Abstract
Background
We compared the incidence of depression, defined by a Geriatric Depression Score (GDS) ≥6, between people with versus without peripheral artery disease (PAD). We determined whether depressive symptoms were associated with increased mortality in people with and without PAD.
Methods and Results
Nine hundred and fifty‐one PAD patients and 478 non‐PAD patients were recruited from Chicago medical centers and followed prospectively. At baseline and annually, participants completed the GDS (0–15 scale, score ≥6=depression) and 6‐minute walk. Cause of death was confirmed with death certificates. The prevalence of a GDS ≥6 at baseline was 186/951 (19.6%) among PAD versus 63/478 (13.2%) among non‐PAD participants (P=0.003). During a mean follow‐up of 2.7±1.2 years, 122/712 (17.1%) of participants with PAD versus 51/403 (12.7%) without PAD developed a GDS ≥6 (P=0.047). Adjusting for age, sex, race, comorbidities, and other confounders, PAD participants had an increased rate of developing a GDS ≥6 compared to non‐PAD participants (hazard ratio=1.54 (95% CI=1.05–2.25, P=0.026). This association was not statistically significant after adjusting for 6‐minute walk (P=0.258). Among PAD participants, a baseline GDS ≥6 was associated with increased all‐cause mortality, adjusting for confounders (hazard ratio=1.57, 95% CI=1.12–2.21, P=0.009). This association was not significant after adjusting for 6‐minute walk (P=0.224).
Conclusions
People with PAD have a higher incidence of depressive symptoms than people without PAD. In PAD, depressive symptoms are associated with increased all‐cause and cardiovascular mortality. These associations are explained in part by poorer 6‐minute walk among people with PAD and among depressed people with PAD, respectively.
Keywords: atherosclerosis, cardiovascular disease, peripheral vascular disease
Subject Categories: Peripheral Vascular Disease, Mental Health, Epidemiology, Clinical Studies
Introduction
The incidence and prognostic significance of depression in people with lower extremity peripheral artery disease (PAD) are unclear. Cross‐sectional studies report a higher prevalence of depression or depressive symptoms among people with PAD compared to those without PAD.1, 2, 3 However, to our knowledge, no prospective studies have compared the incidence of depression or new depressive symptoms among people with PAD compared to those without PAD. Among patients with coronary artery disease, individuals with depression have higher morbidity and mortality rates than people without depression.4, 5 One prior study reported that among symptomatic patients undergoing lower extremity revascularization, those with depression had a higher risk of the combined outcome of death or major adverse cardiovascular events than those without depression.6 However, the association of depression with mortality among PAD patients, including those not planning revascularization, has not been studied previously to our knowledge.
To determine whether patients with PAD have an increased incidence of depressive symptoms compared to people without PAD, we compared rates of new onset of depressive symptoms over time between people with versus without PAD. We hypothesized that people with PAD would have a higher incidence of depressive symptoms compared to people without PAD. To determine whether depressive symptoms are associated with increased all‐cause and cardiovascular disease (CVD) mortality in people with PAD, we compared mortality rates between people with versus without depressive symptoms. We hypothesized that among people with PAD, those with depressive symptoms would have a higher mortality rate than those without depressive symptoms.
Methods
Study Overview
We combined data from 3 prospective observational studies of patients with PAD: The Walking and Leg Circulation Study (WALCS), WALCS II, and WALCS III cohorts.7, 8, 9, 10 WALCS was conducted between 1998 and 2002,7, 8 WALCS II was conducted between 2002 and 2006, and WALCS III between 2005 and 2014.9, 10 In all 3 studies, PAD participants were recruited from among consecutively identified PAD patients at Chicago‐area medical centers and followed longitudinally. The institutional review boards of Northwestern University and all participating medical centers approved the protocol for the 3 studies. All participants gave written informed consent. In each study, participants completed baseline testing and returned annually for follow‐up for up to 4 years. In the WALCS cohort, all participants were age 55 and older at baseline. In WALCS II, all participants were age 59 and older at baseline. In WALCS III, there were no age restrictions.
Participant Identification
Similar recruitment methods were used for all 3 studies.7, 8, 9, 10 In all 3 cohorts, PAD participants were identified from among consecutive patients with PAD in Chicago‐area vascular surgery and noninvasive vascular laboratories.7, 8, 9, 10 Participants were also identified from among lists of consecutive PAD patients in cardiology, general medicine, endocrinology, and geriatric clinics at Northwestern. In the WALCS and WALCS II cohorts, participants without PAD were identified from among consecutive patients in a general internal medicine practice who were screened with the ankle–brachial index (ABI) and found to have an ABI of 0.90 to 1.50. In the WALCS III cohort, participants without PAD were identified (from among consecutive patients age 65 and older in Northwestern's general internal medicine practice) who had no history of smoking, diabetes mellitus, or established cardiovascular disease, including PAD. In addition, participants without PAD in WALCS I and WALCS II were identified (from among consecutive patients in a noninvasive vascular laboratory without PAD) who had an ABI of 0.90 to 1.50 at their baseline study visit.
Inclusion Criterion
All PAD participants in these analyses had a baseline ABI value of <0.90.11 All non‐PAD participants included in these analyses had a baseline ABI of 0.90 to 1.30.11
Exclusion Criteria
In all 3 studies, patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents were excluded because they had severely impaired functioning at baseline. Non‐English‐speaking patients were excluded because investigators were not fluent in non‐English languages. Patients with recent major surgery were excluded because the surgery may have influenced their walking speed, sitting down time, or lying down time. Potential participants who were wheelchair bound or who had a history of leg or foot amputations were excluded because of their severe functional impairment at baseline. In the WALCS III cohort, participants with contraindications to magnetic resonance imaging testing were excluded. Participants with critical limb ischemia were not included in any cohorts.
ABI Measurement
A hand‐held Doppler probe (Nicolet Vascular Pocket Dop II; Nicolet Biomedical Inc, Golden, CO) was used to measure systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries.11, 12 Each pressure was measured twice. For each leg, the ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures by the mean of the 4 brachial pressures.13 Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets and the 2 brachial pressures differed by 10 mm Hg or more in at least 1 measurement set. In these cases, subclavian stenosis was possible.13 The leg with lowest ABI was used in analyses.
Depressive Symptoms
Depressive symptoms were measured at baseline and at annual follow‐up visits with the Geriatric Depression Scale Short Form (GDS‐S).14, 15, 16, 17 The GDS‐S is a well‐validated 15‐item questionnaire, derived from the Center for Epidemiological Studies Depression scale, which measures the number of depressive symptoms. Scores range from 0 to 15 (15=worst score).14, 15, 16, 17, 18 A GDS‐S score ≥6 is 92% sensitive and 81% specific for clinical depression, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM‐IIIR).14 “The GDS‐S improves in response to a therapeutic intervention and changes in the GDS‐S are associated with temporally corresponding changes in the ability to perform Activities of Daily Living and Instrumental Activities of Daily Living.19, 20”
Six‐Minute Walk
The 6‐minute walk test was performed at baseline using a standardized and well‐validated protocol.21, 22 Participants walked up and down a 100‐foot hallway for 6 minutes after instructions to cover as much distance as possible.
Comorbidities
Comorbidities assessed at baseline were diabetes, angina, myocardial infarction, heart failure, cancer, chronic lung disease, and stroke. Disease‐specific algorithms that combine data from patient report, medical record review, medications, laboratory values, and a questionnaire completed by the participant's primary care physician were used to verify and document baseline comorbidities.23
Medications
Participants were asked to bring their medication bottles or a complete list of their medications to their baseline study visit. Names of each medication were recorded. The study principal investigator (M.M.M.) reviewed each medication, blinded to other characteristics, and indicated whether the medication was a statin, angiotensin‐converting enzyme inhibitor, or antiplatelet therapy.
Income
We used participants’ zip code linked to US census data to categorize participants according to the median annual household income in their zip code.24
Education Level
Participants were asked their highest level of education achieved, using a questionnaire administered by trained and certified interviewers.
Mortality Assessment
At baseline, participants provided names of 3 proxies to assist with ascertaining complete follow‐up. Mortality information was obtained from family members, proxies, and primary care physicians. For patients lost to follow‐up, we used the Social Security Administration death database to search for deaths. Death certificates were obtained from the State of Illinois or from medical records. Cardiovascular disease deaths consisted of deaths due to coronary heart disease, stroke, peripheral vascular disease, and other cardiovascular disease. The date of death was obtained from the death certificate. Follow‐up for participants who were not deceased continued until the date of last contact at a study visit or by telephone.
Statistical Analyses
Two‐sample t tests and χ2 tests were used to compare continuous and binary baseline clinical characteristics, respectively, according to the presence versus absence of PAD and according to the presence versus absence of depression at baseline. Two‐sample t tests and χ2 analyses were also used to compare baseline characteristics of participants without depression at baseline who developed depression during follow‐up versus those who did not develop depression during follow‐up among PAD and non‐PAD subgroups separately. We used Kaplan–Meier curves and log‐rank analyses to compare cumulative probabilities of depression among participants with versus without PAD at baseline. Among participants with and without PAD at baseline, we used Kaplan–Meier curves and log‐rank analyses to compare cumulative probabilities of all‐cause mortality between participants with versus without depression at baseline.
In our primary analyses, Cox regression models were used to establish the association of PAD with development of depression during follow‐up, stratifying by study cohort (WALCS I, WALCS II, or WALCS III), and adjusting for age, sex, race, body mass index (BMI), smoking, comorbidities, income, and education. In secondary analyses, we repeated analyses with additional adjustment for baseline 6‐minute walk. We imputed income and/or education for 22 participants missing data on income or education, using the cohort median for imputation.
Cox regression models were used to evaluate the association of baseline characteristics with development of depression during follow‐up among participants with and without PAD, stratifying by study cohort, and adjusting for age, sex, race, 6‐minute walk, BMI, smoking, comorbidities, ABI, income, and education.
In our primary analyses, Cox regression models were used to establish the association of depression at baseline with all‐cause mortality and with cardiovascular disease mortality among participants with and without PAD, respectively, stratifying by study cohort, and adjusting for age, sex, race, BMI, smoking, comorbidities, medications, income, education level, and ABI. In secondary analyses, we repeated analyses with additional adjustment for baseline 6‐minute walk. We tested for an interaction of study cohort with the associations in our primary analyses.
In post‐hoc, exploratory analyses, we analyzed whether greater decline in the 6‐minute walk and whether greater declines in the ABI were each associated with a higher incidence of subsequent depression in participants with and without PAD, respectively, using Cox proportional hazards analyses. In these analyses we stratified participants with and without PAD according to their degree of decline in the 6‐minute walk and their decline in the ABI during the first 2 years of follow‐up and related each independent variable of interest to the subsequent incidence of depression, adjusting for confounders.
Analyses were performed using SAS statistical software (version 9.4, SAS Institute Inc, Cary, NC).
Results
Among 1074 individual participants with PAD enrolled in the WALCS I, WALCS II, and WALCS III cohorts, 29 were lost to follow‐up, 67 did not complete the GDS‐S form at baseline, and 27 were missing covariate data required for analyses (Figure 1). Among 532 individual participants without PAD, 16 were lost to follow‐up, 26 did not complete the GDS‐S form at baseline, and 12 were missing covariate data. The remaining 951 participants with PAD and 478 without PAD were included in analyses.
Figure 1.
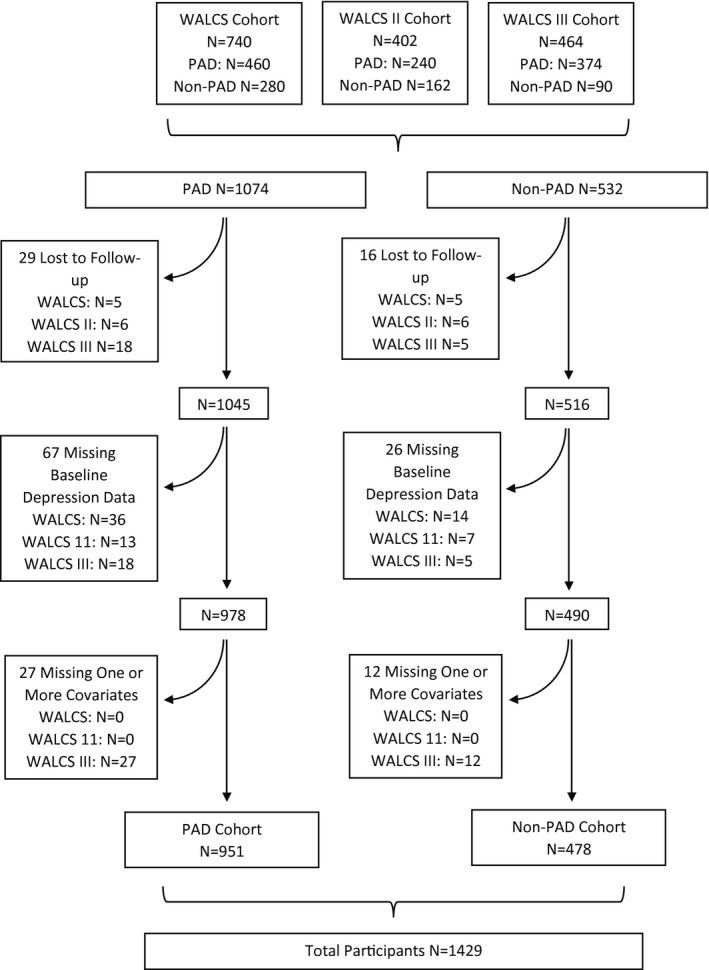
Summary of included participants from the Walking and Leg Circulation Study (WALCS), WALCS II, and WALCS III cohorts. PAD indicates peripheral artery disease.
Characteristics of Participants at Baseline
Overall, participants with PAD were older and had lower BMI and ABI values compared to participants without PAD (Table 1). Participants with PAD included a higher proportion of men and had higher prevalences of current smoking, diabetes, angina, heart failure, prior myocardial infarction, and stroke, compared to people without PAD (Table 1). Participants with PAD had poorer 6‐minute walk performance at baseline and a lower baseline prevalence of spinal stenosis compared to people without PAD. Participants with PAD had higher prevalences of using statins, anti‐platelet therapy, or angiotensin‐converting enzyme inhibitors than those without PAD.
Table 1.
Participant Characteristics According to Presence Versus Absence of PAD and Depression
| Group | PAD | People Without PAD | P Value c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All PAD Participants (N=951) | With Depression at Baseline (N=186) | Without Depression at Baseline (N=765) | P Valuea | All Participants Without PAD (N=478) | With Depression at Baseline (N=63) | Without Depression at Baseline (N=415) | P Valueb | ||
| Age, y | 71.29 (9.14) | 68.62 (9.56) | 71.94 (8.93) | <0.001 | 69.70 (7.71) | 70.25 (8.79) | 69.61 (7.54) | 0.539 | 0.001 |
| Body mass index, kg/m2 | 28.21 (5.40) | 28.67 (6.09) | 28.10 (5.21) | 0.201 | 29.11 (6.20) | 30.00 (7.13) | 28.97 (6.04) | 0.221 | 0.005 |
| Ankle–brachial index | 0.64 (0.15) | 0.64 (0.16) | 0.65 (0.15) | 0.704 | 1.08 (0.11) | 1.06 (0.12) | 1.08 (0.10) | 0.152 | <0.001 |
| Men (%) | 61.1, N=581 | 62.4, N=116 | 60.8, N=465 | 0.692 | 46.2, N=221 | 52.4, N=33 | 45.3, N=188 | 0.294 | <0.001 |
| African American (%) | 22.9, N=218 | 28.5, N=53 | 21.6, N=165 | 0.044 | 20.1, N=96 | 25.4, N=16 | 19.3, N=80 | 0.259 | 0.221 |
| Current smoker (%) | 21.1, N=201 | 33.9, N=63 | 18.0, N=138 | <0.001 | 7.9, N=38 | 15.9, N=10 | 6.7, N=28 | 0.013 | <0.001 |
| Diabetes (%) | 34.3, N=326 | 41.9, N=78 | 32.4, N=248 | 0.014 | 20.9, N=100 | 33.3, N=21 | 19.0, N=79 | 0.009 | <0.001 |
| Pulmonary disease (%) | 36.5, N=347 | 50.0, N=93 | 33.2, N=254 | <0.001 | 32.4, N=155 | 42.9, N=27 | 30.8, N=128 | 0.058 | 0.129 |
| Cancer (%) | 16.8, N=16 | 10.8, N=20 | 18.3, N=140 | 0.014 | 17.8, N=85 | 25.4, N=16 | 16.6, N=69 | 0.09 | 0.65 |
| Angina (%) | 29.0, N=276 | 32.3, N=60 | 28.2, N=216 | 0.278 | 19.7, N=94 | 36.5, N=23 | 17.1, N=71 | <0.001 | <0.001 |
| Myocardial infarction (%) | 23.4, N=223 | 26.9, N=50 | 22.6, N=173 | 0.218 | 13.4, N=64 | 17.5, N=11 | 12.8, N=53 | 0.308 | <0.001 |
| Stroke (%) | 15.2, N=145 | 23.1, N=43 | 13.3, N=102 | <0.001 | 5.6, N=27 | 11.1, N=7 | 4.8, N=20 | 0.07 | <0.001 |
| Heart failure (%) | 19.8, N=188 | 31.2, N=58 | 17.0, N=130 | <0.001 | 12.1, N=58 | 20.6, N=13 | 10.8, N=45 | 0.027 | <0.001 |
| Hip arthritis (%) | 3.2, N=30 | 8.6, N=16 | 1.8, N=14 | <0.001 | 2.5, N=12 | 1.6, N=1 | 2.7, N=11 | 1 | 0.496 |
| Knee arthritis (%) | 9.8, N=93 | 12.4, N=23 | 9.2, N=70 | 0.185 | 12.3, N=59 | 20.6, N=13 | 11.1, N=46 | 0.032 | 0.138 |
| Disk disease (%) | 31.3, N=298 | 39.8, N=74 | 29.3, N=224 | 0.006 | 31.4, N=150 | 33.3, N=21 | 31.1, N=129 | 0.72 | 0.986 |
| Spinal stenosis (%) | 10.1, N=96 | 14.5, N=27 | 9.0, N=69 | 0.026 | 34.1, N=163 | 42.9, N=27 | 32.8, N=136 | 0.116 | <0.001 |
| Six‐minute walk (feet) | 1127.66 (388.38) | 918.11 (389.38) | 1178.61 (370.89) | <0.001 | 1422.27 (399.67) | 1237.76 (465.44) | 1450.28 (381.62) | <0.001 | <0.001 |
| Median annual household income (US dollars) | 53 186 (20 496) | 50 283 (22 019) | 53 892 (20 059) | 0.031 | 54 871 (21 362) | 52 775 (17 306) | 55 189 (21 913) | 0.404 | 0.149 |
| Less than high school (%) | 10.94, N=104 | 17.20, N=32 | 9.41, N=72 | 0.005 | 9.83, N=47 | 20.63, N=13 | 8.19, N=34 | 0.008 | 0.013 |
| High school to college (%) | 69.93, N=665 | 67.74, N=126 | 70.46, N=539 | 64.23, N=307 | 55.56, N=35 | 65.54, N=272 | |||
| Graduate school (%) | 19.14, N=182 | 15.05, N=28 | 20.13, N=154 | 25.94, N=124 | 23.81, N=15 | 26.27, N=109 | |||
| Medication use | |||||||||
| Statin use (%) | 54.9, N=522 | 54.8, N=102 | 54.9, N=420 | 0.988 | 32.8, N=157 | 33.3, N=21 | 32.8, N=136 | 0.929 | <0.001 |
| ACE inhibitor use (%) | 33.9, N=322 | 34.4, N=64 | 33.7, N=258 | 0.860 | 23.6, N=113 | 27.0, N=17 | 23.1, N=96 | 0.503 | <0.001 |
| Anti‐platelet use (%) | 64.8, N=616 | 64.0, N=119 | 65.0, N=497 | 0.800 | 42.5, N=203 | 44.4, N=28 | 42.2, N=175 | 0.733 | <0.001 |
Values shown are means (SD). ACE indicates angiotensin‐converting enzyme; PAD, peripheral artery disease.
P value compares characteristics between participants with depression and those without depression among participants with PAD.
P value compares characteristics between participants with depression and those without depression among participants without PAD.
P value compared characteristics between participants with peripheral artery disease and those without. Depression at baseline was defined based on a Geriatric Depression Scale score ≥6 at baseline.
At baseline, 186/951 (19.6%) of participants with PAD met criteria for depression versus 63/478 (13.2%) of those without PAD (P=0.003). Table 1 shows characteristics associated with presence versus absence of PAD and presence versus absence of depression at baseline. Among participants with PAD, those depressed at baseline were younger, included a higher prevalence of current smokers, and included higher prevalences of African Americans and people with pulmonary disease, diabetes, history of stroke, heart failure, hip arthritis, disk disease, and spinal stenosis (Table 1). PAD participants with depression at baseline had poorer 6‐minute walk performance, a lower prevalence of cancer, achieved less education, and lived in a zip code with lower median income than PAD participants without depression at baseline (Table 1). Participants without PAD who were depressed at baseline had higher prevalences of current smoking, diabetes, angina, and heart failure compared to participants without PAD who were not depressed. Participants without PAD who were depressed at baseline had poorer 6‐minute walk performance and had achieved lower levels of education than those not depressed at baseline (Table 1).
Incidence of Depression During Follow‐Up in People With Versus Without PAD
Among all participants without depression at baseline, the incidence of depression, measured by newly developing a GDS ≥6 after baseline, was 122/712 (17.1%) among participants with PAD and 51/403 (12.7%) among participants without PAD during a mean follow‐up of 2.7±1.2 years. The cumulative probability of developing new depression during follow‐up between participants with versus without PAD at baseline is shown in Figure 2 (log‐rank test P=0.0026). There was no association of PAD severity with incidence of depression (ABI <0.50: 21/125 [16.8%], ABI 0.50 to <0.90: 101/587 [17.2%]; ABI 0.90–1.30: 51/403 [12.7%], P=0.139). Among women, the incidence of depression was 53/279 (19.0%) among participants with PAD versus 29/221 (13.1%) among those without PAD. Among men, the incidence of PAD was 69/433 (15.9%) among those with PAD versus 22/182 (12.1%) among those without PAD. Among participants age >75, the incidence of depression was 47/254 (18.5%) among participants with PAD and 13/91 (14.3%) among participants without PAD. Among participants age ≤75 years, the incidence of depression was 75/458 (16.4%) among those with PAD and 38/312 (12.2%) among those without PAD. Therefore, the higher incidence of depression among people with PAD compared to those without PAD was consistent among men and women and in older and younger participants. There was no interaction of study cohort with the analyses assessed in our primary analyses (data not shown).
Figure 2.

Association of baseline peripheral artery disease status with new onset of depressive symptoms among people without depressive symptoms at baseline.
In analyses stratifying by study cohort, and adjusting for age, sex, race, BMI, smoking, comorbidities, education, and income, PAD was associated with a higher risk of developing new depression compared to people without PAD (hazard ratio [HR]=1.54, 95% CI=1.05–2.25, P=0.026). However, this association was no longer statistically significant when the analyses were additionally adjusted for baseline 6‐minute walk performance (HR=1.26, 95% CI=0.85–1.86, P=0.258).
Among PAD participants, current smoking, heart failure, knee arthritis, and 6‐minute walk were each associated independently with risk of developing depression during follow‐up (Table 2), in stratified analyses adjusting for age, sex race, BMI, comorbidities, ABI, income, and education level. In a similar multivariable analysis among participants without PAD, only 6‐minute walk performance was associated with developing depression during follow‐up (Table 2).
Table 2.
Multivariable Analyses of Baseline Characteristics Associated With Development of Depression During Follow‐Up Among Participants With and Without PAD
| Participants With PAD (N=712) | Participants Without PAD (N=403) | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age, y | 1.01 (0.98–1.03) | 0.638 | 0.98 (0.94–1.02) | 0.320 |
| Male sex | 0.87 (0.59–1.30) | 0.502 | 1.10 (0.58–2.09) | 0.777 |
| African American race | 0.66 (0.38–1.14) | 0.136 | 0.56 (0.23–1.34) | 0.190 |
| Six‐minute walk (feet) baseline (hazard ratio calculated per 100 feet) | 0.94 (0.89–1.00) | 0.047 | 0.86 (0.79–0.93) | <0.001 |
| Body mass index, kg/m2 | 0.98 (0.95–1.02) | 0.413 | 1.02 (0.97–1.07) | 0.458 |
| Current smoker | 1.94 (1.21–3.12) | 0.006 | 1.12 (0.26–4.82) | 0.879 |
| Diabetes | 1.12 (0.74–1.71) | 0.581 | 1.36 (0.62–2.96) | 0.441 |
| Pulmonary disease | 1.05 (0.71–1.55) | 0.817 | 1.29 (0.69–2.43) | 0.426 |
| Cancer | 1.45 (0.91–2.31) | 0.119 | 0.82 (0.33–2.07) | 0.678 |
| Angina | 1.28 (0.82–1.98) | 0.273 | 1.55 (0.66–3.66) | 0.318 |
| Myocardial infarction | 1.05 (0.67–1.67) | 0.822 | 1.14 (0.45–2.90) | 0.782 |
| Stroke | 1.00 (0.58–1.72) | 0.994 | 0.70 (0.16–3.11) | 0.637 |
| Heart failure | 1.66 (1.04–2.66) | 0.034 | 0.97 (0.41–2.30) | 0.946 |
| Hip arthritis | 1.31 (0.44–3.91) | 0.623 | 2.12 (0.54–8.39) | 0.284 |
| Knee arthritis | 2.26 (1.35–3.77) | 0.002 | 1.80 (0.79–4.14) | 0.163 |
| Disk disease | 1.09 (0.72–1.65) | 0.673 | 0.89 (0.48–1.68) | 0.724 |
| Spinal stenosis | 1.46 (0.82–2.59) | 0.194 | 1.42 (0.77–2.63) | 0.258 |
| Ankle brachial index | 1.62 (0.42–6.24) | 0.483 | 1.17 (0.06–24.31) | 0.917 |
| Median annual household income ≤$51 249 vs median annual household income >$51 249 | 0.88 (0.59–1.31) | 0.530 | 0.50 (0.26–0.99) | 0.046 |
| Less than high school vs graduate school | 1.81 (0.90–3.65) | 0.096 | 1.48 (0.49–4.53) | 0.489 |
| High school to college vs graduate school | 1.17 (0.72–1.91) | 0.535 | 0.86 (0.42–1.78) | 0.691 |
PAD indicates peripheral artery disease.
PAD participants with depression at baseline had higher all‐cause mortality rates during follow‐up compared to PAD participants without depression at baseline (54/186 [29.0%] versus 153/765 [20.0%]). The cumulative probability of all‐cause mortality between PAD participants with versus without depression at baseline is shown in Figure 3A (log‐rank test P=0.005). In multivariable analyses, depression at baseline was associated with higher all‐cause mortality, stratifying by study cohort, and adjusting for age, sex, race, comorbidities, ABI, smoking, BMI, medication use, income, and education level (HR=1.57, 95% CI=1.12–2.21, P=0.009) among people with PAD (Table 3). However, this association was not statistically significant after additional adjustment for 6‐minute walk (HR=1.25, 95% CI=0.87–1.79, P=0.224). In multivariable analyses, depression at baseline was associated with higher cardiovascular disease mortality, stratifying by study cohort, and adjusting for age, sex, race, comorbidities, ABI, smoking, BMI, medication use, income, and education (HR=1.80, 95% CI=1.03–3.13, P=0.038) (Table 3) among people with PAD. However, this association was not statistically significant after additional adjustment for 6‐minute walk (HR=1.38, 95% CI=0.77–2.46, P=0.277) (Table 3).
Figure 3.

A, Association of depressive symptoms with all‐cause mortality in participants with peripheral artery disease (N=951); (B) Association of depressive symptoms with all‐cause mortality in participants without peripheral artery disease (N=478).
Table 3.
Adjusted Associations of Depression at Baseline With All‐Cause and Cardiovascular Disease Mortality Among Participants With PAD (N=951)
| All‐Cause Mortality | Cardiovascular Disease Mortality | |||
|---|---|---|---|---|
| Adjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio With Adjustment for 6‐Minute Walk (95% CI) | Adjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio With Adjustment for 6‐Minute Walk (95% CI) | |
| Depressed at baseline | 1.57 (1.12–2.21) | 1.25 (0.87–1.79) | 1.80 (1.03–3.13) | 1.38 (0.77–2.46) |
| Age, y | 1.04 (1.02–1.06) | 1.03 (1.01–1.05) | 1.06 (1.03–1.09) | 1.05 (1.01–1.08) |
| Male sex | 1.52 (1.12–2.07) | 1.73 (1.27–2.37) | 1.51 (0.90–2.54) | 1.85 (1.08–3.16) |
| African American race | 0.85 (0.58–1.25) | 0.79 (0.53–1.17) | 0.99 (0.52–1.88) | 0.91 (0.48–1.74) |
| Body mass index, kg/m2 | 0.96 (0.93–0.99) | 0.95 (0.92–0.98) | 0.97 (0.92–1.02) | 0.95 (0.90–1.01) |
| Current smoker | 1.00 (0.68–1.47) | 0.96 (0.65–1.41) | 1.35 (0.72–2.55) | 1.29 (0.68–2.44) |
| Diabetes | 1.24 (0.91–1.69) | 1.17 (0.86–1.60) | 1.28 (0.77–2.14) | 1.16 (0.69–1.94) |
| Pulmonary disease | 1.20 (0.90–1.62) | 1.09 (0.81–1.48) | 1.09 (0.66–1.79) | 0.97 (0.58–1.62) |
| Cancer | 1.78 (1.28–2.48) | 1.73 (1.24–2.42) | 0.77 (0.39–1.55) | 0.72 (0.36–1.46) |
| Angina | 1.14 (0.82–1.59) | 1.11 (0.80–1.55) | 1.74 (1.02–2.96) | 1.71 (1.00–2.93) |
| Myocardial infarction | 0.91 (0.64–1.29) | 0.90 (0.64–1.28) | 0.84 (0.47–1.52) | 0.84 (0.47–1.51) |
| Stroke | 1.16 (0.80–1.69) | 1.02 (0.70–1.49) | 1.28 (0.68–2.40) | 1.06 (0.55–2.02) |
| Heart failure | 1.63 (1.16–2.29) | 1.52 (1.08–2.14) | 1.59 (0.91–2.77) | 1.39 (0.79–2.45) |
| Ankle–brachial index | 0.58 (0.22–1.51) | 0.95 (0.36–2.49) | 0.58 (0.12–2.69) | 1.05 (0.22–5.00) |
| Statin use | 0.80 (0.59–1.10) | 0.82 (0.60–1.12) | 0.74 (0.44–1.27) | 0.76 (0.44–1.30) |
| ACE inhibitor use | 1.23 (0.90–1.67) | 1.25 (0.92–1.71) | 1.48 (0.88–2.50) | 1.54 (0.92–2.58) |
| Anti‐platelet use | 0.83 (0.61–1.14) | 0.88 (0.64–1.21) | 0.60 (0.36–1.01) | 0.63 (0.37–1.07) |
| Median annual household income ≤$51 249 vs median annual household income >$51 249 | 1.18 (0.87–1.60) | 1.16 (0.85–1.58) | 1.07 (0.64–1.81) | 1.04 (0.61–1.76) |
| Less than high school vs graduate school | 1.25 (0.73–2.14) | 1.15 (0.67–1.98) | 1.28 (0.55–2.98) | 1.14 (0.49–2.67) |
| High school to college vs graduate school | 1.20 (0.83–1.74) | 1.20 (0.83–1.74) | 1.07 (0.58–2.00) | 1.04 (0.56–1.95) |
| Six‐minute walk (feet) baseline (HR calculated at unit=100 feet) | NA | 0.91 (0.87–0.95) | NA | 0.89 (0.83–0.96) |
ACE indicates angiotensin‐converting enzyme; HR, hazard ratio; NA, not available; PAD, peripheral artery disease.
Among participants without PAD, there was no difference in the cumulative probability of all‐cause mortality among those with versus without depression at baseline (Figure 3B). Among participants without PAD, there was no difference in all‐cause or cardiovascular disease mortality among those depressed at baseline compared to those not depressed at baseline, stratifying by study cohort, and adjusting for age, sex, race, comorbidities, ABI, smoking, BMI, education level, income, and medications (HRs=1.32 [95% CI 0.57–3.06] and 0.85 [95% CI 0.14–5.12] respectively). There were no significant interactions of presence versus absence of PAD with the association of depression and all‐cause or cardiovascular mortality (P=0.909 and 0.374, respectively).
Among participants with PAD, an ABI decline >0.15 or a greater decline in the 6‐minute walk during the first 2 years of follow‐up, respectively, were not associated with a higher subsequent incidence of depression, adjusting for age, sex, race, comorbidities, smoking, BMI, baseline ABI, baseline 6‐minute walk, income, or education (Tables 4 and 5, respectively). Among participants without PAD, ABI declines >0.15 and greater declines in the 6‐minute walk, respectively, were associated with greater subsequent risk of depression, adjusting for age, sex, race, comorbidities, smoking, BMI, baseline ABI, baseline 6‐minute walk, income, or education (Tables 4 and 5, respectively).
Table 4.
Adjusted Associations of Decline in the ABI During First 2 Years of Follow‐Up With Subsequent Rate of Depression
| Participants With PAD (N=354) | Participants Without PAD (N=262) | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Decline in ABI >0.15 vs decline in ABI ≤0.15a | 0.94 (0.31–2.86) | 0.913 | 3.88 (1.09–13.89) | 0.037 |
| Age, y | 1.02 (0.97–1.06) | 0.478 | 1.00 (0.94–1.07) | 0.941 |
| Male sex | 0.62 (0.32–1.22) | 0.166 | 1.42 (0.53–3.81) | 0.490 |
| African American race | 0.60 (0.20–1.79) | 0.363 | 0.66 (0.19–2.36) | 0.522 |
| Six‐minute walk (feet) baseline (hazard ratio calculated per 100 feet) | 0.97 (0.87–1.09) | 0.621 | 0.85 (0.76–0.97) | 0.013 |
| Body mass index, kg/m2 | 1.07 (1.00–1.15) | 0.044 | 1.05 (0.97–1.13) | 0.254 |
| Current smoker | 3.08 (1.39–6.80) | 0.005 | 0.00 (0.00–∞) | 0.993 |
| Diabetes | 1.37 (0.68–2.78) | 0.381 | 0.82 (0.21–3.20) | 0.771 |
| Pulmonary disease | 1.22 (0.59–2.54) | 0.589 | 1.31 (0.51–3.36) | 0.580 |
| Cancer | 2.51 (1.15–5.49) | 0.021 | 1.25 (0.32–4.89) | 0.745 |
| Angina | 0.93 (0.42–2.06) | 0.861 | 1.54 (0.39–6.14) | 0.542 |
| Myocardial infarction | 1.17 (0.53–2.61) | 0.694 | 1.29 (0.26–6.33) | 0.750 |
| Stroke | 0.61 (0.21–1.77) | 0.363 | 0.00 (0.00–∞) | 0.994 |
| Heart failure | 2.09 (0.96–4.55) | 0.062 | 1.50 (0.34–6.59) | 0.590 |
| Hip arthritis | 0.80 (0.08–7.66) | 0.844 | 2.48 (0.26–23.31) | 0.428 |
| Knee arthritis | 1.92 (0.73–5.06) | 0.187 | 2.78 (0.90–8.57) | 0.076 |
| Disk disease | 0.77 (0.37–1.57) | 0.468 | 0.99 (0.37–2.61) | 0.983 |
| Spinal stenosis | 0.39 (0.10–1.48) | 0.167 | 1.07 (0.41–2.79) | 0.886 |
| ABI | 1.22 (0.10–14.98) | 0.874 | 3.29 (0.04–286.09) | 0.602 |
| Median annual household income ≤$51 249 vs median annual household income >$51 249 | 0.50 (0.25–1.00) | 0.049 | 0.79 (0.29–2.16) | 0.641 |
| Less than high school vs graduate school | 2.92 (0.81–10.59) | 0.102 | 0.82 (0.15–4.35) | 0.814 |
| High school to college vs graduate school | 1.80 (0.71–4.56) | 0.217 | 0.30 (0.11–0.79) | 0.015 |
ABI indicates Ankle‐Brachial Index; PAD, peripheral artery disease.
Decline in the ABI during the first 2 years of follow‐up was related to subsequent risk of depression.
Table 5.
Adjusted Associations of Decline in the Six‐Minute Walk During First 2 Years of Follow‐Up With Subsequent Depression Among Participants With and Without PAD
| Participants With PAD (N=298) | Participants Without PAD (N=250) | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Decline in 6‐min walk: tertile 3 vs tertile 1a | 2.28 (0.96–5.45) | 0.063 | 4.72 (1.09–20.47) | 0.038 |
| Decline in 6‐min walk: tertile 2 vs tertile 1a | 1.16 (0.45–2.97) | 0.760 | 3.14 (0.70–14.06) | 0.135 |
| Age, y | 1.00 (0.95–1.05) | 0.914 | 0.98 (0.91–1.05) | 0.561 |
| Male sex | 0.75 (0.35–1.59) | 0.448 | 1.20 (0.44–3.30) | 0.717 |
| African American race | 0.63 (0.18–2.24) | 0.475 | 0.42 (0.09–2.03) | 0.279 |
| Six‐minute walk (feet) baseline (hazard ratio calculated per 100 feet) | 0.97 (0.86–1.11) | 0.691 | 0.78 (0.67–0.91) | 0.002 |
| Body mass index, kg/m2 | 1.09 (1.00–1.19) | 0.039 | 1.02 (0.94–1.12) | 0.610 |
| Current smoker | 3.33 (1.34–8.26) | 0.010 | 0.00 (0.00–∞) | 0.995 |
| Diabetes | 1.39 (0.62–3.10) | 0.419 | 1.32 (0.32–5.46) | 0.704 |
| Pulmonary disease | 1.11 (0.48–2.55) | 0.808 | 1.65 (0.63–4.32) | 0.309 |
| Cancer | 1.94 (0.74–5.12) | 0.178 | 1.76 (0.44–7.03) | 0.427 |
| Angina | 1.04 (0.45–2.41) | 0.923 | 2.19 (0.50–9.56) | 0.295 |
| Myocardial infarction | 1.33 (0.57–3.12) | 0.511 | 0.87 (0.15–5.02) | 0.879 |
| Stroke | 1.05 (0.36–3.08) | 0.934 | 0.00 (0.00–∞) | 0.994 |
| Heart failure | 2.22 (0.94–5.21) | 0.068 | 1.57 (0.35–7.15) | 0.557 |
| Hip arthritis | 0.00 (0.00–∞) | 0.991 | 3.48 (0.29–42.11) | 0.326 |
| Knee arthritis | 1.81 (0.66–4.97) | 0.249 | 2.44 (0.72–8.27) | 0.151 |
| Disk disease | 0.93 (0.42–2.05) | 0.854 | 0.88 (0.32–2.39) | 0.799 |
| Spinal stenosis | 0.66 (0.16–2.68) | 0.559 | 0.77 (0.27–2.19) | 0.619 |
| Ankle brachial index | 0.50 (0.03–9.31) | 0.643 | 9.61 (0.07–1357.64) | 0.370 |
| Median annual household income ≤$51 249 vs median annual household income >$51 249 | 0.53 (0.25–1.11) | 0.094 | 0.61 (0.21–1.82) | 0.380 |
| Less than high school vs graduate school | 1.51 (0.33–6.97) | 0.601 | 0.78 (0.13–4.53) | 0.781 |
| High school to college vs graduate school | 1.42 (0.52–3.91) | 0.498 | 0.38 (0.13–1.09) | 0.072 |
PAD indicates peripheral artery disease.
Tertile 1 represents the best (least) decline in the 6‐minute walk between baseline and 2‐year follow‐up. Analyses relate change in 6‐minute walk during the first 2 years of follow‐up with subsequent incidence of depression.
Discussion
Among 951 participants with PAD and 478 without PAD, we report new findings regarding the incidence and clinical significance of depressive symptoms in people with PAD. First, people with PAD had a higher prevalence of depression at baseline and a higher rate of developing new depression during follow‐up compared to people without PAD. The association of PAD with developing new depression was independent of age, sex, race, smoking, BMI, comorbidities, education, and income. However, the association of PAD with increased risk of depressive symptoms was no longer statistically significant after additional adjustment for baseline 6‐minute walk performance. Second, among people with PAD, depression is an independent risk factor for all‐cause mortality, even after adjusting for age, sex, race, comorbidities, BMI, education, income, and smoking. However, this association was no longer significant after adjusting for baseline 6‐minute walk performance.
PAD affects ≈8 million men and women in the United States and nearly 200 million men and women worldwide.25, 26 PAD is associated with increased rates of all‐cause and cardiovascular disease mortality, compared to people without PAD.26, 27, 28 PAD is also associated with greater functional impairment and more rapid functional decline compared to people without PAD.7, 8, 29, 30 We found that 19.2% of PAD participants and 12.9% of non‐PAD participants met criteria for depression at baseline in our cohort. In comparison, the prevalence of depression using the Geriatric Depression Scale, from which the GDS‐S was derived, was 12% among 489 community‐dwelling postmenopausal women without PAD.31 Thus, the prevalence of GDS‐S diagnosed depression in our non‐PAD cohort was comparable to the prevalence of depression diagnosed with the full Geriatric Depression Scale in a community‐dwelling cohort. The prevalence of GDS‐S‐diagnosed depression in our PAD cohort was higher than the prevalence of depression diagnosed with the full GDS in a community‐dwelling cohort.31
Our findings are consistent with prior study in people without PAD showing that depression is more common among people with disability and that depression is associated with disability and mobility loss.32, 33, 34 Similarly, PAD‐related walking impairment may contribute to higher rates of new depression among people with PAD compared to those without PAD. Furthermore, the higher rate of all‐cause mortality in depressed people with PAD may be explained in part by poorer walking endurance among PAD patients with depressive symptoms compared to PAD patients without depressive symptoms. Poorer walking endurance, measured by the 6‐minute walk, is an indicator of PAD severity and an indicator of presence and severity of multiple chronic conditions. The 6‐minute walk may represent a final common pathway for the association of PAD with increased risk of depressive symptoms and for the association of depressive symptoms with increased mortality among people with PAD. However, this observational study cannot delineate causal pathways for the associations reported here. It is possible that poorer 6‐minute walk performance represents psychomotor retardation associated with depression.
Among people with PAD, cross‐sectional studies have documented a high prevalence of depression and poorer functional performance among depressed patients with PAD compared to nondepressed patients with PAD.2, 6, 35 McDermott et al previously reported that greater numbers of depressive symptoms, measured by the GDS‐S, were associated with slower walking velocity and poorer 6‐minute walk performance among 423 men and women with PAD even after adjusting for confounders including the ABI.35 Smolderan et al previously reported that the prevalence of depression among 166 patients with PAD was 16%, measured by a Center for Epidemiological Studies Depression scale score ≥4, and that PAD patients with depression had poorer treadmill walking performance compared to PAD patients without depression.2 In longitudinal analyses, Cherr et al studied patients with PAD undergoing lower extremity revascularization.6, 36 The prevalence of depression at baseline was 35%.6 PAD patients undergoing lower extremity revascularization with depression at baseline had higher rates of failed revascularization, lower patency rates, and higher rates of the combined outcome of all‐cause mortality and cardiovascular disease events during follow‐up compared to PAD patients undergoing revascularization without depression at baseline.6, 37 Depression was also associated with a higher rate of coronary heart disease events but was not associated with higher rates of all‐cause mortality or cerebrovascular events, respectively.6 Previous study from the Heart and Soul study of 1024 men and women with coronary artery disease demonstrated that the presence of depressive symptoms at baseline was associated with a higher incidence of PAD during follow‐up in analyses adjusting for age and sex (HR=2.09, 95% CI=1.09–4.00).34 However, this association was no longer significant after additional adjustment for comorbidities, PAD risk factors, medications, and health behaviors.
To our knowledge, no prior studies have compared the incidence of depression in longitudinal analyses between people with versus without PAD. To our knowledge, no prior studies have compared all‐cause or cardiovascular disease mortality rates between PAD patients with versus without depression, in a cohort of PAD participants not undergoing revascularization. Our study design does not allow us to discern potential mechanisms of our findings. However, previous study suggests that depression is associated with more adverse health behaviors, such as greater inactivity.37 Prior study also suggests that depression is associated with adverse pathophysiologic changes that include increased sympathetic tone, reduced vagal tone, and immunosuppression.38, 39, 40 These pathophysiologic changes may increase risk of mortality among PAD patients with depression. Further study is needed to discern the mechanisms of the associations reported here.
Our study has limitations. First, our data are observational. Results cannot be construed as causal. Second, although our findings suggest that the 6‐minute walk may mediate the associations reported here, these observational data prevent the ability to delineate the causal pathway of findings reported here. Third, we used the GDS‐S to measure depression. We did not collect data on clinical diagnoses of depression. However, previous study demonstrates that the GDS‐S is a well‐validated measure of depression.13, 14, 15, 16, 17, 18 Fourth, we excluded PAD participants who were wheelchair bound and those residing in a nursing home, who may have had a higher prevalence of depression than the participants included here. Our results may not be generalizable to people who did not meet our inclusion criteria. Fifth, our analyses combined data from 3 observational prospective cohorts of participants with PAD, thereby introducing heterogeneity into our analyses. We addressed heterogeneity by stratifying our analyses by study cohort and by testing for a cohort interaction for our primary analyses. Sixth, participants were identified from among patients encountered by physicians in multiple office settings in Chicago. Our findings may not be generalizable to men and women not encountered by clinicians in an office or medical center setting. Seventh, although the GDS‐S is well validated in multiple settings, to our knowledge, it has not been validated against a clinical diagnosis of depression specifically in a population of patients with established cardiovascular disease.
In conclusion, people with PAD are at higher risk for developing depression compared to people without PAD. Among people with PAD, depression is associated with higher mortality rates compared to the absence of depression. Our findings suggest that clinicians should be alert to an increased incidence of depression among people with PAD. Clinicians should also be aware that the presence of depression in people with PAD is associated with increased mortality. Further study is needed to determine whether improving 6‐minute walk performance can prevent depression in PAD and whether treating depression can reduce all‐cause and cardiovascular disease mortality in people with PAD. Further study is also needed to delineate the mechanisms of associations reported here.
Sources of Funding
Funded by the National Heart Lung and Blood Institute (NHLBI); R01‐HL083064, R01‐HL64739, R01‐HL58099, R01‐HL076298, R01‐HL71223, and R01‐HL109244.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002959 doi: 10.1161/JAHA.115.002959)
References
- 1. Arsevan A, Guralnik JM, O'Brien E, Liu K, McDermott MM. Peripheral arterial disease and depressed mood in older men and women. Vasc Med. 2001;6:229–234. [DOI] [PubMed] [Google Scholar]
- 2. Smolderen KG, Aquarius AE, de Vries J, Smith OR, Hamming JF, Denollet J. Depressive symptoms in peripheral arterial disease: a follow‐up study on prevalence, stability, and risk factors. J Affect Disord. 2008;110:27–35. [DOI] [PubMed] [Google Scholar]
- 3. Smolderan KG, Hoeks SE, Pedersen SS, van Domburg RT, de Liefde II, Poldermans D. Lower‐leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med. 2009;14:297–304. [DOI] [PubMed] [Google Scholar]
- 4. Frasure‐Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact of 6‐month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 5. Bush DE, Ziegelstein RC, Tayback M. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–341. [DOI] [PubMed] [Google Scholar]
- 6. Charr G, Zimmerman PM, Wang J, Dosluoglu HH. Patients with depression are at increased risk of secondary cardiovascular events after lower extremity revascularization. J Gen Intern Med. 2008;23:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the walking and leg circulation study. Ann Intern Med. 2002;136:873–883. [DOI] [PubMed] [Google Scholar]
- 8. McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. [DOI] [PubMed] [Google Scholar]
- 9. McDermott MM, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, Liao Y, Criqui MH. Pathophysiological changes in calf muscle predict mobility loss at 2‐year follow‐up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDermott MM, Carroll TJ, Kibbe M, Kramer CM, Liu K, Guralnik JM, Keeling AN, Criqui MH, Ferrucci L, Yuan C, Tian L, Liao Y, Berry J, Zhao L, Carr J. Proximal superficial femoral artery occlusion, collateral vessels, and walking performance in peripheral artery disease. JACC Cardiovasc Imaging. 2013;6:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat‐Jacobson D; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. [DOI] [PubMed] [Google Scholar]
- 12. McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. [DOI] [PubMed] [Google Scholar]
- 13. Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, McDermott MM. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. [DOI] [PubMed] [Google Scholar]
- 14. Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies‐Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157:449–454. [PubMed] [Google Scholar]
- 15. Sheikh JI, Yesavage JA. Clinical Gerontology: A Guide to Assessment and Therapeutic Intervention. Binghamton, NY: The Haworth Press Inc; 1986:165–173. [Google Scholar]
- 16. Greenberg SA. How to try this: the Geriatric Depression Scale: Short Form. Am J Nurs. 2007;107:60–69. [DOI] [PubMed] [Google Scholar]
- 17. Blazer DG. Depression in late life: review and commentary. Focus. 2009;7:118–136. [Google Scholar]
- 18. Ruo B, Liu K, Tian L, Tan J, Ferrucci L, Guralknik JM, McDermott MM. Persistent depressive symptoms and functional decline among patients with peripheral arterial disease. Psychosom Med. 2007;69:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nyuant MS, Lim ML, Yap KB, Ng TP. Changes in depressive symptoms and functional disability among community‐dwelling depressive older adults. Int Psychogeriatr. 2012;24:1633–1641. [DOI] [PubMed] [Google Scholar]
- 20. Huang TT, Liu CB, Tsai YH, Chin YF, Wong CH. Physical fitness exercise versus cognitive behavior therapy on reducing the depressive symptoms among community‐dwelling elderly adults: a randomized controlled trial. Int J Nurs Stud. 2015;52:1542–1552. [DOI] [PubMed] [Google Scholar]
- 21. McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, Pearce WH, Schneider JR, Criqui MH. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51:1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six‐minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fried LP, Kasper JD, Williamson JD, Skinner EA, Morris CD, Hochberg MC. Disease ascertainment algorithms In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, eds. The Women's Health and Aging Study: Health and Social Characteristics of Older Women With Disability. Bethesda, MD: National Institute on Aging; 1995: Appendix E. NIH Pub. NO. 95‐4009. [Google Scholar]
- 24. Jolly M, Mikolaitis RA, Shakoor N, Flogg LF, Block JA. Education, zip code based annualized household income and health outcomes in patients with lupus erythematosus. J Rheumatol. 2010;37:1150–1157. [DOI] [PubMed] [Google Scholar]
- 25. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 26. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics‐2014 update. A report from the American Heart Association. Circulation. 2014;129:399–410. [DOI] [PubMed] [Google Scholar]
- 27. Heald CL, Fowkes FG, Murray GD, Price GF; Ankle Brachial Index Collaboration . Risk of mortality and cardiovascular disease associated with the ankle‐brachial index: systematic review. Atherosclerosis. 2006;189:61–69. [DOI] [PubMed] [Google Scholar]
- 28. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton‐Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM; Ankle Brachial Index Collaboration . Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta‐analysis. JAMA. 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. [DOI] [PubMed] [Google Scholar]
- 30. McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, Sharma L, Criqui MH. Associations of borderline and low normal ankle‐brachial index values with functional decline at 5‐year follow‐up: the WALCS (Walking and Leg Circulation Study). J Am Coll Cardiol. 2009;53:1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012;19:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang M, Phillips C, Coppin AK, van der Linden M, Ferrucci L, Fried L, Guralnik JM. An association between incident disability and depressive symptoms over 3 years of follow‐up among older women: the Women's Health and Aging Study. Aging Clin Exp Res. 2009;21:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pennix BWJH, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJH, Wallace RB. Depressive symptoms and physical decline in community‐dwelling older persons. JAMA. 1998;279:1720–1726. [DOI] [PubMed] [Google Scholar]
- 34. Pennix BWJH, Leveille S, Ferrucci L, van Eijk JTM, Guralnik JM. Exploring the effect of depression on physical disability. Longitudinal evidence from the established populations for the epidemiologic studies of the elderly. Am J Public Health. 1989;99:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDermott MM, Greenland P, Guralnik JM, Liu K, Criqui MH, Pearce WH, Chan C, Schneider J, Sharma L, Taylor LM, Arseven A, Quann M, Celic L. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cherr GS, Wang J, Zimmerman PM, Dosluoglu HH. Depression is associated with worse patency and recurrent leg symptoms after lower extremity revascularization. J Vasc Surg. 2007;45:744–750. [DOI] [PubMed] [Google Scholar]
- 37. Grenon SM, Hiramoto J, Smolderen KG, Vittinghoff E, Whooley MA, Cohen BE. Association between depression and peripheral artery disease insights from the Heart and Soul Study. J Am Heart Assoc. 2012;1:e002667 doi: 10.1161/JAHA.112.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ader R, Cohen N, Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet. 1995;345:99–103. [DOI] [PubMed] [Google Scholar]
- 39. Musselman DL, Nemeroff CB. Depression and endocrine disorders: focus on the thyroid and adrenal system. Br J Psychiatry Suppl. 1996;30:123–128. [PubMed] [Google Scholar]
- 40. Stein M, Miller AH, Trestman RL. Depression, the immune system, and health and illness: findings in search of meaning. Arch Gen Psychiatry. 1991;48:171–177. [DOI] [PubMed] [Google Scholar]


