Abstract
A patient reported to the Florida Spine Institute (Clearwater, Fla., USA) with severe lichen sclerosus of the anogenital region and legs. The patient's pain presentation was neuropathic with hypersensitivity, allodynia, swelling, and weakness. The patient had failed multiple pain management modalities including opioid therapy, anticonvulsants, and antidepressants. The patient completed a standard intravenous ketamine infusion regimen developed at the Florida Spine Institute and reported complete abolishment of her pain syndrome. For the first time, we report that ketamine infusions also dramatically improved a patient's lichen sclerosus. That ketamine is known to have immunomodulatory properties, and given the clinical observations described in this case report, suggests that ketamine should be explored as a possible new therapeutic option for managing lichen sclerosus, especially in cases that are refractory to conventional therapies.
Keywords: Neuropathic pain, Ketamine, Lichen sclerosus
Introduction
Lichen sclerosus (LS) is a chronic inflammatory skin disease of unknown etiology. At present, the typical first line of treatment for LS includes various topical steroids to control inflammation [1]. Its epidemiology is probably underestimated, but it has been calculated to have a prevalence of 0.1–0.3% among all patients referred to a community-based dermatology department [2]. It affects males and females, adults and children. Although the underlying cause is unknown, researchers have linked the disease to autoimmune mechanisms [3]. A recent study analyzed serum autoantibody profiles in patients with LS versus healthy controls [4]. This study found the presence of circulating autoantibodies to a specific skin protein, extracellular matrix protein 1 (ECM1), in most patients with LS. They concluded that ECM1 is a plausible target antigen for autoimmune mechanisms observed in LS pathology.
Ketamine is an anesthetic drug that was introduced into clinical practice in 1970 [5]. Its primary and most well-known pharmacological action is NMDA antagonism. This is the main rationale for the off-label use of ketamine infusions for the treatment of neuropathic pain [6]. However, ketamine exhibits extensive polypharmacological effects, which has led it to be regarded as ‘a pharmacologist's nightmare’ because there is no one clear defining mechanism of action [7]. Ketamine has been shown to limit and even prevent inflammation [8]. It also exhibits robust immunoinhibitory effects that have been studied mainly in the context of developing a novel antidepressant [9]. We hypothesized that this pharmacodynamic profile may produce an ‘entourage effect’ that could be uniquely beneficial for the treatment of painful inflammatory conditions with a possible autoimmune component such as LS.
Case Report
Patient History and Examination
A 66-year-old female patient presented to the Florida Spine Institute (FSI) as a referral from her dermatologist for the treatment of chronic pain in her bilateral lower limbs. She had begun having pain in her lower limbs approximately 8 years prior to her first visit to the FSI. The patient had developed a rash that encompassed large portions of her lower limbs and anus. The patient had been diagnosed with LS et atrophicus at the Department of Dermatology and Cutaneous Surgery of the University of South Florida (Tampa, Fla., USA), where she was referred from. There, the patient had been treated with steroids, with little improvement. Over time, the patient had developed significant skin scarring, skin atrophy, and edema. She had also developed significant neuropathic pain that involved both lower limbs. Her condition made ambulation significantly difficult, and her overall physical activity diminished. The patient underwent physical therapy for deconditioning, with minimal success. Her pain was treated with a common neuropathic pain agent (gabapentin) and an opiate (hydrocodone). This pain regimen gave the patient mild relief, but her ability to perform physical activities was only minimally affected. The patient stated that pain, weakness, and edema in her lower limbs were her main complaints and the reason for her intolerance of physical activity.
The patient was first seen at the FSI in January 2014. Her main complaint was pain in the lower limbs. The pain was described as constant burning with deep aching pain and sporadic sharp pains. On a visual analog scale (VAS), her pain was reported as 6/10 at this first visit. She stated that if her pain were controlled, she would be able to increase her physical activity and performance. On physical exam, the patient's skin revealed skin atrophy, scarring, and plaques consistent with LS et atrophicus located in both legs, thighs, buttocks, and the anus (fig. 1a, b). Her lower limbs also demonstrated edema, color changes, and temperature changes in the distal parts of the legs (when compared to the hands and upper thighs). Her distal parts of the legs also revealed hyperpathia, allodynia, and hypersensitivity. The major muscle groups in her lower limbs were considerably weak, but she had a manual muscle strength grade of at least 4 out of 5. Thus, the patient was treated as a case of possible neuropathic pain, which was likely a complication of the patient's LS. Typically, LS is associated with pruritus, but not necessarily with pain. The patient's pain started after months of having her rash and escalated to a severity at which her ambulation and ability to perform her activities of daily living were significantly affected. In addition, the patient's sensory, vasomotor, and motor dysfunction as well as edema started months after the rash had started.
Fig. 1.
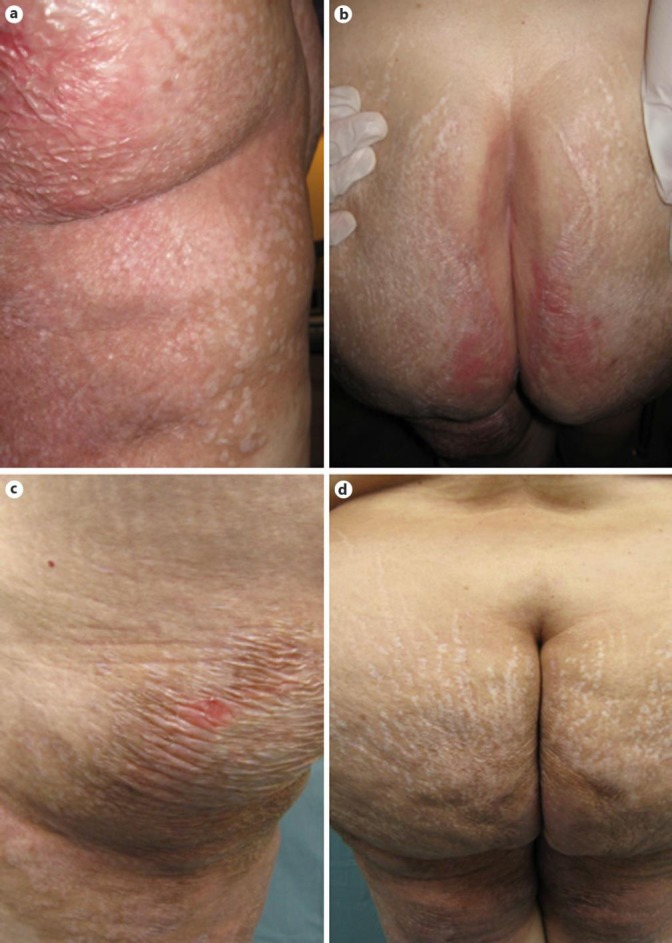
a, b LS plaques just prior to initiating ketamine infusion on the upper thigh (a) and intergluteal cleft (b) of the patient. c, d The patient's upper thigh (c) and intergluteal cleft (d) 2 months after ketamine infusion.
At the time of her first visit, the patient's pain regimen consisted of hydrocodone and gabapentin. This regimen provided inadequate pain relief and, therefore, was changed. Hydrocodone was discontinued and the patient was started on methadone. Methadone was chosen because, in addition to being an opioid analgesic, it acts as a mild NMDA receptor antagonist, a novel target for neuropathic pain. The gabapentin dosage was also increased at this time. The patient's pain improved over the next month (to VAS 5/10). Despite the improvement in the patient's pain, the main goal for the patient and treating physician was ultimately to discontinue opiate medications while maintaining adequate pain control. Therefore, ketamine infusions were discussed at length with the patient. Ketamine is an FDA-approved medication for anesthesia induction and maintenance. It has the ability to provide conscious sedation, but, more importantly, it is a potent NMDA receptor antagonist.
Intravenous Ketamine Administration
In May 2014, the patient was started on the ketamine infusion protocol set forth by the FSI. On day 1, the patient was infused for approximately 4 h with 200 mg ketamine, 200 mg lidocaine, and 6 mg midazolam in a 100-ml bag of saline. The ketamine dosage was increased by 200 mg each day until 800 mg was reached. The patient received intravenous ketamine for 5 consecutive days. She did not demonstrate any complications.
Treatment Outcomes
The overall clinical course of the patient in this case study is plotted in fig. 2. Four days following the last ketamine infusion during a follow-up visit, the patient reported improvement in her pain levels (to VAS 4/10). One month after the ketamine infusions, the patient was seen for another follow-up. Interestingly, the patient's LS rashes had significantly improved. Areas that had previously been causing much discomfort due to sclerotic plaques and lesions showed drastic improvement. In addition, the patient reported decreased allodynia, hyperpathia, and hypersensitivity in her lower limbs. She reported that her pain levels were VAS 4/10. She found that her ambulation ability was improving greatly. The patient volunteered to undergo 2 ketamine booster infusions over the course of 2 days.
Fig. 2.
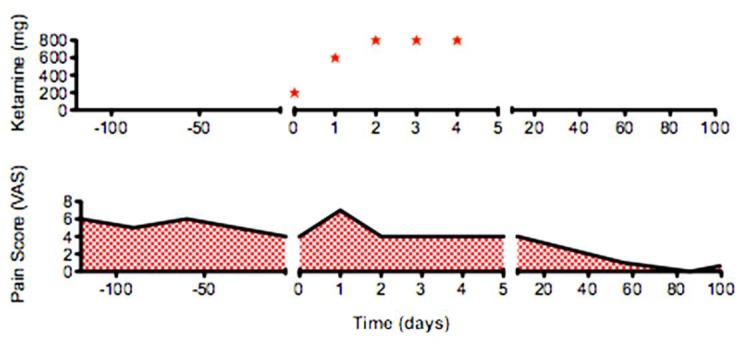
Clinical course. Ketamine dosage plotted with pain scores as reported via standard VAS.
In July 2014, the patient was seen for another follow-up appointment after the ketamine infusions. Her pain had significantly reduced (to VAS 1/10) from her pre-ketamine infusion state, and her LS rash and edema continued to be significantly reduced (fig. 1c, d). The patient's ability to perform activities of daily living and ambulation were greatly improved. She stated that she had become more physically active. She also stated that she neither needed nor wanted to continue opiate pain medication. Therefore, the patient's methadone was weaned appropriately and discontinued. Over the course of 8 months, she received ketamine booster infusions every 2–3 months. Her pain continues to be controlled without the use of opiates (VAS 1/10). The patient's rashes, edema, allodynia, and hypersensitivity continue to be significantly improved compared to the pre-ketamine infusion state. She has undergone physical therapy with significant improvement in ambulation ability and exercise/physical activity tolerance. The patient claims to be at a level of pain control and physical activity similar to her premorbid state.
Discussion and Conclusion
A recent case-control study suggested that LS is characterized by aberrant autoimmune function [10]. That study compared a total of 190 women with adult-onset LS with healthy controls. The authors found that patients with LS more frequently had autoimmune disorders (28 vs. 9%; p < 0.001), supporting an autoimmune association with LS [10]. Thus, pharmacotherapies that address inflammation and autoimmune responses should be particularly useful for the treatment of LS. To our knowledge, there have been no other reports on the effects of ketamine in patients with LS until now.
In our case study, we report that the pain and dermatological pathology associated with LS in one patient were significantly reduced following ketamine infusion therapy that was employed for neuropathic pain. Even though this is an extremely rare case, we felt that these serendipitous observations related to the effects of ketamine in LS might spark further research to better understand its etiology. Moreover, this case study provides evidence that supports intravenous ketamine as an alternative treatment option for patients who cannot manage their LS with topical steroids.
That intravenous ketamine infusions drastically improved symptoms of LS in one patient is scientifically interesting, in part due to its debatable etiology. Ketamine is known to exert analgesic, anesthetic, bronchodilatory, immunomodulatory, neuroprotective, and antidepressant effects, probably via multiple pharmacological modalities [7]. Although extensive efforts are already underway to repurpose ketamine for medical use beyond anesthesia, no other studies have looked at LS. As described in this case study, intravenous ketamine infusion not only provided rapid and sufficient analgesia but it also produced drastic and unexpected improvements in lesions associated with severe LS. We believe that these therapeutic effects could be due to not yet fully understood immunomodulatory properties of ketamine. Nonetheless, the results achieved for this patient merit further investigation. Moreover, we hope that future studies reveal the importance of ketamine's polypharmacological effects in the context of various disease states, rather than fearing the drug's nonspecificity.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Sadowska-Przytocka A, Dańczak-Pazdrowska A, Szewczyk A, Czarnecka-Operacz M, Jenerowicz D, Osmola-Mańkowska A, et al. Treatment of genital lichen sclerosus in women – review. Ginekol Pol. 2012;83:458–461. [PubMed] [Google Scholar]
- 2.Cooper SM, Ali I, Baldo M, Wojnarowska F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: a case-control study. Arch Dermatol. 2008;144:1432–1435. doi: 10.1001/archderm.144.11.1432. [DOI] [PubMed] [Google Scholar]
- 3.Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777–1783. doi: 10.1016/s0140-6736(98)08228-2. [DOI] [PubMed] [Google Scholar]
- 4.Oyama N, Chan I, Neill SM, Hamada T, South AP, Wessagowit V, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet. 2003;362:118–123. doi: 10.1016/S0140-6736(03)13863-9. [DOI] [PubMed] [Google Scholar]
- 5.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H, Miyazaki M, Nanbu T, Yanagida H, Morita S. The NMDA-receptor antagonist ketamine abolishes neuropathic pain after epidural administration in a clinical case. Pain. 1998;75:391–394. doi: 10.1016/s0304-3959(97)00189-9. [DOI] [PubMed] [Google Scholar]
- 7.Potter DE, Choudhury M. Ketamine: repurposing and redefining a multifaceted drug. Drug Discov Today. 2014;19:1848–1854. doi: 10.1016/j.drudis.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62:47–58. [PubMed] [Google Scholar]
- 9.Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol Psychiatry. 2013;18:1236–1241. doi: 10.1038/mp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Paola M, Batsikosta A, Feci L, Benedetti M, Bilenchi R. Granuloma annulare, autoimmune thyroiditis, and lichen sclerosus in a woman: randomness or significant association? Case Rep Dermatol Med. 2013;2013:289084. doi: 10.1155/2013/289084. [DOI] [PMC free article] [PubMed] [Google Scholar]


