were 66 067 leads associated with these devices registered.
The absolute and proportional number of ICD, PM, and CRT device types implanted between 2007 and 2013 are shown in Table 2. Of initial devices placed, 47.6% of ICDs and 14.5% of PMs were single chamber and the majority (92.5%) of all CRTs were defibrillators (Table 2). Interestingly, while the proportion of other devices remained relatively constant, the use of dual‐chamber ICDs decreased by 17.9% (from 62.9% in 2007 to 42.1% in 2013), with a corresponding increase in single‐chamber ICD implants from 37.1% to 58.0%.
Table 2.
Device Type at Initial Implant, 2007–2013
| Device Type | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | Total |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| ICD | ||||||||
| SC | 440 (37.1) | 553 (46.5) | 603 (49.8) | 459 (40.7) | 537 (50.1) | 580 (52.9) | 605 (58.0) | 3777 (47.7) |
| DC | 746 (63.0) | 637 (53.5) | 608 (50.2) | 670 (59.3) | 535 (49.9) | 517 (47.1) | 439 (42.1) | 4152 (52.4) |
| Total | 1186 (100) | 1190 (100) | 1211 (100) | 1129 (100) | 1072 (100) | 1097 (100) | 1044 (100) | 7929 (100) |
| PM | ||||||||
| SC | 547 (16.5) | 523 (15.2) | 573 (16.0) | 432 (12.6) | 496 (14.7) | 459 (13.7) | 409 (12.4) | 3439 (14.5) |
| DC | 2760 (83.5) | 2924 (84.8) | 3006 (84.0) | 2995 (87.4) | 2873 (85.3) | 2891 (86.3) | 2884 (87.6) | 20 333 (85.5) |
| Total | 3307 (100) | 3447 (100) | 3579 (100) | 3427 (100) | 3369 (100) | 3350 (100) | 3293 (100) | 23 772 (100) |
| CRT | ||||||||
| CRT‐D | 398 (92.6) | 308 (91.9) | 312 (91.5) | 354 (91.0) | 308 (93.3) | 321 (93.9) | 347 (93.8) | 2348 (92.6) |
| CRT‐P | 32 (7.4) | 27 (8.1) | 29 (8.5) | 35 (9) | 22 (6.7) | 21 (6.1) | 23 (6.2) | 189 (7.5) |
| Total | 430 (100) | 335 (100) | 341 (100) | 389 (100) | 330 (100) | 342 (100) | 370 (100) | 2537 (100) |
CRT indicates cardiac resynchronization therapy device; CRT‐D, cardiac resynchronization therapy defibrillators; CRT‐P, cardiac resynchronization therapy pacemakers; DC, dual‐chamber device; ICD, implantable cardioverter defibrillator; PM, pacemaker; SC, single‐chamber device.
Outcomes
The early (30‐day) complication rates for the end point of cardiac tamponade were 0.15% ICD, 0.27% PM, and 0.25% CRT; rates of hematoma were 0.27% ICD, 0.19% PM, and 0.38% CRT; and rates of pneumothorax were 0.21% ICD, 0.51% PM, and 0.13% CRT. Device failures requiring revision occurred at a (100‐patient‐year adjusted) rate of 2.17% for ICDs, 0.85% for PMs, and 4.93% for CRTs. Early revision was defined by a procedure to revise, replace, or explant the pulse generator within 36 months of implant (ICD) or 60 months (PM). For ICD or PM implants, the most common reasons for early revision of the device or leads were changes in device type, noted as upgrade/downgrade (41.6% and 34.1%, respectively), followed by infection (19.7% and 22.0%, respectively). For CRT, the most common reasons were infection (30.7%) followed by premature battery depletion (26.7%) (Figure 3). Superficial infection rates for all device types were 0.02% (ICD), 0.03% (PM), and 0.02% (CRT); deep infection rates were 0.60% ICD, 0.51% PM, and 0.98% for CRT (Table 3). Deep infection rates were higher for replacement (mean=0.8% ICD, 0.7% PM, 1.1% CRT) than initial (mean=0.5% ICD, 0.4% PM, 0.9% CRT) implants for all device types.
Figure 3.
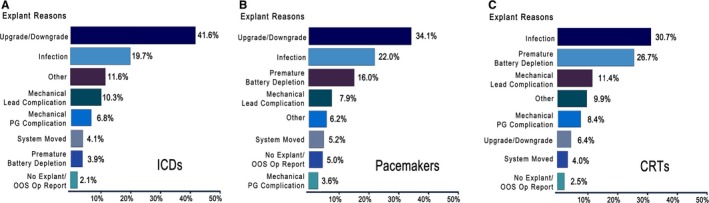
Reasons for early device revision, explantation, or replacement. During 2007–2013, 465 implantable cardioverter defibrillators (ICDs) were explanted, replaced, or revised after having been implanted for less than 36 months (A). 1018 Pacemakers were explanted, replaced, or revised after having been implanted for less than 60 months (B), and 182 cardiac resynchronization therapy devices were explanted, replaced, or revised after having been implanted for less than 36 months (C). Time frame for early explants was determined by electrophysiologist consultants. (1) “Mechanical Lead Complication” refers to devices functioning normally and with ample battery life replaced at the time of lead revision/replacement for a lead malfunction. (2) “Premature Battery Depletion” is classified as a form of device malfunction if the battery reaches elective replacement indicator or end of life and is replaced within 36/60 months of implant. (3) “Other” reasons include Pocket Erosion, Pocket Discomfort/Pain, Patient Requested Removal, and Patient Anatomy Issues. More than one reason may apply to a single revised device. Exclusions: elective replacement indicator, Heart Transplants, and Hospice Care.
Table 3.
Complications Following ICD or PM Implant, 2007–2013
| Complication | ICD | PM | CRT |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total N (initial and replacement) | 11 924 | 33 519 | 4472 |
| Tamponade | 18 (0.15) | 89 (0.27) | 11 (0.25) |
| Pneumothorax | 25 (0.21) | 172 (0.51) | 6 (0.13) |
| Hematoma | 32 (0.27) | 64 (0.19) | 17 (0.38) |
| Infection | |||
| Deep | 71 (0.60) | 170 (0.51) | 44 (0.98) |
| Organ space | 5 (0.04) | 12 (0.04) | 4 (0.09) |
| Superficial | 2 (0.02) | 9 (0.03) | 1 (0.02) |
CRT indicates cardiac resynchronization therapy device; ICD, implantable cardioverter defibrillator; PM, pacemaker.
Registry Reporting and Information Dissemination to Stakeholders
Reports from the KP‐CDR were distributed at regularly scheduled intervals to widespread national and regional KP audiences of implanting physicians, cardiology chiefs of service, quality improvement groups, infection control practitioners, hospital administrators, purchasing leaders, and supporting clinical staff within the healthcare system. The goals of these detailed reports were to identify potential early clinical signals (ie, device or lead failures), regional variation, and temporal changes in practice/utilization. This information allowed implanters to benchmark personal practice with regional and inter‐regional practice in order to identify areas with outlying performance that could then be targeted for practice improvement. Implanting physicians could request medical‐center specific reports to benchmark local practice, as well as make requests for data to support quality improvement or research projects.
Monitoring of Devices and Patient Safety
The KP‐CDR supported 7 recalls associated with ICDs, PMs, or leads during this time period. Four were Class 1 (the highest hazard level, indicating a device that may cause serious health problems and/or death). Examples of recalled leads included the Sprint Fidelis defibrillator lead in 2007 and the Riata/Riata ST silicone defibrillator leads in 2011. Within a day of notification of a recall, the registry identified all patients within our healthcare system with the affected implant and the responsible treating physician. While the device manufacturers recorded devices or leads at the time of implant, the registry followed patients for their lifetime in our system. As a result, information from vendors about recalled devices often contained incomplete patient lists, outdated patient contact information, incorrect physician names, or inaccurate implant data. A national KP recall department, in conjunction with a core group of electrophysiologists, communicated and coordinated clinical recommendations, patient notification, and management of the recall response to the appropriate individuals in an expeditious and independent manner.
Support for New Technology
The device registry offered customized analysis of device performance (eg, evidence of a higher‐than‐expected failure rate benchmarked with comparable devices and leads) when new ICDs, PMs, or leads were introduced to the market. Any device or lead identified as “out of range” was brought to the attention of implanting surgeons for more in‐depth analysis and discussion. Reasons for revision were documented to better understand the mechanism of failure, either related to implant or, possibly, implant technique. Complication rates and cost comparison of new implant designs were compared to well‐established devices or leads, implanted by the same surgeons and at the same facilities. That analysis was used to support clinical and purchasing recommendations about new technology.
Discussion
To our knowledge, the KP‐CDR is one of the first registries of cardiac implantable electronic devices to provide the combination of large numbers of patients and devices, diverse implanting physicians, across a wide geographical distribution, and critically, with robust short‐ and long‐term clinical follow‐up using a single integrated EMR.
Many of the general descriptive findings from the registry were expected and consistent with prior published reports and clinical practice. More males than females received both ICDs and PMs, possibly due to differences in cardiac disease prevalence between the 2; however, under‐recognition of disease in females cannot be excluded.12, 13 Patients were younger in the ICD cohort than the PM cohort, presumably driven by primary prevention ICDs used in patients without symptoms of bradycardia or other indications for pacing that otherwise usually occur later in life.
While our infection rates combined initial and replacement implants, other studies reported higher infection rates with replacement than initial implants.14, 15 Consistent with prior reports, there was a higher infection rate in CRT compared to ICD recipients, most likely due to the greater number of leads, longer procedure length, and adverse patient characteristics associated with advanced heart failure.14, 15 Given that the most common reason for early device revision was for a change in device type due to changes in clinical condition (upgrade/downgrade procedure in 36.7% of all replacement devices), proper device selection can not only improve device utilization but minimize repeat procedures and lessen these infection risks. Thirty‐day procedural complication rates remained low and similar for all device types and were similar to those reported elsewhere.16
The KP‐CDR tracked device/lead longevity, device selection trends, and implant utilization in order to support clinical practice patterns. One example of how implanters used registry data was the change of use of dual‐chamber ICDs towards single‐chamber ICDs over time (Table 2) among centers sharing regional use data. While this practice has evolved nationally with emerging evidence of higher complication rates with dual‐chamber ICDs,16 evolution within our implanters occurred prior to these data becoming widely distributed—due in part to dissemination of utilization reports from the KP‐CDR. One geographic region was able to objectively recognize a larger percentage of dual‐chamber ICDs versus single‐chamber ICDs compared to another region among colleagues. In 2007, single‐chamber initial implants in Southern California comprised 13.9% of all ICDs but in 2013, they comprised 49.7%. This is in contrast to Northern California, which had 64.3% single‐chamber implants in 2007 versus 72.1% in 2013.
Another example was the KP‐CDR's ability to more accurately report the true rate of non‐evidence‐based implants to each center. For example, while it has been reported that non‐evidence‐based primary prevention ICDs from the ACC‐NCDR registry is as high as 22.5%17 nationally, the KP rate from the same ACC‐NCDR criteria was 8.8%.18 However, when the submitted data were validated through the KP‐CDR, which was dependent on clinically active EMR information and not a separate data form or manual submission, the actual rate was 3.1%. This information allowed physicians to further evaluate a true sample of cases for reasons and rationales for either breaking those guidelines based on clinical judgment or effect change in practice patterns.
Finally, the KP‐CDR was designed to identify specific device and lead performance trends important to implanters even before formal advisories and recalls were issued. Quarterly device and lead performance reports monitored whether any device or lead had a higher‐than‐expected failure rate, benchmarked with comparable devices and leads. Any device or lead identified was brought to the attention of implanting physicians for more in‐depth analysis and discussion. Reasons for revision were documented to better understand the mechanism of failure (for example, whether related to implant generator or leads themselves, implant technique, or patient characteristics). For example, a spike in lead perforations in multiple regions was identified in the KP‐CDR with the release of the new, smaller‐diameter Riata‐ST Optim ICD leads, and a group moratorium to stop implants was made months before the “pillow‐topped” lower‐risk Durata lead was released to address this issue. This represents one of several instances where leveraging the KP‐CDR translated into improved quality care.
Strengths
The KP‐CDR offers unique strengths compared to other published CIED registries. The described cohort is sociodemographically diverse and is representative of the geographic population it covers.19, 20 Our population diversity, coupled with its large size, allows for meaningful inter‐regional analyses and comparisons to be conducted. This in turn can be of use to identify specific populations at risk or important practice variations across the health system. Additionally, we have a large number of participating physicians across many medical centers, of various sizes, contributing data to the registry. Because of the volume and diversity of medical centers and implanters, this sample represents a range of device implantation experience, techniques, and settings, as well as patient management practices. Implanters are from several specialties including general cardiac/cardiothoracic surgery, invasive and interventional cardiology, and cardiac electrophysiology. Finally, longitudinal patient and device follow‐up via a robust pan‐institutional EMR allows for clinical tracking of patients years beyond the postdischarge period, overcoming a key limitation of other available registries (ACC‐NCDR, vendor‐sponsored registries).
Limitations
Data from registries are observational in nature, and analyses deriving from such data must account for its limitations (such as confounding factors, missing data, and attrition). In the KP‐CDR, these limitations are mitigated by a comprehensive EMR that is linked across all implanters and regions to track these data in a longitudinal fashion. The KP‐CDR does not track certain data on time variant and CIED‐specific variables such as lead parameters (sensing, thresholds, and impedances), clinical events (such as ICD therapies), and programmable settings of the devices, and is limited on the number of variables and detail of procedures captured in order to minimize data collection burden and ensure high quality.
Conclusions
The KP‐CDR is a robust tool for an integrated healthcare system to monitor postprocedural patient outcomes and postmarket surveillance of CIEDs as well as potentially identify early failures and change practice patterns. The volume and diversity of the KP‐CDR implanters, implanting settings, and patient population along with its completeness in follow‐up through an integrated EMR rather than reliance on self‐reported or Medicare claims data makes it unique from other national registries. It is expected that current and future reports from the KP‐CDR will serve as a valuable resource for physicians and hospitals both within and beyond the KP system.
Disclosures
None.
Supporting information
Appendix S1. Definitions of Reported Outcomes.
(J Am Heart Assoc. 2016;5:e002798 doi: 10.1161/JAHA.115.002798)
An accompanying Appendix S1 is available at http://jaha.ahajournals.org/content/5/3/e002798/suppl/DC1
References
- 1. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter‐defibrillators: calendar year 2009—a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34:1013–1027. [DOI] [PubMed] [Google Scholar]
- 2. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. [DOI] [PubMed] [Google Scholar]
- 3. Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population‐based analysis. J Gen Intern Med. 2008;23:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60:1540–1545. [DOI] [PubMed] [Google Scholar]
- 5. Resnic FS, Normand SL. Postmarketing surveillance of medical devices—filling in the gaps. N Engl J Med. 2012;366:875–877. [DOI] [PubMed] [Google Scholar]
- 6. Rome BN, Kramer DB, Kesselheim AS. FDA approval of cardiac implantable electronic devices via original and supplement premarket approval pathways, 1979–2012. JAMA. 2014;311:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. United States Government Accountability Office . Medical devices: FDA should enhance its oversight of recalls. 2013. Available at: http://www.Gao.Gov/products/gao-11-468. Accessed June 2, 2015.
- 8. Foundation. ACoC . About the National Cardiovascular Data Registry. Available at: https://www.Ncdr.Com/webncdr/home/about-the-ncdr. Accessed June 2, 2015.
- 9. Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, Beachy J, Blum Meisnere L, Conyers del M, Reynolds MR, Heidenreich PA, Al‐Khatib SM, Pina IL, Blake K, Walsh MN, Wilkoff BL, Shalaby A, Masoudi FA, Rumsfeld J. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–e65. [DOI] [PubMed] [Google Scholar]
- 10. Medtronic . Medtronic Paceart System. 2010;2014. Available at: http://www.Medtronic.Com/for-healthcare-professionals/products-therapies/cardiac-rhythm/patient-management-carelink/medtronic-paceart-system/index.htm. Accessed June 2, 2015.
- 11. Lumedx . Lumedx implementation services. 2014;2014. Available at: http://www.Lumedx.Com/implementation.Aspx. Accessed June 2, 2015.
- 12. Wenger NK. Coronary heart disease: the female heart is vulnerable. Prog Cardiovasc Dis. 2003;46:199–229. [DOI] [PubMed] [Google Scholar]
- 13. Yarnoz MJ, Curtis AB. Sex‐based differences in cardiac resynchronization therapy and implantable cardioverter defibrillator therapies: effectiveness and use. Cardiol Rev. 2006;14:292–298. [DOI] [PubMed] [Google Scholar]
- 14. Borleffs CJ, Thijssen J, de Bie MK, van Rees JB, van Welsenes GH, van Erven L, Bax JJ, Cannegieter SC, Schalij MJ. Recurrent implantable cardioverter‐defibrillator replacement is associated with an increasing risk of pocket‐related complications. Pacing Clin Electrophysiol. 2010;33:1013–1019. [DOI] [PubMed] [Google Scholar]
- 15. Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, Gottipaty V, Shinn T, Dan D, Feldman LA, Seide H, Winston SA, Gallagher JJ, Langberg JJ, Mitchell K, Holcomb R. Complication rates associated with pacemaker or implantable cardioverter‐defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. [DOI] [PubMed] [Google Scholar]
- 16. Peterson PN, Varosy PD, Heidenreich PA, Wang Y, Dewland TA, Curtis JP, Go AS, Greenlee RT, Magid DJ, Normand SL, Masoudi FA. Association of single‐ vs dual‐chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA. 2013;309:2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al‐Khatib SM, Hellkamp A, Curtis J, Mark D, Peterson E, Sanders GD, Heidenreich PA, Hernandez AF, Curtis LH, Hammill S. Non‐evidence‐based ICD implantations in the United States. JAMA. 2011;305:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta N, Anthony F, Kiley ML, Phan K, Young C. Potential pitfall of using national databases to monitor non‐evidence‐based treatment. J Am Coll Cardiol. 2013;61:(10_S). [Google Scholar]
- 19. Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. [DOI] [PubMed] [Google Scholar]
- 20. Koebnick C, Langer‐Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Definitions of Reported Outcomes.


