Abstract
Background
Recent literature suggests that blood pressure variability (BPV) predicts outcome beyond blood pressure level (BPL) and that antihypertensive drug classes differentially influence BPV. We compared calcium channel blockers, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockade for effects on changes in self‐measured home BPL and BPV and for their prognostic significance in newly treated hypertensive patients.
Methods and Results
We enrolled 2484 patients randomly allocated to first‐line treatment with a calcium channel blocker (n=833), an angiotensin‐converting enzyme inhibitor (n=821), or angiotensin receptor blockade (n=830). Home blood pressures in the morning and evening were measured for 5 days off treatment before randomization and for 5 days after 2 to 4 weeks of randomized drug treatment. We assessed BPL and BPV changes as estimated by variability independent of the mean and compared cardiovascular outcomes. Home BPL response in each group was significant (P≤0.0001) but small in the angiotensin‐converting enzyme inhibitor group (systolic/diastolic: 4.6/2.8 mm Hg) compared with the groups treated with a calcium channel blocker (systolic/diastolic: 8.3/3.9 mm Hg) and angiotensin receptor blockade (systolic/diastolic: 8.2/4.5 mm Hg). In multivariable adjusted analyses, changes in home variability independent of the mean did not differ among the 3 drug classes (P≥0.054). Evening variability independent of the mean before treatment significantly predicted hard cardiovascular events independent of the corresponding home BPL (P≤0.022), whereas BPV did not predict any cardiovascular outcome based on the morning measurement (P≥0.056). Home BPV captured after monotherapy had no predictive power for cardiovascular outcome (P≥0.22).
Conclusions
Self‐measured home evening BPV estimated by variability independent of the mean had prognostic significance, whereas antihypertensive drug classes had no significant impact on BPV changes. Home BPL should remain the primary focus for risk stratification and treatment.
Clinical Trial Registration
URL: http://www.umin.ac.jp/ctr/index.htm. Unique identifier: C000000137.
Keywords: antihypertensive drugs, blood pressure variability, cardiovascular outcomes, home blood pressure, morning and evening self‐measurement
Subject Categories: Hypertension, Epidemiology, Blood Pressure, Cardiovascular Disease
Introduction
Self‐measurement of blood pressure at home is a more accurate prognosticator than conventionally measured blood pressure because of the greater number of readings, the minimization of the “white‐coat effect,” and the reduction of measurement error through use of automated blood pressure monitors.1, 2 Affordable and validated automated monitors for blood pressure self‐measurement are readily available. Similar to visit‐to‐visit variability in clinic blood pressure,3, 4 multiple home blood pressure measurements provide information on day‐to‐day blood pressure variability in the relatively controlled home environment.5, 6 Although subject to debate, some researchers proposed the idea that antihypertensive drug classes differentially influence blood pressure variability.7, 8
The multicenter Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED‐BP) trial proved the feasibility of adjusting antihypertensive drug treatment based on self‐measured home blood pressure.9, 10 Based on accurate phenotype information, we aimed to compare the effects on blood pressure level and blood pressure variability and then to establish the prognostic significance of calcium channel blockers (CCBs), angiotensin‐converting enzyme inhibitors (ACEIs), and angiotensin receptor blockade (ARB). Home blood pressures, used in the present study, were measured at baseline before treatment and after initiation of CCB, ACEI, or ARB monotherapy.
Methods
Study Population
The HOMED‐BP study was a multicenter clinical trial with a PROBE (prospective, randomized, open‐label, blinded end point, evaluation)11 design. The HOMED‐BP protocol complies with the Declaration of Helsinki regarding investigation of human subjects12 and is registered with the UMIN Clinical Trial Registry (C000000137; http://www.umin.ac.jp/ctr). The institutional review board of the Tohoku University Graduate School of Medicine approved the study protocol, and all study participants gave written informed consent.
In HOMED‐BP,10, 13 participants with mild to moderate hypertension who were aged ≥40 years were recruited from 457 general practices throughout Japan. Participants were both treatment‐naïve and previously treated patients whose antihypertensive drug treatment could be discontinued for at least 2 weeks. Off treatment, participants had to maintain a self‐measured morning home blood pressure of 135 to 179 mm Hg systolic or 85 to 119 mm Hg diastolic. Eligible patients had no contraindication for being administered a CCB, an ACEI, or ARB. In a 2×3 design, 3518 eligible patients were randomized to usual control (ranging from 125 to 134 mm Hg systolic and from 80 to 84 mm Hg diastolic) or tight control (<125 mm Hg systolic and 80 mm Hg diastolic) of morning home blood pressure and to initiation of antihypertensive drug treatment with a CCB, an ACEI, or ARB. The first patient was randomized on June 6, 2001, and the last patient was randomized on October 7, 2009. The primary outcome of HOMED‐BP study was published based on the dataset with follow‐up until April 30, 2010.10 Moreover, the HOMED‐BP management committee decided to continue the operation of the HOMED‐BP system until the end of 2012; therefore, we collected blood pressure data and ascertained outcomes until December 31, 2012.
Of the 3518 randomized patients, we excluded 1034 from analysis because they had obtained <3 morning or evening readings at baseline (n=303) or after monotherapy with the first‐line drug (n=697) or because they did not actually receive an antihypertensive drug or were treated with ≥2 drug classes simultaneously (n=34). A total of 2484 participants were analyzed statistically.
Blood Pressure Measurement and Collection
Patients received spoken and written instructions on blood pressure self‐measurement and use of the validated14 oscillometric Omron HEM‐747IC‐N monitors (Omron Healthcare Co., Ltd.). The monitor stores up to 350 blood pressure and heart rate readings in memory. The home blood pressure used for determining eligibility and treatment adjustments at each visit was the average of the morning readings available over the 5 days immediately preceding the visit. Patients were asked to measure blood pressure in the sitting position once every morning throughout the study period after ≥2 minutes of rest. They had to obtain these measurements within 1 hour of awakening, before breakfast, and before taking antihypertensive medication. They were also asked to measure blood pressure once every evening throughout the study period just before going to bed. The clinic blood pressure was also measured by practitioners twice consecutively at each visit using the validated15 oscillometric Omron HEM‐907IT device (Omron Healthcare Co., Ltd.) after patients had ≥2 minutes of rest in the sitting position. The clinic blood pressure was the average of these 2 readings. At each visit, the central server at Tohoku University received the home and clinic blood pressure data from local practices and immediately displayed these data on the screen of the local computer, along with advice for treatment adjustment based on a computerized algorithm, as described elsewhere.10, 13 A particular drug was also randomly displayed for doctors’ reference; however, doctors were allowed to choose any antihypertensive drug agents within each drug class based on the condition of the patient, the adopted drug at each clinic, or their own judgment, as shown in Table 1.
Table 1.
Detailed Information on Prescribed Medication Among 3 Drug Classes
| Drug Class and Generic Name | Patients, n (%) | Dosage, mg (%) |
|---|---|---|
| Calcium channel blocker | 833 | |
| Amlodipine | 439 (52.7) | 5 (52.6) |
| Benidipine | 183 (22.0) | 4 (59.0) |
| Barnidipine | 65 (7.8) | 10 (38.5) |
| Azelnidipine | 50 (6.0) | 8 (56.0) |
| Cilnidipine | 39 (4.7) | 10 (33.3) |
| Others | 57 (6.8) | N/A |
| Angiotensin‐converting enzyme inhibitor | 821 | |
| Imidapril | 285 (34.7) | 5 (71.6) |
| Perindopril | 193 (23.5) | 4 (51.8) |
| Enalapril | 108 (13.2) | 5 (76.9) |
| Quinapril | 87 (10.6) | 10 (52.9) |
| Temocapril | 55 (6.7) | 2 (78.2) |
| Others | 93 (11.3) | N/A |
| Angiotensin receptor blocker | 830 | |
| Candesartan | 292 (35.2) | 4 (45.9) |
| Valsartan | 232 (28.0) | 80 (48.3) |
| Losartan | 128 (15.4) | 50 (56.3) |
| Telmisartan | 104 (12.5) | 40 (60.6) |
| Olmesartan | 71 (8.6) | 20 (50.7) |
| Irbesartan | 3 (0.4) | 100 (100) |
N/A indicates not available.
In the present study, data on home blood pressure values for, in principle, 5 days before randomization and 5 days after 10 to 28 days of randomized drug treatment were used to calculate level and variability at baseline and after monotherapy, respectively (Figure 1).16 We used this time window because (1) the home blood pressure used for determining eligibility and treatment adjustments at each visit in the HOMED‐BP study was the average of the morning readings available over 5 days immediately preceding the visit,10, 13 (2) the clinical investigators followed the study participants at intervals of ≈2 to 4 weeks in general practice and ≈4 to 8 weeks at hospital outpatient clinics, and (3) the time intervals needed to attain the maximum antihypertensive effects are reported to be ≈9 to 23 days.17 Averaged follow‐up days were calculated as the mean of 5 days of home blood pressure measurements. Patients with home blood pressure data on 3 to 4 days in each interval were also included in the present study, for which there was ≥4.80 average measurement days.
Figure 1.

Time course of home blood pressure measurement before and after randomization. During the study period, morning blood pressure was measured in a sitting position once every morning after ≥2 minutes of rest and within 1 hour of awakening, before breakfast, and before taking antihypertensive medication, if patients were taking antihypertensive medication. Evening blood pressure was measured in a sitting position once every evening just before going to bed and after ≥2 minutes of rest. Data on home blood pressure values for, in principle, 5 days before randomization and for 5 days after 10 to 28 days of randomized drug treatment were used for the calculation; patients with 3 to 4 days of home blood pressure data in each interval were also included. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blockade; CCB, calcium channel blocker.
Definition of Diseases and Events
We coded end points according to the International Classification of Diseases, 10th Revision (ICD‐10). In the current study, the primary hard end point was a composite of cardiovascular death (ICD‐10 codes I00–I99), nonfatal myocardial infarction (I21), and nonfatal stroke (I60, I61, and I63).9, 10 The broader composite cardiovascular end point encompassed the hard end point plus transient ischemic attack (G45), angina pectoris (I20), coronary atherosclerosis (I70), and fatal and nonfatal heart failure (I50).9, 10 The end point committee, which was unaware of patients’ randomization, adjudicated all events. We used the outcome results considering only the first event in individual patients.
Body mass index was calculated as body weight in kilograms divided by height in meters squared. Diabetes mellitus was determined by fasting plasma glucose ≥7.0 mmol/L (≥126 mg/dL), HbA1c ≥6.5%,18 or treatment with antidiabetic agents. Hypercholesterolemia was determined by total cholesterol of ≥5.69 mmol/L (≥220 mg/dL), by a documented history of hypercholesterolemia, or by taking lipid‐lowering drug treatment. We used the World Health Organization's defined daily doses19 (2011 version) to quantify the use of antihypertensive drugs in each group at each visit.
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.4 (SAS Institute Inc). Statistical significance was α < 0.05 on 2‐sided tests. All data are expressed as mean±SD unless otherwise stated. We analyzed the morning and evening blood pressures separately because previous studies showed that they have different prognostic meanings.5, 20
The within‐participant blood pressure variability was represented by variability independent of the mean (VIM),3, 5 which is the standard deviation divided by the mean to the powerx. The power x is obtained by fitting a curve through a plot of SD against mean using the model SD=axmeanx, where x was derived by non‐linear regression analysis as implemented in the PROC NLIN procedure of the SAS package. We also used average real variability (ARV) calculated by the average of the absolute differences between consecutive day blood pressure measurements.21, 22 We further computed blood pressure variability from the standard deviation and the coefficient of variation.
For comparison of means and proportions, we applied the Kruskal–Wallis test and the chi‐square statistic, respectively. We analyzed the difference among groups according to the per‐protocol analysis on first‐line drug prescription to clarify the antihypertensive drug effect on blood pressure level and variability. Changes in blood pressure from baseline to the monotherapy period were tested by analysis of covariance, which accounts for sex, age, body mass index, corresponding blood pressure level or variability and heart rate at baseline, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and defined daily doses after monotherapy. In line with the small differences in home blood pressure, the risks of outcomes were similar in the randomized groups10; therefore, we pooled all participants for the survival analysis. We applied Cox regression to compute hazard ratios (HRs) that expressed the change in risk associated with a 1‐SD increase in mean blood pressure or variability. Covariables were sex, age, body mass index, corresponding heart rate, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and antihypertensive drug classes. For analyzing the risk of hard and broader cardiovascular events, we used the competing risk model by Fine and Gray to account for competing noncardiovascular death.23
Results
Baseline Characteristics
Of 2484 patients, 1257 (50.6%) were women, 507 (20.4%) were current smokers, 1184 (47.7%) used alcohol, 380 (15.3%) had diabetes, 1279 (51.5%) were hypercholesterolemia, and 67 (2.7%) had a history of cardiovascular disease. Age and body mass index averaged 59.9±9.8 years and 24.3±3.3 kg/m2, respectively. For all participants, the clinic blood pressure averaged 154.1±17.3 mm Hg systolic and 90.0±12.0 diastolic. The corresponding home systolic and diastolic blood pressure levels were 151.2±12.3 and 89.6±10.0 mm Hg, respectively, in the morning and 143.7±15.2 and 82.5±10.8 mm Hg, respectively, in the evening.
Table 2 lists the baseline characteristics by the initial antihypertensive drug classes. No significant differences in level and variability were observed among the 3 categories (P≥0.10) except for VIM and ARV derived from morning systolic blood pressure measurements (P=0.0093 and P=0.022, respectively). Tables 3 and 4 list the baseline characteristics by quartiles of VIM based on systolic blood pressure in the morning and evening, respectively. The average morning and evening systolic blood pressures were similar across the corresponding VIM quartiles (P≥0.32), whereas age increased by VIM category increment (P<0.0001).
Table 2.
Baseline Characteristics of 2484 Patients by Antihypertensive Drug Classes
| Characteristic | CCB | ACEI | ARB |
|---|---|---|---|
| Patients, n | 833 | 821 | 830 |
| Women | 424 (50.9) | 420 (51.2) | 413 (49.8) |
| Current smoking | 172 (20.6) | 156 (19.0) | 179 (21.6) |
| Drinking alcohol | 394 (47.3) | 386 (47.0) | 404 (48.7) |
| Diabetes | 123 (14.8) | 132 (16.1) | 125 (15.1) |
| Hypercholesterolemia | 434 (52.1) | 412 (50.2) | 433 (52.3) |
| Previous cardiovascular diseases | 18 (2.2) | 21 (2.6) | 28 (3.4) |
| Tight control group | 433 (52.0) | 393 (47.9) | 425 (51.2) |
| Mean characteristic (SD) | |||
| Age, y | 59.8 (10.0) | 59.9 (9.7) | 60.1 (9.7) |
| Body mass index, kg/m2 | 24.2 (3.2) | 24.5 (3.5) | 24.4 (3.2) |
| Fasting plasma glucose, mmol/L | 5.84 (1.70) | 5.85 (1.68) | 5.79 (1.67) |
| Total serum cholesterol, mmol/L | 5.42 (0.92) | 5.45 (0.88) | 5.49 (0.88) |
| Clinic systolic blood pressure, mm Hg | 154.8 (17.8) | 153.6 (17.1) | 154 (16.9) |
| Clinic diastolic blood pressure, mm Hg | 90.4 (12.2) | 89.7 (12) | 89.8 (11.8) |
| Home blood pressure, mm Hg | |||
| Morning systolic | 151.6 (12.5) | 151.3 (12.2) | 150.8 (12.2) |
| Morning diastolic | 89.9 (9.7) | 89.6 (10.1) | 89.3 (10.4) |
| Evening systolic | 144.1 (15.3) | 143.5 (15.0) | 143.6 (15.3) |
| Evening diastolic | 82.8 (10.6) | 82.6 (10.7) | 82.3 (11.2) |
| Home VIM, unit | |||
| Morning systolic | 8.78 (4.05) | 9.39 (4.37) | 9.30 (4.38) |
| Morning diastolic | 5.07 (2.44) | 5.05 (2.60) | 5.24 (2.72) |
| Evening systolic | 10.71 (4.70) | 10.72 (4.80) | 11.03 (5.26) |
| Evening diastolic | 6.37 (2.98) | 6.44 (3.09) | 6.54 (3.12) |
| Home ARV, mm Hg | |||
| Morning systolic | 10.02 (5.03) | 10.90 (5.90) | 10.64 (5.57) |
| Morning diastolic | 5.78 (3.05) | 5.74 (3.18) | 6.08 (3.50) |
| Evening systolic | 12.65 (6.33) | 12.62 (6.55) | 12.88 (6.65) |
| Evening diastolic | 7.50 (4.05) | 7.68 (4.14) | 7.63 (4.13) |
Values are number of participants (%) or arithmetic mean (SD). Tight control group indicates patients who were allocated to tight control (<125 mm Hg systolic and 80 mm Hg diastolic) of morning home blood pressure. Diabetes mellitus was defined as fasting plasma glucose of ≥7.0 mmol/L (≥126 mg/dL), HbA1c of ≥6.5%, or treatment with oral antidiabetic drugs or insulin. Hypercholesterolemia was defined as total serum cholesterol of ≥5.69 mmol/L (≥220 mg/dL), a history of hypercholesterolemia, or taking lipid‐lowering drugs. Baseline characteristics did not differ between randomized groups (P≥0.10) with the exception of home VIM and ARV calculated by morning systolic measurements (P=0.0093 and P=0.022, respectively). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blockade; ARV, average real variability; CCB, calcium channel blocker; VIM, variability independent of the mean.
Table 3.
Baseline Characteristics of Participants by Distribution of Overall Systolic VIM in the Morning
| Characteristic | Categories of Systolic VIM in the Morning | P Value | |||
|---|---|---|---|---|---|
| Limits, U | 0.84 to 6.16 | 6.16 to 8.42 | 8.42 to 11.3 | 11.3 to 37.4 | |
| Participants, n (%) | |||||
| All participants in category | 621 | 620 | 622 | 621 | |
| Women | 294 (47.3) | 307 (49.5) | 315 (50.6) | 341 (54.9)b | 0.056 |
| Current smoking | 136 (21.9) | 130 (21.0) | 112 (18.0) | 129 (20.8) | 0.36 |
| Drinking alcohol | 303 (48.8) | 306 (49.4) | 290 (46.6) | 285 (45.9) | 0.56 |
| Diabetes | 88 (14.2) | 105 (16.9) | 91 (14.6) | 96 (15.5) | 0.55 |
| Hypercholesterolemia | 314 (50.6) | 307 (49.5) | 331 (53.2) | 327 (52.7) | 0.52 |
| Previous cardiovascular diseases | 14 (2.3) | 11 (1.8) | 15 (2.4) | 27 (4.3) | 0.028 |
| Mean (SD) of characteristic | |||||
| Age, y | 58.8 (9.8) | 59.7 (9.7) | 59.9 (9.5) | 61.4 (9.9)b | <0.0001 |
| Body mass index, kg/m2 | 24.5 (3.2) | 24.6 (3.4) | 24.5 (3.4) | 23.8 (3.3)b | 0.0002 |
| Fasting plasma glucose, mmol/L | 5.81 (1.71) | 5.83 (1.64) | 5.76 (1.51) | 5.92 (1.85) | 0.85 |
| Total serum cholesterol, mmol/L | 5.45 (0.90) | 5.42 (0.86) | 5.48 (0.88) | 5.47 (0.93) | 0.60 |
| Blood pressure, mm Hg | |||||
| Clinic systolic | 154.2 (16.6) | 153.7 (17.6) | 153.7 (17.1) | 154.8 (17.9) | 0.60 |
| Clinic diastolic | 91.1 (11.5) | 90.3 (12.0) | 89.4 (12.1) | 89.1 (12.3) | 0.0032 |
| Home morning systolic | 151.9 (12.6) | 150.7 (12.5) | 150.9 (12.3) | 151.4 (12.0) | 0.32 |
| Home morning diastolic | 90.9 (9.8) | 89.5 (10.0)a | 89.7 (10.0) | 88.3 (10.2)a | 0.0002 |
| Home evening systolic | 144.5 (15.8) | 142.4 (14.1)a | 143.3 (14.6) | 144.7 (16.2) | 0.11 |
| Home evening diastolic | 83.6 (10.8) | 81.7 (10.5)b | 82.5 (11.0) | 82.5 (11.1) | 0.036 |
| Home heart rate, beats per minute | |||||
| Morning | 68.3 (9.4) | 68.2 (8.9) | 68.7 (9.2) | 69.1 (9.5) | 0.28 |
| Evening | 72.9 (9.9) | 73.2 (9.9) | 72.9 (10.1) | 72.5 (9.3) | 0.66 |
Values are number of participants (%) or arithmetic mean (SD). VIM is based on self‐measurement in the morning on up to 5 days (average 4.95 day) within 1 hour after awakening. Body mass index, glucose, and cholesterol level were unavailable in 53, 215, and 81 patients, respectively. P denotes the significance of the linear trend across categories of systolic blood pressure level. VIM indicates variability independent of the mean.
P<0.05, significance of the difference with the adjacent lower fourth.
P<0.01, significance of the difference with the adjacent lower fourth.
Table 4.
Baseline Characteristics of Participants by Distribution of Overall Systolic VIM in the Evening
| Characteristic | Categories of Systolic VIM in the Evening | P Value | |||
|---|---|---|---|---|---|
| Limits, units | 0.99 to 7.44 | 7.44 to 10.1 | 10.1 to 13.5 | 13.5 to 42.0 | |
| Number of participants (%) | |||||
| All participants in category | 621 | 621 | 621 | 621 | |
| Women | 281 (45.2) | 320 (51.5)a | 328 (52.8) | 328 (52.8) | 0.021 |
| Current smoking | 134 (21.6) | 127 (20.5) | 116 (18.7) | 130 (20.9) | 0.62 |
| Drinking alcohol | 291 (46.9) | 289 (46.5) | 287 (46.2) | 317 (51.0) | 0.28 |
| Diabetes | 92 (14.8) | 106 (17.1) | 90 (14.5) | 92 (14.8) | 0.56 |
| Hypercholesterolemia | 311 (50.1) | 323 (52.0) | 326 (52.5) | 319 (51.4) | 0.85 |
| Previous cardiovascular diseases | 16 (2.6) | 18 (2.9) | 14 (2.3) | 19 (3.1) | 0.82 |
| Mean (SD) of characteristic | |||||
| Age, y | 58.2 (10.0) | 59.4 (9.8)a | 60.2 (9.6) | 61.8 (9.4)b | <0.0001 |
| Body mass index, kg/m2 | 24.6 (3.4) | 24.4 (3.4) | 24.3 (3.3) | 24.1 (3.2) | 0.20 |
| Fasting plasma glucose, mmol/L | 5.74 (1.45) | 5.88 (1.64) | 5.91 (2.00) | 5.79 (1.58) | 0.33 |
| Total serum cholesterol, mmol/L | 5.46 (0.90) | 5.45 (0.90) | 5.47 (0.85) | 5.43 (0.93) | 0.88 |
| Blood pressure, mm Hg | |||||
| Clinic systolic | 154.2 (17.1) | 154.6 (16.9) | 153.8 (16.9) | 154.0 (18.3) | 0.79 |
| Clinic diastolic | 90.7 (11.6) | 90.9 (12.2) | 89.9 (12.1) | 88.5 (12.1)a | 0.0023 |
| Home morning systolic | 150.0 (12.1) | 150.5 (12.4) | 150.9 (12.0) | 153.4 (12.6)c | <0.0001 |
| Home morning diastolic | 90.2 (9.6) | 89.6 (10.1) | 89.4 (10.0) | 89.2 (10.5) | 0.28 |
| Home evening systolic | 144.0 (15.1) | 143.7 (15.2) | 143.5 (15.5) | 143.9 (15.0) | 0.83 |
| Home evening diastolic | 83.7 (10.9) | 83.0 (10.8) | 82.4 (10.9) | 81.1 (10.7)a | 0.0004 |
| Home heart rate, beat per minute | |||||
| Morning | 68.4 (9.2) | 69.1 (9.5) | 68.3 (9.0) | 68.4 (9.4) | 0.71 |
| Evening | 72.8 (9.8) | 72.9 (9.5) | 73.0 (10.0) | 72.8 (10.0) | 0.98 |
Values are number of participants (%) or arithmetic mean (SD). VIM is based on self‐measurement in the evening on up to 5 days (average 4.93 days) just before going to bed. Body mass index, glucose, and cholesterol level were unavailable in 53, 215, and 81 patients, respectively. P denotes the significance of the linear trend across categories of systolic blood pressure level. VIM indicates variability independent of the mean.
P<0.05, significance of the difference with the adjacent lower fourth.
P<0.01, significance of the difference with the adjacent lower fourth.
P<0.001, significance of the difference with the adjacent lower fourth.
Blood Pressure Level and Variability at Baseline and After Monotherapy
Home blood pressure level and VIM measured after the monotherapy in 3 antihypertensive drug classes are shown in Table 5. At a median of 25.0 days (interquartile range 13.6–26.0 days) after initiation of monotherapy, reduction of blood pressure was significantly weaker (P≤0.0021) in the ACEI group (systolic/diastolic blood pressure: 4.6/2.8 mm Hg in the morning and 7.0/4.0 mm Hg in the evening) than in the CCB group (systolic/diastolic blood pressure: 8.3/3.9 mm Hg in the morning and 9.4/4.5 mm Hg in the evening) and the ARB group (systolic/diastolic blood pressure: 8.2/4.5 mm Hg in the morning and 9.7/5.0 mm Hg in the evening). In addition, the defined daily doses after first‐line drug prescription were significantly smaller in the ACEI group compared with the other groups (P<0.0001). In multivariable adjusted comparison, changes in VIM and ARV based on morning home blood pressure did not differ among the 3 drug classes (systolic: P≥0.057; diastolic P≥0.054). VIM and ARV changes in evening measurement were also essentially similar (P≥0.091) except for morning diastolic ARV (P=0.012), which was largely reduced in the CCB group (mean reduction of ARV: 0.21±3.87 mm Hg) compared with ACEI (−0.27±4.17 mm Hg) and ARB (0.03±4.14 mm Hg). Results were confirmed when standard deviation and coefficient of variation were used as variability indexes instead of VIM or ARV (Table 6).
Table 5.
Reduction of Blood Pressure Level and Variability After the Monotherapy in 3 Drug Classes Among 2484 Patients With ≥3 Measurements in Both Morning and Evening Home Blood Pressure
| Characteristic | Calcium Channel Blocker | Angiotensin‐Converting Enzyme Inhibitor | Angiotensin Receptor Blocker | Crude P Value | Adjusted P Value |
|---|---|---|---|---|---|
| Average follow‐up days | 25.0 (14.0–26.0) | 24.4 (12.7–26.0) | 25.2 (15.0–26.0) | 0.0022 | N/A |
| Defined daily doses, U | 0.85 (0.38) | 0.64 (0.36) | 0.77 (0.32) | <0.0001 | N/A |
| Δ Home blood pressure, mm Hg | |||||
| Morning systolic | 8.3 (10.7) | 4.6 (10.6) | 8.2 (11.0) | <0.0001 | <0.0001 |
| Morning diastolic | 3.9 (6.2) | 2.8 (5.8) | 4.5 (6.2) | <0.0001 | <0.0001 |
| Evening systolic | 9.4 (11.7) | 7.0 (11.7) | 9.7 (12.6) | <0.0001 | <0.0001 |
| Evening diastolic | 4.5 (6.7) | 4.0 (6.7) | 5.0 (7.5) | 0.016 | 0.0021 |
| Δ Home VIM, U | |||||
| Morning systolic | 0.12 (5.17) | 0.20 (5.72) | 0.25 (5.56) | 0.86 | 0.057 |
| Morning diastolic | 0.14 (3.05) | −0.09 (3.25) | −0.02 (3.12) | 0.16 | 0.054 |
| Evening systolic | 0.69 (5.95) | 0.39 (6.06) | 0.49 (6.14) | 0.48 | 0.12 |
| Evening diastolic | 0.29 (3.70) | 0.09 (3.72) | 0.19 (3.64) | 0.34 | 0.12 |
| Δ Home ARV, mm Hg | |||||
| Morning systolic | −0.08 (6.62) | 0.19 (7.67) | 0.42 (7.08) | 0.34 | 0.091 |
| Morning diastolic | 0.21 (3.87) | −0.27 (4.17) | 0.03 (4.14) | 0.050 | 0.012 |
| Evening systolic | 0.76 (8.28) | 0.35 (8.48) | 0.46 (8.04) | 0.42 | 0.24 |
| Evening diastolic | 0.33 (5.04) | 0.16 (5.12) | 0.15 (5.12) | 0.80 | 0.16 |
Values are arithmetic mean (SD) except averaged follow‐up days as median (interquartile range). Crude P denotes the significance of the difference among drug classes, and adjusted P displays the significance which accounts for sex, age, body mass index, corresponding blood pressure level or variability and heart rate at baseline, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and defined daily doses. ARV indicates average real variability; N/A, not available; VIM, variability independent of the mean.
Table 6.
SD and Coefficient of Variation of Home Blood Pressure at Baseline and After the Monotherapy Among 3 Drug Classes
| Characteristic | Calcium Channel Blocker | Angiotensin‐Converting Enzyme Inhibitor | Angiotensin Receptor Blocker | Crude P Value | Adjusted P Value |
|---|---|---|---|---|---|
| At baseline | |||||
| SD, mm Hg | |||||
| Morning systolic | 8.80 (4.12) | 9.40 (4.52) | 9.27 (4.43) | 0.019 | 0.0009 |
| Morning diastolic | 5.07 (2.45) | 5.04 (2.58) | 5.22 (2.70) | 0.32 | 0.28 |
| Evening systolic | 10.71 (4.75) | 10.71 (4.84) | 11.01 (5.25) | 0.62 | 0.26 |
| Evening diastolic | 6.38 (3.04) | 6.42 (3.13) | 6.52 (3.12) | 0.66 | 0.31 |
| Coefficient of variation, % | |||||
| Morning systolic | 5.81 (2.68) | 6.21 (2.89) | 6.16 (2.90) | 0.0090 | 0.0017 |
| Morning diastolic | 5.68 (2.77) | 5.69 (3.06) | 5.91 (3.18) | 0.27 | 0.21 |
| Evening systolic | 7.48 (3.31) | 7.50 (3.37) | 7.72 (3.74) | 0.68 | 0.25 |
| Evening diastolic | 7.75 (3.63) | 7.85 (3.80) | 8.01 (3.92) | 0.55 | 0.21 |
| After monotherapy | |||||
| Δ SD, mm Hg | |||||
| Morning systolic | 0.19 (5.17) | 0.09 (5.78) | 0.34 (5.48) | 0.80 | 0.0084 |
| Morning diastolic | 0.15 (3.06) | −0.12 (3.25) | 0.01 (3.11) | 0.11 | 0.060 |
| Evening systolic | 0.72 (6.05) | 0.31 (6.11) | 0.54 (6.19) | 0.32 | 0.085 |
| Evening diastolic | 0.29 (3.76) | 0.06 (3.74) | 0.21 (3.64) | 0.31 | 0.11 |
| Δ Coefficient of variation, % | |||||
| Morning systolic | −0.21 (3.51) | −0.15 (3.89) | −0.15 (3.79) | 0.81 | 0.078 |
| Morning diastolic | −0.08 (3.50) | −0.31 (3.82) | −0.28 (3.64) | 0.18 | 0.043 |
| Evening systolic | 0.05 (4.28) | −0.15 (4.38) | −0.10 (4.45) | 0.50 | 0.12 |
| Evening diastolic | −0.05 (4.61) | −0.30 (4.67) | −0.17 (4.62) | 0.35 | 0.12 |
Values are arithmetic mean (SD). Crude P denotes the significance of the difference among drug classes, and adjusted P displays the significance which accounts for sex, age, body mass index, corresponding blood pressure level, variability, and heart rate at baseline, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and defined daily doses.
Reduction of blood pressure level and variability were further compared between patients with amlodipine (n=439) and other CCBs (n=394) prescribed as the first‐line drug (Table 7). Amlodipine significantly lowered blood pressure level more than other CCBs (systolic/diastolic blood pressure: 10.2/5.0 versus 6.0/2.7 mm Hg, respectively, in the morning and 11.3/5.4 versus 7.4/3.5 mm Hg, respectively, in the evening; P<0.0001), and this was confirmed by VIM and ARV calculated by the morning systolic measurement (P≤0.018); however, VIM and ARV derived from morning diastolic, evening systolic, and evening diastolic measurements did not differ (P≥0.19).
Table 7.
Baseline Blood Pressure Level and Variability and Reduction of These Indexes After Monotherapy Among Patients With Amlodipine or Other CCB
| Characteristic | Amlodipine (n=439) | Other CCB (n=394) | Crude P Value | Adjusted P Value |
|---|---|---|---|---|
| Baseline measurement | ||||
| Home blood pressure, mm Hg | ||||
| Morning systolic | 152.9 (12.3) | 150.1 (12.7) | 0.0004 | <0.0001 |
| Morning diastolic | 90.8 (9.6) | 88.9 (9.7) | 0.0016 | 0.0001 |
| Evening systolic | 145.1 (15.0) | 143.0 (15.6) | 0.058 | 0.0023 |
| Evening diastolic | 83.5 (10.6) | 82.0 (10.7) | 0.039 | 0.0061 |
| Home VIM, U | ||||
| Morning systolic | 8.67 (4.24) | 8.90 (3.82) | 0.16 | 0.39 |
| Morning diastolic | 5.05 (2.43) | 5.09 (2.45) | 0.72 | 0.036 |
| Evening systolic | 10.52 (4.47) | 10.92 (4.95) | 0.33 | <0.0001 |
| Evening diastolic | 6.34 (2.74) | 6.42 (3.24) | 0.77 | <0.0001 |
| Home ARV, mm Hg | ||||
| Morning systolic | 10.04 (5.26) | 9.99 (4.77) | 0.83 | 0.090 |
| Morning diastolic | 5.75 (3.03) | 5.81 (3.07) | 0.68 | 0.0053 |
| Evening systolic | 12.48 (5.93) | 12.84 (6.75) | 0.80 | 0.0006 |
| Evening diastolic | 7.46 (3.69) | 7.55 (4.42) | 0.54 | <0.0001 |
| After the monotherapy | ||||
| Δ Home blood pressure, mm Hg | ||||
| Morning systolic | 10.24 (10.63) | 6.03 (10.26) | <0.0001 | <0.0001 |
| Morning diastolic | 5.00 (6.27) | 2.67 (5.78) | <0.0001 | <0.0001 |
| Evening systolic | 11.27 (11.75) | 7.36 (11.27) | <0.0001 | <0.0001 |
| Evening diastolic | 5.39 (6.61) | 3.52 (6.69) | <0.0001 | <0.0001 |
| Δ Home VIM, U | ||||
| Morning systolic | 0.35 (5.18) | −0.14 (5.16) | 0.30 | 0.018 |
| Morning diastolic | 0.23 (2.90) | 0.05 (3.21) | 0.54 | 0.29 |
| Evening systolic | 0.52 (5.90) | 0.89 (6.01) | 0.80 | 0.63 |
| Evening diastolic | 0.27 (3.60) | 0.32 (3.82) | 0.92 | 0.92 |
| Δ Home ARV, mm Hg | ||||
| Morning systolic | 0.44 (6.63) | −0.65 (6.56) | 0.035 | 0.0023 |
| Morning diastolic | 0.32 (3.74) | 0.09 (4.01) | 0.62 | 0.19 |
| Evening systolic | 0.69 (8.11) | 0.84 (8.47) | 0.68 | 0.89 |
| Evening diastolic | 0.37 (4.74) | 0.28 (5.35) | 0.51 | 0.51 |
Values are arithmetic mean (SD). Crude P denotes the significance of the difference between amlodipine and other CCBs, and adjusted P displays the significance which accounts for sex, age, body mass index, corresponding blood pressure level (for VIM and ARV) or variability (for level) at baseline, corresponding heart rate at baseline, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and defined daily doses. ARV indicates average real variability; CCB, calcium channel blocker; VIM, variability independent of the mean.
Incidence of Events
Over a median follow‐up of 7.3 years (interquartile range 4.8–9.1 years; maximum 11.5 years), 61 patients died (3.62 per 1000 person‐years), 43 (2.57 per 1000 person‐years) experienced hard cardiovascular events, and 80 (4.84 per 1000 person‐years) experienced broader cardiovascular events. Considering cause‐specific first cardiovascular events, 32 patients had stroke and 10 had myocardial infarction.
Outcomes in Relation to Blood Pressure Level and Variability
In multivariable adjusted models (Table 8), the systolic morning home blood pressure measured at baseline predicted the hard cardiovascular end point (HR per 1‐SD increment: 1.67; 95% CI 1.29–2.18; P=0.0001) and composite broader cardiovascular events (HR 1.44; 95% CI 1.18–1.75; P=0.0003), which were confirmatory with diastolic pressure (P≤0.040). In models including morning blood pressure level, morning VIM and ARV did not predict any cardiovascular outcome (P≥0.056). Evening VIM in systolic and diastolic blood pressures and evening ARV in diastolic blood pressure significantly predicted future hard end points independent of the mean level of evening blood pressure (P≤0.022). Level and variability did not predict total mortality (P≥0.18).
Table 8.
Adjusted HRs for End Points in Relation to the Level and Variability of Blood Pressure at Baseline
| End Point | Basic Model, BP Level | Full Model, VIM | Full Model, ARV |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Morning systolic measurement | |||
| Hard end point | 1.67 (1.29–2.18)c | 1.03 (0.76–1.39) | 1.09 (0.81–1.45) |
| Composite CV event | 1.44 (1.18–1.75)c | 0.99 (0.81–1.22) | 1.02 (0.82–1.26) |
| Total mortality | 0.95 (0.72–1.24) | 0.99 (0.77–1.28) | 1.11 (0.87–1.41) |
| Morning diastolic measurement | |||
| Hard end point | 1.48 (1.02–2.15)a | 0.93 (0.67–1.29) | 0.95 (0.73–1.24) |
| Composite CV event | 1.38 (1.06–1.78)a | 0.84 (0.66–1.08) | 0.82 (0.67–1.01) |
| Total mortality | 0.81 (0.60–1.10) | 0.97 (0.75–1.24) | 1.07 (0.84–1.36) |
| Evening systolic measurement | |||
| Hard end point | 1.36 (1.03–1.79)a | 1.31 (1.04–1.64)a | 1.11 (0.87–1.43) |
| Composite CV event | 1.32 (1.10–1.59)b | 1.07 (0.88–1.29) | 1.01 (0.83–1.22) |
| Total mortality | 0.93 (0.71–1.20) | 1.06 (0.83–1.35) | 1.13 (0.89–1.43) |
| Evening diastolic measurement | |||
| Hard end point | 1.30 (0.95–1.78) | 1.25 (1.04–1.49)a | 1.27 (1.06–1.53)a |
| Composite CV event | 1.32 (1.07–1.63)b | 1.14 (0.96–1.35) | 1.17 (1.00–1.38) |
| Total mortality | 0.91 (0.69–1.21) | 1.01 (0.79–1.30) | 1.09 (0.86–1.39) |
Home blood pressure level and variability are based on self‐measurement in the morning and in the evening for 3 to 5 days before treatment (average 4.95 and 4.93 days, respectively). The numbers of patients with hard end points, composite CV events, and total mortality were 43, 80, and 61, respectively. The basic model includes in addition to blood pressure level, sex, age, body mass index, corresponding heart rate, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of CV disease, and antihypertensive drug classes. Full models include the aforementioned covariables plus VIM or ARV. HRs given with 95% CIs express the risk associated with a 1‐SD increase in the explanatory variables. ARV indicates average real variability; CV indicates cardiovascular; HR, hazard ratio; VIM, variability independent of the mean.
P<0.05.
P<0.01.
P<0.001.
HRs for end points in relation to mean and variability of systolic and diastolic blood pressures measured during monotherapy are shown in Table 9. In systolic measurements, morning blood pressure was a significant predictor of hard (HR 1.56; 95% CI 1.15–2.11, P=0.0041) and broader (HR 1.37; 95% CI 1.11–1.69; P=0.0029) cardiovascular events. This was also the case with diastolic pressure and measurements captured in the evening (P≤0.048). None of the variability indexes, however, predicted cardiovascular end points in models with blood pressure level, regardless of morning or evening measurements after treatment initiation (P≥0.22). Total mortality was not associated with blood pressure information (P≥0.10).
Table 9.
Adjusted HRs for End Points in Relation to the Level and Variability of Blood Pressure After Monotherapy
| End Point | Basic Model, BP Level | Full Model, VIM | Full Model, ARV |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Morning systolic measurement | |||
| Hard end point | 1.56 (1.15–2.11)b | 1.07 (0.82–1.41) | 1.08 (0.80–1.44) |
| Composite CV event | 1.37 (1.11–1.69)b | 0.92 (0.74–1.14) | 0.92 (0.73–1.14) |
| Total mortality | 1.09 (0.85–1.39) | 1.08 (0.84–1.38) | 1.19 (0.96–1.49) |
| Morning diastolic measurement | |||
| Hard end point | 1.55 (1.09–2.22)a | 1.19 (0.90–1.57) | 1.14 (0.87–1.50) |
| Composite CV event | 1.32 (1.02–1.70)a | 1.00 (0.77–1.30) | 1.03 (0.82–1.29) |
| Total mortality | 0.89 (0.67–1.20) | 0.81 (0.61–1.07) | 0.79 (0.59–1.05) |
| Evening systolic measurement | |||
| Hard end point | 1.51 (1.18–1.92)c | 1.10 (0.83–1.47) | 1.10 (0.86–1.41) |
| Composite CV event | 1.30 (1.08–1.56)b | 1.03 (0.84–1.26) | 1.05 (0.88–1.27) |
| Total mortality | 1.22 (0.95–1.57) | 0.94 (0.73–1.21) | 0.96 (0.75–1.23) |
| Evening diastolic measurement | |||
| Hard end point | 1.40 (1.03–1.91)a | 1.00 (0.81–1.25) | 1.11 (0.90–1.36) |
| Composite CV event | 1.24 (1.00–1.54)a | 0.91 (0.73–1.13) | 0.99 (0.81–1.21) |
| Total mortality | 1.15 (0.87–1.50) | 0.96 (0.75–1.22) | 0.97 (0.76–1.25) |
Home blood pressure level and variability are based on self‐measurement in the morning and in the evening for 3 to 5 days after the monotherapy (average 4.88 and 4.80 days, respectively). The numbers of patients with hard end points, composite CV events, and total mortality were 43, 80, and 61, respectively. The basic model includes in addition to blood pressure level, sex, age, body mass index, corresponding heart rate, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of CV disease, and antihypertensive drug classes. Full models include the aforementioned covariables plus VIM or ARV. Results were confirmatory when defined daily doses of the monotherapy were further included in the models. HRs given with 95% CIs express the risk associated with a 1‐SD increase in the explanatory variables. ARV indicates average real variability; CV indicates cardiovascular; HR, hazard ratio; VIM, variability independent of the mean.
P<0.05.
P<0.01.
P<0.001.
Figures 2 and 3 illustrate the multivariable adjusted 10‐year risk of hard cardiovascular diseases in relation to the mean level and VIM of morning and evening systolic blood pressures measured at baseline (Figure 2) and after monotherapy (Figure 3). Home blood pressure consistently predicted cardiovascular events (P≤0.043). Evening home VIM predicted hard cardiovascular diseases significantly (P=0.043), independent of evening home blood pressure level; however, VIM derived from morning measurement at baseline was not a meaningful scale for hard cardiovascular end points (P=0.86), and VIM captured during monotherapy did not predict outcome (P≥0.54). As shown in Figure 4, home evening ARV also predicted hard cardiovascular events (P=0.045), but morning ARV did not (P=0.55). Home ARV under monotherapy had little impact on cardiovascular outcome (P≥0.52) (Figure 5).
Figure 2.
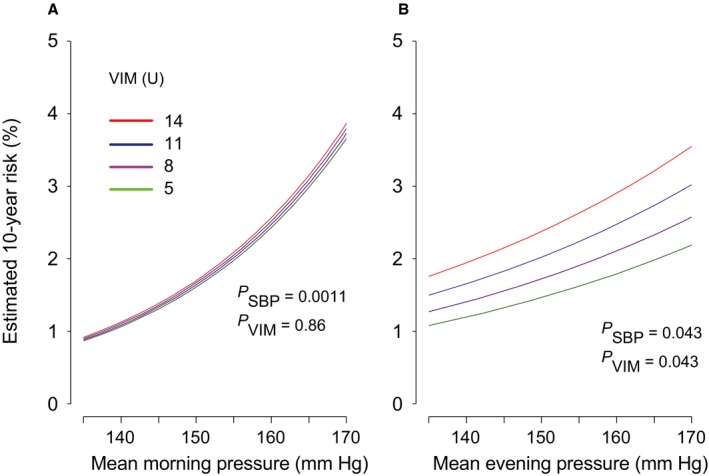
The 10‐year estimated absolute risk of hard cardiovascular events associated with the mean level of systolic home blood pressure and VIM in the morning (A) or the evening (B), measured at baseline. The risk functions were standardized to the distribution (mean or ratio) of sex, age, body mass index, corresponding heart rate, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and antihypertensive drug classes. Among 2484 patients, 43 hard cardiovascular events occurred. Mean systolic blood pressure along the horizontal axis covers 135 to 170 mm Hg. Four continuous lines represent the risk independently associated with VIM equal to 5, 8, 11, and 14 U. P values are for the independent effect of systolic blood pressure (PSBP) and VIM (PVIM). VIM indicates variability independent of the mean.
Figure 3.
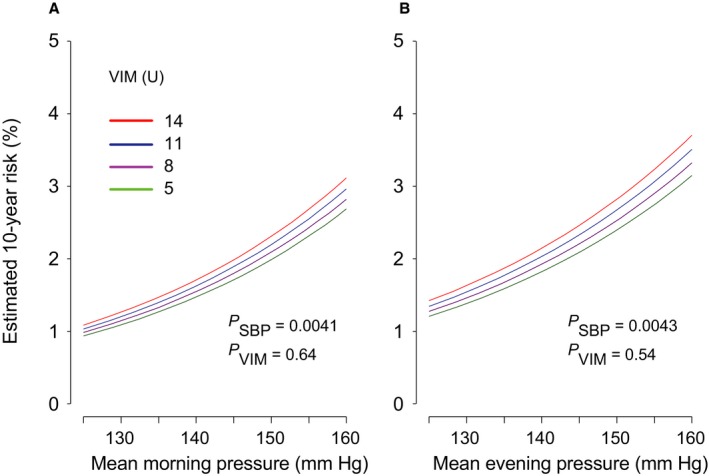
The 10‐year estimated absolute risk of hard cardiovascular events associated with the mean level of systolic home blood pressure and VIM in the morning (A) or the evening (B), measured after monotherapy. The risk functions were standardized to the distribution (mean or ratio) of sex, age, body mass index, corresponding heart rate, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and antihypertensive drug classes. Among 2484 patients, 43 hard cardiovascular events occurred. Mean systolic blood pressure along the horizontal axis covers 125 to 160 mm Hg. Four continuous lines represent the risk independently associated with VIM equal to 5, 8, 11, and 14 U. P values are for the independent effect of systolic blood pressure (PSBP) and VIM (PVIM). VIM indicates variability independent of the mean.
Figure 4.

The 10‐year estimated absolute risk of hard cardiovascular events associated with the mean level of systolic home blood pressure and ARV in the morning (A) or the evening (B), measured at baseline. The risk functions were standardized to the distribution (mean or ratio) of sex, age, body mass index, corresponding heart rate, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and antihypertensive drug classes. Among 2484 patients, 43 hard cardiovascular events occurred. Mean systolic blood pressure along the horizontal axis covers 135 to 170 mm Hg. Four continuous lines represent the risk independently associated with ARV equal to 6, 9, 12, and 15 mm Hg. P values are for the independent effect of systolic blood pressure (PSBP) and ARV (PARV). ARV indicates average real variability.
Figure 5.
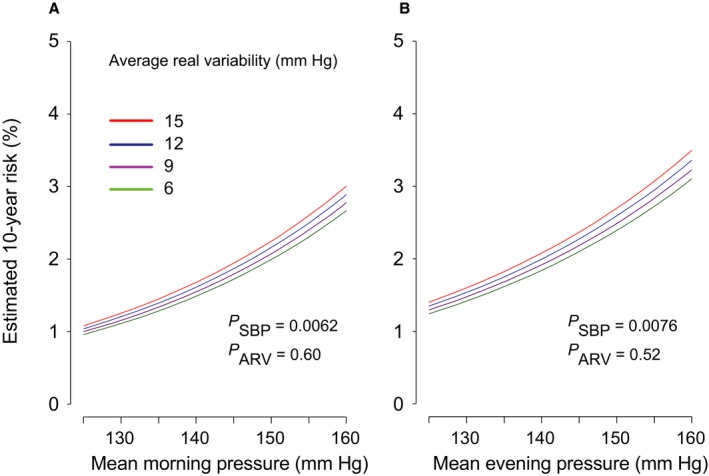
The 10‐year estimated absolute risk of hard cardiovascular events associated with the mean level of systolic home blood pressure and ARV in the morning (A) or the evening (B), measured after monotherapy. The risk functions were standardized to the distribution (mean or ratio) of sex, age, body mass index, corresponding heart rate, current smoking and drinking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, and antihypertensive drug classes. Among 2484 patients, 43 hard cardiovascular events occurred. Mean systolic blood pressure along the horizontal axis covers 125 to 160 mm Hg. Four continuous lines represent the risk independently associated with ARV equal to 6, 9, 12, and 15 mm Hg. P values are for the independent effect of systolic blood pressure (PSBP) and ARV (PARV). ARV indicates average real variability.
Discussion
Our study population comprised treatment‐naïve or washout patients on antihypertensive drugs at baseline; after inclusion, they were followed up over a median of 7.3 years with adjustment of antihypertensive drug treatment based on self‐measured home blood pressure. We investigated whether blood pressure variability is influenced by antihypertensive drug classes and adds to risk prediction beyond mean blood pressure level. We found (1) that the effects of antihypertensive drug therapy on blood pressure variability changes did not differ; (2) that morning blood pressure variability at baseline did not predict any outcomes in this population; (3) that evening blood pressure variability captured at baseline predicted hard cardiovascular end points independent of blood pressure level; and (4) that blood pressure variability, calculated 10 to 28 days after monotherapy started, had no predictive power for cardiovascular events, regardless of morning or evening measurement.
Recent studies suggest that stroke risk can be reduced more effectively by targeting systolic blood pressure variability along with blood pressure level using CCBs.24, 25 Webb and colleagues assessed the differences in home systolic blood pressure variability from 3 to 10 days before to 8 to 15 days after starting or increasing combinations of CCBs and diuretics, renin and angiotensin system inhibitors, or both drug classes among 288 patients with transient ischemic attack or minor stroke.8 Home blood pressure were measured 3 times on 1 occasion and at 3 different times of day (ie, awakening, midmorning, and before sleep). Day‐to‐day home blood pressure variability was represented by the residual coefficient of variation with a moving average of >5 days for the mean of all clusters and the mean of clusters at each time of day. Variability at 3 to 10 days before randomization was similar for all clusters and for morning measurements regardless of the 3 antihypertensive drug groups (P≥0.62), whereas the residual coefficient of variation in patients with CCBs and diuretics was significantly decreased when compared with the other 2 groups (P≤0.015) at 8 to 15 days after the intervention. Nevertheless, we are opposed to their suggestion8, 25, 26 on the ground that the effect of antihypertensive drug therapy on blood pressure variability changes did not differ in this HOMED‐BP population (n=2484). Our results were based on precisely tracked information about antihypertensive drug agents during treatment initiation. We acknowledged that amlodipine significantly reduced morning systolic VIM and ARV more than other CCBs (Table 7) (0.35 versus −0.14 U in VIM and 0.44 versus −0.65 mm Hg in ARV; adjusted P≤0.018). Amlodipine is known to have a long elimination half‐life—34 hours, according to a review.26 This long‐acting effect may settle the variability of morning blood pressure. Nevertheless, we would emphasize that at randomization, selection of a drug agent within a drug class (eg, amlodipine or other CCBs within the CCB group) was based entirely on the doctor's judgment.10, 13 Favorable effects of amlodipine were reported during the HOMED‐BP trial, for example, a comparably powerful blood pressure–lowering effect27 and a preventive effect for cardiovascular events28 that might be achieved by central blood pressure reduction.29 Consequently, we cannot eliminate the potential bias for the judgment of amlodipine usage in the study population. Changes in morning diastolic and evening systolic and diastolic blood pressure variability indexes did not differ (P≥0.19), and variability derived from the morning measurement did not predict any outcomes before and after treatment initiation (Tables 8 and 9). Those results suggest that differences in antihypertensive drug classes regarding morning blood pressure variability have less impact in clinical practice.
James was among those who observed the circadian pattern of blood pressure regarding work stress.30, 31 His team investigated 110 normotensive young women aged 29.7±7.2 years who were employed at a hospital with a day shift.31 Along with ambulatory blood pressure monitoring, each woman self‐rated her perceived stress in work and home environments. Women who perceived greater stress at work had significantly higher blood pressure at work than women who perceived equal or greater stress at home (123 versus 115 mm Hg systolic and 78 versus 74 mm Hg diastolic, P<0.01), whereas no significant differences in pressures were measured in the home environment between the 2 groups. These effects were independent of weight, age, and menstrual cycle. James et al suggested that the perception of stress has a significant effect on the circadian pattern of blood pressure variation.31 Although it should not be pushed so far, instability of blood pressure in the late evening may reflect the diversity of daily activities, and the introduction of antihypertensive drug treatment can preclude residual impact of instability, resulting in the disappearance of differences of prognostic impact on blood pressure variability after the treatment initiated in the present patients.
Our current study must be interpreted within the context of several potential limitations. First, because the patients in HOMED‐BP received home blood pressure–guided therapy,10 treatment was adjusted according to self‐measured home blood pressure level, not variability. Second, we based our analyses on only a single blood pressure reading in the morning and the evening over 5 days; there were 5 consecutive home blood pressure readings in a European study population32 and readings over 7 days in the Finn‐Home study6 and a median of 26 days in the Ohasama study.5 Regarding the home blood pressure level, we reported that even a single measurement is a potent predictor of stroke both in the morning33 and the evening,34 and a longer period of measurement could increase diagnostic accuracy.33, 34, 35 Little information, however, is available on how many days are required to capture blood pressure variability in a reliable way. Third, we excluded 1034 of the original 3518 randomized patients. Although baseline characteristics were balanced properly, randomization might not be a given in this post hoc analysis. Fourth, we measured home blood pressure with an upper arm cuff oscillometric device,14 which could not capture information on central blood pressure. Blood pressure variability based on central blood pressure might have different prognostic significance. Finally, although our results are representative for health care provided to middle‐aged and older Japanese participants, they might not be applicable to other settings or ethnic groups with different distributions of risk factors.
Perspectives
We found that the independent prognostic significance of home blood pressure variability was derived from evening systolic pressure before treatment allocation; however, we were unable to modify blood pressure variability consciously by choosing a drug class of antihypertensive agents, at least among CCBs, ACEIs, and ARB, according to the current findings. Blood pressure variability derived from morning home blood pressure did not substantially refine risk profiling beyond the blood pressure level. Consequently, we emphasize that home blood pressure level is the predominant risk factor and should remain the primary focus for risk stratification and treatment in clinical practice because home blood pressure is manageable by lifestyle modifications and adequate antihypertensive drug treatment.10 Nevertheless, we may deal with evening home blood pressure variability as the independent risk factor, not as the treatment target but rather for assessment of the comprehensive cardiovascular disease risk.
Sources of Funding
This work was supported by the Japan Cardiovascular Research Foundation; the Japan Arteriosclerosis Prevention Fund; Tohoku University 21st Center of Excellence Program “Comprehensive Research and Education Center for Planning of Drug Development and Clinical Evaluation”; Grant‐in‐Aid for Scientific Research (23390171, 25253059, 26860093) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Grant‐in‐Aid for Japan Society for the Promotion of Science (JSPS) fellows (25.7756 and 25.9328).
Disclosures
Omron Healthcare gave research support to Y Imai. None of the other authors has a conflict of interest.
Supporting information
Appendix S1. Hypertensive Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED‐BP) study investigators.
Acknowledgments
We are grateful all HOMED‐BP study collaborators as listed in Appendix S1 for their valuable contribution. We thank the staff of Tohoku University and Teikyo University for their valuable help.
(J Am Heart Assoc. 2016;5:e002995 doi: 10.1161/JAHA.115.002995)
Part of the work was presented at the 2013 Scientific Meeting of the Belgian Hypertension Committee held on October 5, 2013 in Gent, Belgium.
References
- 1. Staessen JA, Thijs L, Ohkubo T, Kikuya M, Richart T, Boggia J, Adiyaman A, Dechering DG, Kuznetsova T, Thien T, de Leeuw P, Imai Y, O'Brien E, Parati G. Thirty years of research on diagnostic and therapeutic thresholds for the self‐measured blood pressure at home. Blood Press Monit. 2008;13:352–365. [DOI] [PubMed] [Google Scholar]
- 2. Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
- 4. Hara A, Thijs L, Asayama K, Jacobs L, Wang JG, Staessen JA. Randomised double‐blind comparison of placebo and active drugs for effects on risks associated with blood pressure variability in the Systolic Hypertension in Europe trial. PLoS One. 2014;9:e103169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asayama K, Kikuya M, Schutte R, Thijs L, Hosaka M, Satoh M, Hara A, Obara T, Inoue R, Metoki H, Hirose T, Ohkubo T, Staessen JA, Imai Y. Home blood pressure variability as cardiovascular risk factor in the population of Ohasama. Hypertension. 2013;61:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home‐measured blood pressure and heart rate: the Finn‐Home Study. Hypertension. 2012;59:212–218. [DOI] [PubMed] [Google Scholar]
- 7. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375:906–915. [DOI] [PubMed] [Google Scholar]
- 8. Webb AJ, Wilson M, Lovett N, Paul N, Fischer U, Rothwell PM. Response of day‐to‐day home blood pressure variability by antihypertensive drug class after transient ischemic attack or nondisabling stroke. Stroke. 2014;45:2967–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noguchi Y, Asayama K, Staessen JA, Inaba M, Ohkubo T, Hosaka M, Satoh M, Kamide K, Awata T, Katayama S, Imai Y; the HOMED‐BP study group . Predictive power of home blood pressure and clinic blood pressure in hypertensive patients with impaired glucose metabolism and diabetes. J Hypertens. 2013;31:1593–1602. [DOI] [PubMed] [Google Scholar]
- 10. Asayama K, Ohkubo T, Metoki H, Obara T, Inoue R, Kikuya M, Thijs L, Staessen JA, Imai Y. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self‐measured home blood pressure. Hypertens Res. 2012;35:1102–1110. [DOI] [PubMed] [Google Scholar]
- 11. Hansson L, Hedner T, Dahlof B. Prospective randomized open blinded end‐point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End‐Point. Blood Press. 1992;1:113–119. [DOI] [PubMed] [Google Scholar]
- 12. World Medical Association declaration of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- 13. Fujiwara T, Nishimura T, Ohkuko T, Imai Y. Rationale and design of HOMED‐BP Study: hypertension objective treatment based on measurement by electrical devices of blood pressure study. Blood Press Monit. 2002;7:77–82. [DOI] [PubMed] [Google Scholar]
- 14. Chonan K, Kikuya M, Araki T, Fujiwara T, Suzuki M, Michimata M, Hashimoto J, Ohkubo T, Hozawa A, Yamamoto N, Miyawaki Y, Matsubara M, Imai Y. Device for the self‐measurement of blood pressure that can monitor blood pressure during sleep. Blood Press Monit. 2001;6:203–205. [DOI] [PubMed] [Google Scholar]
- 15. White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM‐907 monitor in adults. Blood Press Monit. 2001;6:107–110. [DOI] [PubMed] [Google Scholar]
- 16. Kamide K, Asayama K, Katsuya T, Ohkubo T, Hirose T, Inoue R, Metoki H, Kikuya M, Obara T, Hanada H, Thijs L, Kuznetsova T, Noguchi Y, Sugimoto K, Ohishi M, Morimoto S, Nakahashi T, Takiuchi S, Ishimitsu T, Tsuchihashi T, Soma M, Higaki J, Matsuura H, Shinagawa T, Sasaguri T, Miki T, Takeda K, Shimamoto K, Ueno M, Hosomi N, Kato J, Komai N, Kojima S, Sase K, Miyata T, Tomoike H, Kawano Y, Ogihara T, Rakugi H, Staessen JA, Imai Y; GEANE study group, HOMED‐BP study group . Genome‐wide response to antihypertensive medication using home blood pressure measurements: a pilot study nested within the HOMED‐BP study. Pharmacogenomics. 2013;14:1709–1721. [DOI] [PubMed] [Google Scholar]
- 17. Metoki H, Ohkubo T, Kikuya M, Asayama K, Inoue R, Obara T, Hirose T, Sato M, Imai Y. The velocity of antihypertensive effect of losartan/hydrochlorothiazide and angiotensin II receptor blocker. J Hypertens. 2012;30:1478–1486. [DOI] [PubMed] [Google Scholar]
- 18. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes M , Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . World Health Organization Collaborating Centre for Drug Statistics Methodology System of Defined Daily Doses. 2011. Available at: http://www.whocc.no/atc_ddd_index/. Accessed August 23, 2011.
- 20. Asayama K, Ohkubo T, Kikuya M, Obara T, Metoki H, Inoue R, Hara A, Hirose T, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. Prediction of stroke by home “morning” versus “evening” blood pressure values: the Ohasama study. Hypertension. 2006;48:737–743. [DOI] [PubMed] [Google Scholar]
- 21. Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–511. [DOI] [PubMed] [Google Scholar]
- 22. Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund‐Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp‐Pedersen C, Dolan E, Kuznetsova T, Stolarz‐Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka‐Jaszcz K, Imai Y, Wang J, Ibsen H, O'Brien E, Staessen JA. Prognostic value of reading‐to‐reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. [DOI] [PubMed] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24. Webb AJ, Rothwell PM. Effect of dose and combination of antihypertensives on interindividual blood pressure variability: a systematic review. Stroke. 2011;42:2860–2865. [DOI] [PubMed] [Google Scholar]
- 25. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Poulter NR, Sever PS. Effects of beta blockers and calcium‐channel blockers on within‐individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. [DOI] [PubMed] [Google Scholar]
- 26. Abernethy DR. The pharmacokinetic profile of amlodipine. Am Heart J. 1989;118:1100–1103. [DOI] [PubMed] [Google Scholar]
- 27. Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 28. Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 29. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M; the CAFE Investigators, for the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT) Investigators, CAFE Steering Committee and Writing Committee . Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 30. James GD, Cates EM, Pickering TG, Laragh JH. Parity and perceived job stress elevate blood pressure in young normotensive working women. Am J Hypertens. 1989;2:637–639. [DOI] [PubMed] [Google Scholar]
- 31. James GD. Race and perceived stress independently affect the diurnal variation of blood pressure in women. Am J Hypertens. 1991;4:382–384. [DOI] [PubMed] [Google Scholar]
- 32. Kuznetsova T, Staessen JA, Kawecka‐Jaszcz K, Babeanu S, Casiglia E, Filipovsky J, Nachev C, Nikitin Y, Peleska J, O'Brien E. Quality control of the blood pressure phenotype in the European Project on Genes in Hypertension. Blood Press Monit. 2002;7:215–224. [DOI] [PubMed] [Google Scholar]
- 33. Ohkubo T, Asayama K, Kikuya M, Metoki H, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. How many times should blood pressure be measured at home for better prediction of stroke risk? Ten‐year follow‐up results from the Ohasama study. J Hypertens. 2004;22:1099–1104. [DOI] [PubMed] [Google Scholar]
- 34. Asayama K, Ohkubo T, Hara A, Hirose T, Yasui D, Obara T, Metoki H, Inoue R, Kikuya M, Totsune K, Hoshi H, Satoh H, Imai Y. Repeated evening home blood pressure measurement improves prognostic significance for stroke: a 12‐year follow‐up of the Ohasama study. Blood Press Monit. 2009;14:93–98. [DOI] [PubMed] [Google Scholar]
- 35. Niiranen TJ, Asayama K, Thijs L, Johansson JK, Hara A, Hozawa A, Tsuji I, Ohkubo T, Jula AM, Imai Y, Staessen JA; IDHOCO Investigators . Optimal number of days for home blood pressure measurement. Am J Hypertens. 2015;28:595–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Hypertensive Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED‐BP) study investigators.


