Abstract
Immunotherapy strategies against cancer are emerging as powerful weapons for treatment this disease. The success of checkpoint inhibitors against metastatic melanoma and adoptive T-cell therapy with CARTs against B-cell derived leukemias and lymphomas are only two examples of developments that are changing the paradigms of clinical cancer management. These changes are a result of many years of intense research into complex and interrelated cellular and molecular mechanisms controlling immune responses. Promising advances come from the discovery of cancer mutation-encoded neoantigens, improvements in vaccine development, progress in delivery of cellular therapies and impressive achievements in biotechnology. As a result, radical transformation of cancer treatment is taking place in which conventional cancer treatments are being integrated with immunotherapeutic agents. Many clinical trials are in progress testing potential synergistic effects of treatments combining immunotherapy with other therapies. Much remains to be learned about the selection, delivery and off-target effects of immunotherapy used alone or in combination. The existence of numerous escape mechanisms from the host immune system that human tumors have evolved still is a barrier to success. Efforts to understand the rules of immune cell dysfunction and of cancer-associated local and systemic immune suppression are providing new insights and fuel the enthusiasm for new therapeutic strategies. In the future, it might be possible to tailor immune therapy for each cancer patient. The use of new immune biomarkers and the ability to assess responses to therapy by non-invasive monitoring promise to improve early cancer diagnosis and prognosis. Personalized immunotherapy based on individual genetic, molecular and immune profiling is a potentially achievable future goal. The current excitement for immunotherapy is justified in view of many existing opportunities for harnessing the immune system to treat cancer.
Introduction
CD8 T lymphocytes, NK cells and certain CD4 T helper lymphocytes are the only cell types in the organism that acquire the ability to kill sister cells as a mechanism of defense for eradicating or controling intracellular pathogens. As immunotherapists, our efforts are focused on harnessing and redirecting these cell-killing mechanisms to destroy malignant tissues and thus improve therapeutic efficacy against cancer. In modern oncology, attempts to harness and direct the power of the immune system against cancer are best exemplified by therapeutic vaccines. These are formulations of tumor antigens that are expected to elicit immune responses able to arrest cancer progression and prevent it from recurring. Vaccine development has required extensive preclinical and clinical research and has unraveled pro- and anti- cancer immune mechanisms, but has delivered very little to clinical practice (1). This has created skepticism towards cancer immunotherapy among clinical oncologists. In the last 20 years, two lines of research have dramatically changed this unfavorable view of immune therapies: (i) modulation of immune cells with immunostimulatory monoclonal antibodies (mAbs) (2) and (ii) adoptive T cell therapy (3).
The development of immunostimulatory mAbs (4) owes much to the pioneering work of James Allison (5), Lieping Chen (6), Tasuko Honjo (7) and Gordon Freeman (8), who discovered the critical role of surface receptor- ligand pairs, now known as checkpoint inhibitors, in downregulating T-cell immunity. Checkpoint inhibition could be interfered with by mAbs able to restore T-cell activation and enable T cells to control cancer progression. This line of research has resulted in unprecedented objective clinical efficacy against cancer starting with CTLA-4 blockade in metastatic melanoma (9, 10) and with PD-1/PD-L1 blockade in NSCLC, extending to a growing list of other malignancies, including RCC (11), bladder cancer (12), refractory Hodgkin lymphoma (13), head and neck cancer (14), ovarian cancer (15), MSI colon cancer (16), etc. Table 1 lists recent FDA approvals for clinical use of agents blocking immune checkpoints.
Table 1.
Chronologic FDA approvals of novel immunotherapies.
| Agent | Target/class | indication | Evidence | FDA approval date | Pivotal trial references |
|---|---|---|---|---|---|
| Ipilimumab | CTLA-4 Fully human MAb |
Metastatic melanoma | OS | 25 March 2011 | (9, 10) |
| Pembrolizumab | PD-1 Humanized mAb |
Metastatic melanoma | OS | 4 Sept 2014 | (99, 100) |
| Nivolumab | PD-1 Fully human MAb |
Metastatic melanoma | OS | 22 Dec 2014 | (101, 102) |
| Nivolumab | PD-1 Fully human MAb |
Stage IV NSCLC (squamous) Second line |
OS | 24 March 2015 | (103) |
| Ipilimumab+Nivolumab (Combination) | CTLA-4 and PD-1 Fully human MAbs |
Metastatic melanoma | PFS | 30 September 2015 | (32–34) |
| Pembrolizumab | PD-1 Humanized mAb |
Stage IV NSCLC PD-L1+<50% |
OS | 2 October 2015 | (104) |
| Nivolumab | PD-1 Fully human MAb |
Stage IV NSCLC (adenocarcinoma) Second line |
OS | 9 October 2015 | (105) |
| Tamoligene laherparepvec (T-vec) | HSV-1 replicative viral vector engineered to express GM-CSF | Locally advanced or metastatic melanoma accessible for intratumoral delivery. | OS | 27 October 2015 | (97) |
| Ipilimumab | CTLA-4 Fully human MAb |
Surgically resectable melanoma | OS | 28 October 2015 | (106) |
| Nivolumab | PD-1 Fully human MAb |
Renal cell carcinoma Second line |
OS | 23 November 2015 | (11) |
The other strategy that has improved efficacy of cancer immunotherapy is adoptive transfer of T cells. This field was pioneered by Steven Rosenberg whose team developed methods for isolation and culture of tumor-infiltrating lymphocytes (TILs) which can be re-infused together with exogenous IL-2 to patients rendered lymphopenic by preconditioning regimens (17). Durable response rates of TIL-based adoptive therapies are remarkable and are being replicated in cancer centers worldwide (18). Adoptive T-cell therapy has benefited from Zelig Esshar’s seminal work (19). By engineering T-cells with transmembrane receptors encompassing extracellular single-chain Abs and intracellular signaling domains, impressive efficacy has been attained in clinical trials against B-cell derived malignancies (20). The most successful chimeric receptors pioneered by Carl June and Michael Sadelain include anti-CD19 mAb and the intracellular signaling domains of CD3ζ plus either CD137 or CD28. Results in pediatric ALL, CLL, non-Hodgkin’s lymphoma and myeloma(21–24) have introduced well justified optimism for broader applicability of this therapy to hematological and solid malignancies (20).
Many other recent developments in immunotherapy have contributed to making it “popular” among oncologists and patients. The most promising developments are discussed in this CCR Focus and include: (i) characterization of non- synonymous mutations in cancer giving rise to neoantigens (25); (ii) discovery of new checkpoints and other targetable immunosuppressive mechanisms (26); (iii) progress in the field of T cell trafficking to tumors (27); (iv) an enlarged repertoire of immunologic biomarkers for monitoring responses to therapy and understanding the underlying biology(28); (v) potentiation by immunotherapy of abscopal effects of radiotherapy (see below); and (vi) re-invigoration of therapeutic cancer vaccines by improving tumor antigen presentation and cross-priming (29).
A potential barrier to wide application of immunotherapy has been a concern about toxicities. The concern is legitimate, as most immunotherapies, whether with cells, antibodies or cytokines, are associated with adverse events. These can be readily managed. However, in cancer one additional concern is critical, and this is a possibility of accelerated tumor growth as a result of immune therapy. Therapeutic disturbance of the relationship between the tumor and immune system could result in tumor growth, e.g., if re-activated immune cells produce an excess of factors that will favor proliferation of residual tumor cells or cancer stem cells. For this reason, combinatorial therapies designed to first eliminate these cells and then re-juvenate anti-tumor immunity are under development. More important, the immune system is calibrated to prevent excessive activation that could damage tissues. Hence, Treg and MDSC and other regulatory cells play a key role in maintaining the balance. Its disturbance by re- activating T cells with, e.g., checkpoint inhibitors, is likely to call on regulatory cells to dampen this activation. This is a “rebound effect” which naturally occurs after T-cell activation and leads to expansion of regulatory elements in the immune system. When initiated and/or maintained by therapeutic T-cell activation, it could result in temporary or permanent suppression of anti-tumor activity by endogenous immune regulation. Thus, disturbing the immune balance with the intention of restoring potent anti-tumor responses might induce resistance to further activation. This and other aspects of interference with the physiology of the immune system by immunotherapies may be one of the major challenges that the field will have to overcome.
Although the use of antibodies in cancer has a relatively long history, and clinicians have learned how to deal with related toxicities, therapies with immune cells are much less familiar to oncologists. The widely prevalent perception that cellular therapies for cancer, e.g., with TILs or CARTs, are difficult to manage and costly has limited the production of cells for therapy and their use to few specialized centers. This perception is persisting despite the fact that technological advances in the production, transport and delivery to patients of therapeutic cells have made this therapy more affordable, safe and more widely available. Expectations are that this barrier will disappear, as oncologists become more familiar with cellular therapies and their use.
Current enthusiasm for immunotherapy is justified because overwhelming evidence indicates that it is effective, albeit not in all cases, where conventional therapies were not. Nevertheless, many challenges still exist and will have to be overcome to make it universally available to those patients with cancer who need immune intervention in addition to other therapies.
Immunotherapy Combinations: The Land of Opportunity
Immunotherapeutic synergy defined as a therapeutic effect superior to the additive effect of each of the components in a combination is generally perceived as the most potent engine for progress (30, 31). The first immunotherapy combination which has received FDA approval for metastatic melanoma has been the double CTLA-4 and PD-1 blockade (32–34) (Table 1).
Building on successes of the PD-1/PD-L1 blockade, numerous clinical trials of immunotherapy combinations are in progress (162 entries in Clinical Trials gov. and more under preparation). Combinations include various immunotherapy agents as well as combinations of immunotherapy agents with standard-of-care treatments (30, 31). It would be very surprising if these combinations do not deliver success. However, in some instances, combinations might give positive results at the expense of safety concerns (32–34) and thus become non-tolerable. One promising approach undergoing clinical trials is the combination of co-stimulatory agents and checkpoint inhibitors. As indicated in Figure 1 immunomodulation relies on the presence of an ongoing baseline immune response to cancer neoantigens (25) and our abilities to remove the brakes as well as press gas pedals driving this response (35).
Figure 1. A conceptual palette of immune interventions designed to mix potentially effective combined immunotherapies.
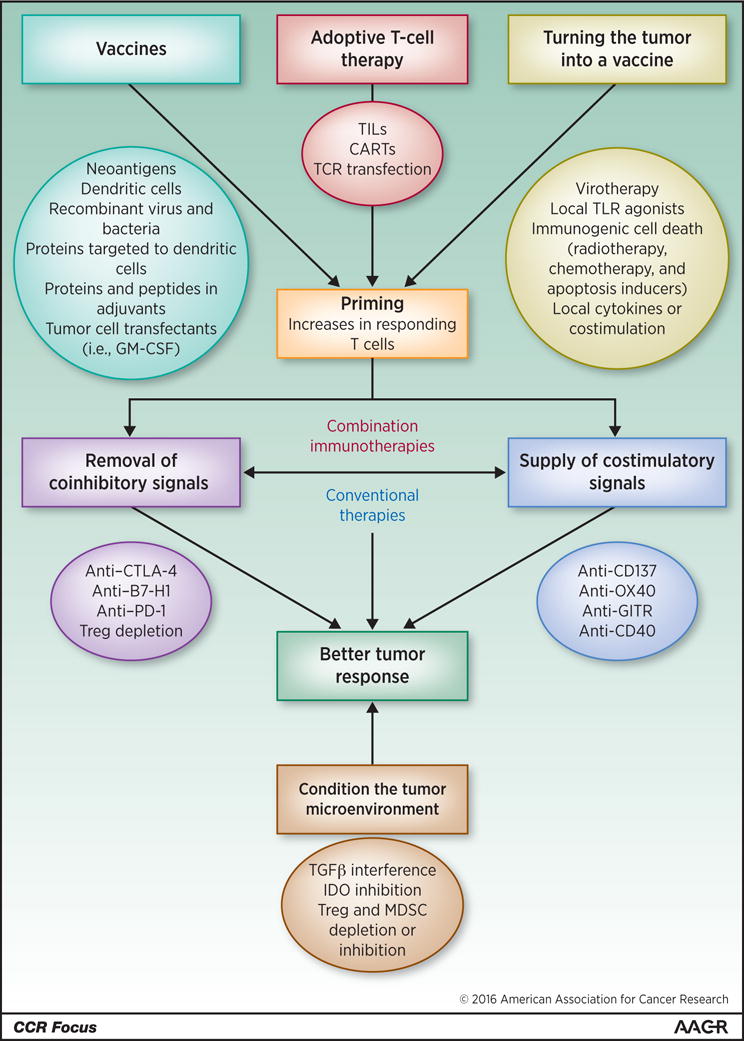
For immunomodulatory interventions to be effective a baseline immune response must be available. Such antitumor responses can be built up by means of vaccines, adoptive cell transfers or by enhancing tumor tissue immunogenicity using one or more of the f listed strategies. Manipulation of the tumor microenvironment appears to be most important to achieve the goal. Adapted from Meleroand colelagues (35).
Concomitant and sequential use of the palettes of new treatments in various combinations is likely to lead to much needed synergistic efficacy. For instance, recently disclosed results from the combination of an IDO inhibitor and PD-1 blockade with excellent safety and efficacy profiles in a phase I/II trial further justify optimism for this and similar therapeutic strategies (36).
Interestingly, the aforementioned brightest stars in immunotherapy (immunomodulatory monoclonal antibodies and adoptive T-cell therapy) are clearly synergistic in animal models (37).
Understanding Immunosuppression in the Tumor Microenvironment
The tumor microenvironment (TME) consisting of tumor cells, stroma, vascular elements and tumor-draining lymph nodes is a milieu in which multiple and complex cellular interactions take place that shape anti-tumor immune responses and determine eventual efficacy of immunotherapy. The immunosuppressive nature of TME is well known (38, 39), and the realization that each tumor creates its own, unique TME and orchestrates interactions between various cells present in the TME is likely to individualize our strategies for cancer immunotherapy. Immune cells infiltrating the TME are instructed to preferentially adopt the functional phenotypes and activities that support tumor progression. The instructive signals are delivered by the tumor in the form of soluble factors (cytokines, chemokines, inhibitory factors) or exosomes (virus-size vesicles) which alter the behavior of local or distant immune and tissue cells and/or invite the entry of regulatory immune cells into the tumor milieu. As Table 2 summarizes, many immunosuppressive factors and cells lurk in the TME; however, not all are present in all tumors. For example, some human tumors express COX-2 and secrete PGE2, others produce adenosine or express idoleamine-2,3 dioxygenase (IDO) and still others are avid TGF-β or IL-10 producers (40). The immunosuppressive profile appears to be related to tumor aggressiveness and determines the presence and degree of T-cell activation or exhaustion/dysfunction prevailing in the TME (26, 40).
Table 2.
Immunosuppressive factors and cells that contribute to T-cell dysfunction in the TME and immune therapies for restoration of anti-tumor immune competence a)
| Factor/cell/cell product | Effects in T cells | Potential immune therapy | References |
|---|---|---|---|
| Inhibitory receptor ligands | T-cell exhaustion via IRs signaling plus the TCR-mediated chronic stimulation with TAs | Checkpoint inhibition: partial or complete restoration of T-cell functions translating into clinical responses | (32, 99, 107, 108) |
|
| |||
| Soluble factors: IL-10 TGF-β IL-35 IDO Galectin-9 Arginase COX-2/PGE2 Adenosine Oxygen radicals |
Alone or in cooperation with PD-1 inhibit functions of TA-specific CD8+ T cells by utilizing various relevant molecular pathways (referenced in the last column) | Neutralizing Abs; Abs targeting/blocking receptors, pharmacologic inhibitors; selective drug blockade | (109–116) |
|
| |||
| Regulatory cells: iTreg MDSC |
Down-regulation of effector T-cell functions by contact-dependent or contact-independent delivery of inhibitory proteins, killing-inducing mediators or oxygen radicals |
Treg or MDSC depletion or inhibition of their suppressor activities with blocking antibodies, immune checkpoint inhibitors or pharmacological agents |
(40–42, 44) (115–130) |
| Tumor-derived immunoinhibitory exosomes |
Negative signals inhibit Teff functions but promote regulatory cell expansion; inhibitory miRNA transfer |
Removal of exosomes (plasmapheresis); blockade of signaling or inhibition of exosome release | (131) |
|
| |||
| MHC class I down-regulation/loss; β2-microglogulin inactivation on tumor cells | Interferes with Ag presentation by silencing Ag presenting machinery by tumors and with tumor recognition by T cells | Up-regulation of MHC-I expression by interferons or other immune therapies | (132, 133) |
|
| |||
| Metabolic checkpoints, e.g., glucose deprivation | Limits aerobic glycolysis in TILs; decreases the mTOR pathway activity and the ability to produce IFN-ɣ; | Up-regulation of metabolites regulating aerobic glycolysis in the TME | (134, 135) |
The table lists the best known inhibitors of T-cell functions in the TME. The list is not comprehensive, as additional blocking factors may be present. Each tumor develops its own unique immunosuppressive signature and the degree of T-cell dysfunction in the TME varies broadly from one tumor to another depending on the prevailing signature. Abbreviations used: TCR, T-cell receptor; TAs, tumor antigens; IDO, idoleamine-2,3 dioxygenase; PGE2, prostaglandin E2; iTreg, inducible regulatory T cells; MDSC, myeloid-derived suppressor cells; MHC, the major histocompatibility complex; APM, antigen processing machinery.
Much has been learned recently about Treg and MDSC accumulating in the TME (41–43). Emerging data suggest that these regulatory cells are unlike ordinary garden variety T cells or monocytes/macrophages that reside in tissues or blood of normal donors. MDSC in the hypoxic TME are programmed to produce an excess of iNOS, ROS, arginase-1, TGF-β and PGE2, the factors known to interfere with differentiation of DC, effector functions of T cells and to alter the tumor stroma. A high burden of MDSC in the chronically inflamed TME favors tumor progression(43). Therefore, strategies to eliminate MDSC or block their functions are being actively translated into the clinic, including, pharmacologic interference with the major suppressive pathways, e.g., by inhibition of the IDO and tryptophan pathway with indoximod or regulation of the myelopoiesis, e.g., by the administration of all-trans-retinoic acid (ATRA) alone or together with IL-2 to promote differentiation of myeloid cells. Alternatively, prevention of myeloid cells trafficking to tumors by direct targeting chemokines (including CCL2, CCL3, CCL4 and CCL5) or blocking their production by the tumor can be pursued. Other approaches involve reduction in the frequency or blocking functions of MDSC, e.g., by utilizing chemotherapies, which when delivered at lower doses deplete MDSC and induce anti-tumor immunity. Not surprisingly, MDSC accumulation in tumors appears to interfere with anti-PD1 immunotherapy, and targeting of CXCR2+ MDSC with antibodies was reported to improve efficiency of the checkpoint blockade (44). Other approaches already in clinical development involve targeting the CSF1-R (45). Neutralization of MDSC as an adjunct strategy to other immunotherapies is a significant component of the novel anti-tumor therapeutics.
Treg present in the TME are highly suppressive and, in contrast to other tumor-infiltrating T cells (TILs) are not dysfunctional. Intra-tumoral CD4+CD25hiCD39+ FOXP3+ Treg up-regulate immunosuppressive molecules (e.g., CD39 or TGF-β-associated molecules, LAP and GARP) and inhibitory receptors (46). Treg isolated from patients’ peripheral blood or tumor tissues co-expressed several inhibitory receptors and their suppressive activity within tumor-infiltrating lymphocytes (TIL) far exceeded that of Treg in the periphery (47). As these Treg had high expression levels of PD-1, it was expected that strong negative signaling via this receptor would inhibit Treg functions. However, early studies in mice showed that PD-L1 signaling via PD-1 promoted Treg cell development and functions, synergized with TGF-β to enhance conventional T-cell conversion to iTreg, maintained FOXP3 expression and increased Treg survival. It appears that PD-1, and perhaps other checkpoint receptors, function not as inhibitory but as stimulatory receptors in Treg (48). These data suggest that in Treg, PD-1 is programmed to function differently than in conventional T cells. Thus, anti-PD-1 antibodies, which release the break in conventional T cells restoring their functions, would be expected to block Treg-mediated suppression and further enhance anti-tumor responses benefiting the host. However, there is a concern that in Treg, which overexpress PD-1 in the TME, PDL-1 signaling up-regulates PTEN expression, blocks the Akt/mTOR pathway and activates STAT5/STAT3 signaling (49), leading to expansion of Treg and promoting their suppressive functions. This scenario, based on unique molecular signaling in Treg, implies that anti-PD-1 antibody therapies could have unexpected effects on Treg. Already evidence emerges that ipilimumab targeting CTLA-4 is not completely effective in eliminating Treg by ADCC (T.L. Whiteside; unpublished data) as suggested in mouse models (50). Depending on conditions prevailing in the TME, the surviving Treg might expand and interfere with benefits of checkpoint inhibitors. Despite many approaches used in the clinic for Treg depletion [reviewed in (51, 52) ] their persistence and resistance to chemotherapies (53) have been a problem. In addition, considerable functional heterogeneity of these cells and their essential role in preventing autoimmunity, compels us to think of how to deplete or muzzle “bad” iTreg operating in the TME without sacrificing “good” natural Treg necessary for maintaining homeostasis and keep autoimmunity at bay. Successful management of cancer-associated iTreg remains one of the challenges of cancer immunotherapies today.
Reversal of T- cell Dysfunction at the Tumor Microenvironment and Checkpoint Inhibitors
There is ample evidence in experimental models and in humans that CD8+ T cells become exhausted/dysfunctional upon chronic antigen exposure in the tumor microenvironment (TME). These dysfunctional/exhausted T cells exhibit defective proliferative capacities and cytokine production (54). However, they are not totally inert and appear capable of exerting lytic functions (26). Dysfunctional CD8+ T cells upregulate a number of inhibitory receptors (IRs)/immune checkpoints (55) that bind to their ligands expressed by tumor cells and antigen-presenting cells (APCs)(56) in TME, including PD-1, CTLA-4, Tim-3 (57), LAG-3, BTLA (58) and TIGIT (59). Hence, dual immune checkpoint blockade appear to better enhance T cell expansion and functions and promote tumor rejection in vitro and in vivo. The recent success of dual CTLA-4/PD-1 blockade, which has been approved by the FDA (Table 1) in advanced melanoma underlines the clinical efficacy of such strategy.
While CD8+ TILs in the TME appear to upregulate IRs, they also upregulate a number of activating receptors (ARs) like 4-1BB, OX40 and GITR (60). These are members of the TNFR family that can readily co-stimulate T cell functions upon ligation. Agonist monoclonal antibodies show promising therapeutic effects against cancer mouse models are under development in clinical trials (61–63). At least in preclinical models these agonist agents are strongly synergistic with checkpoint inhibitors (30, 31).
One important question is to determine among cancer patients who is more likely to respond to immunotherapies targeting immunoregulatory pathways and when additional strategies may be needed to induce T cell responses to tumors. The answer to this question may come from the gene signature studies of metastatic melanoma, which propose to classify tumors into “inflamed” and “non-inflamed” phenotypes (28). While inflamed tumors are spontaneously immunogenic and may be more likely to respond to immune interventions to counteract the mechanisms of tumor-induced T cell dysfunction, non-inflamed tumors lack tumor-infiltrating T cells and may likely need to be treated with novel targeted therapies (sting agonists, inhibitors of β catenin pathway) to induce T-cell activation and migration into the tumors (64–66).
Radiotherapy and Immune-Mediated Abscopal Effects
The above mentioned successes of immune checkpoint inhibitors have clearly demonstrated that treating the host immune system in addition to killing the neoplastic cells can be very effective at achieving long-term tumor control. However, responses are limited to patients with some degree of pre-existing tumor-reactive T cells infiltrating the tumor. In this context, ionizing radiation therapy (RT), a local cancer treatment used for almost a century to kill cancer cells is finding a new role. The convergence of technological progress in the precise delivery of RT with improved understanding of the inflammatory signals associated with various cell death pathways triggered by radiation (67, 68) has enabled a conceptual transformation whereby RT is considered a promising partner for immunotherapy due to its ability to induce a cell death that is immunogenic potentially converting the tumor into an in situ vaccine (69–71).
The ability of RT to enlist the help of the immune system against the tumor has important implications not only for improved local control of the irradiated tumor (72, 73), but most importantly for systemic tumor control (74) (Figure 2). The regression of metastases outside the field of radiation after irradiation of one tumor site is known as “abscopal effect”. It is a rare but well-documented phenomenon that has been reported more frequently in patients with more immunogenic tumor types (75). Sensing of tumor-derived DNA by tumor-infiltrating dendritic cells activates type I interferon (IFN) production via the stimulator of IFN genes (STING) pathway, a mechanism critical for generation of spontaneous anti-tumor T cells responses to immunogenic tumors (76). Importantly, recent data show that the same pathway is amplified by RT (77), providing a possible explanation for the occurrence of abscopal effects. However, the ability of RT to induce T cell responses in less immunogenic tumors is limited by immunosuppressive networks operating in the TME., This explains why abscopal effects are very rare. For example, TGF-β is a critical barrier to RT-induced priming of T-cell responses to multiple endogenous tumor antigens, exacerbated by the conversion of TGF-β from its latent to active form by RT-generated ROS (78). Other barriers include regulatory T cells and MDSC (79, 80). Pre-clinical studies have demonstrated that multiple immunotherapies that either block immunosuppressive mechanisms or improve immune activation can work in concert with RT to generate an in situ tumor vaccine and induce abscopal effects (81).
Figure 2. Concept of immune mediated abscopal effects.
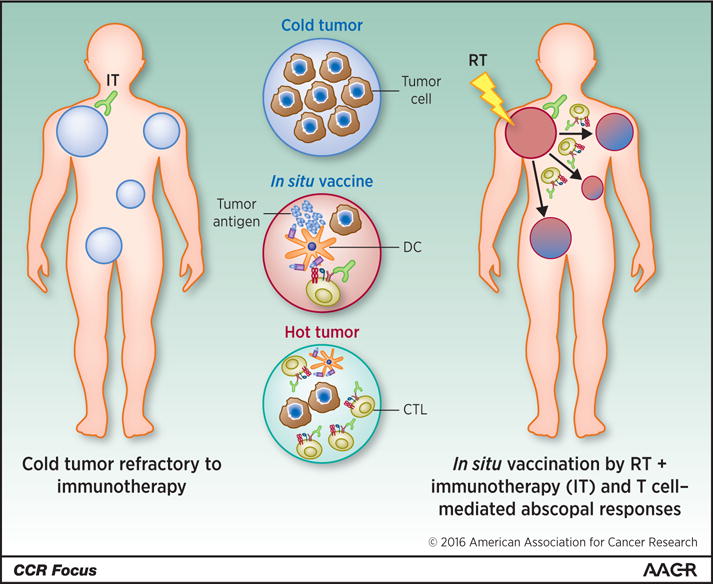
Schematic representation of immune mediated effects. The scheme describes the systemic pro-inflamatory effects of gamma irradiation of the irradiated tumor lesion well that become hot and acts as an in situ tumor-attenuated vaccine against distant non-irradiated tumors. Such local response can be enhanced by immunostimulatory monoclonal antibodies to attain a systemic effect. Exploiting the systemic immune-mediated effects of radiotherapy offers opportunity to maximize the effect of novel immunotherapies. DC, dendritic cell; CTL, cytotoxic T cell.
Importantly, these pre-clinical data are beginning to show clinical relevance. Combination of RT with cytokines that enhance dendritic cell numbers and function or TLR agonists that improve immune activation within the irradiated tumor induced abscopal responses in close to 30% of the patients in early clinical trials (82, 83). In another phase I study markedly improved response rate to high dose IL-2 was seen in melanoma and renal cell carcinoma patients treated with RT (84). Several trials are ongoing to test RT in combination with various immunotherapy agents, including OX40 agonist and TGFβ neutralizing antibodies (85).
Perhaps the most exciting hypothesis being tested in the clinic is that RT can “raise the roof” of responders to immune checkpoint inhibitors. Extensive pre-clinical evidence and a growing number of clinical reports in melanoma patients unresponsive to anti-CTLA-4 support this hypothesis (86–88). Importantly, a striking synergy of RT with anti-CTLA-4 has also been seen in a patient with non-small cell lung cancer (NSCLC), a tumor type where anti-CTLA-4 alone has no activity (89, 90), raising hope that RT could be used to extend the benefits of this treatment to multiple tumor types. Recent results of a prospective clinical trial support the synergy of RT with anti-CTLA-4 in NSCLC (91). However, in another large study in metastatic castrate-resistant prostate cancer the addition of anti-CTLA-4 to RT failed to improve responses (92). While reasons for this difference are unclear, the RT dose and fractionation used (93), the tumor type or the site chosen for irradiation may all play a role in determining the responses, and need to be further investigated. Several trials testing the synergy of PD-1/PD-L1 targeting agents with RT are ongoing, and will provide important results.
Overall, RT has a strong appeal as a commonly available, cost-effective treatment to generate T cells specific for neo-antigens expressed by each individual patient’s tumor (94). Research is ongoing to define the antigenic targets of T cell responses at the irradiated and abscopal tumor sites, the optimal RT doses and fractionation and the optimal partnerships with immunotherapy.
The Road Ahead of Us and Our Patients
In the cancer immunotherapy community, the overall state of mind is optimistic. Much knowledge painstakingly accumulated over the years is driven to clinical translation at an incredibly fast pace. Big pharmaceutical and biotechnology companies are committing their best resources to the field and we expect good news in the following months and years. In this climate, the following points should be considered:
We will be mainly constructing and developing drug combinations based on the success of PD-1 and PD-L1 blockade. And we will especially focus on the non-responders to PD-1 blockade monotherapy.
There are interesting opportunities in targeting engineered biomolecules to the tumor microenvironment (95) and in intratumoral delivery of immunotherapeutic compounds (96).
Local and systemic virotherapy (96) will become more widely used as the best way to alert the immune system and render tumors immunogenic hold great promise especially regarding combinations (30, 31). An agent of this kind based on HSV-1 has recently received FDA approval for melanoma (Table 1) to be used by direct intra-tumoral injections (97).
We will concentrate efforts on strategies to improve therapy of tumors endowed with low antigenicity (25) or those which are refractory to T- cell infiltration (27, 66).
We will be developing better, more predictive preclinical models to test immunotherapies including humanized mice implanted with human tumors and human immune systems (98).
Access to ever-improving personalized genetic and molecular profiling of tumors together with assessments of the patients’ immune status will provide a basis for individualized and potentially more effective selective immunotherapy
Numerous clinical trials will be needed to demonstrate efficacy and learn the biology necessary for building most effective combinations and addressing malignant diseases that are classically considered to be non-amenable to immunotherapy.
Acknowledging that our knowledge of the immune system functions in cancer patients is incomplete, we will increase discovery efforts and focus attention on the development of new biomarkers that could improve early diagnosis, serve as surrogates of response to immune therapies and predict responses.
Looking at the impressive Kaplan Meier survival plots of pivotal immunotherapy clinical trials, we are encouraged to remember that there are many opportunities for making improvements in terms of both patients’ survival and the quality of life. Hence it will be acceptable to take balanced risks in the pursuit of improvements.
Reviews in this in CCR Focus have been selected to concentrate on the new trends and challenges in cancer immunotherapy. We should “never underestimate the dark side of the force”, but if we are doing the right things now, the eyes of our medical students of today will see in their patients things that we would have never dreamt of only fifteen years ago.
Acknowledgments
Grant Support
T.L. Whiteside was supported by the NIH under award numbers R01CA16862 and P30CA047904. S. Demaria was supported by the NIH under award number R01CA201246, the U.S. Department of Defense Breast Cancer Research Program (W81XWH-11-1-0532), the Breast Cancer Research Foundation, and the Chemotherapy Foundation. M.E. Rodriguez-Ruiz was supported by the MICINN (SAF2011-22831 and SAF2014-52361-R) and a Rio Hortega contract from ISCIII. H.M. Zarour was supported by the NIH under award numbers R01CA157467 and P50CA121973 (Spore in Skin Cancer). I. Melero was supported by the MICINN (SAF2011-22831 and SAF2014-52361-R), Departamento de Salud del Gobierno de Navarra, Redes temáticas de investigación cooperativa RETICC, European Commission VII Framework and Horizon 2020 programs (AICR and PROCROP), SUDOE-IMMUNONET, Fundación de la Asociación Española Contra el Cáncer (AECC), Fundación BBVA, and Fundación Caja Navarra.
Footnotes
Disclosure of Potential Conflicts of Interest
S. Demaria is a consultant/advisory board member for Eisai, Lytix Biopharma, and Nanobiotix. H.M. Zarour reports receiving commercial research grants from Bristol-Myers Squibb and Merck. I. Melero reports receiving commercial research grants from Bristol-Myers Squibb and Pfizer and is a consultant/advisory board member for Alligator Bioscience, AstraZeneca, BiOncoTech Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, and Novartis. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–24. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murillo O, Arina A, Tirapu I, Alfaro C, Mazzolini G, Palencia B, et al. Potentiation of therapeutic immune responses against malignancies with monoclonal antibodies. Clin Cancer Res. 2003;9:5454–64. [PubMed] [Google Scholar]
- 5.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 7.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 13.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. The New England journal of medicine. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen EEW, Machiels JPH, Harrington KJ, Burtness B, Shin SW, Gause CK, et al. KEYNOTE-040: A phase III randomized trial of pembrolizumab (MK-3475) versus standard treatment in patients with recurrent or metastatic head and neck cancer. J Clin Oncol. 2015;33(suppl) abstr TPS6084. [Google Scholar]
- 15.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:4015–22. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 16.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer–what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577–85. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19:4792–800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 19.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maus MV, June CH. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res. 2016;22:xxxx–xxxx. doi: 10.1158/1078-0432.CCR-15-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science translational medicine. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. The New England journal of medicine. 2015;373:1040–7. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Türeci Ö, Vormehr M, Diken M, Kreiter S, Huber C, Sahin U. Targeting the heterogeneity of cancer with individualized neoepitope vaccines. Clin Cancer Res. 2016;22:xxxx–xxxx. doi: 10.1158/1078-0432.CCR-15-1509. [DOI] [PubMed] [Google Scholar]
- 26.Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016;22:xxxx–xxxx. doi: 10.1158/1078-0432.CCR-15-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–6. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22:xxxx–xxxx. doi: 10.1158/1078-0432.CCR-15-1507. [DOI] [PubMed] [Google Scholar]
- 29.Bol KF, Schreibelt G, Gerritsen WR, de Vries IJM, Figdor CG. Dendritic cell–based immunotherapy: state of the art and beyond. Clin Cancer Res. 2016;22:xxxx–xxxx. doi: 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- 30.Melero I, Berman DM, Aznar MA, Korman AJ, Perez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–72. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 31.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2015;13:143–58. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 32.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373:1270–1. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melero I, Martinez-Forero I, Dubrot J, Suarez N, Palazon A, Chen L. Palettes of vaccines and immunostimulatory monoclonal antibodies for combination. Clin Cancer Res. 2009;15:1507–9. doi: 10.1158/1078-0432.CCR-08-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangadhar TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Luke JJ, et al. Preliminary results from a phase I/II study of epacadostat (incb024360) in combination with pembrolizumab in patients with selected advanced cancers. J Immunother Cancer. 2015;3(Suppl 2):07. [Google Scholar]
- 37.Morales-Kastresana A, Labiano S, Quetglas JI, Melero I. Better performance of CARs deprived of the PD-1 brake. Clin Cancer Res. 2013;19:5546–8. doi: 10.1158/1078-0432.CCR-13-2157. [DOI] [PubMed] [Google Scholar]
- 38.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2015;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteside TL. Induced regulatory T cells in inhibitory microenvironments created by cancer. Expert Opin Biol Ther. 2014;14:1411–25. doi: 10.1517/14712598.2014.927432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell DJ. Control of Regulatory T Cell Migration, Function, and Homeostasis. J Immunol. 2015;195:2507–13. doi: 10.4049/jimmunol.1500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Science translational medicine. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–59. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Schuler PJ, Schilling B, Harasymczuk M, Hoffmann TK, Johnson J, Lang S, et al. Phenotypic and functional characteristics of CD4+ CD39+ FOXP3+ and CD4+ CD39+ FOXP3neg T-cell subsets in cancer patients. Eur J Immunol. 2012;42:1876–85. doi: 10.1002/eji.201142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–35. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeiser R, Negrin RS. Interleukin-2 receptor downstream events in regulatory T cells: implications for the choice of immunosuppressive drug therapy. Cell Cycle. 2008;7:458–62. doi: 10.4161/cc.7.4.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Whiteside TL. The role of regulatory T cells in cancer immunology. ImmunoTargets and Therapy. 2015;4:159–71. doi: 10.2147/ITT.S55415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuler PJ, Harasymczuk M, Schilling B, Saze Z, Strauss L, Lang S, et al. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clin Cancer Res. 2013;19:6585–96. doi: 10.1158/1078-0432.CCR-13-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apetoh L, Smyth MJ, Drake CG, Abastado JP, Apte RN, Ayyoub M, et al. Consensus nomenclature for CD8 T cell phenotypes in cancer. Oncoimmunology. 2015;4:e998538. doi: 10.1080/2162402X.2014.998538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer research. 2012;72:887–96. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turnis ME, Andrews LP, Vignali DA. Inhibitory receptors as targets for cancer immunotherapy. Eur J Immunol. 2015;45:1892–905. doi: 10.1002/eji.201344413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer immunology research. 2014;2:393–8. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 58.Pasero C, Olive D. Interfering with coinhibitory molecules: BTLA/HVEM as new targets to enhance anti-tumor immunity. Immunol Lett. 2013;151:71–5. doi: 10.1016/j.imlet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol. 2015;15:243–54. doi: 10.1038/nri3799. [DOI] [PubMed] [Google Scholar]
- 60.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–53. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Seminars in oncology. 2010;37:508–16. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 62.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–7. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol. 2012;24:217–24. doi: 10.1016/j.coi.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol. 2015;42:663–71. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015;11:1018–30. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 67.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med. 2013;19:565–82. doi: 10.1016/j.molmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25:11–7. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 71.Formenti SC, Demaria S. Radiotherapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012;84:879–80. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63:1229–35. [PubMed] [Google Scholar]
- 74.Demaria S, Ng B, Devitt M-L, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 75.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–10. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity. 2014;41:830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33:7415–22. doi: 10.1016/j.vaccine.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2010;11:2435–66. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–32. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 82.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–32. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and GM-CSF in patients with metastatic solid tumors: a proof of principle trial to generate abscopal responses. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 84.Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2–tumor and immunological responses. Sci Transl Med. 2012;4:137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 85.Crittenden M, Kohrt H, Levy R, Jones J, Camphausen K, Dicker A, et al. Current clinical trials testing combinations of immunotherapy and radiation. Semin Radiat Oncol. 2015;25:54–64. doi: 10.1016/j.semradonc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pilones KA, Vanpouille-Box C, Demaria S. Combination of radiotherapy and immune checkpoint inhibitors. Semin Radiat Oncol. 2015;25:28–33. doi: 10.1016/j.semradonc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 87.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non-Small Cell Lung Cancer. Cancer Immunol Res. 2013;1:365–72. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zatloukal P, Heo DS, Park K, Kang J, Butts C, Bradford D, et al. Randomized phase II clinical trial comparing tremelimumab (CP-675, 206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2009;27:8071. [Google Scholar]
- 91.Golden EB, Chachoua A, Fenton-Kerimian MB, Demaria S, Formenti SC. Abscopla responses in metastatic non-small cell lung cancer (NSCLC) patients treatded on a phase 2 study of combined radiation therapy and ipilimumab: evidence for the in situ vaccination hypothesis of radiation. Int J Radiat Oncol Biol Phys. 2015;93:S66–7. [Google Scholar]
- 92.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 95.Przepiorka D, Ko CW, Deisseroth A, Yancey CL, Candau-Chacon R, Chiu HJ, et al. FDA Approval: Blinatumomab. Clin Cancer Res. 2015;21:4035–9. doi: 10.1158/1078-0432.CCR-15-0612. [DOI] [PubMed] [Google Scholar]
- 96.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20:1747–56. doi: 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:2780–8. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 98.Sanmamed MF, Rodriguez I, Schalper KA, Onate C, Azpilikueta A, Rodriguez-Ruiz ME, et al. Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2−/−IL2Rgammanull Immunodeficient Mice. Cancer research. 2015;75:3466–78. doi: 10.1158/0008-5472.CAN-14-3510. [DOI] [PubMed] [Google Scholar]
- 99.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 100.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. The Lancet Oncology. 2015;16:908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 102.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. The Lancet Oncology. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 103.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2015 Dec 18; doi: 10.1016/S0140-6736(15)01281-7. pii: S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 105.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. The Lancet Oncology. 2015;16:522–30. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 107.Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421–7. doi: 10.1038/bjc.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun Z, Fourcade J, Pagliano O, Chauvin JM, Sander C, Kirkwood JM, et al. IL10 and PD-1 Cooperate to Limit the Activity of Tumor-Specific CD8+ T Cells. Cancer Res. 2015;75:1635–44. doi: 10.1158/0008-5472.CAN-14-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fujii S, Shimizu K, Shimizu T, Lotze MT. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood. 2001;98:2143–51. doi: 10.1182/blood.v98.7.2143. [DOI] [PubMed] [Google Scholar]
- 111.Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-beta pathway for cancer therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:4514–21. doi: 10.1158/1078-0432.CCR-11-3224. [DOI] [PubMed] [Google Scholar]
- 112.Sawant DV, Hamilton K, Vignali DA. Interleukin-35: Expanding Its Job Profile. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2015;35:499–512. doi: 10.1089/jir.2015.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Soliman HH, Jackson E, Neuger T, Dees EC, Harvey RD, Han H, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5:8136–46. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochimica et biophysica acta. 2015;1855:235–47. doi: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunological reviews. 2008;222:180–91. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mandapathil M, Whiteside TL. Targeting human inducible regulatory T cells (Tr1) in patients with cancer: blocking of adenosine-prostaglandin E(2) cooperation. Expert opinion on biological therapy. 2011;11:1203–14. doi: 10.1517/14712598.2011.581225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaminska K, Szczylik C, Lian F, Czarnecka AM. The role of prostaglandin E2 in renal cell cancer development: future implications for prognosis and therapy. Future oncology. 2014;10:2177–87. doi: 10.2217/fon.14.152. [DOI] [PubMed] [Google Scholar]
- 118.Whiteside TL, Jackson EK. Adenosine and prostaglandin e2 production by human inducible regulatory T cells in health and disease. Frontiers in immunology. 2013;4:212. doi: 10.3389/fimmu.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:5626–35. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 120.Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, et al. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74:3652–8. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 121.Tekiner-Gulbas B, Westwell AD, Suzen S. Oxidative stress in carcinogenesis: new synthetic compounds with dual effects upon free radicals and cancer. Current medicinal chemistry. 2013;20:4451–9. doi: 10.2174/09298673113203690142. [DOI] [PubMed] [Google Scholar]
- 122.Tong L, Chuang CC, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer letters. 2015;367:18–25. doi: 10.1016/j.canlet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 123.Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:134ra62. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192–201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peraino JS, Zhang H, Rajasekera PV, Wei M, Madsen JC, Sachs DH, et al. Diphtheria toxin-based bivalent human IL-2 fusion toxin with improved efficacy for targeting human CD25(+) cells. Journal of immunological methods. 2014;405:57–66. doi: 10.1016/j.jim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 127.Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Seminars in cancer biology. 2012;22:319–26. doi: 10.1016/j.semcancer.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 128.Pico de Coana Y, Poschke I, Gentilcore G, Mao Y, Nystrom M, Hansson J, et al. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer immunology research. 2013;1:158–62. doi: 10.1158/2326-6066.CIR-13-0016. [DOI] [PubMed] [Google Scholar]
- 129.Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Critical reviews in oncology/hematology. 2011;77:12–9. doi: 10.1016/j.critrevonc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 131.Gyorgy B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annual review of pharmacology and toxicology. 2015;55:439–64. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. International journal of cancer Journal international du cancer. 2010;127:249–56. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]
- 133.Seliger B, Ritz U, Ferrone S. Molecular mechanisms of HLA class I antigen abnormalities following viral infection and transformation. International journal of cancer Journal international du cancer. 2006;118:129–38. doi: 10.1002/ijc.21312. [DOI] [PubMed] [Google Scholar]
- 134.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–28. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


