Abstract
Background
The role of kidney hypoxia is considered pivotal in the progression of chronic kidney disease. A widely used method to assess kidney oxygenation is blood oxygen level dependent (BOLD)–magnetic resonance imaging (MRI), but its interpretation remains problematic. The BOLD‐MRI signal is the result of kidney oxygen consumption (a proxy of glomerular filtration) and supply (ie, glomerular perfusion). Therefore, we hypothesized that with pharmacological modulation of kidney blood flow, renal oxygenation, as assessed by BOLD‐MRI, correlates to filtration fraction (ie, glomerular filtration rate/effective renal plasma flow) in healthy humans.
Methods and Results
Eight healthy volunteers were subjected to continuous angiotensin‐II infusion at 0.3, 0.9, and 3.0 ng/kg per minute. At each dose, renal oxygenation and blood flow were assessed using BOLD and phase‐contrast MRI. Subsequently, “gold standard” glomerular filtration rate/effective renal plasma flow measurements were performed under the same conditions. Renal plasma flow decreased dose dependently from 660±146 to 467±103 mL/min per 1.73 m2 (F[3, 21]=33.3, P<0.001). Glomerular filtration rate decreased from 121±23 to 110±18 mL/min per 1.73 m2 (F[1.8, 2.4]=6.4, P=0.013). Cortical transverse relaxation rate (R2*; increases in R2* represent decreases in oxygenation) increased by 7.2±3.8% (F[3, 21]=7.37, P=0.001); medullar R2* did not change. Cortical R2* related to filtration fraction (R 2 0.46, P<0.001).
Conclusions
By direct comparison between “gold standard” kidney function measurements and BOLD MRI, we showed that cortical oxygenation measured by BOLD MRI relates poorly to glomerular filtration rate but is associated with filtration fraction. For future studies, there may be a need to include renal plasma flow measurements when employing renal BOLD‐MRI.
Keywords: blood oxygen level–dependent, chronic kidney disease, magnetic resonance imaging, renal oxygenation, renal perfusion, renin angiotensin system
Subject Categories: Magnetic Resonance Imaging (MRI), Nephrology and Kidney, Blood Pressure, ACE/Angiotension Receptors/Renin Angiotensin System, Hemodynamics
Introduction
Based on extensive animal research, disturbances of renal oxygenation are considered pivotal in the progression of chronic kidney disease (CKD).1, 2, 3 Activation of the renin–angiotensin–aldosterone system is one of the major determinants of renal perfusion and oxygenation.4, 5 In an elegant proof‐of‐concept study, Schachinger et al studied renal oxygenation by blood oxygen level dependent (BOLD)–magnetic resonance imaging (MRI) and demonstrated an acute decrease in cortical oxygenation during supraphysiological bolus injections of angiotensin II (Ang‐II) in healthy volunteers.6 Subsequently, in 2 more recent studies, Blankestijn and coworkers showed that blocking the renin–angiotensin–aldosterone system in CKD patients increases renal oxygenation. This effect was predominantly present in the renal medulla.7, 8 In a similar study in healthy subjects, angiotensin‐converting enzyme inhibition increased oxygenation in both cortex and medulla.9 However, none of these studies were able to relate BOLD measures of kidney oxygenation among CKD patients to kidney function.10, 11 This implies that either the hypothesis on the role of hypoxia in the progression of CKD in humans is not correct, or the interpretation of the kidney BOLD signal should be revised. In this study we focus on the latter.
The BOLD‐MRI signal is expressed by the transverse relaxation rate or R2*. This value represents the ratio between oxy‐ and deoxyhemoglobin and increases in R2* value indicate decreases in oxygenation. Therefore, it is the result of the oxygen extraction rate from the blood (ie, oxygen demand, a proxy of glomerular filtration rate) and blood supply (perfusion).12 BOLD‐MRI does not differentiate between the two. Against this background, assessing oxygen demand and supply, respectively, is essential to understand the relation between renal oxygenation as measured by BOLD‐MRI and kidney function. Others have also suggested that measurement of renal blood flow (RBF) could be a prerequisite in interpreting renal BOLD‐MRI;10, 13 however, this has not yet been shown.
In an attempt to improve the interpretation of renal BOLD, we choose to translate kidney oxygenation and perfusion into terms corresponding to the physical properties of the MRI‐derived parameters. By doing so, we accepted a vast simplification of the very complex regulatory mechanism of RBF and oxygenation a priori. Considering a stable plasma sodium concentration (eg, during an acute intervention without fluid or electrolyte intake), glomerular filtration rate (GFR) indirectly represents the filtered sodium load. If GFR increases, then water and sodium reabsorption will increase to maintain volume homeostasis. While water reabsorption is metabolically neutral, sodium reabsorption is not and with increasing GFR, the tubular oxygen demand increases. Therefore, we consider GFR and effective renal plasma flow (ERPF) to represent oxygen demand and supply, respectively, and then the oxygenation balance should be represented by the filtration fraction (FF).14 Because FF is the calculated GFR/ERPF ratio, it should depend on demand and supply, thus implying that FF might be one of the main determinants of renal oxygenation status.2 Therefore, we employed a combination of renal BOLD‐MRI, MRI‐based renal flow measurements, and radioisotope measurements of kidney function during standardized RBF modulation by continuous, steady‐state Ang‐II infusion in healthy human subjects.15 We hypothesized that in healthy humans (1) Ang‐II infusion causes a dose‐dependent decrease in both RBF and oxygenation, (2) oxygenation changes induced by Ang‐II spatially differ between cortex and medulla, and finally (3) renal oxygenation directly correlates to FF.
Methods
Study Population
Eight healthy subjects were studied (age 20±1 years, 5 males). Their medical histories revealed no significant disease, none used medication, and physical examination was unremarkable. Blood pressure was 120±5/63±6 mm Hg (systolic/diastolic). Plasma creatinine was 71±11 μmol/L, hemoglobin level was 8.9±0.6 mmol/L, and hematocrit was 0.47±0.03. Spot urine samples tested negative for proteinuria by dipstick. Baseline characteristics are given in Table.
Table 1.
Baseline Characteristics
| Subject No. | Hemodynamics | Kidney Function | MRI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP (mm Hg) | HR (bpm) | CO (L/min) | SVR (dyn/cm5 per second) | GFR (mL/min per 1.73 m2) | ERPF (mL/min per 1.73 m2) | FF | RVR (mL/min per mm Hg) | CR2* | MR2* | PC‐RPF (mL/min per 1.73 m2) | |
| 1 | 88 | 66 | 8.1 | 874 | 124 | 310 | 0.40 | 0.07 | 17.1 | 32.9 | 635 |
| 2 | 85 | 61 | 5.8 | 1158 | 104 | 348 | 0.30 | 0.09 | 18.4 | 26.8 | 533 |
| 3 | 80 | 60 | 6.1 | 1055 | 138 | 306 | 0.45 | 0.06 | 20.8 | 33.9 | 598 |
| 4 | 84 | 60 | 7.0 | 1004 | 151 | 391 | 0.39 | 0.05 | 20.7 | 28.8 | 825 |
| 5 | 74 | 57 | 6.5 | 915 | 101 | 336 | 0.44 | 0.06 | 10.7 | 31.5 | 561 |
| 6 | 86 | 56 | 6.0 | 1155 | 110 | 381 | 0.29 | 0.08 | 17.3 | 32.4 | 491 |
| 7 | 83 | 71 | 6.5 | 1016 | 89 | 307 | 0.29 | 0.06 | 15.7 | 20.0 | 742 |
| 8 | 78 | 63 | 6.5 | 963 | 147 | 453 | 0.43 | 0.05 | 17.6 | 27.8 | 898 |
| Mean (SEM) | 82 (1.5) | 62 (1.6) | 6.6 (0.24) | 1018 (34) | 121 (7.6) | 354 (17) | 0.37 (0.023) | 0.07 (0.0046) | 17.3 (1.1) | 29.3 (1.5) | 660 (48) |
CO indicates cardiac output; CR2*, cortical R2*; ERPF, effective renal plasma flow; FF, filtration fraction; GFR, glomerular filtration rate; HR, heart rate; MAP, mean arterial pressure; MR2*, medullary R2*; MRI, magnetic resonance imaging; PC‐RPF, phase contrast MRI renal plasma flow; RVR, renal vascular resistance; SVR, systemic vascular resistance.
Written informed consent was obtained from all participants and the study was approved by the institutional review board of the Academic Medical Center at the University of Amsterdam.
Study Design
All subjects visited our institution on 2 occasions. MRI was performed on visit 1 while visit 2 involved GFR and ERPF measurements. Directly before each visit, subjects collected 24‐hour urine samples. To ensure a comparable sodium balance between visits, all subjects were instructed to follow their normal diet and refrain from the intake of high caloric and/or salty foods, caffeinated beverages, and smoking for 12 hours before each visit. Adherence to dietary instructions was evaluated between visits by 24‐hour sodium/creatinine ratio. Mean sodium/creatinine ratio was 8.9±3.9 at visit 1 and 9.4±5.2 at visit 2. Both the MRI and GFR/ERPF measurements were performed at baseline and during infusion of Ang‐II (Angiotensin II acetate; Clinalfa, Merck Biosciences AG, Läufelfingen, Switzerland) at 0.3, 0.9, and 3.0 ng/kg per minute.16 The half‐life of Ang‐II is 15 to 20 s; therefore after ≈100 s a steady state exists.
Hemodynamic Monitoring
During MRI, brachial artery blood pressure was measured intermittently (Accutor Plus; Datascope Corp., Montvale, New Jersey, USA). During GFR/ERPF measurements, blood pressure was monitored by continuous noninvasive finger artery blood pressure monitoring (Nexfin, BMEye, Amsterdam, The Netherlands). Nexfin continuous blood pressure monitoring is not MRI compatible, but has been extensively validated against intermittent brachial artery pressure measurements, making both methods comparable.17 From the continuous blood pressure recordings, estimations of cardiac output (CO, L/min) and systemic vascular resistance (dyn·s/cm5) were calculated using a validated pulse wave analysis algorithm (FloTrac; BMEye, Amsterdam, The Netherlands).18
Magnetic Resonance Imaging
MRI was performed on a 3.0‐Tesla MRI system (Ingenia; Philips Healthcare, Best, The Netherlands). Survey scans, including three‐dimensional Dixon, were used to locate the position of the kidneys. Subsequently, BOLD and phase contrast (PC) MRI scans were acquired at baseline and at each Ang‐II dose with an initial run‐in period of at least 120 s after each Ang‐II dose increase. Acquisition time for each set of scans was ≈10 minutes (Figure 1), and Ang‐II infusion time was 12 minutes per dose.
Figure 1.
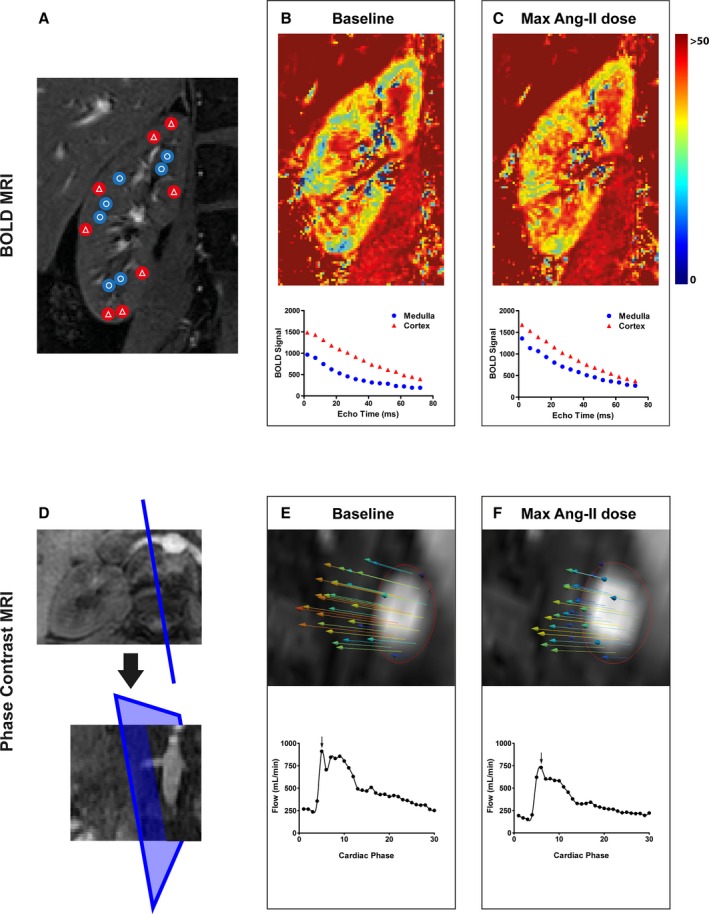
Diagram of BOLD and PC MRI acquisition and analysis. A through C, BOLD MRI scan and segmentation (A—blue with circles in the medulla, red with triangles in the cortex), followed by R2* heat maps at baseline (B) and maximal Ang‐II dose (C—3.0 ng/kg per minute) with cortical and medullary T2* relaxation curves. D through F, PC MRI of the right renal artery, plane positioning perpendicular to the renal artery in transversal and coronal planes (D), followed by profiles of renal artery blood flow velocity (vectors) and curves at baseline (E) and maximal Ang‐II dose (F—3.0 ng/kg per minute). Depicted velocity contours show maximal flow indicated by the arrows in the graphs below for each condition. Ang‐II indicates angiotensin II; BOLD, blood oxygen level dependent; MRI, magnetic resonance imaging; PC, phase contrast.
Three‐directional blood flow velocity was measured by phase contrast (PC) MRI in a slice placed perpendicular to the right proximal renal artery, as described previously.15 The number of ECG‐triggered cardiac phases was 30. PC MRI sequence parameters were as follows: field of view 200×200 mm, resolution=0.65×0.65 mm2, slice thickness=3 mm, repetition time/echo time=8.5/5.7 ms, flip angle=10°, sensitivy encoding factor=2, velocity encoding=100 cm/s in all directions.
Offline image processing for PC MRI was performed using dedicated software (GTFlow version 2.2.9, GyroTools LLC, Zürich, Switzerland). After correction for background phase‐offset errors and aliasing, mean RBF (mL/s) was calculated in the right proximal renal artery using manual vessel segmentation in each cardiac phase (Figure 1D through 1F). Further RBF analysis assumed equal perfusion of both right and left kidneys.19 To make direct comparison between the radioisotope ERPF‐ and MRI‐derived RBF measurements possible, MR RBF was corrected for body surface area (according to Haycock) and hematocrit (Ht) to calculate a PC MRI‐based renal plasma flow (PC‐RPF). Simultaneously PC‐RPF was extrapolated to both kidneys, according to the following formula: MRI flow [mL/s]·60 [s]·2 [kidneys]·(1−Ht)·1.73 [m2]/√(body weight [kg]·height [cm]/3600)=PC‐RPF [mL/min/1.73 m2].
Changes in the BOLD‐MRI signal were quantitatively assessed by measuring the transverse relaxation rate (R2*) within different regions of interest. The R2* value is the rate of exponential signal decay during MRI acquisition, partly as result of the presence of local field inhomogeneities. The R2* value is influenced by magnetically active particles such as deoxyhemoglobin. A decrease in oxygenation therefore leads to a relative increase in deoxyhemoglobin and an increase in R2*. We measured R2* using a multiecho single‐slice fast field‐echo MRI sequence with the following parameters: field of view=400×400, resolution=1.2×1.2 mm2, slice thickness=4 mm, repetition time=140 ms, flip angle=70°, echo time=2 ms, Δecho time=5 ms; number of echoes=16.20 Image acquisition was performed during a single expiratory breath hold in a coronal slice where the kidney cross section was largest and cortical/medullary definition best visible on the survey scans (Figure 1A).
Offline image processing for BOLD imaging was performed using routines written in Matlab (The MathWorks, Natick, MA). In each kidney, 8 circular regions of interest with an 8‐voxel diameter were identified at regular intervals throughout the renal cortex and medulla (Figure 1A) in the baseline scan. For each subject, the resulting masks were then applied to the 3 subsequent BOLD images acquired during staged Ang‐II infusion. Where necessary, regions of interest were adjusted vertically or horizontally to match any movement of the subject during the scan. Renal R2* values were calculated for cortex and medulla separately, via mono‐exponential fitting to the multiecho data (Figure 1B and 1C).
Glomerular Filtration Rate
GFR and ERPF were measured from the clearance of the constantly intravenously infused tracers 125I‐iothalamate (Glofil‐125, ISO‐TEX diagnostics, Friendswood, TX) and 131I‐Hippuran (Radioisotope Centre POLATOM, Otwock, Poland), respectively, as described previously.21, 22 After loading doses, tracer clearances were calculated at baseline and during stepwise increased Ang‐II doses (ie, 0.3, 0.9, and 3.0 ng/kg per minute for 40 minutes each). Plasma 125I‐iothalamate and 131I‐Hippuran were assumed to have reached new steady states at the end of each 40‐minute phase. Ang‐II infusion time was 40 minutes per dose.
ERPF was calculated according to plasma clearance of 131I‐Hippuran: IH·V/PH, where IH is the 131I‐Hippuran concentration in the infusion solution in counts/min per mL, V the volume infused in mL/min, and PH the plasma concentration of 131I‐Hippuran in counts/min per mL. GFR was calculated by the renal clearance of 125I‐iothalamate: UT·V/PT, where UT is the 125I‐iothalamate concentration in the urine in counts/min per mL and PT the plasma concentration of 125I‐iothalamate in counts/min per mL. GFR was corrected for inaccurate urine collections according to: (IH·V/PH)/(UH·V/PH) as described previously.23 ERPF and GFR values were corrected for body surface area and are presented as mL/min per 1.73 m2. FF is calculated as the GFR/ERPF ratio. Renal vascular resistance (mL/min per mm Hg) was calculated as RBF/mean arterial blood pressure.
Statistical Analysis
The primary outcome of this study was cortical and medullary R2* during staged Ang‐II infusion. The effect size was estimated at 0.5, based on data reported by Visser et al and Gloviczki et al16, 24 A priori, the study was powered at 0.8 to detect a significant R2* effect during Ang‐II infusion with an α of 0.05 by ANOVA for repeated measures. Levine's test was used to assess normal distribution of the data. Normally distributed variables are presented as mean±SE, and non‐normally distributed as median (range) or as proportion where appropriate.
One‐way ANOVA for repeated measures was used to assess the dose‐dependent effect of Ang‐II on systemic (blood pressure, heart rate, CO, and systemic vascular resistance) and renal (GFR, ERPF, FF, and renal vascular resistance) hemodynamic parameters, renal artery blood flow, and cortical and medullar R2*. In case the sphericity assumption was violated, degrees of freedom were corrected according to Greenhouse‐Geisser. Pairwise comparison between baseline and each Ang‐II dose was performed using post hoc analyses with Bonferroni correction.
Taking into account the clustered nature of the repeated measures, we used linear mixed model analysis to test the association between cortical or medullar R2*and radioisotope measurements of GFR, ERPF, or FF. The model included the Ang‐II dose as covariate and the measurement number as factor, in order to take the within‐subject relation of the measurements into account. All variables were included as fixed variables into the model, which was corrected for random intercept variation. All statistical analyses were performed using SPSS Statistics 22 (IBM, Chicago, IL). A P‐value <0.05 was considered significant.
Results
Systemic and Renal Hemodynamic Changes Induced by Ang‐II
Systemic and renal hemodynamic changes during Ang‐II infusion are shown in Figure 2. On both study visits, there was a significant effect of Ang‐II infusion on the mean arterial blood pressure increased dose dependently during Ang‐II infusion. During visit 1, mean arterial blood pressure increased from 82±2 at baseline to 90±2 mm Hg at maximal Ang‐II dosage, F(3, 21)=27.9, P<0.001. At visit 2, mean arterial blood pressure increased similarly from 82±2 to 98±2 mm Hg at maximal Ang‐II dosage (F[3, 21]=24.0, P<0.001, Figure 2A). There was a dose‐dependent increase in systemic vascular resistance, from 1018±34 dyn·s/cm5 by 22.7±6.5%, F(1.8, 12.7)=5.1, P=0.05 and renal vascular resistance, from 0.07±0.005 mL/min per mm Hg by 72.5±10.7% (F[3, 21]=28.9, P<0.001, Figure 2D and 2H). CO showed no significant change (baseline 6.5±0.24 L/min; during 3.0 ng/kg per minute Ang‐II 6.4±0.2 L/min, F[3, 21]=0.7, P=0.56, Figure 2C).
Figure 2.
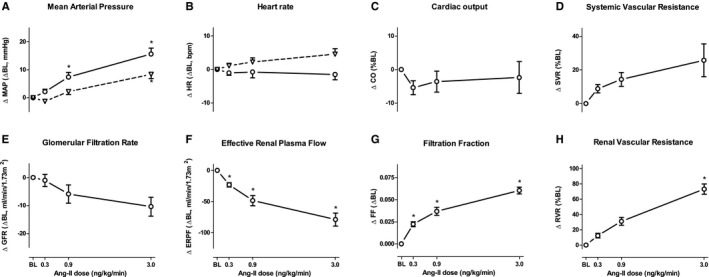
Systemic and renal hemodynamics during staged Ang‐II infusion. Lines represent the absolute mean or mean percentage difference to baseline; error bars indicate SEM. In (A and B) dashed lines represent measurements at visit 1 (Accutor Plus), solid lines at visit 2 (Nexfin). C and D, respone of cardiac output and systemic vascular resistance to Ang‐II infusion, measured by Nexfin. E‐G response of GFR, ERPF and FF measured by radioisotope clearance tests. H, response in renal vascular resistance (RBF/mean arterial pressure). *Significant difference from baseline P<0.05. Ang‐II indicates angiotensin II.
MRI‐derived PC‐RPF decreased dose dependently from 660±48 to 467±36 mL/min per 1.73 m2 (F[3, 21]=33.3, P<0.001, Figure 3C). Radioisotopic ERPF decreased dose dependently from 354±17 mL/min per 1.73 m2 at baseline to 275±12 mL/min per 1.73 m2, at maximal Ang‐II dosage (F[1.62, 11.36]=38.3, P<0.001, Figure 2F). FF increased from 0.37±0.02 at baseline to 0.44±0.03 (F[3, 21]=53.4, P<0.001, Figure 2G). GFR change was less overt (121±7.6–110±6.6 mL/min per 1.73 m2, F[1.8, 2.4]=6.4, P=0.013, Figure 2E).
Figure 3.

Cortical (A), medullary (B) BOLD, and flow MRI data (C). Lines represent mean percentage difference to baseline±SEM. *Significant difference from baseline P<0.05. BOLD indicates blood oxygen level dependent; MRI, magnetic resonance imaging.
Renal Oxygenation Changes Induced by Ang‐II
At baseline, cortical R2* (CR2*) value was 17.4±1.1 and medullary R2* 29.3±1.5 s−1 (Figure 3A and 3B). CR2* increased to 20.0±0.8 s−1 (ie, 7.2±5.4%) at maximal Ang‐II dose (F[3, 21]=7.37, P=0.001, Figure 3A). Medullary R2* did not change during Ang‐II infusion (F[3, 21]=1.38, P=0.29, Figure 3B). There was no association between CR2* and GFR (F[20.3, 142]=0.014, P=0.58) or between CR2* and ERPF (F[25.9, 182]=−5.1×10−4, P=0.97). Also, there were no associations between medullary R2* and GFR or ERPF.
To account for the influence of tissue perfusion on kidney function, we explored the association between CR2* and radioisotope measured FF. This resulted in a positive association depicted in Figure 4A (F [7.69, 53.8]=18.15, P=0.049). In order to make it possible to associate BOLD‐MRI to GFR, we corrected CR2* for renal plasma flow by substituting the MRI derived parameters: CR2* and PC‐RPF for the FF and ERPF in the following formula for the FF (FF=GFR/ERPF). With these assumptions we calculated the CR2*∙PC‐RPF product; subsequently this product did associate positively to GFR (F[15.9, 111]=69.3, P=0.022, Figure 4B).
Figure 4.
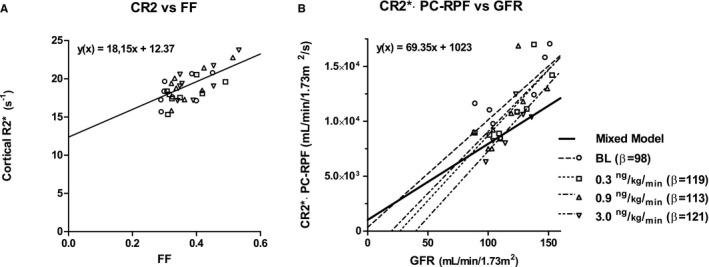
BOLD MRI and kidney function correlations. A, Correlation between cortical R2* and filtration fraction, with fitted mixed model regression line (black) (F[7.69, 53.8]=18.15, P=0.049). B, Correlation between the product of PC‐RPF and cortical R2* vs GFR, with fitted regression lines for mixed model analysis (black line, F[15.9, 111]=69.3, P=0.02) and for each separate Ang‐II dose (dotted lines). Shapes of data points correspond to Ang‐II doses. Ang‐II indicates angiotensin II; BOLD, blood oxygen level dependent; MRI, magnetic resonance imaging; PC‐RPF, phase contrast MRI‐based renal plasma flow.
To evaluate the dependence of the CR2*∙PC‐RPF product's relation to GFR on the Ang‐II dose, additional regression analyses at each Ang‐II dose are included in Figure 4B. The figure shows regression lines for each Ang‐II dose with similar β coefficients; 98, 119, 113, and 121 for baseline, 0.3, 0.9, and 3.0 ng/kg per minute Ang‐II, respectively.
Discussion
Major Findings
In the present study, we corroborate that continuous steady‐state Ang‐II infusion causes a dose‐dependent decrease in RBF in healthy humans. However, the flow reduction is only accompanied by a minor decrease of oxygenation in the cortex, and not in the medulla. As a composite of oxygen supply and demand, the FF is an important determinant of cortical oxygenation. These data imply that kidney BOLD‐MRI on its own is most associated with FF and RBF measurement may be a prerequisite to interpret renal BOLD‐MRI in terms of glomerular filter function.
Ang‐II and Renal Hypoxia
The systemic effects of the Ang‐II infusion that we found are, although not all statistically significant, similar to those reported previously in young healthy volunteers.16 As to the magnitude of the RBF decrease (which was highly statistically significant): This was adequate to test our hypotheses regarding changes in renal oxygenation. BOLD‐MRI‐derived R2* values were also comparable to previously reported values in the kidney cortex and medulla, both in magnitude and range.7, 8, 10, 13 Ang‐II induced an increase in cortical R2*, reaching a plateau phase that did not change between 0.9 and 3.0 ng/kg per minute Ang‐II. Interestingly, this maximal cortical R2* change is similar to those found by Schachinger et al during bolus infusion with 8 ng/kg Ang‐II and by Gloviczki et al in patients with severe renal artery stenosis.6, 13
These observations suggest that the effect of RBF on renal oxygenation is restricted. Possibly this is due to a simultaneous and proportional effect of RBF on the metabolic workload. This is further illustrated by separate linear regression analysis at baseline and each Ang‐II dose of the relation between GFR and the flow corrected CR2*, showing similar β coefficients for each condition. This could indicate that both the afferent and efferent renal vasculature is equally affected with increasing Ang‐II dose.
As to the spatial differentiation of the effects of impaired RBF, our data also indicate that RBF restriction does not affect medullary oxygenation but manifests as a decreased cortical oxygenation similar to observations in critical renal artery stenosis.13 Therefore, these observations are not in line with the proposed role of Ang‐II in the development of hypoxic damage in the renal medulla in CKD. However, deterioration of kidney function is a slow and chronic process. In this respect, our results represent the reaction to acute kidney blood flow reduction and management of a kidney‐specific hemodynamic stressor in healthy humans as opposed to chronically impaired sodium homeostasis and subsequent renal dysfunction in CKD patients.
Interpretation of Renal BOLD‐MRI
Ever since its introduction, the interpretation of renal BOLD‐MRI has been challenging. BOLD imaging remains an indirect measure of tissue oxygenation, resulting from the complex interplay of blood flow, hemoglobin oxygen saturation, and other biological factors such as vessel geometry, hydration status, hematocrit, and pH. It cannot differentiate between oxygen delivery, oxygen consumption, and efficiency of arteriovenous oxygen diffusion. Inherently, baseline BOLD signal intensity cannot be translated directly to an absolute quantitative measure of oxygenation.12
To improve the interpretation of renal BOLD‐MRI, we compared R2* measurements to radioisotope kidney function measurements (GFR, ERPF, and FF). We were unable to show an association between renal R2* values and GFR. However, we found a clear relation between cortical oxygenation measured by BOLD‐MRI and the FF. Therefore, for correct renal BOLD‐MRI interpretation, simultaneous assessment of RBF measurements may be obligatory. MRI‐based flow measurements for such a purpose are widely used and easily implemented.
In this study, we adhered to the most frequently used single‐slice BOLD‐MRI analysis method with observer‐selected regions of interest in the renal cortex and medulla, in order to allow the comparison of our results to those reported previously. In future studies, renal BOLD‐MRI interpretation could be further improved upon by the implementation of new analysis algorithms that are more observer independent and provide a more detailed insight into the oxygenation gradient across the medulla and cortex.25
Study Limitations
This study has several potential limitations that merit discussion. First, PC MRI measurements of RBF were performed in the right renal artery only and assumed equal perfusion of both kidneys. However, this can be justified since kidney perfusion should amount to ≈20% of CO in healthy individuals19 and we measured a baseline RBF of 19±4.4% of CO. Secondly, we studied healthy volunteers and this limits the generalizability of our findings to CKD patients. Further studies in kidney disease require validation of the combined BOLD and PC MRI measurements. Also, we did not assess alterations in sodium hemostasis in these individuals, which might have influenced renal oxygenation.
Lastly, we acknowledge the discrepancy between the radioisotope‐ and MRI‐measured renal plasma flow. The 131I‐Hippuran‐derived ERPF values we found are comparable to those reported by others.16 However, these values underestimate the MRI‐derived PC‐RPF. This discrepancy can be attributed to two possible causes: (1) The renal extraction of Hippuran is not 100%, but about 85% to 90%.26 (2) The remaining 25% underestimation could be attributed to extranephronic perfusion as Hippuran measures effective RPF only (ie, the amount of plasma that passes through the glomeruli). However, in proportional changes, both methods show satisfactory concurrence.
Perspectives
In conclusion, Ang‐II causes a dose‐dependent decline in RBF. The observed oxygenation change differs between cortex and medulla. During an ≈30% perfusion reduction of the kidneys, medullary oxygenation is maintained. By direct comparison among radioisotope GFR, ERPF, and FF measurements and BOLD‐MRI of the kidneys, we showed that, out of these 3 renal perfusion parameters, renal BOLD‐MRI associates most with kidney FF and not with GFR. As the FF is the product of both the GFR and RPF, the interpretation of renal BOLD‐MRI might be improved with simultaneous RBF measurements in future studies.
Sources of Funding
This project was funded by the Dutch Kidney Foundation (Project KJPB 12.029 to Krediet). Krediet is supported by the Netherlands Organisation for Health Research (Clinical Fellowship 40007039712461). This support is gratefully acknowledged.
Disclosures
None.
Acknowledgments
We thank Hessel Peters Sengers, MSc for his expert statistical advice.
(J Am Heart Assoc. 2016;5:e003185 doi: 10.1161/JAHA.115.003185)
References
- 1. Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol. 2013;40:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans RG, Ince C, Joles JA, Smith DW, May CN, O'Connor PM, Gardiner BS. Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin Exp Pharmacol Physiol. 2013;40:106–122. [DOI] [PubMed] [Google Scholar]
- 3. Pruijm M, Hofmann L, Vogt B, Muller ME, Piskunowicz M, Stuber M, Burnier M. Renal tissue oxygenation in essential hypertension and chronic kidney disease. Int J Hypertens. 2013;2013:696598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macia‐Heras M, Del Castillo‐Rodriguez N, Navarro Gonzales JF. The renin‐angiotensin‐aldosterone system in renal and cardiovascular disease and the effects of its pharmacological blockade. J Diabetes Metab. 2012;3:24. [Google Scholar]
- 5. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin‐angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. [DOI] [PubMed] [Google Scholar]
- 6. Schachinger H, Klarhofer M, Linder L, Drewe J, Scheffler K. Angiotensin II decreases the renal MRI blood oxygenation level‐dependent signal. Hypertension. 2006;47:1062–1066. [DOI] [PubMed] [Google Scholar]
- 7. Siddiqi L, Hoogduin H, Visser F, Leiner T, Mali WP, Blankestijn PJ. Inhibition of the renin‐angiotensin system affects kidney tissue oxygenation evaluated by magnetic resonance imaging in patients with chronic kidney disease. J Clin Hypertens (Greenwich). 2014;16:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vink EE, de Boer A, Hoogduin HJ, Voskuil M, Leiner T, Bots ML, Joles JA, Blankestijn PJ. Renal BOLD‐MRI relates to kidney function and activity of the renin‐angiotensin‐aldosterone system in hypertensive patients. J Hypertens. 2015;33:597–604. [DOI] [PubMed] [Google Scholar]
- 9. Stein A, Goldmeier S, Voltolini S, Setogutti E, Feldman C, Figueiredo E, Eick R, Irigoyen M, Rigatto K. Renal oxygen content is increased in healthy subjects after angiotensin‐converting enzyme inhibition. Clinics (Sao Paulo). 2012;67:761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pruijm M, Hofmann L, Piskunowicz M, Muller ME, Zweiacker C, Bassi I, Vogt B, Stuber M, Burnier M. Determinants of renal tissue oxygenation as measured with BOLD‐MRI in chronic kidney disease and hypertension in humans. PLoS One. 2014;9:e95895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI. Renal BOLD‐MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012;81:684–689. [DOI] [PubMed] [Google Scholar]
- 12. Liss P, Cox EF, Eckerbom P, Francis ST. Imaging of intrarenal haemodynamics and oxygen metabolism. Clin Exp Pharmacol Physiol. 2013;40:158–167. [DOI] [PubMed] [Google Scholar]
- 13. Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC. Blood oxygen level‐dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension. 2011;58:1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neugarten J, Golestaneh L. Blood oxygenation level‐dependent MRI for assessment of renal oxygenation. Int J Nephrol Renovasc Dis. 2014;7:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bax L, Bakker CJ, Klein WM, Blanken N, Beutler JJ, Mali WP. Renal blood flow measurements with use of phase‐contrast magnetic resonance imaging: normal values and reproducibility. J Vasc Interv Radiol. 2005;16:807–814. [DOI] [PubMed] [Google Scholar]
- 16. Visser FW, Boonstra AH, Titia Lely A, Boomsma F, Navis G. Renal response to angiotensin II is blunted in sodium‐sensitive normotensive men. Am J Hypertens. 2008;21:323–328. [DOI] [PubMed] [Google Scholar]
- 17. Eeftinck Schattenkerk DW, Van Lieshout JJ, van den Meiracker AH, Wesseling KR, Blanc S, Wieling W, Van Montfrans GA, Settels JJ, Wesseling KH, Westerhof BE. Nexfin noninvasive continuous blood pressure validated against Riva‐Rocci/Korotkoff. Am J Hypertens. 2009;22:378–383. [DOI] [PubMed] [Google Scholar]
- 18. Ameloot K, Van De Vijver K, Broch O, Van Regenmortel N, De Laet I, Schoonheydt K, Dits H, Bein B, Malbrain ML. Nexfin noninvasive continuous hemodynamic monitoring: validation against continuous pulse contour and intermittent transpulmonary thermodilution derived cardiac output in critically ill patients. ScientificWorldJournal. 2013;2013:519080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hura C, Stein JH. Renal blood flow. Comprehensive physiology. John Wiley & Sons, Inc.; 2011:1129–1184. [Google Scholar]
- 20. Rossi C, Sharma P, Pazahr S, Alkadhi H, Nanz D, Boss A. Blood oxygen level‐dependent magnetic resonance imaging of the kidneys: influence of spatial resolution on the apparent R2* transverse relaxation rate of renal tissue. Invest Radiol. 2013;48:671–677. [DOI] [PubMed] [Google Scholar]
- 21. Donker AJ, Arisz L, Brentjens JR, van der Hem GK, Hollemans HJ. The effect of indomethacin on kidney function and plasma renin activity in man. Nephron. 1976;17:288–296. [DOI] [PubMed] [Google Scholar]
- 22. Apperloo AJ, de Zeeuw D, Donker AJ, de Jong PE. Precision of glomerular filtration rate determinations for long‐term slope calculations is improved by simultaneous infusion of 125I‐iothalamate and 131I‐hippuran. J Am Soc Nephrol. 1996;7:567–572. [DOI] [PubMed] [Google Scholar]
- 23. Michels WM, Grootendorst DC, Rozemeijer K, Dekker FW, Krediet RT. Glomerular filtration rate measurements by 125I‐iothalamate should be corrected for inaccurate urine collections with 131I‐hippuran. Clin Nephrol. 2009;72:337–343. [DOI] [PubMed] [Google Scholar]
- 24. Gloviczki ML, Saad A, Textor SC. Blood oxygen level‐dependent (BOLD) MRI analysis in atherosclerotic renal artery stenosis. Curr Opin Nephrol Hypertens. 2013;22:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piskunowicz M, Hofmann L, Zuercher E, Bassi I, Milani B, Stuber M, Narkiewicz K, Vogt B, Burnier M, Pruijm M. A new technique with high reproducibility to estimate renal oxygenation using BOLD‐MRI in chronic kidney disease. Magn Reson Imaging. 2015;33:253–261. [DOI] [PubMed] [Google Scholar]
- 26. Houwen B, Donker A, Woldring M. Simultaneous determination of glomerular filtration rate with 125I‐iothalamate and effective renal plasma flow with 131I‐hippuran. Dyn Stud Radioisotopes Med. 1970;1:331–336. [Google Scholar]


