Abstract
Background
The majority of women with angina‐like chest pain have no obstructive coronary artery disease when evaluated with coronary angiography. Coronary microvascular dysfunction is a possible explanation and associated with a poor prognosis. This study evaluated the prevalence of coronary microvascular dysfunction and the association with symptoms, cardiovascular risk factors, psychosocial factors, and results from diagnostic stress testing.
Methods and Results
After screening 3568 women, 963 women with angina‐like chest pain and a diagnostic coronary angiogram without significant coronary artery stenosis (<50%) were consecutively included. Mean age (SD) was 62.1 (9.7). Assessment included demographic and clinical data, blood samples, questionnaires, and transthoracic echocardiography during rest and high‐dose dipyridamole (0.84 mg/kg) with measurement of coronary flow velocity reserve (CFVR) by Doppler examination of the left anterior descending coronary artery. CFVR was successfully measured in 919 (95%) women. Median (IQR) CFVR was 2.33 (1.98–2.76), and 241 (26%) had markedly impaired CFVR (<2). In multivariable regression analysis, predictors of impaired CFVR were age (P<0.01), hypertension (P=0.02), current smoking (P<0.01), elevated heart rate (P<0.01), and low high‐density lipoprotein cholesterol (P=0.02), but these variables explained only a little of the CFVR variation (r 2=0.09). CFVR was not associated with chest pain characteristics or results from diagnostic stress testing.
Conclusion
Impaired CFVR was detected in a substantial proportion, which suggests that coronary microvascular dysfunction plays a role in the development of angina pectoris. CFVR was associated with few cardiovascular risk factors, suggesting that CFVR is an independent parameter in the risk evaluation of these women. Symptom characteristics and results from stress testing did not identify individuals with impaired CFVR.
Keywords: angina pectoris, coronary artery disease, echocardiography, microvascular dysfunction, women
Subject Categories: Risk Factors, Women, Echocardiography, Coronary Artery Disease
Introduction
More than half of women with angina‐like chest pain referred for clinical coronary angiography (CAG) have no obstructive coronary artery disease (CAD), and this is twice as often seen in women compared with men after the CAG.1 While previously considered a benign condition, recent studies have found the condition to be associated with persistent chest pain, repeated angiograms, reduced quality of life, and increased cardiovascular morbidity and mortality.1, 2, 3 A possible explanation for the discrepancy between symptoms and CAG findings could be ischemia caused by coronary microvascular dysfunction (CMD). Recent studies have convincingly demonstrated that CMD is a strong predictor of cardiovascular prognosis,4, 5, 6 and CMD is common in both men and women with no obstructive CAD.4
Previous studies of CMD among subjects with angina with no obstructive CAD have been based on relatively small, selected populations and little is known about the burden of CMD. CMD can be assessed as reduced coronary flow velocity reserve (CFVR) invasively during the CAG,5 by positron emission tomography7, 8 or by transthoracic Doppler echocardiography (TTDE) of the left anterior descending coronary artery (LAD) during dipyridamole or adenosine infusion. With perhaps as little as 5% of patients with angina needing revascularization, as found in the PROspective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial,9 the need for assessment of CMD is expanding and it is important to also evaluate CMD outside of the few dedicated centers that routinely perform invasive assessment of CMD during the CAG. Although there is an increasing interest in CMD, several gaps in knowledge remain. Thus, little is known about the burden of CMD among women with angina and no obstructive CAD and the correlation with symptoms, results of stress tests, and risk factors, since this has never been systematically assessed in an unselected population. Also, knowledge regarding the prognostic implications of CMD and effective treatment targeting both symptoms and prognosis are needed.
The iPOWER study (ImProve diagnOsis and treatment of Women with angina pEctoris and micRovessel disease) aims to investigate diagnostic possibilities and prognosis of impaired CFVR in women with angina‐like chest pain and no obstructive CAD.10 We investigated CMD prevalence measured with TTDE‐assessed CFVR and the association with cardiovascular risk factors in women with angina who were consecutively sampled after diagnostic invasive CAG. Further, we examined whether CMD was associated with characteristics, severity, and frequency of angina‐like chest pain and results of diagnostic stress testing.
Methods
Population
Participants were recruited from the database PATS (Patient Analysis & Tracking System; Dendrite Clinical Systems), which covers eastern Denmark with ≈3 million inhabitants. All women (18–80 years) referred between March 2012 and September 2014 for a clinically indicated diagnostic CAG due to angina‐like chest pain and suspected obstructive CAD were screened according to previously described well‐defined inclusion and exclusion criteria (Figure 1).10 We included both women referred for stable angina and women hospitalized suspected of unstable angina, since the latter may be first manifestation of stable angina. Women with elevated cardiac markers or ST‐segment elevation were excluded (Figure 1). All women were included within 1 year of their CAG.
Figure 1.
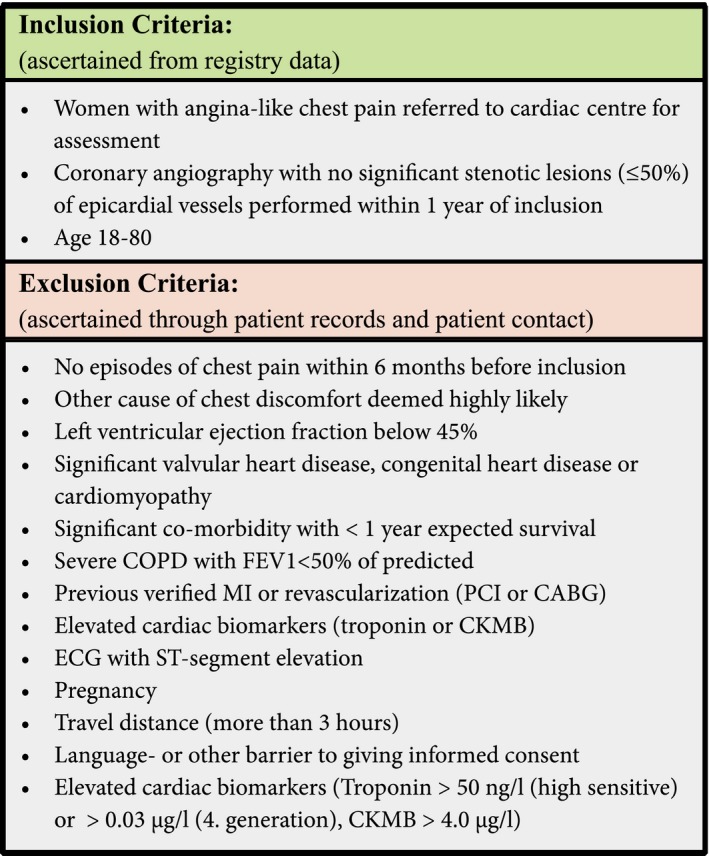
Inclusion and exclusion criteria in the iPOWER study.
Basic Examination
Basic assessment included clinical and demographic data. Trained health professionals interviewed participants regarding cardiac symptoms with respect to location, character, duration, radiation, frequency, and provoking and alleviating factors. According to the classical classification of chest pain symptoms were classified as typical angina pectoris, atypical angina pectoris and non‐cardiac chest pain.11, 12 Questionnaires regarding chest pain symptoms included the Seattle Angina Questionnaire, which evaluates 5 dimensions of functional status,13 and the World Health Organization's Rose's Angina Questionnaire, which evaluates symptoms as definite angina or not, further subdividing those with definite angina as severe or nonsevere.14
We obtained information regarding cardiovascular risk factors (age, body mass index [BMI], diabetes, hypertension, hyperlipidemia, smoking, family history of cardiovascular disease, and menopausal status), comorbidity, previous hospital admissions, and previous diagnostic tests, which included noninvasive cardiac computed tomography–angiography (CTA), exercise electrocardiography (ECG), and single‐photon emission computed tomography (SPECT) performed within 6 months before CAG from interviews and charts. ECG, blood pressure, and heart rate measures were obtained at rest, and abdominal circumference was measured. The ECG was analyzed for signs of ischemia (inverted T wave, pathological Q wave, ST‐segment depression), bundle‐branch block, and atrial fibrillation. Blood samples were analyzed for cholesterol levels (total, low‐density lipoprotein [LDL], and high‐density lipoprotein [HDL] cholesterol), triglycerides, creatinine level, and glycated hemoglobin (HbA1c). A spot urine sample was analyzed for microalbuminuria.
Echocardiographic Examination
All participants underwent standard transthorarcic echocardiography and TTDE of the LAD during rest and high‐dose dipyridamole stress (0.84 mg/kg) over 6 minutes to obtain coronary flow velocities (CFVs) at baseline and at maximal hyperemia. Different vasodilators can be used to induce hyperemia, but the majority of TTDE studies of CFVR have used dipyridamole. Adenosine and dipyridamole are regarded as equal to achieve peak coronary vasodilation15, 16 and are used interchangeably in clinical practice. Echocardiographic examinations were performed by using the GE Healthcare Vivid E9 cardiovascular ultrasound system (GE Healthcare) with a 1.3‐ to 4.0‐MHz transducer (GE Vivid 5S probe) for standard echocardiography and a 2.7‐ to 8‐MHz transducer (GE Vivid 6S probe) for TTDE. Images were stored for offline analysis (GE EchoPac v.112). The same 4 experienced echocardiographers performed all examinations in the same settings. Before examination, participants were instructed to be abstinent from caffeine or food containing significant amount of methylexanthine (coffee, tea, chocolate, cola, and banana) for 24 hours. Medication containing dipyridamole was paused for 48 hours; long‐lasting nitroglycerin, β‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin type II antagonists, calcium antagonists, and diuretics were paused for 24 hours; and short‐lasting nitroglycerin was paused for 1 hour before the examination. Participants were studied in the left lateral decubitus position. The octave was set at 3.1/6.2 MHz, frequency at 8 MHz for B‐mode (2D), while a baseline color scale between 1.00–2.50 kHz (velocity range ±10–24 cm/s) was chosen according to low or high flow velocities respectively. Color gain was adjusted to provide optimal 2‐dimensional imaging quality. LAD was visualized with color Doppler in an apical modified foreshortened 2‐ or 4 chamber view or in a modified short‐axis view of the left ventricle. CFV was measured with pulsed‐wave Doppler as a laminar flow toward the transducer. We aimed to align the ultrasound beam direction to the LAD flow by adjusting probe position during recording of 2‐dimensional and pulse‐wave images. In case of difficulty in visualization of the LAD, a microbubble contrast agent was used (SonoVue; Bracco Imaging). Acquisitions of CFV during dipyridamole infusion were obtained throughout the infusion or up to 3 minutes after the infusion had terminated until flow had reached peak velocity. Blood pressure and heart rate were measured every 3 minutes during the examination of CFVR. After the examination, intravenous theophylline (maximum dose 220 mg) was administered to relieve potential side effects of dipyridamole.
For the analysis of CFVR, diastolic peak flow velocities were measured at rest and at peak hyperemia (Figure 2). CFVR was calculated as the ratio between peak velocities during stress and during rest. Two experts, blinded to participant data, analyzed every CFVR examination independently. The first reading was used, except for estimates that differed by >0.2, in which case the 2 analyzers reanalyzed the CFVR examination and reached agreement. In our previous validation study with repeated TTDE CFVR examinations in 10 young, healthy subjects by the same observer, we found an intraclass correlation coefficient of 0.97 (95% CI 0.92–1.00) and coefficient of variation of 7% (95% CI 3–10%) for repeat examinations. In a subsample of 50 participants from the iPOWER study, CFVR readings for the 2 observers were highly reproducible.10
Figure 2.
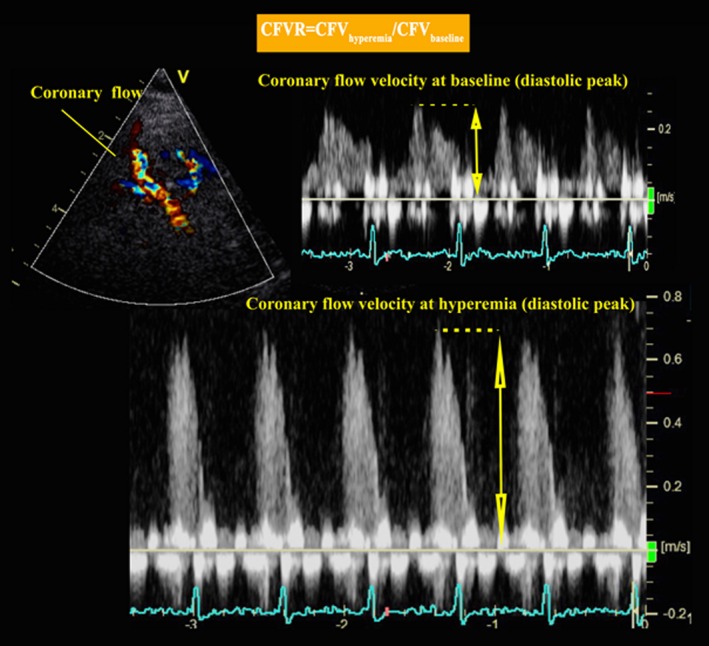
Measurement of coronary flow velocity. DPV indicates diastolic peak velocity.
Left ventricular ejection fraction (LVEF) was analyzed by a skilled echocardiographer as an automated biplane calculation (Auto‐EF tool; GE EchoPac v.112).
Statistical Analyses
Continuous variables with a Gaussian distribution are expressed as mean±SD (standard deviation) values. Median±IQR (interquartile range) values are used for variables with a non‐Gaussian distribution. Count in percent values is used for categorical variables. Distribution was assessed graphically. Difference between participants and nonparticipants was tested by using 1‐way ANOVA or χ2 test for continuous and categorical variables, respectively. Missing values in the Seattle Angina Questionnaire were imputed according to the validated scoring system.13
Participants were divided into 3 groups according to CFVR, based on current guidelines for determination of CMD by using a cutoff point of 2.017 and a previously used cutoff point of 2.5.18 Age‐adjusted trend tests by multivariable adjusted logistic or linear regression analysis were used to evaluate the distribution of variables (cardiovascular risk factors, clinical assessment, laboratory tests, results from diagnostic stress testing, medical history including medication, and psychosocial factors) among the 3 CFVR groups. Dependent variables with skewed distribution (smoking duration and menopause duration) were logarithmically transformed to base 2. Symptom characteristics from questionnaires were evaluated by using age‐adjusted trend tests or χ2 if parameters of interest were divided into 3 categories. Moreover, to explore whether each symptom variable was a predictor of reduced CFVR, age‐adjusted linear regression analyses were performed with natural logarithmically transformed CFVR as outcome variable because of non‐Gaussian distribution. Interaction analysis was performed to investigate possible differences in association between participants regarded as having stable or unstable angina at the time of the CAG.
To explore predictors of reduced CFVR, multivariable linear regression analyses were performed with natural logarithmically transformed CFVR. All potential explanatory variables with an a priori defined hypothesis (age, hypertension, smoking status, diabetes, BMI, cholesterol, postmenopausal status, systolic blood pressure, resting heart rate, nonobstructive atherosclerosis at CAG, HDL, non‐high‐density lipoprotein cholesterol (non‐HDL), and triglycerides) were tested in a prioritized order as determinants of CFVR and discarded at a cutoff level of P≥0.10. Assumptions of linearity, variance homogeneity, and Gaussian distribution of residuals were assessed graphically. The logarithmically transformed parameter estimates in the regression equation were converted back to the original scale for interpretation as an expected percentage change in CFVR value for each parameter by using the equation: 1−(eparameterestimate)×100%. Interaction analysis was performed to investigate difference in association between participants with stable or unstable angina. A likelihood ratio test was used to test difference between the final model and a model including interactions.
Confidence interval (CI) refer to 95% intervals, and a 2‐sided P value <0.05 was considered significant. All analyses were performed by using STATA/IC 13.1 (StataCorp LP).
Ethics
This study was performed in accordance with the Helsinki Declaration and was approved by the Danish Regional Committee on Biomedical Research Ethics (H‐3‐2012‐005). All participants have given written informed consent on oral and written information.
Results
Study Population
Of the 5288 women with angina undergoing CAG in eastern Denmark between March 2012 and September 2014, 2159 were eligible for the study, 963 were included, and 919 had successfully measured CFVR (Figure 3). Of the included participants, 72% were categorized as having stable angina and 28% as having unstable angina at the time of CAG. Median interval (IQR) between diagnostic clinical CAG and CFVR examination was 71 days (51–97 days). A microbubble contrast agent (SonoVue; Bracco Imaging) was used in 59 (6%) participants. Almost all participants experienced side effects during the CFVR examination (98%), and on a visual analog scale from 1 to 10, the mean (SD) severity of symptoms reported by the participants was 5.7 (2.6). Two participants had an inherent atrial fibrillation induced by dipyridamole, and one experienced a delayed universal urticarial reaction. A higher proportion of nonparticipants had hypertension, diabetes mellitus, or nonobstructive atherosclerosis at CAG and stable angina pectoris as CAG indication, and more were currently smoking compared with participants (Table 1). This was similar when including only participants referred with stable angina.
Figure 3.
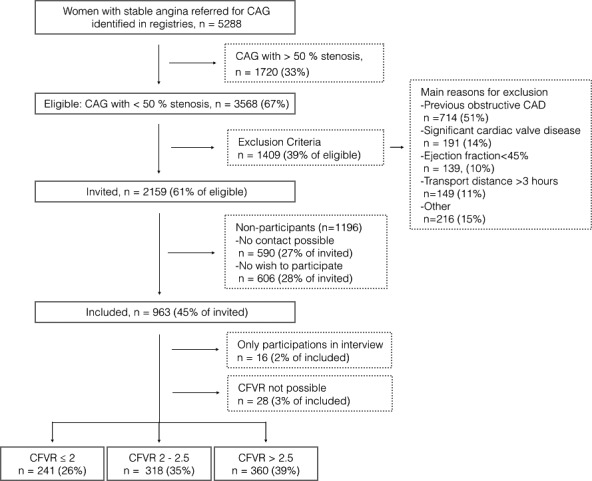
Participant flow chart. CAD indicates coronary artery disease; CAG, coronary angiography; CFVR, coronary flow reserve.
Table 1.
Background Characteristics on Included Participants and Nonparticipants
| Participants (n=963) | Nonparticipants (n=1196) | P Value | |
|---|---|---|---|
| Age, y, mean (SD) | 62.1 (9.7) | 62.7 (10.8) | 0.19 |
| Body mass index, kg/m2, mean (SD) | 27.3 (5.4) | 27.4 (6.0) | 0.71 |
| Hypertension, n (%) | 487 (51) | 598 (59) | 0.001 |
| Hyperlipidemia, n (%) | 604 (63) | 672 (66) | 0.16 |
| Diabetes mellitus, n (%) | 127 (13) | 177 (17) | 0.02 |
| Family history of CAD, n (%) | 496 (53) | 501 (51) | 0.34 |
| Smoking (current), n (%) | 152 (16) | 231 (22) | <0.001 |
| Stable angina pectoris, n (%) | 693 (72) | 918 (77) | 0.01 |
| Atherosclerosis at CAG, n (%) | 335 (35) | 513 (43) | <0.001 |
| Resident outside the capital region, n (%) | 293 (30) | 366 (31) | 0.93 |
P value from 1‐way ANOVA or χ2 test. CAD indicates coronary artery disease; CAG, coronary angiography.
Characteristics of Participants With CMD
Median (IQR) CFVR was 2.33 (1.98–2.76) and did not differ between participants with stable angina and those with unstable angina (P=0.89). A total of 241 (26%) participants had a CFVR ≤2, 318 (35%) had a CFVR between 2 and 2.5, and 360 (39%) had a CFVR >2.5. Table 2 displays characteristics of the study population by CFVR level. Participants with lower CFVR were significantly older. After age adjustment, participants with impaired CFVR had significantly more history of hypertension, diabetes mellitus, current smoking, elevated heart rate and more nonobstructive atherosclerosis at CAG. Participants with low CFVR also had significantly lower HDL cholesterol levels (P=0.02) whereas CFVR was not associated with other serum lipid measures. Low CFVR was associated with a higher use of acetylsalicylic acid (P=0.02), which was partly explained by a higher proportion with nonobstructive CAD. CFVR was not associated with cardiovascular medication such as β‐blockers, calcium antagonists, angiotensin‐converting enzyme inhibitors, or statins (Table 2), and there was no relation between CFVR and comorbidities such as musculoskeletal, pulmonary, thyroid, gastrointestinal, or gynecologic disease. There was no significant association between CFVR and results from a resting 12‐lead study ECG (signs of ischemia, bundle‐branch block, or atrial fibrillation). However, only 25 (3%) participants had atrial fibrillation. LVEF was not associated with impaired CFVR, but participants with LVEF <45% were excluded.
Table 2.
Participant Characteristics According to Coronary Flow Velocity Reserve Level
| CFVR≤2.0 (n=241) | 2<CFVR≤2.5 (n=318) | CFVR>2.5 (n=360) | P Value | |
|---|---|---|---|---|
| Age, y, mean (SD) | 65.0 (9.2) | 62.0 (9.7) | 60.0 (9.5) | <0.001 |
| Stable angina pectoris, n (%) | 64 (27) | 91 (29) | 107 (30) | 0.89 |
| Hypertension, n (%) | 143 (59) | 168 (53) | 156 (44) | 0.02 |
| Hyperlipidemia, n (%) | 161 (67) | 205 (65) | 214 (60) | 0.51 |
| Diabetes mellitus, n (%) | 40 (17) | 42 (13) | 35 (10) | 0.02 |
| Family history of CAD, n (%) | 117 (49) | 170 (55) | 190 (54) | 0.64 |
| Smoking (current), n (%) | 46 (19) | 57 (18) | 46 (13) | 0.001 |
| Pack‐years, [20 cigarettes/d]·y,a median (IQR) | 20 (2–35) | 15 (2–30) | 10 (0–20) | <0.001 |
| Peripheral or cerebral vascular disease, n (%) | 28 (12) | 38 (12) | 35 (10) | 0.84 |
| Atherosclerosis at CAG, n (%) | 100 (41) | 116 (36) | 100 (28) | 0.05 |
| Postmenopausal status, n (%) | 134 (85) | 150 (74) | 159 (68) | 0.38 |
| Menopause duration, y,b median (IQR) | 18 (10–23) | 15 (8–21) | 13.5 (7–20) | 0.65 |
| Clinical assessment, mean (SD) | ||||
| Body mass index, kg/m2 | 27.4 (5.8) | 27.3 (5.4) | 26.9 (5.1) | 0.11 |
| Abdominal circumference, cm | 98 (14) | 98 (15) | 97 (14) | 0.27 |
| Systolic blood pressure, mm Hg | 134 (22) | 133 (21) | 133 (22) | 0.37 |
| Heart rate at rest, bpm | 71 (12) | 71 (11) | 69 (11) | 0.001 |
| LVEF, % | 59 (6) | 59 (6) | 58 (6) | 0.19 |
| Medication, n (%) | ||||
| Acetylsalicylic acid | 123 (51) | 150 (48) | 134 (38) | 0.02 |
| β‐Blockers | 82 (35) | 101 (32) | 88 (25) | 0.12 |
| Calcium antagonists | 57 (24) | 68 (22) | 73 (21) | 0.69 |
| Angiotensin‐converting enzyme inhibitor | 35 (15) | 61 (20) | 40 (11) | 0.36 |
| Statins | 139 (58) | 155 (50) | 164 (46) | 0.11 |
| Angiotensin type II receptor blockers | 59 (25) | 51 (16) | 56 (16) | 0.08 |
| Pantoprazole | 48 (20) | 65 (20) | 62 (17) | 0.67 |
| Previous diagnostic stress tests, n (%) | ||||
| Positive exercise testc (n=317) | 22 (28) | 37 (33) | 40 (32) | 0.52 |
| Positive SPECTc (n=99) | 11 (33) | 12 (36) | 8 (24) | 0.41 |
P value from age‐adjusted trend test (multivariable regression and logistic regression). CAD indicates coronary artery disease; CAG, coronary angiography; LVEF, left ventricular ejection fraction.
Only including previous and current smokers.
Only postmenopausal participants with natural menopause.
Only participants with stable angina pectoris who had a diagnostic stress test before CAG.
Baseline CFV correlated with CFVR (r=−0.42, P<0.001), but CFV was not associated with the same cardiovascular risk factors as was CFVR. CFV was like CFVR associated with smoking (P=0.004) and heart rate (P<0.001). Cardiovascular risk factor associations with CFVR were similar for participants characterized as having stable (n=693) versus unstable angina (all P values for interaction >0.05).
Determinants of CFVR
In multivariable regression analyses, CFVR remained associated with age, hypertension, smoking, resting heart rate, and HDL cholesterol in the final model (Table 3). However, the model explained only a minor part of the variation in CFVR (r 2=0.09). Adjusting for baseline CFV, which was strongly associated with CFVR, did not alter associations (results not shown). There was no significant interaction effect of stable versus unstable angina on the associations between cardiovascular risk factors and CFVR and no significant difference between our final model and a model including full interaction analysis (P=0.54).
Table 3.
Final Multivariable Regression Model in 885 Women
| Expected Change of CFVR Valuea | 95% CI | P Value | |
|---|---|---|---|
| Age (for 10 y of aging) | −6.2 | −8.0 to −4.4 | <0.001 |
| Hypertension | −4.0 | −7.2 to −0.8 | 0.016 |
| Smoking | |||
| Previous | −1.9 | −5.2 to +1.6 | 0.29 |
| Current | −8.6 | −12.7 to −4.0 | <0.001 |
| Heart rate (for increase of 10 bpm) | −2.3 | −3.8 to −0.8 | 0.002 |
| High‐density lipoprotein (per 1‐mmol/L increase) | +4.1 | +0.8 to +7.2 | 0.016 |
P value obtained by multivariable linear regression analyses with ln base logarithmic transformed coronary flow velocity reserve (CFVR) as outcome variable.
Percent increase (indicated by +) or decrease (indicated by −) in percent per unit increase of independent variables.
When looking at smoking amount as a determinant of CFVR, adjusting only for age, CFVR decreased 4.6% (95% CI 2.0–7.2%) per 10 pack‐year ([20 cigarettes/d]·10 y) for current smokers and 2.4% (95% CI 0.8–4.0%) per 10 pack‐year ([20 cigarettes/d]·10 y) for previous smokers.
Symptoms
Of the participants, 471 (53%) had symptoms weekly and 306 (32%) had typical angina symptoms according to the classic characterization of chest pain.11, 12 There was no association between CFVR level and symptom burden or symptom characteristics according to the classic classification of chest pain11, 12 and Rose's Angina Questionnaire. In addition, there was no association between CFVR level and angina frequency, angina stability, and treatment satisfaction evaluated by using the Seattle Angina Questionnaire, but participants with low CFVR had a significantly higher degree of physical limitation and a higher self‐perception of disease as assessed by using the Seattle Angina Questionnaire (Figure 4). There was no association between impaired CFVR and whether angina pectoris occurred during rest, exertion, rest and exertion, or dipyridamole infusion. Further, we found no difference in number of hospital admissions or contacts with general practitioner (Table 4).
Figure 4.
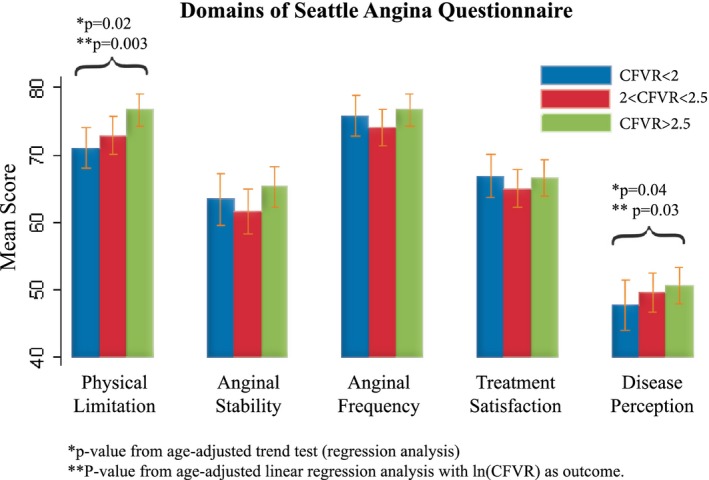
Seattle Angina Questionnaire. Higher scores represent higher/better function of each variable in Seattle Angina Questionnaire. *P value from trend‐test (age‐corrected multivariate regression). ‡ P value from regression analysis with natural logarithmically transformed CFVR as outcome. CFVR indicates coronary flow velocity reserve.
Table 4.
Classification of Chest Pain Variables According to CFVR Level
| CFVR≤2.0 (n=241) | 2<CFVR≤2.5 (n=318) | CFVR>2.5 (n=360) | P Valuea | P Valueb | |
|---|---|---|---|---|---|
| Symptom characteristics for classic chest pain classification, n (%) | |||||
| Typical AP | 71 (30) | 102 (32) | 122 (34) | 0.17 | 0.96 |
| Atypical AP | 129 (54) | 150 (47) | 156 (43) | ||
| Noncardiac chest pain | 41 (17) | 66 (21) | 82 (23) | ||
| Rose's angina classification, n (%) | |||||
| Severe definite AP | 45 (19) | 56 (18) | 61 (17) | 0.91 | 0.49 |
| Nonsevere definite AP | 56 (24) | 73 (24) | 94 (27) | ||
| Nondefinite AP | 130 (56) | 174 (57) | 195 (56) | ||
| Symptom burden by Seattle Angina Questionnaire, mean (SD) | |||||
| Physical limitation | 71 (22) | 73 (24) | 77 (22) | 0.02‡ | 0.003 |
| Angina stability | 63 (29) | 62 (29) | 65 (28) | 0.17 | 0.05 |
| Angina frequency | 76 (23) | 74 (24) | 77 (22) | 0.33 | 0.25 |
| Treatment satisfaction | 67 (25) | 65 (24) | 67 (24) | 0.42 | 0.56 |
| Perception/quality of life | 48 (29) | 50 (26 | 51 (25) | 0.04‡ | 0.03 |
| Further chest pain classification, n (%) | |||||
| Chest pain at exertion | 41 (19) | 50 (18) | 51 (17) | 0.88 | 0.79 |
| Chest pain at rest | 67 (32) | 92 (33) | 106 (36) | ||
| Chest pain at exertion and rest | 104 (49) | 137 (49) | 139 (47) | ||
| Chest pain during dipyridamole infusion | 70 (31) | 112 (36) | 119 (35) | 0.91 | 0.76 |
| Reproduced symptoms during dipyridamole infusion | 68 (30) | 96 (32) | 95 (28) | 0.22 | 0.11 |
| Weekly chest discomfort | 120 (53) | 164 (56) | 173 (51) | 0.30 | 0.24 |
| Contact with health care system, mean (SD) | |||||
| Previous hospitalization as a result of AP | 1 (1) | 1 (2) | 1 (1) | 0.60 | 0.84 |
| Previous contacts with general practitioner as a result of AP | 3 (4) | 3 (4) | 3 (3) | 0.33 | 0.14 |
In some categories, there are missing data. AP indicates angina pectoris.
P value from age‐adjusted trend test (logistic or regression analyses) or chi‐square test when symptom parameters of interest are divided into 3 categories.
P value from age‐adjusted linear regression analysis with natural logarithmically transformed coronary flow velocity reserve (CFVR) as outcome.
Among participants referred for stable angina, 317 (47%) had previously undergone an exercise ECG and 101 (15%) had undergone SPECT. A positive stress test (exercise ECG or SPECT) could not identify participants with CMD assessed by using TTDE (Table 2).
Discussion
In this large study in which we systematically investigated the association between symptoms, cardiovascular risk factors, and noninvasively assessed CMD in women with angina‐like chest pain and no obstructive CAD, we found that CFVR was impaired in a large proportion and was associated with age, hypertension, current smoking, elevated resting heart rate, and low HDL cholesterol. No convincing correlation between severity or characterization of chest pain and impaired CFVR was found, and a positive diagnostic stress test did not identify participants with CMD.
The burden of symptoms was similar to that of previous studies of obstructive CAD. Prevalence of typical angina in our population was identical to that of women with obstructive CAD from the CONFIRM registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry).19 In our study, 50% of all participants had angina‐like chest pain at least once a week. For comparison, in the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) study of subjects with obstructive CAD, ≈40% had angina at least once a month at 3‐year follow‐up.20 Participants in the COURAGE study also scored higher on all 5 elements in the Seattle Angina Questionnaire compared with the participants in the current study, indicating better function.20 Another study found that participants with nonobstructive atherosclerosis at CAG have more persistent angina compared with participants with obstructive CAD evaluated by using the Seattle Angina Questionnaire.3 Together, the findings indicate an overall limited association between symptoms and angiographic CAD severity. We further found that degree of CFVR impairment was not associated with symptom characteristics and that the classic characterization of chest pain could not be used to identify CMD.11 The results may question whether CMD is the cause of symptoms in these women. However, we have only assessed 1 dimension of CMD. In this present study, we have not assessed the endothelium‐dependent epicardial dysfunction or mechanisms pertaining to pain perception, epicardial disease, or noncardiac causes of chest pain such as gastric pain, musculoskeletal disorders, or pulmonary disease. This might as well explain the lack of association between angina pectoris and CFVR. Studies simultaneously targeting symptoms and CMD are needed to clarify this.
We found that 26% of the women had CFVR <2 and, thus, CMD according to current guidelines.17 This is in agreement with several other studies. In a previous study of TTDE‐assessed CFVR in 394 participants (48% men) with angina pectoris and no angiographic stenosis, 22% of participants had CMD, and further, CMD was associated with a hazard ratio of 16 for death or nonfatal myocardial infarction.6 Another study in 65 women with angina and no obstructive CAD found a TTDE‐measured CFVR <2 in 40%.21 Other studies assessing CMD invasively or by positron emission tomography with different cutoffs have indicated that 39% to 54% have CMD.4, 5, 22 In a recent large study with invasive assessment of CMD in 1439 men and women with chest pain and nonobstructive CAD included over a period of 19 years, 30% had abnormal CFVR in response to adenosine.23
In the present study, the prevalence of risk factors and the use of medication were similar to results from another large Danish study of 2253 women with angina pectoris and no obstructive CAD.1 Prevalence of risk factors was also comparable to the National Heart, Lung, and Blood Institute Women's Ischemia Syndrome Evaluation (WISE) study of women with chest pain and no signs of obstructive CAD. However, our population was ≈8 years older and fewer were current smokers.24 Overall, the prevalence of cardiovascular risk factors was relatively high compared with that of a general Danish population of women at a similar age.25
Reduced CFVR was associated with some, but not all, traditional cardiovascular risk factors. This was comparable to other studies that have found age,5, 6, 21, 22, 26, 27 hypertension,6, 27, 28 diabetes mellitus,27, 28 smoking status,26 resting heart rate,29 and HDL cholesterol26 to be determinants of CFVR, including in multiple adjusted models. One study in obese men and women with no obstructive CAD found impaired CFVR to be related to a high BMI.29 In another report, CFVR was associated with years since menopause and the absence of hormone therapy postmenopause.22 We did not find relations between CFVR and BMI, menopause or lack of hormonal therapy and CFVR in this large cohort and one reason may be that none of the mentioned studies adjusted for age, which is highly correlated to CFVR. However, the explanatory effect of the variation of CFVR in our final model was low, indicating that although associations are present, traditional cardiovascular risk factors account for little of the variation in CFVR, which is in accordance with previously published results from the National Heart, Lung, and Blood Institute WISE study24 and another recent large study.23
It is often assumed that a positive stress test in the presence of no obstructive CAD is indicative of microvascular dysfunction. One study of 68 women found that significantly more women with low CFVR had a positive clinical stress test.21 This could not be corroborated in our study: among the 47% of participants who had a stress test performed, a positive stress test was not predictive of CMD. A recent large study that included 1439 subjects with angina and invasive assessment of CMD also found no association between results of stress testing and CMD.23 The cause of the positive stress tests is unclear; however, it is plausible that CMD can cause ischemia without positive stress testing since these are mostly based on demonstration of regional rather than diffuse ischemia. Other cardiovascular indices might explain more of the variation in CFVR than traditional cardiovascular risk factors. Studies that include factors of vessel stiffness such as brachial–ankle pulse‐wave velocity, augmentation index, and aortic pulse‐wave velocity achieve a greater explanatory effect of the CFVR variation with r 2 values ranging from 0.36 to 0.52.30, 31
A full invasive assessment including both endothelial and nonendothelial mechanisms is regarded as the “gold standard” for assessing CMD. However, given that only a minority of patients with angina will ultimately need revascularization; noninvasive methods for assessment of CMD deserve wider application. Recent evidence has shown that CMD assessed by positron emission tomography among patients with no visual evidence of CAD on rest/stress positron emission tomography–myocardial perfusion imaging was a powerful predictor of major cardiovascular events with a hazard ratio of 0.8 per 10% increase in CFVR.4 Thus, noninvasive assessment of CMD may prove an important means of risk‐stratifying this group. The present study demonstrates that this is feasible in the vast majority of unselected subjects and that the results are similar to those found by using invasive assessment. In addition, TTDE assessment of CMD is easily available given appropriate training and can be repeatedly assessed with no concern of radiation.
Strengths and Limitations
A main strength of this study is the systematic inclusion of participants who represent women with clinically assessed angina pectoris and no obstructive CAD in a region covering almost 3.0 million inhabitants. All women referred to CAG for angina were systematically screened and invited. Participants represent both the capital and rural areas. Nonparticipants had a greater burden of some cardiovascular risk factors and more nonobstructive atherosclerosis at CAG compared with participants, and this may have led us to underestimate the prevalence of CMD. The most common reason not to participate in the study was exhaustion and lack of energy, and this could also explain why nonparticipants had more cardiovascular risk factors. However, the internal validity is not likely to be affected by nonparticipants. Prevalence of CMD was comparable to previous studies, but more information on whether CFVR differs between clinically determined symptomatic and atypically symptomatic women or asymptomatic women not referred could have been addressed by including a matched control group of asymptomatic women. Future studies will address this.
We have assessed 1 dimension of CMD; the adenosine‐induced reduced CFVR, which is mainly caused by dysfunction of the endothelium‐independent vasodilation of the microcirculation. We have not assessed the endothelium‐dependent epicardial dysfunction or microvascular spasm, which can be detected by using invasive acetylcholine provocation test.32, 33, 34 The latter might have been the main mechanism responsible for chest pain in a subgroup of participants included in this study, in particular those with unstable angina pectoris. Therefore, the burden of CMD might be underestimated in this study.
Conclusion
In the iPOWER study, CMD was systematically assessed by TTDE with high feasibility. One‐third of the women had impaired CFVR and this was not associated with symptom characteristics or severity, or with results from diagnostic stress testing. CFVR was associated with only a few traditional cardiovascular risk factors. Further follow‐up will determine whether assessment of CMD is useful to risk stratify the large population of women with angina and no obstructive CAD and to monitor effects of intervention.
Sources of Funding
This work was supported by the Danish Heart Foundation (grant 11‐10‐R87‐B‐A3628‐22678) and by the University of Copenhagen.
Disclosures
None.
Acknowledgments
The authors would like to thank the Danish Heart Foundation and the University of Copenhagen for financial support, all collaborators in the iPOWER group, the Department of Cardiology at Bispebjerg Hospital, University of Copenhagen, Denmark where the examinations have taken place, and all the participating women in iPOWER for their time and willingness to contribute to the research.
(J Am Heart Assoc. 2016;5:e003064 doi: 10.1161/JAHA.115.003064)
References
- 1. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 2. Jespersen L, Abildstrom SZ, Hvelplund A, Galatius S, Madsen JK, Pedersen F, Hojberg S, Prescott E. Symptoms of angina pectoris increase the probability of disability pension and premature exit from the workforce even in the absence of obstructive coronary artery disease. Eur Heart J. 2013;34:3294–3303. [DOI] [PubMed] [Google Scholar]
- 3. Jespersen L, Abildstrom SZ, Hvelplund A, Prescott E. Persistent angina: highly prevalent and associated with long‐term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571–581. [DOI] [PubMed] [Google Scholar]
- 4. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near‐normal coronary arteries. Am J Cardiol. 2009;103:626–631. [DOI] [PubMed] [Google Scholar]
- 7. El FG, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. J Nucl Med. 2005;46:1264–1271. [PubMed] [Google Scholar]
- 8. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di CG, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al‐Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prescott E, Abildstrom SZ, Aziz A, Merz NB, Gustafsson I, Halcox J, Hansen HS, Hansen PR, Kastrup J, Michelsen M, Mygind ND, Ong P, Pena A, Rosengren A, Sechtem U, Sogaard P. Improving diagnosis and treatment of women with angina pectoris and microvascular disease: the iPOWER study design and rationale. Am Heart J. 2014;167:452–458. [DOI] [PubMed] [Google Scholar]
- 11. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di MC, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 12. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB III, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 13. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 14. Rose G, McCartney P, Reid DD. Self‐administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med. 1977;31:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim HE, Shim WJ, Rhee H, Kim SM, Hwang GS, Kim YH, Seo HS, Oh DJ, Ro YM. Assessment of coronary flow reserve with transthoracic Doppler echocardiography: comparison among adenosine, standard‐dose dipyridamole, and high‐dose dipyridamole. J Am Soc Echocardiogr. 2000;13:264–270. [DOI] [PubMed] [Google Scholar]
- 16. Picano E. Stress Echocardiography. 5th ed Springer‐Verlag Berlin Heidelberg: Springer; 2009. [Google Scholar]
- 17. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Mario C, Feddeira R, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, Wall E, Vrints CJ. 2013 ESC guidelines on the management of stable coronary artery disease‐addenda. 2013:1–32. Available at: https://www.escardio.org/static_file/Escardio/Guidelines/publications/ANGINA2013_Stable_Coronary_Artery_Disease_web_addenda.pdf. Accessed October 23, 2015.
- 18. Graf S, Khorsand A, Gwechenberger M, Novotny C, Kletter K, Sochor H, Pirich C, Maurer G, Porenta G, Zehetgruber M. Typical chest pain and normal coronary angiogram: cardiac risk factor analysis versus PET for detection of microvascular disease. J Nucl Med. 2007;48:175–181. [PubMed] [Google Scholar]
- 19. Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, Achenbach S, Al‐Mallah M, Berman DS, Budoff MJ, Callister TQ, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Maffei E, Cademartiri F, Kaufmann P, Shaw LJ, Raff GL, Chinnaiyan KM, Villines TC, Cheng V, Nasir K, Gomez M, Min JK. Coronary computed tomographic angiography and risk of all‐cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry). Circulation. 2012;126:304–313. [DOI] [PubMed] [Google Scholar]
- 20. Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O'Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. [DOI] [PubMed] [Google Scholar]
- 21. Sade LE, Eroglu S, Bozbas H, Ozbicer S, Hayran M, Haberal A, Muderrisoglu H. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204:580–585. [DOI] [PubMed] [Google Scholar]
- 22. Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. [DOI] [PubMed] [Google Scholar]
- 23. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 24. Wessel TR, Arant CB, McGorray SP, Sharaf BL, Reis SE, Kerensky RA, von Mering GO, Smith KM, Pauly DF, Handberg EM, Mankad S, Olson MB, Johnson BD, Merz CN, Sopko G, Pepine CJ. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women's Ischemia Syndrome Evaluation (WISE). Clin Cardiol. 2007;30:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The National Institute of Public Health . The National Health Interview Surveys. 2013. Available at: http://www.sundhedsprofil2010.dk/. Accessed April 29, 2015.
- 26. Lee DH, Youn HJ, Choi YS, Park CS, Park JH, Jeon HK, Kim JH. Coronary flow reserve is a comprehensive indicator of cardiovascular risk factors in subjects with chest pain and normal coronary angiogram. Circ J. 2010;74:1405–1414. [DOI] [PubMed] [Google Scholar]
- 27. Tuccillo B, Accadia M, Rumolo S, Iengo R, D'Andrea A, Granata G, Sacra C, Guarini P, Al‐Kebsi M, De MM, Ascione L. Factors predicting coronary flow reserve impairment in patients evaluated for chest pain: an ultrasound study. J Cardiovasc Med (Hagerstown). 2008;9:251–255. [DOI] [PubMed] [Google Scholar]
- 28. Ahmari SA, Bunch TJ, Modesto K, Stussy V, Dichak A, Seward JB, Pellikka PA, Chandrasekaran K. Impact of individual and cumulative coronary risk factors on coronary flow reserve assessed by dobutamine stress echocardiography. Am J Cardiol. 2008;101:1694–1699. [DOI] [PubMed] [Google Scholar]
- 29. Tona F, Serra R, Di AL, Osto E, Scarda A, Fabris R, Montisci R, Famoso G, Tellatin S, Foletto M, Giovagnoni A, Iliceto S, Vettor R. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis. 2014;24:447–453. [DOI] [PubMed] [Google Scholar]
- 30. Saito M, Okayama H, Nishimura K, Ogimoto A, Ohtsuka T, Inoue K, Hiasa G, Sumimoto T, Higaki J. Possible link between large artery stiffness and coronary flow velocity reserve. Heart. 2008;94:e20. [DOI] [PubMed] [Google Scholar]
- 31. Nichols WW, Denardo SJ, Davidson JB, Huo T, Bairey Merz CN, Pepine CJ. Association of aortic stiffness and wave reflections with coronary flow reserve in women without obstructive coronary artery disease: an ancillary study from the National Heart, Lung, and Blood Institute–sponsored Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. 2015;170:1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010;74:1745–1762. [DOI] [PubMed] [Google Scholar]
- 33. Lanza GA, Camici PG, Galiuto L, Niccoli G, Pizzi C, Di MA, Sestito A, Novo S, Piscione F, Tritto I, Ambrosio G, Bugiardini R, Crea F, Marzilli M. Methods to investigate coronary microvascular function in clinical practice. J Cardiovasc Med (Hagerstown). 2013;14:1–18. [DOI] [PubMed] [Google Scholar]
- 34. Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, Nagayoshi Y, Yamamuro M, Izumiya Y, Iwashita S, Matsui K, Jinnouchi H, Kimura K, Umemura S, Ogawa H. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. [DOI] [PubMed] [Google Scholar]


