Abstract
Keloidal atypical fibroxanthoma (KAF) has recently been categorized as a variant of atypical fibroxanthoma. This paper will emphasize the importance of including KAF in both clinical and histological differential diagnosis of benign and malignant lesions which exhibit keloidal collagen and will also review the current literature on epidemiology, pathogenesis, histology, immunochemistry and treatments.
Key Words: Atypical fibroxanthoma, Sarcoma, Dermatopathology, Spindle cell, Immunohistochemistry, Keloidal atypical fibroxanthoma
Introduction
Atypical fibroxanthoma (AFX) is an asymptomatic low-grade sarcoma that was first described by Helwig [1] in 1961.
Numerous histological variants of AFX have been described, including clear cell [2,3,4], granular cell [5,6,7], chondroid [8], hemosiderin pigmented [9], osteoid [10] AFX with osteoclast-like giant cells [11, 12] and sclerotic forms [13]. Keloidal AFX (KAF) is a recently described variant that has often been misdiagnosed [14]. KAF was first mentioned in the study by Welsh [15] in 2006. KAF is a variant of AFX that consists of thick bands of hyalinized keloid-like collagen [16].
Case Report
We present a 75-year-old Caucasian male with a 1.3-cm pink-to-brown dome-shaped nodule on the helix of the left ear. A shave biopsy was performed, which demonstrated a cytologically malignant spindle cell neoplasm diffusely replacing the dermis and involving the biopsy margins. The spindle cells revealed enlarged and hyperchromatic nuclei with prominent nucleoli and irregular nuclear contours arranged in vaguely intersecting fascicles which effaced the dermal-epidermal junction. A scant chronic inflammatory cell infiltrate was present which was admixed with the tumor cells. Scattered multinucleated bizarre tumor cells, as well as scattered atypical mitotic figures, were noted. A sclerotic background with hyalinized keloidal-like collagen bundles was admixed with the proliferation of spindle cells. The spindle cells were positive for CD68 and negative for S100, Melan-A, pancytokeratin, smooth muscle actin (SMA) and p63. Ki-67 showed a high proliferation rate labeling 40% of tumor cells.
The second case is a 69-year-old male who presented with an atypical lesion on his right ear. A shave biopsy also demonstrated a cytologically malignant spindle cell neoplasm, with diffuse replacement by malignant spindle cells. The spindle cells demonstrated a marked degree of pleomorphism with hyperchromatic and enlarged nuclei with prominent nucleoli and scattered bizarre multinucleated giant cells. A prominent sclerotic component was present with a hyalinized keloid-like collagen bundle. Scattered chronic inflammation was noted in the background. Immunohistochemical studies were performed on the spindle cells, and the results were as follows: positive for CD10, factor XIIIa, SMA (60% staining) and CD68 focally positive. The spindle cells were negative for S100 and cytokeratin.
The immunohistochemical findings of both of these lesions combined with the morphology of the tumors are consistent with a new variant of AFX known as ‘keloidal atypical fibroxanthoma’. Immunostaining was performed to exclude other pathologies, such as leiomyosarcoma, sarcomatoid carcinoma or spindle cell melanoma.
KAF is a new, recent variant of AFX that was first described in 2006 [15]. It is clinically and histologically similar to a variety of pathologies. Dermatologists and dermatopathologists need to include KAF in their differential diagnosis of keloidal scarring lesions. Additionally, our goal is to further acknowledge this variant of AFX and to advocate for this variant to become more officially accepted in the literature. We feel that recognition of KAF represents an important step in distinguishing between benign and malignant lesions which exhibit keloidal collagen, including keloidal dermatofibroma (KDF), leiomyosarcoma, fibrosarcoma and fibromatosis (fig. 1, fig. 2, fig. 3, fig. 4).
Fig. 1.
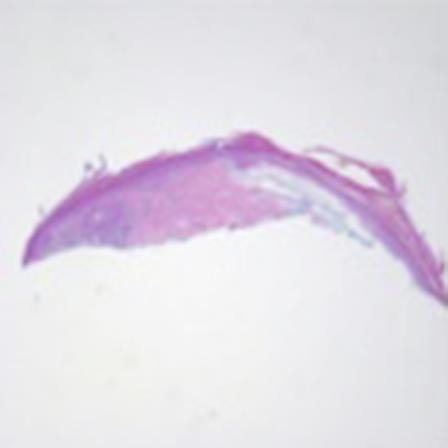
Hematoxylin and eosin. ×2.5.
Fig. 2.
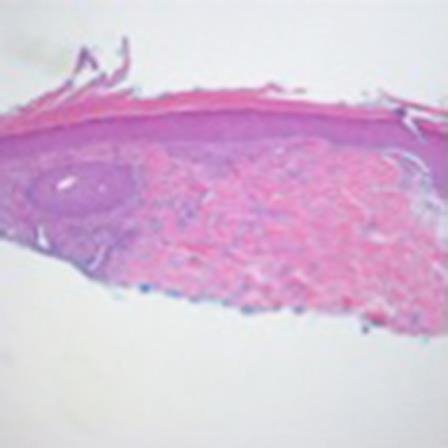
Hematoxylin and eosin. ×5.
Fig. 3.
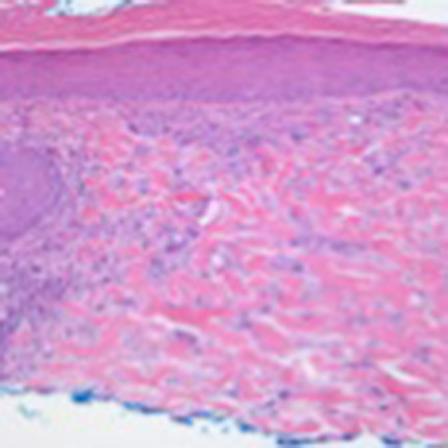
Hematoxylin and eosin. ×10.
Fig. 4.
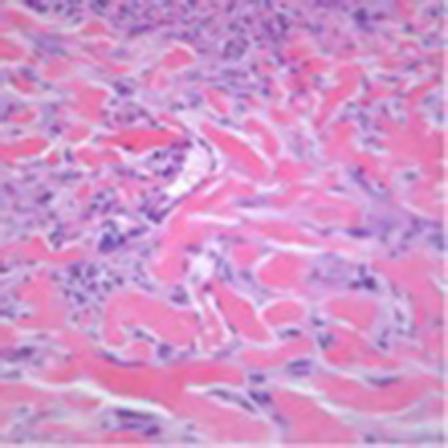
Hematoxylin and eosin. ×20.
Differential Diagnosis
To diagnose KAF, other benign and malignant lesions with keloidal collagen must be excluded, such as KDF, leiomyosarcoma, fibrosarcoma and fibromatosis. The clinical differential diagnosis of 9 cases of KAF in the study of Kim and McNiff [16] included basal cell carcinoma (5 cases), squamous cell carcinoma (SCC; 4 cases), melanoma (1 case), angioma (1 case) and actinic keratosis (1 case). There are also case reports on keloidal collagen histologically presenting as other benign and malignant lesions including basal cell carcinomas [17, 18], sclerotic nevi [19], Spitz nevi, solitary fibrous tumor, scleroderma, sclerotic nevi and histiocytoma [17, 19,20,21,22,23].
Histology and Immunohistochemistry
Diagnosis of KAF is made based on histological findings and immunohistochemistry. Histologically, it is characterized by a thin atrophic epidermis and a storiform proliferation of large fibrocystic [24], atypical spindle-shaped cells with moderate amounts of cytoplasm and large atypical neoplastic cells with abundant pale-staining vacuolated cytoplasm. The neoplastic cells are bizarre, large, round polyhedral, and are multinucleated giant cells with hyperchromatic nuclei. Numerous atypical mitotic figures are present within the keloidal sclerotic collagen [25]. Sometimes ulceration may be present [4]. KAF may also have vascular proliferation in the superficial portion of the tumor [16]. A small amount of coarse, glassy eosinophilic, keloid-like collagen fibers may be present in AFX. However, histologically, KAF has thick bands of hyalinized collagen/keloidal fibers prominent in the dermis admixed with pleomorphic, atypical and multinucleated cells [16, 25]. The keloidal collagen can present in an interstitial pattern and focally form ring-like structures that preferentially deposit around small vessels which can be demonstrated by CD31 immunostaining [14, 16]. Bland hypocellular sclerosis replacing >50% of the tumor may represent a sclerotic variant of AFX [14]. In our case, sclerosis was present but did not replace >50% of the tumor.
The immunohistochemical profile of KAF is similar to that of AFX. AFX is chiefly defined by a diagnosis of exclusion, but there are several markers that can be used to help making an accurate diagnosis. The summary provided in table 1 can be used in this respect. AFX expresses CD68 [26, 27], CD10 [26], vimentin, α1-antitypsin, 1-antichymotrypsin, CD99 [29, 30], CD74 and CD163 [31]. The presence of CD74 (LN-2 antibody) has been proposed to indicate more aggressive behavior. AFX needs to be differentiated from spindle cell SCC and desmoplastic melanoma [4]. S100 is positive in desmoplastic melanoma and negative in AFX [2, 32, 33]. Cytokeratin, p63, high- and low-molecular-weight keratin are positive in spindle cell SCC and negative in AFX [34, 35]. KAF will have identical immunohistochemistry findings with AFX, with the histological description given above.
Table 1.
Immunohistochemical markers
| Immunohistochemistry | KAF/AFX | Spindle cell SCC | Desmoplastic melanoma | KDF | Leiomyosarcoma | Fibrosarcoma | Fibromatosis |
|---|---|---|---|---|---|---|---|
| ɑ1-Antitypsin | √ | ||||||
| 1-Antichymotrypsin | √ | ||||||
| Vimentin | √ | √ | √ | √ | √ | √ | |
| S100 | √ | ||||||
| Neuron-specific enolase | √ | ||||||
| CD68 | √ | √ | |||||
| Microphthalmia transcription factor | √ | ||||||
| Melanoma cell adhesion molecule | √ | ||||||
| CD10 | √ | √ | √ | ||||
| p63 | √ | ||||||
| Cytokeratin | √ | ||||||
| LN-2 (CD74) | √ | ||||||
| CD99 | √ | ||||||
| KiM1P | √ | ||||||
| MAC387 | √ | ||||||
| Actin | √ | √ | √ | ||||
| Desmin | √ |
Other benign and malignant lesions which histologically resemble KAF must also be excluded. These are KDF, cutaneous leiomyosarcoma, fibromatosis and fibrosarcoma. KDF is a variant of dermatofibroma characterized by the presence of keloidal collagen in a typical dermatofibroma [36]. Within the keloid collagen, multinucleated giant cells, hemorrhage, hemosiderin deposits and scattered KiM1P-positive histiocytes are present. However, factor XIIIa- or CD34-positive cells and S100 are absent. Factor XIIIa-positive dendrocytes are present in the surrounding areas where keloidal collagen is not present. Histiocytic markers KP1 (CD68), KiM1P, Mac387 and vimentin are present in the keloidal areas and the surrounding dermatofibroma areas [20]. Also, in all of these benign entities, the cytological blandness and general paucity of mitotic figures helps to differentiate from KAF.
There are many case reports which demonstrate that leiomyosarcomas have mistakenly been diagnosed as keloids [37,38,39]. Leiomyosarcomas present with a fascicular arrangement of hyperchromatic spindle cells and atypical mitotic figures. Immunohistochemical studies are positive for actin, desmin and vimentin and negative for S100. SMA and keratin are not consistently expressed [40]. Fibrosarcomas demonstrate atypical fibroblasts and collagen with proliferation of atypical spindle-shaped cells arranged as intersecting fascicles in a herringbone growth pattern. High-grade fibrosarcomas may be positive for vimentin and actin [41]. Fibromatosis lesions are composed of long-sweeping fascicles of bland fibroblast in a collagenous stromal background. Atypical mitotic features are absent. Lesions are positive for vimentin and negative for S100 and desmin [42]. Scars are typically negative for CD34, factor XIIIa and S100 [43]. To distinguish KAF from a keloid or scar, Ki-67 staining will demonstrate a high proliferation rate as evidenced by our case.
Discussion
Kim and McNiff [16] were the first authors to write an article on KAF, where they analyzed 9 cases of AFX with keloidal tumoral sclerosis. Histology demonstrated pleomorphic tumor cells within hyalinized keloidal collagen bands. Some cases revealed keloidal collagen-forming ring-shaped structures around CD31-positive vascular structures. Their study indicates that pleomorphic cells were negative for the S100 protein and cytokeratin, but all cases were positive for antibodies targeted towards CD68. The average age of patients in their study was 80 years and ranged from 66 to 91 years. Seven were males and 2 were females, with all lesions originating from the head and neck. Histologically, their studies demonstrated atrophic epidermis, confirmed in 2 out of 9 cases. The tumors contained both spindle and pleomorphic cells with atypical mitosis, admixed within keloidal collagen. CD31 staining demonstrated the keloidal collagen deposition around small vessels in a ring-like fashion within the tumor, in addition to being present in an interstitial pattern. This is the first case of keloidal collagen being deposited preferentially around vascular structures and focally at the basement membrane in AFX. Keloidal collagen deposition in this fashion is not specific to KAF [16].
Stefanato et al. [44] studied 8 cases of AFX in order to analyze tumor architecture, degree of pattern of fibrosis and inflammatory cell infiltrate. Three of 8 cases exhibited a keloidal variant without any specific pattern. The KAF observed in this study had changed only focally and did not reveal the perivascular deposition of keloidal collagen that was observed in the study of Kim and McNiff [16]. However, all 8 cases, which included other variants of AFX, did present a sclerotic stroma similar to the cases in our study.
Offman et al. [14] analyzed 91 cases of AFX which were diagnosed in their institution over a 10-year period, and 17 (19%) of them were identified as KAF. Their study suggested that collagen is a product of tumor cells and not part of a stromal response to the lesion, supporting the purported fibrohistiocytic or myofibroblastic lineage of AFX. They concluded that the presence of keloidal collagen does not promote involution of AFX. There was no difference in demographic data from patients who had KAF compared to other variants of AFX. Their cases displayed keloidal collagen dispersed between tumor cells, as well as similar collagen in thinner strands encircling the vessels. These authors indicated that there is some morphological overlap between the keloidal variant of AFX and the sclerotic variant. This is further supported by Bruecks et al. [13] with their report on sclerosis and hyalinization. Similarly, both of our cases had also revealed sclerosis.
Wide local excision with frozen section control or Mohs micrographic surgery is the treatment of choice for AFX. When AFX is diagnosed, the potential for metastasis should always be considered [4]. Due to the possibility of local recurrence and a spread to the lymph nodes, complete tumor removal is required [45,46,47,48,49].
Conclusion
KAF may be confused with benign and malignant lesions which exhibit keloidal collagen. The 2 cases we present fit within the clinical and histopathological profile of previously reported cases. They demonstrate that keloidal collagen may be present in AFX lesions establishing a variant of the classical histopathological presentation. Histologically, both our cases presented with a sclerotic background with hyalinized keloid-like collagen bundles admixed with the proliferation of spindle cells. Our case and the few previously reported cases represent a distinct and under-recognized variant of AFX which may be misdiagnosed as KDF, leiomyosarcoma, fibrosarcoma, fibromatosis scars, spindle cell SCC and desmoplastic melanoma. The accurate diagnosis of KAF differentiating it from other keloidal lesions can be accomplished through conventional histopathology and immunohistochemical markers. Nevertheless, the cases described herein emphasize the importance of diagnosing KAF and differentiating it from other malignant and benign lesions with keloidal collagen. Dermatologists and dermatopathologists should be aware of this variant to avoid improper diagnoses.
Statement of Ethics
The research ‘Keloidal Atypical Fibroxanthoma: Case and Review of the Literature’ complied with the guidelines for human studies. The subjects have given their informed consent and the study protocol has been approved by our institute's committee for human research.
Disclosure Statement
There are no financial disclosures to be made.
References
- 1.Helwig E. Atypical fibroxanthoma. San Antonio: Semin Proc 18th Annu Semin San Antonio Soc Pathol; 1961. p. 664. [Google Scholar]
- 2.Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301–309. doi: 10.1111/j.1600-0560.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- 3.Huether MJ, Zitelli JA, Brodland DG. Mohs micrographic surgery for the treatment of spindle cell tumors of the skin. J Am Acad Dermatol. 2001;44:656–659. doi: 10.1067/mjd.2001.112381. [DOI] [PubMed] [Google Scholar]
- 4.Iorizzo LJ, 3rd, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146–157. doi: 10.1111/j.1524-4725.2010.01843.x. [DOI] [PubMed] [Google Scholar]
- 5.Orosz Z. Atypical fibroxanthoma with granular cells. Histopathology. 1998;33:88. doi: 10.1046/j.1365-2559.1998.0415d.x. [DOI] [PubMed] [Google Scholar]
- 6.Rudisaile SN, Hurt MA, Santa Cruz DJ. Granular cell atypical fibroxanthoma. J Cutan Pathol. 2005;32:314. doi: 10.1111/j.0303-6987.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 7.Rios-Martin JJ, Delgado MD, Moreno-Ramirez D, Garcia Escudero A, Gonzalez-Campora R. Granular cell atypical fibroxanthoma: report of two cases. Am J Dermatopathol. 2007;29:84. doi: 10.1097/01.dad.0000246175.73447.3a. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PR, Strutton GM, Stewart MR. Atypical fibroxanthoma: two unusual variants. J Cutan Pathol. 1989;16:93. doi: 10.1111/j.1600-0560.1989.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Cascajo C, Borghi S, Bonczkowitz M. Pigmented atypical fibroxanthoma. Histopathology. 1998;33:537. doi: 10.1046/j.1365-2559.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen KTK. Atypical fibroxanthoma of the skin with osteoid production. Arch Dermatol. 1980;116:113–114. [PubMed] [Google Scholar]
- 11.Val-Bernal JF, Corral J, Fernandez F, Gomez-Bellvert C. Atypical fibroxanthoma with osteoclast-like giant cells. Acta Derm Venereol. 1994;74:467. doi: 10.2340/0001555574467470. [DOI] [PubMed] [Google Scholar]
- 12.Tomaszewski MM, Lupton GP. Atypical fibroxanthoma. An unusual variant with osteoclast-like giant cells. Am J Surg Pathol. 1997;21:213. doi: 10.1097/00000478-199702000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Bruecks AK, Medlicott SA, Trotter MJ. Atypical fibroxanthoma with prominent sclerosis. J Cutan Pathol. 2003;30:336–339. doi: 10.1034/j.1600-0560.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 14.Offman S, Pasternak S, Walsh N. Keloidal and other collagen patterns in atypical fibroxanthomas. Am J Dermatopathol. 2010;32:326–332. doi: 10.1097/dad.0b013e3181c183f9. [DOI] [PubMed] [Google Scholar]
- 15.Welsh N. 27th Annu Meet Australasian Dermatopathol Soc Clinicopathol Conf. Sydney: 2006. Aug, Atypical fibroxanthoma, keloidal variant. [Google Scholar]
- 16.Kim J, McNiff JM. Keloidal atypical fibroxanthoma: a case series. J Cutan Pathol. 2009;36:535–539. doi: 10.1111/j.1600-0560.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JE. Keloidal basal cell carcinoma. Am J Dermatopathol. 2007;29:485. doi: 10.1097/DAD.0b013e3181469195. [DOI] [PubMed] [Google Scholar]
- 18.Requena L, Martin L, Farina MC, et al. Keloidal basal cell carcinoma. A new clinicopathological variant of basal cell carcinoma. Br J Dermatol. 1996;134:953–957. [PubMed] [Google Scholar]
- 19.Harris GR, Shea CR, Horenstein MG, et al. Desmoplastic (sclerotic) nevus: an underrecognized entity that resembles dermatofibroma and desmoplastic melanoma. Am J Surg Pathol. 1999;23:786–794. doi: 10.1097/00000478-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kuo TT, Hu S, Chan HL. Keloidal dermatofibroma: report of 10 cases of a new variant. Am J Surg Pathol. 1998;22:564. doi: 10.1097/00000478-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Barzilai A, Lyakhovitsky A, Horowitz A, Trau H. Keloid-like scleroderma. Am J Dermatopathol. 2003;25:327. doi: 10.1097/00000372-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Lee JR, Hancock SM, Martindale RG. Solitary fibrous tumors arising in abdominal wall hernia sacs. Am Surg. 2001;67:577. [PubMed] [Google Scholar]
- 23.Kaddu S, McMenamin ME, Fletcher CD. Atypical fibrous histiocytoma of the skin: clinicopathologic analysis of 59 cases with evidence of infrequent metastasis. Am J Surg Pathol. 2002;26:35. doi: 10.1097/00000478-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Rapini RP. Fibrohistiocytic proliferations and neoplasms. In: Rapini RP, editor. Practical Dermatopathology. Mosby: Elsevier; 2005. pp. 341–356. [Google Scholar]
- 25.Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. A clinicopathologic study of 140 cases. Cancer. 1973;31:1541–1552. doi: 10.1002/1097-0142(197306)31:6<1541::aid-cncr2820310635>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Silvis NG, Swanson PE, Manivel JC, et al. Spindle-cell and pleomorphic neoplasms of the skin. A clinicopathologic and immunohistochemical study of 30 cases, with emphasis on ‘atypical fibroxanthomas’. Am J Dermatopathol. 1988;10:9–19. doi: 10.1097/00000372-198802000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Kuwano H, Hashimoto H, Enjoji M. Atypical fibroxanthoma distinguishable from spindle cell carcinoma in sarcoma-like skin lesions: a clinicopathologic and immunohistochemical study of 21 cases. Cancer. 1985;55:172–180. doi: 10.1002/1097-0142(19850101)55:1<172::aid-cncr2820550127>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Clarke LE, Frauenhoffer E, Fox E, et al. CD10-positive myxofibrosarcomas: a pitfall in the differential diagnosis of atypical fibroxanthoma. J Cutan Pathol. 2010;37:737–743. doi: 10.1111/j.1600-0560.2010.01532.x. [DOI] [PubMed] [Google Scholar]
- 29.Hartel PH, Jackson J, Ducatman BS, Zhang P. CD99 immunoreactivity in atypical fibroxanthoma and pleomorphic malignant fibrous histiocytoma: a useful diagnostic marker. J Cutan Pathol. 2006;33(suppl 2):24–28. doi: 10.1111/j.1600-0560.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- 30.Monteagudo C, Calduch L, Navarro S, et al. CD99 immunoreactivity in atypical fibroxanthoma: a common feature of diagnostic value. Am J Clin Pathol. 2002;117:126–131. doi: 10.1309/2EXB-70CW-3U6P-VQ6H. [DOI] [PubMed] [Google Scholar]
- 31.Pouryazdanparast P, Yu L, Cutlan JE, et al. Diagnostic value of CD163 in cutaneous spindle cell lesions. J Cutan Pathol. 2009;36:859–864. doi: 10.1111/j.1600-0560.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- 32.Leinweber B, Hofmann-Wellenhof R, Kaddu S, et al. Procollagen 1 and Melan-A expression in desmoplastic melanomas. Am J Dermatopathol. 2009;31:173–176. doi: 10.1097/DAD.0b013e3181930b85. [DOI] [PubMed] [Google Scholar]
- 33.Ma CK, Zarbo RJ, Gown AM. Immunohistochemical characterization of atypical fibroxanthoma and dermatofibrosarcoma protuberans. Am J Clin Pathol. 1992;97:478–483. doi: 10.1093/ajcp/97.4.478. [DOI] [PubMed] [Google Scholar]
- 34.Gleason BC, Calder KB, Cibull TL, et al. Utility of p63 in the differential diagnosis of atypical fibroxanthoma and spindle cell squamous cell carcinoma. J Cutan Pathol. 2009;36:543–547. doi: 10.1111/j.1600-0560.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 35.Dotto JE, Glusac EJ. p63 is a useful marker for cutaneous spindle cell squamous cell carcinoma. J Cutan Pathol. 2006;33:413–417. doi: 10.1111/j.0303-6987.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 36.Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98:827. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Valeriani M, Ribuffo D, et al. Recurrent cutaneous leiomyosarcoma. J Exp Clin Cancer Res. 1998;17:83–85. [PubMed] [Google Scholar]
- 38.Yamada S, Guo X, et al. Primary desmoplastic cutaneous leiomyosarcoma associated with high MIB-1 labeling index: a teaching case giving rise to diagnostic difficulties on a small biopsy. Pathol Res Pract. 2011;207:728–732. doi: 10.1016/j.prp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Kraft S, Fletcher CD. Atypical intradermal smooth muscle neoplasms: clinicopathologic analysis of 84 cases and a reappraisal of cutaneous ‘leiomyosarcoma’. Am J Surg Pathol. 2011;35:599–607. doi: 10.1097/PAS.0b013e31820e6093. [DOI] [PubMed] [Google Scholar]
- 40.Bellezza G, Sidoni A, et al. Primary cutaneous leiomyosarcoma: a clinicopathological and immunohistochemical study of 7 cases. Int J Surg Pathol. 2004;12:39–44. doi: 10.1177/106689690401200106. [DOI] [PubMed] [Google Scholar]
- 41.Bahrami A, Folpe AL. Adult-type fibrosarcoma: a reevaluation of 163 putative cases diagnosed at a single institution over a 48-year period. Am J Surg Pathol. 2010;34:1504–1513. doi: 10.1097/PAS.0b013e3181ef70b6. [DOI] [PubMed] [Google Scholar]
- 42.Busam K. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. ed 1. New York: Saunders; 2010. [Google Scholar]
- 43.Kamath NV, Ormsby A, Bergfeld WF, House NS. A light microscopic and immunohistochemical evaluation of scars. J Cutan Pathol. 2002;29:27–32. doi: 10.1034/j.1600-0560.2002.290105.x. [DOI] [PubMed] [Google Scholar]
- 44.Stefanato CM, Robson A, Calonje JE. The histopathologic spectrum of regression in atypical fibroxanthoma. J Cutan Pathol. 2010;37:310–315. doi: 10.1111/j.1600-0560.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 45.Seavolt M, McCall M. Atypical fibroxanthomas: review of the literature and summary of 13 patients treated with Mohs micrographic surgery. Dermatol Surg. 1999;32:435–441. doi: 10.1111/j.1524-4725.2006.32087.x. [DOI] [PubMed] [Google Scholar]
- 46.Ang GC, Roenigk RK, Otley CC, et al. More than 2 decades of treating atypical fibroxanthoma at Mayo Clinic: what have we learned from 91 patients? Dermatol Surg. 2009;35:765–772. doi: 10.1111/j.1524-4725.2009.01126.x. [DOI] [PubMed] [Google Scholar]
- 47.Zalla MJ, Randle HW, Brodland DG, et al. Mohs surgery vs wide excision for atypical fibroxanthoma: follow-up. Dermatol Surg. 1997;23:1223–1224. doi: 10.1111/j.1524-4725.1997.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 48.Chilukuri S, Alam M, Goldberg L. Two atypical fibroxanthomas of the ear. Dermatol Surg. 2003;29:408–410. doi: 10.1046/j.1524-4725.2003.29095.x. [DOI] [PubMed] [Google Scholar]
- 49.Davis JL, Randle HW, Zalla MJ, et al. A comparison of Mohs micrographic surgery and wide excision for the treatment of atypical fibroxanthoma. Dermatol Surg. 1997;23:105–110. doi: 10.1111/j.1524-4725.1997.tb00670.x. [DOI] [PubMed] [Google Scholar]


