Abstract
Background
Contrast medium–induced acute kidney injury (CIAKI) is a leading cause of acquired renal impairment. The effects of antioxidants have been conflicting regarding the prevention of CIAKI. We performed a study of vitamin E use to decrease CIAKI in patients undergoing elective coronary angiography.
Methods and Results
In a placebo‐controlled randomized trial at 2 centers in Iran, 300 patients with chronic kidney disease—defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2—were randomized 1:1 to receive 0.9% saline infusion 12 hours prior to and after intervention combined with 600 mg vitamin E 12 hours before plus 400 mg vitamin E 2 hours before coronary angiography or to receive placebo. The primary end point was the development of CIAKI, defined as an increase ≥0.5 mg/dL or ≥25% in serum creatinine that peaked within 72 hours. Based on an intention‐to‐treat analysis, CIAKI developed in 10 (6.7%) and 21 (14.1%) patients in the vitamin E and placebo groups, respectively (P=0.037). Change in white blood cell count from baseline to peak value was greater in the vitamin E group compared with the placebo group (−500 [−1500 to 200] versus 100 [−900 to 600]×103/mL, P=0.001). In multivariate analysis, vitamin E (odds ratio 0.408, 95% CI 0.170–0.982, P=0.045) and baseline Mehran score (odds ratio 1.257, 95% CI 1.007–1.569; P=0.043) predicted CIAKI.
Conclusions
Prophylactic short‐term high‐dose vitamin E combined with 0.9% saline infusion is superior to placebo for prevention of CIAKI in patients undergoing elective coronary angiography.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT02070679.
Keywords: chronic kidney disease, contrast‐induced acute kidney injury, coronary angiography, vitamin E
Subject Categories: Nephrology and Kidney, Clinical Studies, Angiography, Inflammation, Pharmacology
Introduction
Contrast medium–induced acute kidney injury (CIAKI) is the third most frequent cause of hospital‐acquired acute kidney injury (AKI), compromising 10% of all in‐hospital nephropathies1, 2 and contributing to increased hospital length of stay and cost of care.3, 4 CIAKI is usually defined as an absolute increase of ≥0.5 mg/dL or a relative increase of ≥25% in serum creatinine concentration within 48 to 72 hours following contrast medium exposure and may peak up to 3 to 5 days after exposure.1, 3, 4, 5, 6
Several risk factors have been proposed to be associated with CIAKI, including previous renal insufficiency, diabetes mellitus, age >75 years, hypertension, congestive heart failure, volume‐depleted conditions, contrast medium osmolality and volume, and nephrotoxic drugs.1, 3, 6 Pathogenesis has been linked to transient increase in renal blood flow with subsequent prolonged decrease in blood flow, necrosis of epithelial cells, medullary hypoxia, enhancement of renal vasoconstriction, and decreased activity of renal vasodilators.1 In addition, in experimental models, a decrease in antioxidant activity and the direct cytotoxic effect of reactive oxygen species have been shown to cause CIAKI.6
To prevent CIAKI, many therapeutic strategies aimed at different pathological mechanisms have been implemented; however, the role of different strategies is still controversial.7 Therapeutic modalities mainly include risk factor modification, hydration, diuresis, tubular alkalization, vasodilators, anti‐inflammatory and antioxidant agents, and adjustment of contrast medium volume and osmolality.8, 9, 10, 11, 12, 13, 14 Vitamin E is a lipid‐soluble vitamin with antioxidative and anti‐inflammatory properties in the form of α‐tocopherol, which has been found to be effective in the prevention and treatment of cardiovascular complications and cancer.15, 16 Moreover, in some animal studies, it has been shown that vitamin E decreased cisplatin‐induced oxidative damage to renal tissue17, 18 and development of CIAKI.19 A clinical study showed 2 forms of vitamin E, α‐ and γ‐tocopherol, combined with 0.9% saline infusion to be associated with a decrease in the incidence of CIAKI.20 In contrast, vitamin E plus 0.45% saline infusion before administration of contrast medium in patients with chronic kidney disease (CKD) undergoing elective computed tomography with nonionic radiocontrast agents was not protective against CIAKI on imaging evaluation.21
The purpose of the present study was to determine whether vitamin E could protect the kidneys from developing CIAKI in the setting of coronary angiography. In a prospective placebo‐controlled randomized trial, for the first time, we examined the effect of high‐dose preprocedural oral α‐tocopherol in a cohort of CKD patients undergoing elective coronary angiography.
Methods
Study Design
In a double‐blind, placebo‐controlled, 2‐center, randomized clinical trial, 300 consecutive CKD patients who met inclusion criteria and who underwent coronary angiography were randomized into 2 groups to receive either vitamin E or placebo from February 21, 2014, to June 13, 2015, at the Seyyed‐al‐Shohada Heart Center, which is a tertiary referral center, and Taleghani teaching hospital in Urmia, West Azerbaijan Province, Iran. The study was approved by the ethics committee of Urmia University of Medical Sciences. Before any study procedures were performed, consent was obtained from all participants. This study was registered at ClinicalTrials.gov (identifier NCT02070679).
All patients aged ≥18 years with baseline estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2 (based on the Modification of Diet in Renal Disease study group formula)22 who underwent coronary angiography were included in the study if they met inclusion criteria, including stable angina with ischemia and indication for coronary angiography or non–ST‐segment elevation (NSTE) acute coronary syndrome (ACS) requiring an early invasive strategy. Exclusion criteria included acute ST‐segment elevation myocardial infarction, high‐risk NSTE‐ACS warranting emergency coronary angiography (<2 hours), cardiogenic shock, pulmonary edema, overt heart failure and/or ejection fraction <30%, ACS undergoing coronary angiography or angioplasty during the previous 5 days, sensitivity to contrast medium, recent administration of contrast medium for any reason, AKI, history of dialysis, pregnancy, newly prescribed angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and bleeding and/or coagulopathy diseases. The consumption of nephrotoxic drugs, vitamin E, vitamin C, or N‐acetylcysteine at least 48 hours before intervention was also considered an exclusion criterion.
A total of 300 patients who met the inclusion criteria were randomized into 2 groups at a 1:1 ratio, using a random number table and block randomization, to ensure that equal numbers of participants were assigned to each group. All drug bottles were labeled from 1 to 300. At each hospital, 150 drug bottles were given to the randomized patients. The series of paired random numbers were generated by an independent statistician and sent to our 2 centers where the patients were enrolled in the study. All patients were randomly assigned to prophylaxis administration, with 0.9% saline infusions (1 mL/kg) for 12 hours prior to and after coronary angiography combined with 600 mg oral vitamin E at 12 hours before plus 400 mg 2 hours before intervention, or to placebo, which was administered in a similar fashion. An adjusted dose of iodixanol (Visipaque, GE Healthcare; 320 mg iodine/mL), a nonionic iso‐osmolar (290 mOsm/kg water) contrast medium, was used in all patients. Both patients and care providers were blinded to the study groups and were unaware of the allocations. Of note, based on previous clinical trials, the safe dose of vitamin E for adults has been found to be 1073 mg/day for α‐tocopherol or the molar equivalent of its esters23; therefore, we used a maximum of 1000 mg vitamin E containing α‐tocopherol. Coronary interventions were routinely performed with the Judkins technique, using 6F right and left heart catheters through the femoral or radial approach, by attending cardiologists at both centers. All conditions and facilities at the 2 centers were similar because both are educational and research institutions affiliated with the same university.
Serum Laboratory Values and CIAKI Definition
All blood samples were obtained before angiography and within 72 hours postoperatively via venipuncture. Samples were analyzed within 30 minutes of sampling by an automated blood cell counter (Sysmex). The methods and instruments for providing laboratory samples were similar in our 2 centers during the study period.
CIAKI was defined as an absolute increase ≥0.5 mg/dL or a relative increase ≥25% over baseline serum creatinine concentration within 72 hours after administration of contrast media, and the peak value within 72 hours was used in this study. Because the coronary angiographies were elective and some patients were willing to discharge at <72 hours, the evaluation of serum biochemistry was performed in our outpatient clinic in such cases.
Primary and Secondary End Points
The primary end point of the study was the development of CIAKI, as defined above, in the patients receiving vitamin E compared with the patients receiving placebo within 72 hours after coronary angiography.
The following outcomes were considered secondary end points: (1) changes in the levels of serum creatinine and eGFR within 72 hours after angiography; (2) postprocedure levels of complete blood cell count components, including white blood cell (WBC) count, platelet count, hemoglobin, and hematocrit; (3) changes in laboratory values from baseline to follow‐up; (4) side effects of study medication; (5) hospital stay in days; (6) requirement of renal replacement therapy; (7) postprocedure ACS; (8) cerebrovascular events; and (9) in‐hospital mortality.
Statistical Analysis
The purpose of the trial was to assess the effect of vitamin E on the incidence of CIAKI as the primary end point. The sample size was based on assuming 5% incidence of CIAKI in the patients receiving vitamin E and 15% in those receiving placebo.20 To achieve a statistical power of 80% with 2‐sided α=0.05, a total of 141 patients were required in each group; to allow for dropouts, we randomized 300 patients. Demographic and procedural characteristics were compared between the study arms. All variables were also compared between the groups of patients with and without CIAKI and for subgroups by fasting blood glucose (FBG) values. Continuous variables were presented as mean±SD in normally distributed data and as median with interquartile range (IQR; 25th and 75th percentiles) in nonnormally distributed data. All continuous variables were analyzed using a t test or Mann–Whitney U test, as appropriate. Categorical variables were presented as number (percentage). The Pearson χ2 or Fisher exact tests were used, as appropriate, for categorical data. To detect the predictors of CIAKI, univariate analysis was conducted. In the multivariate logistic regression analysis, we included all variables showing a significant relationship with CIAKI in the univariate analysis and potentially relevant variables that were previously demonstrated to have an impact on the risk of CIAKI, including CKD, age >75 years, high contrast volume >140 mL, statin use, and diabetes mellitus, as well as study site. In the logistic regression model, the CKD variable was entered as a dichotomous variable of CKD stage 3 or 4 (all patients in our study had CKD stage 3 or 4). All analyses were performed using SPSS statistical software, version 21.0 (IBM Corp). Two‐sided P values were calculated.
Results
Baseline Characteristics
After excluding patients who did not meet our criteria, a total of 300 patients (14.8% recruitment rate) were randomized into 2 groups. During follow‐up, 298 patients (99.3%) were analyzed based on an intention‐to‐treat approach. The diagnosis of CIAKI was not provided during 72 hours for 3 patients in the vitamin E group and for 6 patients in the placebo group; however, results for detecting CIAKI were obtained during the fourth and fifth days after angiography, and none of these patients had CIAKI. The causes of deviation from the study protocol at follow‐up are depicted in Figure 1. The clinical characteristics and biochemistry results are summarized in Tables 1 and 2. The mean age of patients was 67±11 years, and 46% of patients were male. Baseline Mehran risk score was comparable for the placebo and vitamin E groups (7.5 [IQR 5–10] and 7.35 [IQR 5.3–9.5], respectively, P=0.6). There were no statistically significant differences regarding the baseline characteristics and biochemistry results between the study groups (Table 1).
Figure 1.
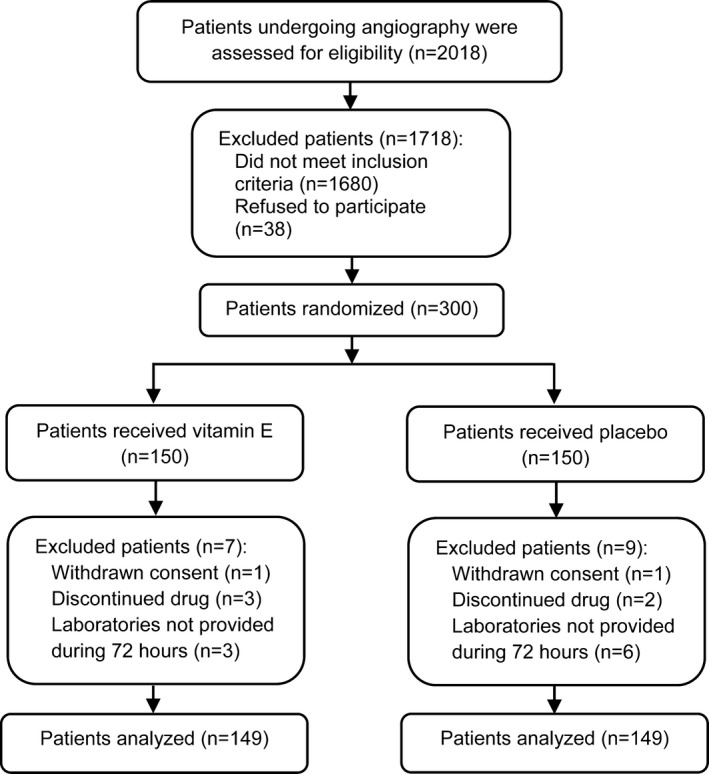
Flow diagram of patient selection.
Table 1.
Clinical Characteristics of Patients in the Study Groups
| Placebo Group (n=149) | Vitamin E Group (n=149) | P Value | |
|---|---|---|---|
| Baseline clinical characteristics | |||
| Age, y | 67±10 | 66±11 | 0.4 |
| Male | 69 (46.3) | 68 (45.6) | 0.9 |
| Height, m | 1.62 (1.53–1.7) | 1.63 (1.54–1.7) | 0.4 |
| Weight, kg | 78 (67–83) | 75 (67–86) | 0.8 |
| Body mass index, kg/m2 | 28.8 (26–32.1) | 28.7 (25.2–32.3) | 0.7 |
| Waist circumference, cm | 102 (90–112) | 101 (90–110) | 0.4 |
| Systolic BP, mm/Hg | 130 (115–140) | 130 (110–140) | 0.3 |
| Diastolic BP, mm/Hg | 80 (70–80) | 78 (70–80) | 0.2 |
| Heart rate, beats/min | 78 (70–80) | 78 (70–80) | 1 |
| LVEF, % | 50 (45–55) | 50 (40–50) | 0.1 |
| Hypertension | 120 (80.5) | 119 (79.9) | 0.8 |
| Diabetes mellitus | 53 (35.6) | 53 (35.6) | 1 |
| Hypercholesterolemia | 25 (16.8) | 26 (17.4) | 0.8 |
| Current smoking | 34 (22.8) | 29 (19.5) | 0.4 |
| Congestive heart failure | 15 (10.1) | 7 (4.7) | 0.076 |
| Prior MI | 15 (10.1) | 18 (12.1) | 0.5 |
| Prior coronary stenting | 10 (6.7) | 9 (6) | 0.8 |
| Prior cerebrovascular events | 2 (1.3) | 4 (2.7) | 0.4 |
| Metabolic syndromea | 82 (55) | 75 (50.3) | 0.4 |
| Mehran risk score | 7.5 (5–10) | 7.35 (5.3–9.5) | 0.6 |
| Medications | |||
| Beta blockers | 74 (49.7) | 77 (51.7) | 0.7 |
| ACEIs | 31 (20.8) | 37 (24.8) | 0.4 |
| ARBs | 56 (37.6) | 54 (36.2) | 0.8 |
| Statins | 80 (53.7) | 75 (50.3) | 0.5 |
| Diuretics | 41 (27.5) | 50 (33.6) | 0.2 |
| Aspirin | 110 (73.8) | 104 (69.8) | 0.4 |
| Nitrates | 77 (51.7) | 71 (47.7) | 0.4 |
| Procedural features | |||
| Presentation | 0.093 | ||
| Stable IHD | 62 (41.6) | 48 (32.2) | |
| NSTE‐ACS | 87 (58.4) | 101 (67.8) | |
| Multivessel disease | 45 (30.2) | 48 (32.2) | 0.7 |
| Contrast volume, mL | 50 (50–100) | 50 (40–100) | 0.6 |
| Contrast volume ≥140 mL | 32 (21.5) | 28 (18.8) | 0.5 |
| Baseline serum laboratories | |||
| HDL, mg/dL | 37 (32–45) | 37 (32–45) | 0.7 |
| Triglyceride, mg/dL | 147 (112–191) | 135 (103–187) | 0.1 |
| Total cholesterol, mg/dL | 146 (120–185) | 150 (123–180) | 0.6 |
| Fasting blood glucose, mg/dL | 98 (80–130) | 100 (81–130) | 0.5 |
| Blood sugar, mg/dL | 113 (94–165) | 122 (102–177) | 0.1 |
Values are presented as mean±SD, median (interquartile range), or number (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; HDL, high‐density lipoprotein; IHD; ischemic heart disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome.
Metabolic syndrome was detected as a criterion defined by the Third Report of the National Cholesterol Education Program.24
Table 2.
Serum Values Measured at Baseline and Follow‐up According to the Study Groups
| Placebo Groupa (n=149) | Vitamin E Groupa (n=149) | P Value | |
|---|---|---|---|
| Serum creatinine, mg/dL | |||
| Baseline | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) | 0.2 |
| Within 72 hours | 1.3 (1.1–1.5) | 1.3 (1.1–1.4) | 0.082 |
| Serum urea, mg/dL | |||
| Baseline | 52 (40–69) | 48 (38–65) | 0.2 |
| Within 72 hours | 45 (35–60) | 41 (33–53) | 0.073 |
| eGFR, mL/min per 1.73 m2 | |||
| Baseline | 44 (37–51) | 45 (39–53) | 0.3 |
| Within 72 hours | 49 (39–55) | 49 (41–59) | 0.1 |
| Serum Na+, mEq/L | |||
| Baseline | 141 (139–143) | 141 (140–144) | 0.057 |
| Within 72 hours | 141 (139–143) | 141 (139–142) | 0.5 |
| Serum K+, mEq/L | |||
| Baseline | 4.3 (3.9–4.6) | 4.3 (4–4.6) | 0.6 |
| Within 72 hours | 4.2 (4–4.4) | 4.2 (4–4.6) | 0.2 |
| WBC, ×103/mL | |||
| Baseline | 7.9 (6.26–9.7) | 7.8 (6.5–9) | 0.5 |
| Within 72 hours | 7.5 (6.3–8.9) | 7 (6–8.4) | 0.1 |
| Platelet count, ×103/L | |||
| Baseline | 215 (180–261) | 205 (168–245) | 0.077 |
| Within 72 hours | 189 (157–238) | 186 (156–222) | 0.3 |
| Hematocrit, % | |||
| Baseline | 39.3 (36.3–43.4) | 39.1 (36–41.6) | 0.4 |
| Within 72 hours | 38.3 (34–41.5) | 37.1 (34.3–41.1) | 0.5 |
| Hemoglobin, mg/dL | |||
| Baseline | 12.8 (11.3–13.5) | 12.3 (11.2–13.6) | 0.3 |
| Within 72 hours | 12.2 (10.9–13.2) | 12 (10.8–13.2) | 0.5 |
Values are presented as median (interquartile range). eGFR indicates estimated glomerular filtration rate; WBC, white blood cell.
Values were not provided for 6 and 3 patients during 72 hours in the placebo and vitamin E groups, respectively.
Median baseline serum creatinine concentration for all patients was 1.3 mg/dL (IQR 1.2–1.5 mg/dL). For placebo versus vitamin E, baseline serum creatinine (1.3 [IQR 1.2–1.5] versus 1.3 [IQR 1.2–1.5] mg/dL, respectively; P=0.2) and eGFR (44 [IQR 37–51] versus 45 [39–53] mL/min per 1.73 m2, respectively; P=0.4) were not significantly different between groups. Comparing the placebo and vitamin E groups, serum creatinine level (1.3 [IQR 1.1–1.5] versus 1.3 [IQR 1.1–1.4] mg/dL, respectively; P=0.2) and eGFR (49 [IQR 39–55] versus 49 [IQR 41–59] mL/min per 1.73 m2, respectively; P=0.2) within 72 hours were comparable. Other biochemical tests, including complete blood count components, were not significant between the study groups (Table 2).
Primary End Point
CIAKI developed in 31 (10.4%) patients across groups. Incidence of CIAKI was significantly higher in the placebo group (21 of 149, 14.1%) than in the vitamin E group (10 of 149, 6.7%; P=0.037) (Table 3). Additional definition of CIAKI (an efficacy end point) as an eGFR decrease of ≥25% over the baseline value was comparable between the study groups (13.4% for placebo versus 6.7% for vitamin E, P=0.054) (Table 3).
Table 3.
CIAKI Incidence and In‐Hospital Outcomes in the Study Groups
| Placebo Group (n=149) | Vitamin E Group (n=149) | P Value | |
|---|---|---|---|
| CIAKI definitions | |||
| Serum creatinine increase by ≥25% | 21 (14.1) | 10 (6.7) | 0.037 |
| Serum creatinine increase by ≥0.5 mg/dL | 20 (13.4) | 8 (5.4) | 0.017 |
| Serum creatinine increase by ≥25% or Serum creatinine increase by ≥0.5 mg/dL | 21 (14.1) | 10 (6.7) | 0.037 |
| eGFR decrease by ≥25% | 20 (13.4) | 10 (6.7) | 0.054 |
| In‐hospital outcomes | |||
| Medication side effect | 0 (0) | 0 (0) | 1 |
| Hospital stay, day | 2 (2–3) | 2 (2–3) | 0.2 |
| Renal replacement therapy | 0 (0) | 0 (0) | 1 |
| NSTE‐ACS | 0 (0) | 2 (1.4) | 0.5 |
| AMI | 0 (0) | 1 (0.7) | 1 |
| Cerebrovascular events | 0 (0) | 0 (0) | 1 |
| Death | 0 (0) | 1 (0.7) | 1 |
Values are presented as number (%). AMI indicates acute myocardial infarction; CIAKI, contrast medium–induced acute kidney injury; eGFR, estimated glomerular filtration rate; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome.
Secondary End Points
No side effects related to the interventions were observed. Median of hospital stay was 2 days and was comparable between groups (P=0.2) (Table 3). None of the study patients needed renal replacement therapy. Two cases (0.7%) of NSTE‐ACS developed in the vitamin E group; however, the difference between groups was not significant (P=0.5). In the vitamin E group, 1 patient (0.35%) with acute ST‐segment elevation myocardial infarction died in the hospital (Table 3).
The decrease in creatinine level was greater in the vitamin E group but not significantly. Similarly, the change in eGFR was higher in the vitamin E group but also not significantly. The median changes in serum creatinine and eGFR improved more in the placebo group, even though these patients had a higher risk of AKI compared with the vitamin E group. Furthermore, decrease in WBC count was significantly greater in the vitamin E group compared with the placebo group (Figure 2).
Figure 2.

Change in serum creatinine (A), eGFR (B), and WBC count (C) in vitamin E and placebo groups. eGFR indicates estimated glomerular filtration rate; WBC, white blood cell.
Subgroup Analysis
To compare the risk of CIAKI among patients with diabetes mellitus, we divided the patients into 2 groups according to median FBG. Among diabetic patients with FBG ≥100 mg/dL, the rate of CIAKI was significantly higher compared with those with FBG <100 mg/dL (17.4% versus 0%, respectively; P=0.044) (Table 4). Among all patients, more CIAKI developed in those with FBG ≥100 versus <100 mg/dL, regardless of study group (15.7% versus 4.8%, respectively; P=0.002) (Table 4).
Table 4.
CIAKI Incidence in the Study Groups According to Preoperative FBG Values
| CIAKI Definitions | FBG <100 | FBG ≥100 | P Value |
|---|---|---|---|
| All patients, n | 145 | 153 | |
| Serum creatinine increase by ≥25% | 7 (4.8) | 24 (15.7) | 0.002 |
| Serum creatinine increase by ≥0.5 mg/dL | 7 (4.8) | 21 (13.7) | 0.009 |
| Serum creatinine increase by ≥25% or serum creatinine increase by ≥0.5 mg/dL | 7 (4.8) | 24 (15.7) | 0.002 |
| eGFR decrease by ≥25% | 7 (4.8) | 23 (15) | 0.003 |
| Diabetic patients, n | 20 | 86 | |
| Serum creatinine increase by ≥25% | 0 (0) | 15 (17.4) | 0.044 |
| Serum creatinine increase by ≥0.5 mg/dL | 0 (0) | 13 (15.1) | 0.063 |
| Serum creatinine increase by ≥25% or serum creatinine increase by ≥0.5 mg/dL | 0 (0) | 15 (17.4) | 0.044 |
| eGFR decrease by ≥25% | 0 (0) | 14 (16.3) | 0.053 |
Values are presented as number (%). CIAKI indicates contrast medium–induced acute kidney injury; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose.
We also compared the risk of CIAKI in patients with regard to history of preoperative statin use. Accordingly, the rates of CIAKI incidence in the study groups were comparable among patients with or without history of preoperative statin use and were similar to the risk of CIAKI development (Figure 3).
Figure 3.
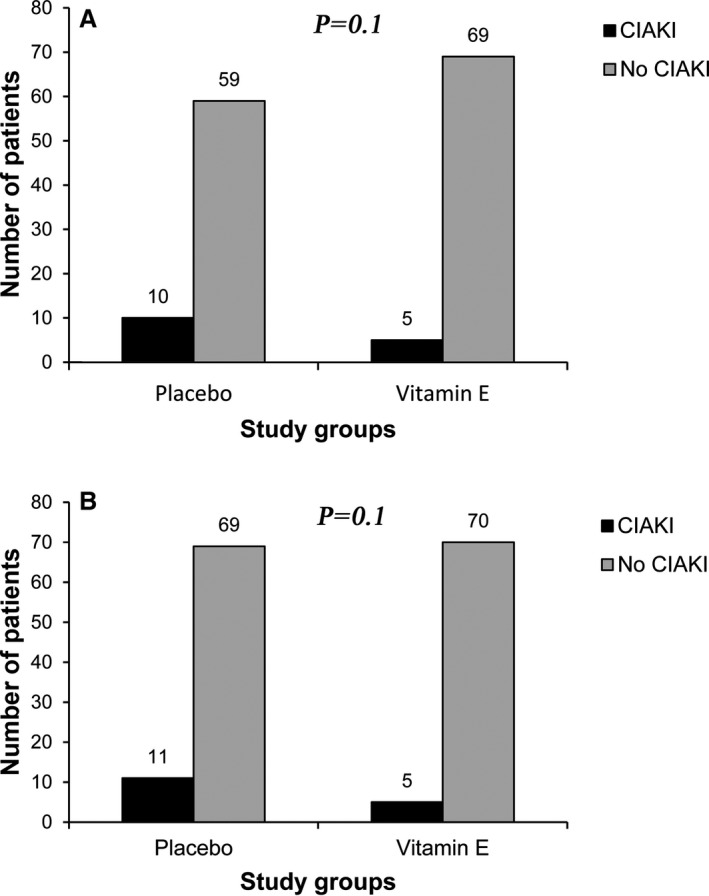
Incidence of CIAKI in the subgroups (A) without history of preoperative statin use and (B) with history of preoperative statin use, divided by treatment groups. CIAKI indicates contrast medium–induced acute kidney injury.
Patients with CIAKI had higher baseline Mehran risk scores compared with patients without CIAKI (11.3 [IQR 5.5–16.5] versus 7.5 [IQR 5.25–9.3], P=0.002). The patients with CIAKI had more metabolic syndrome (71% versus 50.6%), more congestive heart failure (16.1% versus 6.4%), higher median of baseline WBC count (8.9 [IQR 7.6–10.6] versus 7.7 [IQR 6.2–9.3]), and lower median of baseline hemoglobin (12 [IQR 9.3–12.4] versus 12.6 [IQR 11.3–13.8]). All mentioned variables were significantly different between the patients with and without CIAKI (Table 5).
Table 5.
Predictors of CIAKI According to Univariate and Multivariate Analyses
| Univariate Analysis | Multivariate Analysisa | |||||
|---|---|---|---|---|---|---|
| With CIAKI (n=31) | Without CIAKI (n=267) | P Value | OR | 95% CI | P Value | |
| Vitamin E (vs placebo) | 6.7% vs 14.1% | 93.3% vs 85.9% | 0.037 | 0.408 | 0.170–0.982 | 0.045 |
| Mehran risk score | 11.3 (5.5–16.5) | 7.5 (5.25–9.3) | 0.002 | 1.257 | 1.007–1.569 | 0.043 |
| Metabolic syndrome | 22 (71) | 135 (50.6) | 0.031 | — | — | — |
| Congestive heart failure | 5 (16.1) | 17 (6.4) | 0.049 | — | — | — |
| Baseline WBC count, ×103/mL | 8.9 (7.6–10.6) | 7.7 (6.2–9.3) | 0.009 | — | — | — |
| Baseline hemoglobin, mg/dL | 12 (9.3–12.4) | 12.6 (11.3–13.8) | <0.001 | — | — | — |
Values are presented as median (interquartile range) or number (%). AMI indicates acute myocardial infarction; CIAKI, contrast medium–induced acute kidney injury; OR, odds ratio, WBC, white blood cell.
Only factors that predicted CIAKI are reported.
The Mehran risk score groups ≤5, 6 to 10, 11 to 15, and ≥16 included 69 (23.2%), 169 (56.7%), 47 (15.8%), and 13 (4.4%) patients, respectively, and 4 (5.8%), 10 (5.9%), 7 (14.9%), and 10 (76.9%) patients in the respective Mehran risk score groups developed CIAKI (P<0.001). In the low‐risk groups with Mehran risk scores ≤15, the incidence of CIAKI was lower in patients receiving vitamin E than placebo; however, for the patients with the highest risk of ≥16, no evidence showed that vitamin E was effective (Figure 4).
Figure 4.
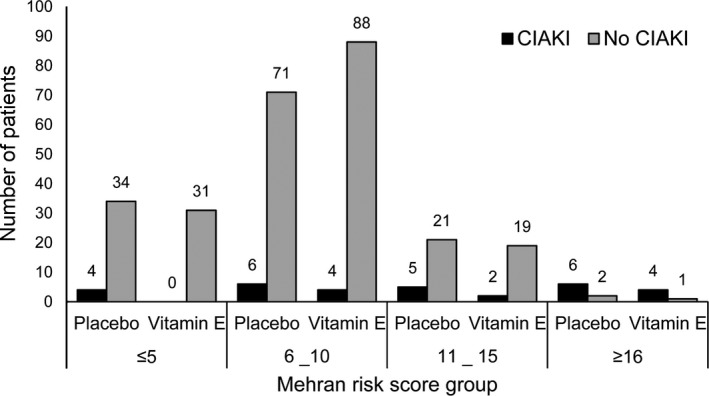
Incidence of CIAKI in the vitamin E and placebo groups based on baseline Mehran risk score in all patients, divided by treatment groups. None of paired comparisons were statistically significant. CIAKI indicates contrast medium–induced acute kidney injury.
Multivariable Factors for Predicting CIAKI
In the logistic regression analysis, we entered the study treatment groups, Mehran risk score as a continuous variable, metabolic syndrome, congestive heart failure, statin use, CKD, age >75 years, high contrast volume >140 mL, diabetes mellitus, study site, and baseline hemoglobin and WBC values as covariates in our model. The only independent predictors of CIAKI were vitamin E (odds ratio 0.408, 95% CI 0.170–0.982, P=0.045) and baseline Mehran risk score (odds ratio 1.257, 95% CI 1.007–1.569; P=0.043) (Table 5).
Discussion
The key findings of the present study are that prophylactic oral administration of 1000 mg vitamin E (α‐tocopherol) combined with hydration of 0.9% saline infusion appears to decrease CIAKI incidence in CKD patients undergoing elective coronary angiography compared with normal saline alone, with a number needed to treat of 13. Concordantly, the consumption of vitamin E and baseline Mehran risk scores independently predicted CIAKI incidence.
In general, contrast media are well tolerated; however, they can cause AKI, especially in high‐risk patients such as those with CKD. Because of an increase in cardiac interventional procedures, the rate of CIAKI has steadily increased and become a leading cause of hospital‐acquired AKI.6 The rate of complications attributable to CIAKI is high: In‐hospital mortality has been found to be about 5‐fold higher in patients who received contrast medium and developed CIAKI compared with those who received contrast medium but did not develop CIAKI, and 1‐ and 5‐year mortality rates are about 4‐fold higher.4, 25 Despite the great impact of CIAKI in daily clinical practice, its pathophysiology is still a matter of debate. It has been speculated that contrast media exposure leads to AKI through 2 main mechanisms: cytotoxicity and higher viscosity. The cytotoxic effect of contrast media damages endothelial and tubular cells, causing decreases in nitic oxide levels and elevated oxidative stress and consequent vasoconstriction and medullary hypoxia. In addition to that effect, a decreased glomerular filtration rate and medullary hypoperfusion develop that are attributable to the exponential increase in tubular fluid viscosity.26
Many risk factors have been reported to be associated with CIAKI after coronary interventions and can predict patients at risk for CIAKI.27 The important factors include calculated creatinine clearance, diabetic status, and contrast media volume prior to a proposed coronary intervention.2 For better risk stratification, some risk scores have been useful; the Mehran risk score is a simple and widely used tool.28 It is based on the presence of 8 factors (hypotension, use of an intra‐aortic balloon pump, congestive heart failure, CKD, diabetes, age >75 years, anemia, and volume of contrast). In our study, we found that metabolic syndrome, congestive heart failure, and elevated WBC count and decreased hemoglobin at baseline were associated with CIAKI. Despite relationships between these factors and CIAKI in univariate analysis, these effects disappeared in multivariate analysis. Nevertheless, baseline Mehran score directly predicted CIAKI incidence with an odds ratio of 1.257 (95% CI1.007–1.569, P=0.043). Individual risk factors did not predict CIAKI, but the predictive role of the Mehran score demonstrated that these factors can be used for risk stratification. We think that the relatively small sample size of our cohort, despite being sufficiently powered to detect primary outcome of interest, might be the main obstacle to showing the individual impact of risk factors on the incidence of CIAKI.
CIAKI was detected mainly by biomarkers, which are dependent on underlying diseases. Consequently, the results of one study cannot be generalizable to another study under different settings. In such a situation, finding the proper diagnostic tool for early detection of AKI may be of great importance in clinical trials.29 The measurements of serum creatinine and urine output are considered the primary tools for diagnosing AKI in daily practice because use of biomarkers with unproven reliability might result in incorrect interpretation of clinical trials.29 Despite the shortcomings of serum creatinine to estimate GFR, with very few exceptions, it is the only diagnostic marker used in clinical trials for CIAKI.30 The absolute change in serum creatinine within 48 to 72 hours after contrast medium exposure has been routinely used as a definition of CIAKI, as in our study; we defined CIAKI as an absolute increase ≥0.5 mg/dL or a 25% relative increase in the baseline serum creatinine concentrations measured within 72 hours after intervention. The serum creatinine concentration has been shown to peak within 2 to 3 days after exposure, although the majority of studies have reported serum levels at 48 hours, which can underestimate the incidence of CIAKI or underpower studies.1 We believe that in daily practice, serum creatinine is a simple, inexpensive, and available marker until a new biomarker is proven beneficial for such circumstances.
The preventive strategies are focused on the possible pathophysiological mechanisms involved in CIAKI. Increase in tubular viscosity and resultant decrease in urine flow rate lead to diminishing GFR by activation of the renin–angiotensin system. Hydration therapy has been demonstrated to be the main preventive strategy by increasing tubular urine flow and consequently diminishing contrast medium concentration and viscosity of tubular lumens.26 Furthermore, contrast media lead to generation of reactive oxygen species, which may lead to vasoconstriction,31 and hypoperfusion of the medulla, promoting mitochondrial generation of reactive oxygen species.32 Accordingly, use of antioxidants (eg, vitamin C, N‐acetylcysteine, vitamin E) represents an effective strategy to prevent CIAKI, considering their roles in attenuating the oxidative damage from radiocontrast.
A protective effect of oral vitamin C against CIAKI was found in a cohort of CKD patients undergoing coronary angiography or intervention.12 Furthermore, a recent meta‐analysis revealed conflicting results regarding the efficacy of N‐acetylcysteine infusion against CIAKI.13 In contrast, studies of vitamin E have been associated with inconsistent findings. Kitzler et al demonstrated that intravenous vitamin E in addition to 0.45% saline infusion was not effective against CIAKI in CKD patients undergoing computed tomography scanning compared with 0.45% saline infusion alone.21 In another study, however, α‐ or γ‐tocopherol combined with administration of 0.9% saline resulted in a decrease in CIAKI after elective coronary angiography.20 Vitamin E protects against oxidative damage by stabilizing cell membranes and scavenging lipid peroxyl radicals; therefore, it is able to break peroxyl chain propagation reactions.33 The antioxidant activity of vitamin C is mediated by direct effects on hydroxyl radicals and hydrogen peroxide to reduce lipid peroxidation.34 Moreover, N‐acetylcysteine, a glutathione precursor, has been found to enhance intracellular glutathione levels, as detoxifying hydroxyl groups, in rats but not in human studies.35 Given these different mechanisms of action among antioxidants, further studies are required to establish a more robust conclusion about their effects in clinical practice, especially for prevention of CIAKI.
Statins are antilipid agents with anti‐inflammatory action in cardiovascular disease and have been used for the prevention of CIAKI. A recently published meta‐analysis showed that preprocedure statin therapy reduced the risk of CIAKI compared with control groups; however, patients with CKD stage ≥3 were largely underrepresented in published studies, and statin therapy did not significantly decrease CIAKI development in trials that enrolled patients with eGFR <60 mL/min per 1.73 m2. The authors concluded that further trials were required to better define the role of statins for protection against CIAKI in the setting of coronary angiography, particularly in patients with CKD stage ≥3.36 Moreover, in another meta‐analysis, Lee et al found that short‐term high‐dose statin (≥40 mg atorvastatin) significantly reduced the incidence of CIAKI compared with low‐dose statin or placebo in patients undergoing coronary angiography.37 In our study, we were unable to show the benefits of statin use beyond the study intervention. We think that the main limitation of this study in this regard was the small sample size to detect statin effects. We also did not prescribe a high‐dose statin preprocedurally for our cohort, and that dose can have more impact than a low‐dose statin, as demonstrated previously.
The glycemic status of patients has been found to influence renal protection of patients on exposure to contrast medium. Patients with elevated FBG are at higher risk of developing CIAKI than nondiabetic patients.38 The main biomarkers indicating glycemic status are FBG, blood sugar, and hemoglobin A1c levels. Among patients undergoing coronary catheterization, elevated baseline hemoglobin A1c, but not blood sugar, was also associated with increased risk of CIAKI in nondiabetic patients.39 In contrast, another study showed that elevated preprocedural blood glucose correlated with greater risk for CIAKI in nondiabetic patients undergoing coronary angiography.40 In addition, Yoshikawa et al found that hemoglobin A1c ≥6.5% was associated with changes in renal function after contrast exposure on computed tomography angiography.41 In line with the mentioned findings, we also demonstrated that CIAKI incidence was significantly higher in patients with elevated preprocedural FBG, and CIAKI developed more often in those who also had diabetes mellitus. We did not measure other markers indicating nephropathy in diabetic patients, especially hemoglobin A1c and albuminuria, which have been shown to be associated with the risk of CIAKI in diabetic patients.39, 42, 43
In our study, the patients who developed CIAKI had higher peak levels of WBCs, representing the possible role of inflammation in the setting of CIAKI; WBC count is a marker for acute inflammation. Interestingly, we found that WBC change from baseline to its peak value within 72 hours was significantly greater in the patients receiving vitamin E compared with those receiving placebo. These findings confirm that inflammatory mechanisms may be involved in the pathogenesis of CIAKI incidence. It may be speculated that the effects of vitamin E are applied, in some part, through attenuation of inflammation in addition to scavenging free oxygen radicals.44 A recent meta‐analysis that evaluated the effects of vitamin E–coated dialyzer on oxidative status and inflammation revealed that dialyzer membrane containing vitamin E decreased inflammatory markers, reflecting the anti‐inflammatory property of vitamin E.45
Limitations
In interpreting these data, some limitations should be considered. First, the results of this study cannot be extended to very high‐risk patients, particularly those who need to undergo renal replacement therapy, because the number of patients with a Mehran score ≥16 was insufficient and no patients needed dialysis during our study. Second, we did not measure cystatin C level, which seems to be a more reliable marker than serum creatinine. Moreover, we used the Modification of Diet in Renal Disease formula to calculate eGFR.46 Nonetheless, these limitations are observed in most of the previous clinical trials in this setting. Third, we did not measure the serum levels of vitamin E at baseline and follow‐up to evaluate the effect of patients’ vitamin E status on our findings. Fourth, 2 patients developed NSTE‐ACS, and 1 patient had acute ST‐segment elevation myocardial infarction and died during hospitalization; all of these events occurred in the vitamin E group. Because of the low numbers of events and very short‐term follow‐up, we were unable to assess why these events happened in the vitamin E group. Finally, changes in WBC levels were used to show the probable anti‐inflammatory role of vitamin E, whereas C‐reactive protein could be a more accurate biomarker than WBCs for demonstrating such an effect in the prevention of the CIAKI.
Conclusion
The results of this study showed that pretreatment 1000 mg vitamin E (α‐tocopherol) combined with 0.9% saline infusion is a safe, well‐tolerated, efficacious, and readily available antioxidant to prevent CIAKI incidence in moderate‐ to high‐risk CKD patients undergoing elective coronary angiography.
Sources of Funding
This study was funded by a grant from our university, supported by the vice chancellor for research at Urmia University of Medical Sciences. Institutional support was also provided by Seyyed‐al‐Shohada Heart Center and Taleghani Hospital, Urmia, West Azerbaijan Province, Iran. No other sources are present to mention. The sponsors had no role in study design, data analysis, or interpretation of findings.
Disclosures
None.
Acknowledgments
The authors are grateful to the nursing department of Seyyed‐al‐Shohada Heart Center and Taleghani hospital, which fully supported our team in gathering the study data. Moreover, we greatly thank to Dr Harri Hemilä, a faculty member at the University of Helsinki, for his consultation on the statistical aspects of this study.
(J Am Heart Assoc. 2016;5:e002919 doi: 10.1161/JAHA.115.002919)
References
- 1. Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. [DOI] [PubMed] [Google Scholar]
- 3. Maeder M, Klein M, Fehr T, Rickli H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004;44:1763–1771. [DOI] [PubMed] [Google Scholar]
- 4. Rihal C, Textor S, Grill D, Berger P, Ting H, Best P, Singh M, Bell M, Barsness G, Mathew V. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 5. Brown JR, Thompson CA. Contrast‐induced acute kidney injury: the at‐risk patient and protective measures. Curr Cardiol Rep. 2010;12:440–445. [DOI] [PubMed] [Google Scholar]
- 6. Gami AS, Garovic VD. Contrast nephropathy after coronary angiography. Mayo Clin Proc. 2004;79:211–219. [DOI] [PubMed] [Google Scholar]
- 7. Caixeta A, Nikolsky E, Mehran R. Prevention and treatment of contrast‐associated nephropathy in interventional cardiology. Curr Cardiol Rep. 2009;11:377–383. [DOI] [PubMed] [Google Scholar]
- 8. Firouzi A, Eshraghi A, Shakerian F, Sanati HR, Salehi N, Zahedmehr A, Kiani R, Madani M, Pedarzadeh A. Efficacy of pentoxifylline in prevention of contrast‐induced nephropathy in angioplasty patients. Int Urol Nephrol. 2012;44:1145–1149. [DOI] [PubMed] [Google Scholar]
- 9. Landoni G, Biondi‐Zoccai GG, Tumlin JA, Bove T, De Luca M, Calabro MG, Ranucci M, Zangrillo A. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: a meta‐analysis of randomized clinical trials. Am J Kidney Dis. 2007;49:56–68. [DOI] [PubMed] [Google Scholar]
- 10. Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA III, Rittase RA, Norton HJ, Kennedy TP. Prevention of contrast‐induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–2334. [DOI] [PubMed] [Google Scholar]
- 11. Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. [DOI] [PubMed] [Google Scholar]
- 12. Spargias K, Alexopoulos E, Kyrzopoulos S, Iokovis P, Greenwood DC, Manginas A, Voudris V, Pavlides G, Buller CE, Kremastinos D, Cokkinos DV. Ascorbic acid prevents contrast‐mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110:2837–2842. [DOI] [PubMed] [Google Scholar]
- 13. Sun Z, Fu Q, Cao L, Jin W, Cheng L, Li Z. Intravenous N‐acetylcysteine for prevention of contrast‐induced nephropathy: a meta‐analysis of randomized, controlled trials. PLoS One. 2013;8:e55124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vasheghani‐Farahani A, Sadigh G, Kassaian SE, Khatami SM, Fotouhi A, Razavi SA, Mansournia MA, Yamini‐Sharif A, Amirzadegan A, Salarifar M, Sadeghian S, Davoodi G, Borumand MA, Esfehani FA, Darabian S. Sodium bicarbonate plus isotonic saline versus saline for prevention of contrast‐induced nephropathy in patients undergoing coronary angiography: a randomized controlled trial. Am J Kidney Dis. 2009;54:610–618. [DOI] [PubMed] [Google Scholar]
- 15. Engelen W, Keenoy BM, Vertommen J, De Leeuw I. Effects of long‐term supplementation with moderate pharmacologic doses of vitamin E are saturable and reversible in patients with type 1 diabetes. Am J Clin Nutr. 2000;72:1142–1149. [DOI] [PubMed] [Google Scholar]
- 16. Kaegi E. Unconventional therapies for cancer: 5. Vitamins A, C and E. The task force on alternative therapies of the Canadian Breast Cancer Research Initiative. CMAJ. 1998;158:1483–1488. [PMC free article] [PubMed] [Google Scholar]
- 17. Dillioglugil MO, Maral Kir H, Gulkac MD, Ozon Kanli A, Ozdogan HK, Acar O, Dillioglugil O. Protective effects of increasing vitamin E and a doses on cisplatin‐induced oxidative damage to kidney tissue in rats. Urol Int. 2005;75:340–344. [DOI] [PubMed] [Google Scholar]
- 18. Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin‐induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195:221–230. [DOI] [PubMed] [Google Scholar]
- 19. Kongkham S, Sriwong S, Tasanarong A. Protective effect of alpha tocopherol on contrast‐induced nephropathy in rats. Nefrologia. 2013;33:116–123. [DOI] [PubMed] [Google Scholar]
- 20. Tasanarong A, Vohakiat A, Hutayanon P, Piyayotai D. New strategy of alpha‐ and gamma‐tocopherol to prevent contrast‐induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. Nephrol Dial Transplant. 2013;28:337–344. [DOI] [PubMed] [Google Scholar]
- 21. Kitzler TM, Jaberi A, Sendlhofer G, Rehak P, Binder C, Petnehazy E, Stacher R, Kotanko P. Efficacy of vitamin E and N‐acetylcysteine in the prevention of contrast induced kidney injury in patients with chronic kidney disease: a double blind, randomized controlled trial. Wien Klin Wochenschr. 2012;124:312–319. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 23. Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, Johnston CS, Kelly FJ, Kraemer K, Packer L, Parthasarathy S, Sies H, Traber MG. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–745. [DOI] [PubMed] [Google Scholar]
- 24. National Cholesterol Education Program (NCEP) Expert Panel on Detection , (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high Blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143‐421. [PubMed] [Google Scholar]
- 25. McCullough PA. Radiocontrast‐induced acute kidney injury. Nephron Physiol. 2008;109:p61–p72. [DOI] [PubMed] [Google Scholar]
- 26. Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast‐induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–2015. [DOI] [PubMed] [Google Scholar]
- 27. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Weintraub WS, Curtis JP, Messenger JC, Rumsfeld JS, Spertus JA. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath‐PCI Registry. J Am Heart Assoc. 2014;3:e001380 doi: 10.1161/JAHA.114.001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 29. Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in‐depth review of the literature. Nephrol Dial Transplant. 2013;28:254–273. [DOI] [PubMed] [Google Scholar]
- 30. Reddan D, Laville M, Garovic VD. Contrast‐induced nephropathy and its prevention: what do we really know from evidence‐based findings? J Nephrol. 2009;22:333–351. [PubMed] [Google Scholar]
- 31. Bakris GL, Lass N, Gaber AO, Jones JD, Burnett JC Jr. Radiocontrast medium‐induced declines in renal function: a role for oxygen free radicals. Am J Physiol. 1990;258:F115–F120. [DOI] [PubMed] [Google Scholar]
- 32. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia‐inducible factor‐1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Quinn PJ. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38:309–336. [DOI] [PubMed] [Google Scholar]
- 34. Cox CD, Tsikouris JP. Preventing contrast nephropathy: what is the best strategy? A review of the literature. J Clin Pharmacol. 2004;44:327–337. [DOI] [PubMed] [Google Scholar]
- 35. Fishbane S, Durham JH, Marzo K, Rudnick M. N‐acetylcysteine in the prevention of radiocontrast‐induced nephropathy. J Am Soc Nephrol. 2004;15:251–260. [DOI] [PubMed] [Google Scholar]
- 36. Thompson K, Razi R, Lee MS, Shen A, Stone GW, Hiremath S, Mehran R, Brar SS. Statin use prior to angiography for the prevention of contrast‐induced acute kidney injury: a meta‐analysis of 19 randomised trials. EuroIntervention. 2015;19:11. [DOI] [PubMed] [Google Scholar]
- 37. Lee JM, Park J, Jeon KH, Jung JH, Lee SE, Han JK, Kim HL, Yang HM, Park KW, Kang HJ, Koo BK, Jo SH, Kim HS. Efficacy of short‐term high‐dose statin pretreatment in prevention of contrast‐induced acute kidney injury: updated study‐level meta‐analysis of 13 randomized controlled trials. PLoS One. 2014;9:e111397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toprak O, Cirit M, Yesil M, Bayata S, Tanrisev M, Varol U, Ersoy R, Esi E. Impact of diabetic and pre‐diabetic state on development of contrast‐induced nephropathy in patients with chronic kidney disease. Nephrol Dial Transplant. 2007;22:819–826. [DOI] [PubMed] [Google Scholar]
- 39. Barbieri L, Verdoia M, Schaffer A, Cassetti E, Di Giovine G, Marino P, Suryapranata H, De Luca G. Pre‐diabetes and the risk of contrast induced nephropathy in patients undergoing coronary angiography or percutaneous intervention. Diabetes Res Clin Pract. 2014;106:458–464. [DOI] [PubMed] [Google Scholar]
- 40. Stolker JM, McCullough PA, Rao S, Inzucchi SE, Spertus JA, Maddox TM, Masoudi FA, Xiao L, Kosiborod M. Pre‐procedural glucose levels and the risk for contrast‐induced acute kidney injury in patients undergoing coronary angiography. J Am Coll Cardiol. 2010;55:1433–1440. [DOI] [PubMed] [Google Scholar]
- 41. Yoshikawa D, Isobe S, Sato K, Ohashi T, Fujiwara Y, Ohyama H, Ishii H, Murohara T. Importance of oral fluid intake after coronary computed tomography angiography: an observational study. Eur J Radiol. 2011;77:118–122. [DOI] [PubMed] [Google Scholar]
- 42. de Jong PE, Gansevoort RT, Bakker SJ. Macroalbuminuria and microalbuminuria: do both predict renal and cardiovascular events with similar strength? J Nephrol. 2007;20:375–380. [PubMed] [Google Scholar]
- 43. Yang JQ, Ran P, Chen JY, He YT, Li LW, Tan N, Li G, Sun S, Liu Y, Zhan JX, Zheng JY, Zhou YL. Development of contrast‐induced acute kidney injury after elective contrast media exposure in patients with type 2 diabetes mellitus: effect of albuminuria. PLoS One. 2014;9:e106454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saboori S, Shab‐Bidar S, Speakman JR, Yousefi Rad E, Djafarian K. Effect of vitamin E supplementation on serum C‐reactive protein level: a meta‐analysis of randomized controlled trials. Eur J Clin Nutr. 2015;69:867–873. [DOI] [PubMed] [Google Scholar]
- 45. Yang SK, Xiao L, Xu B, Xu XX, Liu FY, Sun L. Effects of vitamin E‐coated dialyzer on oxidative stress and inflammation status in hemodialysis patients: a systematic review and meta‐analysis. Ren Fail. 2014;36:722–731. [DOI] [PubMed] [Google Scholar]
- 46. Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318. [DOI] [PubMed] [Google Scholar]


