Abstract
Background
Racial differences in electrocardiographic (ECG) characteristics and prognostic significance among Whites and Asians are not well described.
Methods and Results
We studied 2677 White Framingham Heart Study participants (57% women) and 2972 Asian (64% women) Singapore Longitudinal Aging Study participants (mean age 66 years in both) free of myocardial infarction or heart failure. Racial differences in ECG characteristics and effect on mortality were assessed. In linear regression models, PR interval was longer in Asians compared with Whites (multivariable‐adjusted β±SE 5.0±1.4 ms in men and 6.6±0.9 ms in women, both P<0.0006). QT interval was shorter in Asian men (β±SE −6.2±1.2 ms, P<0.0001) and longer in Asian women (β±SE 3.6±0.9 ms, P=0.02) compared to White men and women, respectively. Asians had greater odds of having ECG left ventricular hypertrophy (LVH) compared with Whites (odds ratio [OR] 3.56, 95% confidence interval [CI] 1.36–9.35 for men, OR 1.93, 95% CI 1.35–2.76 for women, both P<0.02). Over a mean follow‐up of 11±3 years in Framingham and 8±3 years in Singapore, mortality rates were 24.5 and 13.4 per 1000 person‐years among Whites and Asians, respectively. In Cox models, the presence of LVH had a greater effect on all‐cause mortality in Asians compared with Whites (hazard ratio [HR] 2.66, 95% CI 1.83–3.88 vs HR 1.30, 95% CI 0.90–1.89, P for interaction=0.02).
Conclusion
Our findings from two large community‐based cohorts show prominent race differences in ECG characteristics between Whites and Asians, and also suggest a differential association with mortality. These differences may carry implications for race‐specific ECG reference ranges and cardiovascular risk.
Keywords: electrocardiography, epidemiology, left ventricular hypertrophy, mortality, race
Subject Categories: Race and Ethnicity, Electrocardiology (ECG)
Introduction
There are important differences between Asians and Caucasians in cardiovascular disease risk factors, incidence, and survival.1, 2, 3, 4 Asians have a higher prevalence of risk factors such as diabetes, hypertension, renal insufficiency, and narrower coronary arteries.2, 5, 6 These risk factors differentially affect left ventricular function between these two races.7 Despite the higher prevalence of traditional cardiovascular risk factors among Asians, the overall death rate due to cardiovascular disease is strikingly higher in White compared to Asian Americans.3
Electrocardiographic (ECG) characteristics are helpful in diagnosing cardiovascular disease, reflect cardiac structure and remodeling, and also predict mortality.8, 9, 10, 11, 12 There is a widespread perception that ECG reference ranges should differ in Asians compared to Whites given smaller heart13 and body sizes14 in Asians and the dependence of ECG parameters on heart and chest size. However, prior studies were restricted to single races, highly selected individuals from clinical settings, or small subgroups of different races from the same country. Few studies have directly compared ECG characteristics between Asians and Whites from the general community, and none have examined differences in prognostic capability of ECG features. We therefore sought to compare ECG traits between White and Asian adults from the large prospective community‐based cohorts of the Framingham Heart Study (FHS) and Singapore Longitudinal Aging Study (SLAS), respectively, and to investigate whether ECG features may differentially impact prognosis between Whites and Asians.
Methods
Study Participants
Participants with ages ranging from 55 to 90 years from two large community‐based cohorts were included: Whites from FHS and Asians from SLAS. FHS is a longitudinal population‐based study intended to examine risk factors of cardiovascular disease. Individuals attending the following baseline examinations were included: original cohort examination 26 (1999–2001), offspring examination 7 (1998–2001), and generation 3 examination 1 (2002–2005).15, 16, 17 Of 8192 eligible FHS individuals, we excluded 4835 due to age <55 or >90 years, 433 without ECG data due to offsite exams, 200 due to history of myocardial infarction or heart failure, 30 due to missing covariates, 14 due to loss to follow‐up, and 3 due to paced rhythm, leaving 2677 White participants for this analysis. Similarly, SLAS is a population‐based longitudinal cohort study of aging and health.18 Participants were community‐living Asian adults recruited from the Singapore general population, identified by a door‐to‐door census of all residents within five districts in the southeastern region of Singapore. Of 3116 eligible participants recruited between the years of 2004 and 2014, we excluded 18 for age <55 and >90 years, 91 due to history of MI or heart failure, 30 for missing covariates, and 5 due to paced rhythm, leaving 2972 Asian participants for analysis. Both studies were approved by the respective institutional review boards, and informed consent was obtained from all the individual participants.
Clinical Assessment and Follow‐Up
All participants underwent comprehensive clinical examination, medical history, anthropometrics, and fasting laboratory assessment at the baseline examination. Diabetes was defined as a fasting glucose level ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or the use of hypoglycemic drugs or insulin. Smoking was defined based on self‐reported use of ≥1 cigarettes/day in the year prior to the examination.
Participants in both studies were followed longitudinally with periodic examinations and health questionnaire updates. Vital status was obtained and verified via medical records and death certificates.
ECG Measurement
Standard resting 12‐lead ECGs were performed. ECG characteristics were measured using the Marquette (now General Electric, Fairfield, CT) MAC 5000 machine (for FHS) and the 5500 machine (for SLAS) using identical definitions and coding sheets. Measurements were made in both studies using Marquette 12SL ECG (General Electric) analysis program for P‐wave indices quantification. QRS voltage was defined as sum of R in aVL and S in V3. We defined left ventricular hypertrophy (LVH) by Cornell criteria (QRS voltage >28 mm in men and >20 mm in women).19 Left atrial enlargement (LAE), QTc by Framingham formula (QTcFram=QT+0.154×[1000−RR]), and QTc by Bazett's formula (QTcBaz=QT/√RR) used established criteria.9, 20, 21 Reproducibility was ascertained in a subset of 50 randomly selected ECGs from FHS and 50 ECGs from SLAS by one reader. Between the two cohorts, the intraclass correlation coefficient ranged from 0.78 to 1.00, with a coefficient of variation from 0% to 6.5% for continuous ECG traits (PR interval, QRS duration, QT interval, QRS voltage components). For dichotomous traits (LVH and LAE), the percentage agreement between observers ranged from 88% to 100%, with a κ of 0.45 to 0.79.
Statistical Analysis
Baseline characteristics were examined by race and sex (White men, Asian men, White women, and Asian women). Multivariable linear regression was used to examine the association of race with the following ECG characteristics: PR interval, QRS duration, QT interval, and QRS voltage, presence of LVH, LAE, left bundle branch block (LBBB), and right bundle branch block (RBBB). Models were adjusted first for age and sex, and additionally for body mass index, systolic blood pressure, antihypertensive therapy, diabetes, smoking status, and heart rate. QTcFram and QTcBaz were adjusted for all the above variables except heart rate. In secondary analyses, height and weight were substituted for body‐mass index.
The association of individual ECG characteristics and all‐cause mortality was then examined in the pooled sample using Cox regression models and adjusting for the same covariates as in cross‐sectional analyses. We specifically tested for ECG×race and ECG×sex interaction terms. In secondary analyses, stratified analyses were performed by race and by sex. Given 4 primary ECG traits (PR interval, QRS duration, QT interval, and QRS voltage), after Bonferroni correction, a 2‐tailed P value threshold of <0.05/4=0.0125 was considered statistically significant for primary analyses. Age‐adjusted Kaplan‐Meier curves22 and a 3‐degree‐of‐freedom Wald test were used to compare the survival in the 4 participant groups. All statistical analyses were conducted using SAS version 9.2 for Windows (SAS Institute, Cary, NC).
Results
A total of 2677 White participants (57% women, mean age 66±9 years) were compared with 2972 Asian participants (64% women, mean age 66±7 years). Asian participants were predominantly of Chinese origin, with a smaller proportion of Malay and Indian origin. Baseline clinical characteristics are presented by race and sex in Table 1, and they show higher body mass index among Whites compared with Asians (28.1±5.3 vs 23.8±3.8 kg/m2, P<0.0001). In contrast, Asians had higher systolic blood pressure (131±19 vs 125±18 mm Hg, P=0.005) and greater prevalence of diabetes mellitus (15% vs 12%, P=0.002) compared with Whites. Within each race group, there were no significant sex differences in clinical characteristics except for smoking among Asians (higher rates of smoking among Asian men than women).
Table 1.
Clinical and Electrocardiographic Characteristics in Asian and Western Adults
| FHS Men (n=1158) | SLAS Men (n=1059) | FHS Women (n=1519) | SLAS Women (n=1913) | |
|---|---|---|---|---|
| Age, y | 66±8 | 67±8a | 67±9 | 66±7b |
| Heart rate, bpm | 64±11 | 68±12a | 67±11 | 69±11b |
| Systolic blood pressure, mm Hg | 131±18 | 133±17a | 131±20 | 132±17 |
| Body mass index, kg/m2 | 28.7±4.5 | 23.6±3.5a | 27.6±5.7 | 23.9±4.0b |
| Smoking status, n (%) | 116 (10) | 403 (38)a | 151 (10) | 91 (5)b |
| Diabetes mellitus, n (%) | 172 (15) | 169 (16)a | 156 (10) | 284 (15)b |
| Race, n (%) | ||||
| White | 1158 (100) | — | 1519 (100) | — |
| Chinese | — | 970 (91.6) | — | 1763 (92.1) |
| Malay | — | 48 (4.5) | — | 91 (4.8) |
| Indian | — | 36 (3.4) | — | 40 (2.1) |
| Others | — | 5 (0.5) | — | 19 (1) |
| ECG variables | ||||
| PR interval, ms | 175±30 | 175±24 | 165±27 | 169±22b |
| QRS duration, ms | 90±17 | 91±15a | 82±14 | 86±13b |
| QT interval, ms | 402±35 | 387±34a | 396±31 | 394±36 |
| QTcFram | 406±22 | 401±23a | 409±21 | 411±26b |
| QTcBaz | 410±24 | 408±26 | 415±24 | 420±28b |
| QRS voltage, mV | 14.2±5.1 | 13.8±6.3 | 11.8±4.6 | 11.4±5.5 |
| LVH, n (%) | 8 (1) | 23 (2)a | 60 (4) | 120 (6)b |
| Left atrial enlargement, n (%) | 42 (4) | 171 (16)a | 37 (2) | 138 (7)b |
| LBBB, n (%) | 16 (1) | 2 (<1) | 27 (2) | 6 (<1)b |
| RBBB, n (%) | 59 (5) | 43 (4) | 33 (2) | 35 (2) |
Data are means±standard deviation unless otherwise noted. ECG indicates electrocardiogram; FHS, Framingham Heart Study; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; RBBB, right bundle branch block; SBP, systolic blood pressure; SLAS, Singapore Longitudinal Aging Study.
P<0.05 vs FHS men.
P<0.05 vs FHS women.
Baseline ECG characteristics are presented by race and sex in Table 1. LAE and LVH were more common among Asians than Whites. Prevalent conduction defects, including LBBB and RBBB, were uncommon. In both race groups, clinical correlates of ECG traits included age, sex, heart rate, body mass index, systolic blood pressure, antihypertensive therapy, diabetes, and smoking.
Differences in ECG Characteristics between Whites and Asians
We examined race differences in continuous and dichotomous ECG traits after accounting for potential confounders, including age, blood pressure, antihypertensive therapy, heart rate, body mass index, smoking status, and diabetes mellitus (Table 2). The PR interval was longer among Asian men and women than among White men and women (β estimate 5.0±1.4 ms and 6.6±0.9 ms, respectively, both P<0.0006). Asian men and women had a longer QRS duration than White men and women respectively (β estimate 2.1±0.8 ms, P=0.01 in men; 4.6±0.5 ms, P<0.0001 for women). QT interval and QTcFram were shorter in Asian men compared with White men (β estimate −6.2±1.2 and −5.7±1.2, respectively, P<0.0001 for both). In contrast, among women, QT interval, QTcFram, and QTcBaz were longer in Asian women than in White women (β estimate 3.6±0.9, 3.4±0.9, and 5.7±1.0 ms, respectively, P<0.0003 for all). There was no difference in QRS voltage between Asians and Whites.
Table 2.
Cohort Differences in ECG Variables
| Variables | Asian Men vs White Men | Asian Women vs White Women | ||
|---|---|---|---|---|
| β Estimate±SE | P Value | β Estimate±SE | P Valuea | |
| PR interval | 4.98±1.43 | 0.0005 | 6.60±0.89 | <0.0001 |
| QRS duration | 2.13±0.83 | 0.01 | 4.56±0.51 | <0.0001 |
| QT interval | −6.21±1.23 | <0.0001 | 3.58±0.91 | <0.0001 |
| QTcFram | −5.65±1.17 | <0.0001 | 3.39±0.90 | 0.0002 |
| QTcBaz | −1.57±1.28 | 0.2 | 5.74±0.97 | <0.0001 |
| QRS voltage | 0.15±0.30 | 0.6 | 0.20±0.18 | 0.3 |
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| LVH | 3.56 (1.36, 9.35) | 0.01 | 1.93 (1.35, 2.76) | 0.0003 |
| Left atrial enlargement | 5.31 (3.51, 8.02) | <0.0001 | 3. 28 (2.20, 4.91) | <0.0001 |
| LBBB | b | 0.001 | b | <0.0001 |
| RBBB | b | 0.2 | b | 0.002 |
Estimated β represents difference of ECG variable in SLAS compared with FHS adjusted for age, systolic blood pressure, hypertension treatment, heart rate (except QTc), body mass index, smoking, and diabetes. CI indicates confidence interval; ECG, electrocardiogram; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; OR, odds ratio; RBBB, right bundle branch block; SE, standard error.
P<0.0125 is considered significant (after Bonferroni correction for 4 variables).
Multivariable analyses were not performed there were very few events; Fisher exact test P value was calculated.
With respect to dichotomous ECG traits, Asian men and women had increased odds of LVH compared with Whites (OR 3.6, 95% CI 1.4–9.4, P=0.01 for men; OR 1.9, 95% CI 1.4–2.8, P=0.0003 for women), and higher odds of LAE (OR 5.3, 95% CI 3.5–8.0 in men and OR 3.3, 95% CI 2.2–4.9 in women, P<0.0001 for both). Multivariable analyses were not performed for LBBB and RBBB due to low prevalence among both races, although LBBB appeared to be more common among Whites than among Asians, with P‐values for Fisher's exact test presented in Table 2.
ECG Characteristics Predict All‐Cause Mortality
Over a mean follow‐up period of 11±3 years in FHS and 8±3 years in SLAS, there were 660 (24.7%) deaths among Whites and 286 (10.3%) deaths among Asians. The cumulative incidence plots of death in men and women adjusted for age are displayed in Figure. Age‐ and sex‐standardized mortality rates were 24.5 per 1000 person‐years (95% CI 22.6–26.4) among Whites and 13.4 per 1000 person‐years (95% CI 11.8–15.0) among Asians.
Figure 1.
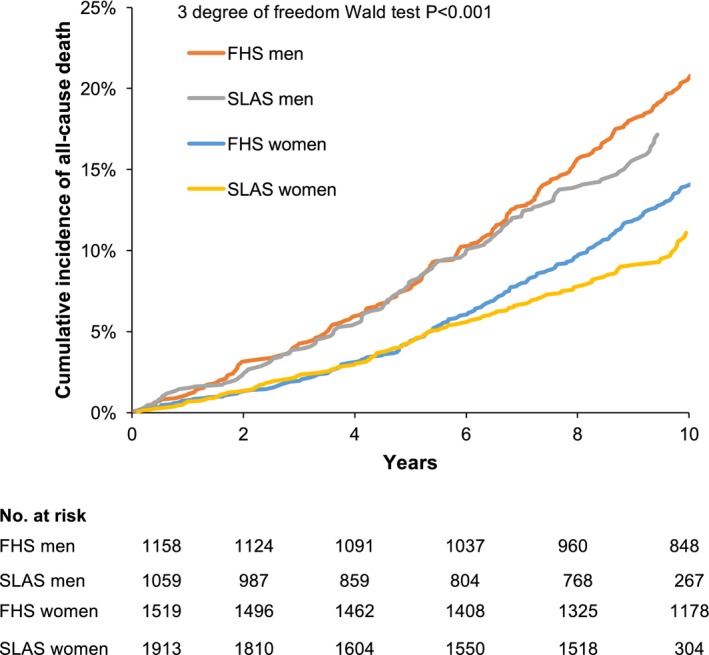
Cumulative incidence of mortality by cohort and sex adjusted for age. SLAS men had the highest incidence of mortality, followed by FHS men, SLAS women, and FHS women.
In the overall sample, a longer QRS duration was associated with all‐cause mortality in multivariable adjusted Cox models (hazard ratio [HR] 1.09 per 1 standard deviation [SD] increase in QRS duration, 95% CI 1.03–1.15, P=0.003, Table 3). Longer QTcFram and QTcBaz were also associated with mortality (P<0.0001 for both), as was higher QRS voltage (HR 1.12, 95% CI 1.05–1.20, P=0.0009). The presence of LVH was associated with a 73% increased mortality (HR 1.73, 95% CI 1.33–2.24, P<0.0001). Similarly, the presence of LAE conferred a higher risk of mortality (HR 1.41, 95% CI 1.12–1.77, P=0.004). We found no significant association of RBBB or LBBB with mortality, although hazard ratio point estimates were greater than 1.
Table 3.
ECG Variables Related to All‐Cause Mortality
| Variables | Age‐, Sex‐ and Cohort‐ Adjusted | Multivariable Adjusteda | ECG×Cohort | ECG×Sex | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | Interaction P | Interaction P | |
| PR interval | 1.03 (0.97, 1.09) | 0.35 | 1.07 (1.01, 1.13) | 0.03 | 0.79 | 0.48 |
| QRS duration | 1.09 (1.04, 1.15) | 0.001 | 1.09 (1.03, 1.15) | 0.003 | 0.49 | 0.67 |
| QT interval | 1.04 (0.97, 1.10) | 0.26 | 1.25 (1.15, 1.36) | <0.0001 | 0.53 | 0.44 |
| QTcFram | 1.15 (1.09, 1.22) | <0.0001 | 1.15 (1.08, 1.22) | <0.0001 | 0.98 | 0.01 |
| QTcBaz | 1.20 (1.13, 1.27) | <0.0001 | 1.18 (1.11, 1.25) | <0.0001 | 0.56 | 0.004 |
| QRS voltage | 1.15 (1.08, 1.23) | <0.0001 | 1.12 (1.05, 1.20) | 0.0009 | 0.045 | 0.28 |
| LVH | 1.93 (1.49, 2.50) | <0.0001 | 1.73 (1.33, 2.24) | <0.0001 | 0.02 | 0.14 |
| Left atrial enlargement | 1.58 (1.26, 1.99) | <0.0001 | 1.41 (1.12, 1.77) | 0.004 | 0.06 | 0.91 |
| LBBB | 1.41 (0.87, 2.28) | 0.16 | 1.35 (0.83, 2.18) | 0.2 | 0.68 | 0.91 |
| RBBB | 1.29 (0.99, 1.68) | 0.06 | 1.21 (0.93, 1.58) | 0.2 | 0.64 | 0.90 |
Hazard ratio is per SD change in continuous variables, and from comparison of the presence of dichotomous variables to their absence. CI indicates confidence interval; ECG, electrocardiogram; HR, hazard ratio; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; RBBB, right bundle branch block.
Adjusted for body mass index, heart rate (except QTc), systolic blood pressure, antihypertensive therapy, diabetes, and smoking.
The Associations of ECG Characteristics with Mortality Are Modified by Race and Sex
We tested for ECG×cohort and ECG×sex interactions (Table 3). Race appeared to be an effect modifier for the association of LVH and mortality, with a suggestive P value for interaction of 0.02. The QRS voltage×race interaction term was borderline significant (P=0.045). In race‐stratified analyses, QRS voltage appeared to predict mortality in Asians but not Whites (HR 1.23, 95% CI 1.12–1.36, P<0.0001 in Asians, and HR 1.06, 95% CI 0.97–1.15, P=0.21 in Whites). Similarly, the presence of LVH predicted mortality in Asians but not Whites (HR 2.66, 95% CI 1.83–3.88, P<0.0001 in Asians, and HR 1.30, 95% CI 0.90–1.89, P=0.16 in Whites; Table 4).
Table 4.
Stratified Analysis of All‐Cause Mortality by Cohort and by Sex
| Age‐ and Sex‐Adjusted | Multivariable Adjusteda | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ECG by cohort | ||||
| FHS | ||||
| QRS voltage | 1.09 (1.003–1.19) | 0.04 | 1.06 (0.97–1.15) | 0.21 |
| LVH | 1.43 (0.99–2.06) | 0.06 | 1.30 (0.90–1.89) | 0.16 |
| SLAS | ||||
| QRS voltage | 1.25 (1.13–1.38) | <0.0001 | 1.23 (1.12–1.36) | <0.0001 |
| LVH | 2.91 (2.02–4.20) | <0.0001 | 2.66 (1.83–3.88) | <0.0001 |
| ECG by sex | ||||
| Men | ||||
| QTcFram | 1.24 (1.15–1.34) | <0.0001 | 1.23 (1.14–1.33) | <0.0001 |
| QTcBaz | 1.30 (1.20–1.41) | <0.0001 | 1.28 (1.18–1.39) | <0.0001 |
| Women | ||||
| QTcFram | 1.07 (0.98–1.16) | 0.15 | 1.07 (0.98–1.16) | 0.14 |
| QTcBaz | 1.10 (1.01–1.20) | 0.04 | 1.10 (1.01–1.20) | 0.03 |
CI indicates confidence interval; ECG, electrocardiogram; HR, hazard ratio; LVH, left ventricular hypertrophy.
Adjusted for age, sex, body mass index, heart rate (except QTc), systolic blood pressure, antihypertensive therapy, diabetes, and smoking.
In the overall population, we also noted that the effect of QTc on mortality appeared to be modified by sex (P for interaction=0.01 for QTcFram and 0.004 for QTcBaz; Table 3). In analyses stratified by sex, QTcBaz appeared to predict mortality in both men and women (P<0.0001 and P=0.03, respectively, Table 4). In contrast, QTcFram predicted all‐cause death only in men (HR 1.23, 95% CI 1.14–1.33, P<0.0001) but not women (HR 1.07, 95% CI 0.98–1.16, P=0.14).
Discussion
We provide the first ECG data from two large prospective longitudinal community‐based studies showing significant differences in ECG characteristics between White and Asian adults with no apparent cardiovascular disease. Specifically, PR interval was longer among Asians than Whites. Asians had about 2‐fold the prevalence of both LAE and LVH than Whites. QT interval was shorter in Asian men but longer in Asian women compared with White men and women, respectively. These ECG differences were not explained by differences in known cardiovascular disease risk factors. Furthermore, the effect of some ECG characteristics on prognosis appeared to be modified by race. Specifically, LVH was associated with a greater increased risk of death in Asians compared with Whites.
Historically, ECG reference limits have been derived from Western White populations. Limited studies23, 24 have described the distribution of ECG traits in Asians and examined their associations with cardiovascular disease. However, there are no comparative data that investigate differences in ECG characteristics and their prognostic significance in Asians versus Whites in population‐based samples. Interestingly, we found divergent race differences among men and women for QT interval, which was shorter in Asian men and longer in Asian women compared with White men and women, respectively. This difference held true for both QTcFram and QTcBaz. Sex differences in QT interval have previously been described,25, 26 and we now show that this sex difference is even more pronounced among Asians. The underlying mechanism of this difference is not well understood but may be related to the effect of sex hormones on myocyte conduction. In an experimental model, inhibition of L‐type calcium current in the cardiac cell by testosterone shortened the action potential in men, contributing to short‐QT interval.27 Several studies have shown that dietary, environmental,28 and genetic polymorphisms29 are associated with differences in the testosterone production and metabolism between Asians and Whites. Furthermore, in a genome‐wide association study on QT interval in a multiethnic population including Asians and Whites, there were 4 single nucleotide polymorphisms (rs10919071, rs2968863, rs2968864, and rs17779747) associated with QT interval that were noted to have significant heterogeneity in effect between Asians and Whites.30 Thus, heterogeneity in genetic determinants may contribute to the difference in QT interval distribution between Asians and Whites observed in our study. Future studies will be needed to explore whether race‐specific reference ranges for QT interval may be appropriate.
Although the QT interval did not differentially predict prognosis among races, we noted sex differences wherein a longer QTc was associated with a worse prognosis in men but not in women. Sex differences in QT interval and prognosis have been noted previously: Nielsen et al studied the risk prediction of cardiovascular disease based on QT interval subgroups in men and women of age groups 50 to 70 years and 70 to 90 years separately.10 He found that in people with very long QT intervals (≥99th percentile), the incidence of cardiovascular disease and all‐cause mortality was higher in men than women. Further, adding QTcFram to a model predicting cardiovascular disease resulted in greater improvements in the c‐statistic in men compared with women between 50 and 70 years of age. Our data are consistent with these prior studies showing that QT interval has a greater prognostic significance in men compared to women.
We found that the proportion of subjects with LVH was higher in Asians (both men and women) than in Whites. Chahal et al31 showed that the prevalence of LVH by echocardiography was higher in UK Indian Asians compared with European Whites. In contrast, another study32 including South Asians and Whites showed that there was no difference in the proportion of ECG‐diagnosed LVH between these two groups after adjustment for body mass index. However, this prior study was limited to hypertensive individuals, potentially explaining the difference with our current findings in community‐based adults. Our findings therefore extend the prior data showing that ECG LVH was more prevalent among Asians than Whites in the general community, a finding independent of racial differences in body size or cardiovascular risk factors.
Furthermore, we found that LVH was more strongly associated with mortality in Asians compared with Whites. This is consistent with ethnic differences in LVH‐related mortality risk reported in African Americans, Whites, and Latinos,33 where ECG‐defined LVH was associated with a higher risk for mortality in African Americans compared with the other two groups after adjustment for cardiovascular risk factors. Intriguingly, the standard Cornell ECG criteria for LVH (which was derived in Whites) may need to be scaled downward in Asians, consistent with the smaller normal heart sizes and correspondingly smaller ECG complexes in Asian compared with White adults.34 Thus, fulfillment of standard Cornell ECG criteria for LVH in Asians may signify greater deviation from normal compared with the same criteria applied in Whites, and this may explain the observed higher mortality risk in Asians than in Whites with standard Cornell‐positive LVH. Further studies are needed to elucidate potential drivers of differences in survival and also to evaluate the potential need for race‐specific ECG reference ranges.
There are several limitations of our study worth noting. We acknowledge that despite adjusting for traditional cardiovascular risk factors, we were unable to adjust for renal function, dietary, socioeconomic, and environmental differences, and cannot rule out residual confounding in the observed associations. Systematic ECG measurement differences between our two cohorts are unlikely given the divergent sex differences observed for a single measurement variable (eg, QT interval) and the demonstrated high reproducibility of measurements. We only report all‐cause mortality; ECG changes may be reasonably expected to relate more closely to cardiovascular than to noncardiovascular deaths. Last, ethnic diversity within the Asian population studied needs to be acknowledged. Approximately 90% of our sample was Chinese, with fewer Malay and Indian participants. Given the diversity in cardiovascular disease incidence and mortality among different South Asian races,35 our result may not be generalizable to all Asians.
Conclusion
In summary, we demonstrate significant differences in ECG characteristics between White and Asian adults with no apparent cardiovascular disease from two large prospective longitudinal community‐based studies. Compared with Whites, Asians had longer PR intervals and about 2‐fold greater odds of LVH. The QT interval was shorter in Asian men but longer in Asian women compared with White men and women, respectively. These ECG differences were not explained by differences in body size or prevalence of known cardiovascular disease risk factors. ECG characteristics exhibited interracial differences in their relationships to prognosis. LVH predicted a greater increased risk of death in Asians than Whites. These differences carry implications for race‐specific ECG reference ranges and prediction of cardiovascular risk.
Sources of Funding
This work was partially supported by the National Heart, Lung, and Blood Institute's Framingham Heart Study (Contract No. N01‐HC‐25195 and HHSN268201500001I). This work was supported by the National Institutes of Health—K23‐HL116780 (Dr Ho). Dr Ho is supported by a Boston University School of Medicine, Department of Medicine Career Investment award (Boston, MA).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002956 doi: 10.1161/JAHA.115.002956)
This article was handled independently by Viola Vaccarino, MD, PhD as a Guest Editor. The editors had no role in the evaluation of this manuscript or in the decision about its acceptance.
References
- 1. Lloyd‐Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel‐Smoller S, Wong N, Wylie‐Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. [DOI] [PubMed] [Google Scholar]
- 2. Xu W, Holmes DN, Becker RC, Roe MT, Peterson ED, Wang TY. Comparison of long‐term outcomes between older Asian and White patients with non‐ST‐segment elevation myocardial infarction: findings from CRUSADE‐CMS database. Am Heart J. 2013;166:1050–1055. [DOI] [PubMed] [Google Scholar]
- 3. Pleis JR, Lethbridge‐Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2005. Vital Health Stat 10. 2007;232:1–153. [PubMed] [Google Scholar]
- 4. Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JAC. Differences in the incidence of congestive heart failure by ethnicity: the Multi‐Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tillin T, Dhutia H, Chambers J, Malik I, Coady E, Mayet J, Wright AR, Kooner J, Shore A, Thom S, Chaturvedi N, Hughes A. South Asian men have different patterns of coronary artery disease when compared with European men. Int J Cardiol. 2007;129:406–413. [DOI] [PubMed] [Google Scholar]
- 6. Gholap NN, Khunti K, Davies MJ, Bodicoat DH, Squire IB. Survival in South Asian and White European patients after acute myocardial infarction. Heart. 2015;101:630–636. [DOI] [PubMed] [Google Scholar]
- 7. Park CM, Tillin T, March K, Ghosh AK, Jones S, Wright A, Heasman J, Francis D, Sattar N, Mayet J, Chaturvedi N, Hughes AD. Hyperglycemia has a greater impact on left ventricle function in South Asians than in Europeans. Diabetes Care. 2013;37:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crawford MH, Bernstein SJ, Deedwania PC, DiMarco JP, Ferrick KJ, Garson A, Green LA, Greene HL, Silka MJ, Stone PH, Tracy CM, Gibbons RJ, Alpert JS, Eagle KA, Gardner TJ, Gregoratos G, Russell RO, Ryan TJ, Smith SC. ACC/AHA guidelines for ambulatory electrocardiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee to revise the guidelines for ambulatory electrocardiography). Circulation. 1999;100:886–893. [DOI] [PubMed] [Google Scholar]
- 9. Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e251–e261. [DOI] [PubMed] [Google Scholar]
- 10. Nielsen JB, Graff C, Rasmussen PV, Pietersen A, Lind B, Olesen MS, Struijk JJ, Haunsø S, Svendsen JH, Køber L, Gerds TA, Holst AG. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur Heart J. 2014;35:1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai CS, Ning H, Lloyd DM. Competing cardiovascular outcomes associated with electrocardiographic left ventricular hypertrophy: the Atherosclerosis Risk in Communities Study. Heart. 2011;98:330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alonso A, Chen LY. PR interval, P‐wave duration, and mortality: new insights, additional questions. Heart Rhythm. 2014;11:99–100. [DOI] [PubMed] [Google Scholar]
- 13. Wong RC, Yip JW, Gupta A, Yang H, Ling LH. Echocardiographic left ventricular mass in a multiethnic Southeast Asian population: proposed new gender and age‐specific norms. Echocardiography. 2008;25:805–811. [DOI] [PubMed] [Google Scholar]
- 14. Tan K. Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 15. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 16. Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 18. Niti M, Yap KB, Kua EH, Tan CH, Ng TP. Physical, social and productive leisure activities, cognitive decline and interaction with APOE‐epsilon 4 genotype in Chinese older adults. Int Psychogeriatr. 2008;20:237–251. [DOI] [PubMed] [Google Scholar]
- 19. Casale PN, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. [DOI] [PubMed] [Google Scholar]
- 20. Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol. 1992;70:797–801. [DOI] [PubMed] [Google Scholar]
- 21. Bazett HC. An analysis of the time‐relations of electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2:177–194. [Google Scholar]
- 22. Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. [DOI] [PubMed] [Google Scholar]
- 23. Chen CY, Chiang BN, Macfarlane PW. Normal limits of the electrocardiogram in a Chinese population. J Electrocardiol. 1989;22:1–15. [DOI] [PubMed] [Google Scholar]
- 24. Wu J, Kors JA, Rijnbeek PR, van Herpen G, Lu Z. Normal limits of the electrocardiogram in Chinese subjects. Int J Cardiol. 2003;87:37–51. [DOI] [PubMed] [Google Scholar]
- 25. Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–695. [PubMed] [Google Scholar]
- 26. Vicente J, Johannesen L, Galeotti L, Strauss DG. Mechanisms of sex and age differences in ventricular repolarization in humans. Am Heart J. 2014;168:749–756. [DOI] [PubMed] [Google Scholar]
- 27. Bai C, Kurokawa J, Tamagawa M, Nakaya H, Furukawa T. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112:1701–1710. [DOI] [PubMed] [Google Scholar]
- 28. Santner SJ, Albertson B, Zhang GY, Zhang GH, Santulli M, Wang C, Demers LM, Shackleton C, Santen RJ. Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. J Clin Endocrinol Metab. 1998;83:2104–2109. [DOI] [PubMed] [Google Scholar]
- 29. Tang YM, Green BL, Chen GF, Thompson PA, Lang NP, Shinde A, Lin DX, Tan W, Lyn‐Cook BD, Hammons GJ, Kadlubar FF. Human CYP1B1 Leu432Val gene polymorphism: ethnic distribution in African‐Americans, Caucasians and Chinese; oestradiol hydroxylase activity; and distribution in prostate cancer cases and controls. Pharmacogenetics. 2001;10:761–766. [DOI] [PubMed] [Google Scholar]
- 30. Seyerle AA, Young AM, Jeff JM, Melton PE, Jorgensen NW, Lin Y, Carty CL, Deelman E, Heckbert SR, Hindorff LA, Jackson RD, Martin LW, Okin PM, Perez MV, Psaty BM, Soliman EZ, Whitsel EA, North KE, Laston S, Kooperberg C, Avery CL. Evidence of heterogeneity by race/ethnicity in genetic determinants of QT interval. Epidemiology. 2014;25:790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. The increased prevalence of left ventricular hypertrophy and concentric remodeling in U.K. Indian Asians compared with European Whites. J Hum Hypertens. 2012;27:288–293. [DOI] [PubMed] [Google Scholar]
- 32. Spencer CGC, Beevers DG, Lip GYH. Ethnic differences in left ventricular size and the prevalence of left ventricular hypertrophy among hypertensive patients vary with electrocardiographic criteria. J Hum Hypertens. 2004;18:631–636. [DOI] [PubMed] [Google Scholar]
- 33. Havranek EP, Froshaug DB, Emserman CDB, Hanratty R, Krantz MJ, Masoudi FA, Dickinson LM, Steiner JF. Left ventricular hypertrophy and cardiovascular mortality by race and ethnicity. Am J Med. 2008;121:870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu CF, Tan ES, Feng L, Santhanakrishnan R, Chan MM, Nyunt SZ, Ng TP, Ling LH, Richards AM, Lam CS, Lim TW. Electrocardiographic criteria for left ventricular hypertrophy in Asians differs from criteria derived from Western populations—community‐based data from an Asian population. Ann Acad Med Singapore. 2015;44:274–283. [PubMed] [Google Scholar]
- 35. Palaniappan LP, Araneta MR, Assimes TL, Barrett‐Connor EL, Carnethon MR, Criqui MH, Fung GL, Narayan KM, Patel H, Taylor‐Piliae RE, Wilson PW, Wong ND. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122:1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]


