Abstract
Mesenchymal stem/stromal cells (MSCs) have been extensively investigated for their regenerative, immune-modulatory, and wound healing properties. While the laboratory studies have suggested that MSC’s have a unique potential for modulating the etiopathology of multiple diseases, the results from clinical trials have not been encouraging or reproducible. One of the explanations for such variability is explained by the “art” of isolating and propagating MSCs. Therefore, establishing more than minimal criteria to define MSC would help understand best protocols to isolate, propagate and deliver MSCs. Developing a calibration standard, a database and a set of functional tests would be a better quality metric for MSCs. In this review, we discuss the importance of selecting a standard, issues associated with coming up with such a standard and how these issues can be mitigated.
Introduction
The Mesenchymal stem/stromal cell (MSCs) field has been rapidly advancing towards translational medicine. The time it has taken to go from the initial observations of their therapeutic potential in vitro to the translation of MSC based cell therapy has been extremely rapid, a rate perhaps only matched by the discovery of iPSC. The enthusiasm for using MSCs for cell therapy can be partly explained by the potential for autologous transplantation and ease by which they can be isolated, expanded and characterized. At last count, MSCs have been used in over 420 clinical trials in the USA (www.clinicaltrials.gov). Although encouraging, the outcomes from most clinical trials are not consistent and the magnitude of the response has tended to decline as one moves from early stage science to more rigorous double blind studies. It is not entirely clear why such inconsistent results have been obtained, though many explanations have been offered.
Some explanations offered for the inconsistencies are; a) variability in the quality of the MSC preparation between donors’ b) variability in the protocols for cell isolation, expansion and processing for clinical trials and c) variability in defining the end product use, which is generally a composite product. The minimal criteria as recommended by ISCT in 2006, to define MSCs are too basic and non-specific that do not define many functionally distinct cells that have been labeled as MSCs [1]. Added variability is introduced to the results when different tests are used to confirm the quality of the cells or the clinical readouts are variable. These compounding factors have made interpretation of results from the human studies difficult and have raised doubts as to the utility of MSCs. While these concerns are legitimate it is also likely that MSC per se could be different if derived from different tissue sources have variable properties. Additional compounding factors are variability in protocols adapted by different laboratories, genetic and epigenetic variability in the cells of origin - rather than a lack of functional utility of the cells. The fact that other fields of cell therapy face similar issues demonstrates that the variability and inconsistencies are not unique to MSCs [2, 3]. Distinguishing between numbers of such possibilities is not easy, but it is critical for the directionality of advancing the field of cell therapy. Developing protocols to obtain specific preparation of cells that work and identifying surrogate marker and functions would help overcome these technical hurdles and improve the utility of MSCs in cell therapy. While this sounds simple, implementing such a plan is fraught with difficulty. Cells change with time and are asynchronous in a continuous cell culture environment and thus defining a preparation is not as simple as defining its passage number or expression of markers or response in an assay. Defining such simple metrics while is not adequate enough, even such tests are not standardized and hence variable results due to insufficient controls.
Two recent papers authored by experts in the MSC field, highlight the need for a reference material [4, 5] to overcome some of these hurdles. The authors address all the current limitations in using MSCs in cell therapy context and propose a three step path forward to overcome this limbo; (a) uniformity in nomenclature (b) develop a reference cell type to be distributed from one center for MSC researchers heading towards pre-clinical or clinical path (c) A set of minimal information on the process of generating MSC. Prockop’s team further highlight the need for in process data to be included in all publications [4]. In this review, we further propose and not only support the need for generating such a reference standard MSC line (one cell type) but also creating a virtual database from different researchers using the reference MSC line for a prospective validation and better acceptable quality metric for MSCs.
Uniformity in nomenclature
Learning from the lessons from cluster of differentiation nomenclature and miRNA nomenclature, a lack of definition to derive a name for cell type creates multiple problems: (1) Same cells with different names; for example – MSCs are defined in multiple ways as mesenchymal stem cells, multipotential stromal cells, more recently medicinal signaling cells. (2) Cells of similar phenotype are labeled differently based on a slightly different isolation technique: A quick Pubmed search for adult stem cells reveals in the least 25 different names such as very small embryonic-like stem cells (VSEL), multipotent adult progenitor cells(MAPC), human umbilical cord tissue(HUCT) stem cells, Wharton’s jelly-derived mesenchymal stem cells(WJ-MSCs)SC, adipose-derived stem cells (ASCs) [6] [7] [8] [9, 10]. Most of these are similar cell types isolated from different organ system and some of the cell types are unique to the lab that isolated them originally. (3) Defining the cells based on the functionality, For example - Freidenstein defined MSCs as CFU-F based on their property to form colonies [11] or more recently certain groups refer to them as pericytes based on their location [12, 13].
Every investigator believes his/her cell type is different from other – they may well be. However, due to the inherent variability in the MSCs, it becomes an impossibility to define a cell type with 100% accuracy. Therefore, having a list of criteria to define any cell type would benefit the field both basic research and translational research alike. With the advent of new technologies and maturity in the field we believe it is feasible to develop a specific criteria for both defining a cell type and naming the cell type.
Develop a reference cell type to be distributed from one center
Historically, development of any drug involved a stringent definition of the drug that is defined relative to specific variables. The numbers of variables in case of pharmaceutical agents are few and easily tested. An ideal situation for cell therapy would be to develop such a resource, to which every MSC preparation can be compared. However, the number variables to be considered in using cells as drug are in billions. A less than an ideal situation of having a completely characterized drug called MSCs, would be to develop a reference cell line.
Recent draft guidance for industry released by FDA, suggests that any adherent cells – which includes MSCs and ASCs that are manipulated in vitro should be considered as drugs [14]. Furthermore, recent retractions of several high profile papers in the field of stem cell have opened up the discussion of the need for reproducibility of basic science research and also standards for clinical usage of these cells. The supporters argue that the reproducibility is required especially when the research involves humans and translation. Having a ruler or standard would alleviate problems associated with technical variability of controls. The mindset of any biological scientist begins at the graduate school’s training that teaches to compare the results to a “standard”. For example – compare DNA preparations to relative to standard ladder, western blot for proteins relative to marker: it is intuitive to develop a standard for stem cells that have so many variables. The argument and need for a standard is pretty obvious and uncontested, however the devil is in the details of defining a standard for MSCs and/or any other adult stem cell material used for research and therapeutic purposes. The number one output factor to be considered is the mechanisms that MSCs employ to provide the therapeutic benefits. Understanding this would allow us to both define the attractive features of the cells and develop a model system to evaluate.
Mechanisms for therapeutic benefits
MSCs therapeutic benefits range from cell engraftment to effects through secretory mechanisms, i.e. paracrine and juxtacrine. The first clinical use of MSCs was to accelerate hematopoietic recovery after bone marrow ablation in the context of post chemotherapy hematopoietic stem cell transplant [15]. Demonstration of clinical feasibility and multiple animal models providing rationale for therapeutic efficacy of MSCs in non-hematopoietic indications, gave rise to a series of clinical trials of MSCs in a wide range of major diseases including stroke, heart failure, chronic obstructive pulmonary disease(COPD) and liver failure [15–18]. The role of MSCs in reconstructing wounded skin is shown by Sasaki et al. Barzilay’s review provides a comprehensive review of transcription factors involved in trans-differentiation ([19–21]. Rare diseases treated with MSCs such as Osteogenesis Imperfecta, most of the therapeutic benefits in such models are attributed to MSCs engraftment and its juxtacrine mechanisms [22, 23]. A recent suggestion to define MSCs is medicinal secretory cells, supporting the notion that the therapeutic benefits of MSCs are primarily associated with their secretory phenotype. An elegant review by Yi et al summarizes the role of the mechanisms of immunomodulatory effects of MSCs and examples of animal and clinical uses of their immunomodulatory effects. [24, 25]. The increasing evidence for the role of secretome in the functional properties of MSCs is extremely important and relevant to the ongoing clinical application of the MSCs. The majority of MSCs’s clinical outcomes are attributed to the secretome of MSCs [26–29]. Furthermore, the role of extra vesicular communication in both wound healing and cancer justifies the need for secretome profiling of various preparations [30–32]. The variability of secretome is extensively studies in developmental model systems, which suggests that the contents of secretome are dictated by the microenvironment.
Definition for standard/ruler
The ability to generate clinically significant numbers of well-defined MSCs starting with small clinical samples, feasible administration without the need for haplotype matching, and excellent safety profile of the cells has resulted in a broad interest in the clinical use of MSCs. The data obtained from clinical trials warrants the need for standard or a ruler. The most effective therapies are dependent on the type of cells or specific phenotype of cells: For example – cardiovascular therapies are more effective when the cells expressed pericyte like phenotype. Cancers are aggravated when cells expressed senescent like phenotype. GVHD therapies are effective with early passage cells (actively proliferating).
The definition of a standard/reference material: The information from the standard cell type would have;
Optimal culture conditions – that are universally accepted
A gene expression profile including more than 1000 genes, with a conducive set of genes
A secretome profile including extracellular RNA expression
In vitro assays to determine the quality of cells which include colony forming unit assays, differentiation assays
Genomic stability of cells as they are passaged
An important hurdle in the development of such a standard cell type is the ability to manufacture these cells on a large scale while retaining their MSC like properties. Since this is difficult to achieve with adult tissue derived MSCs, other sources of MSCs like embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) have been explored. Although MSCs were derived from human ESCs over 10 years ago [33], the trilineage differentiation ability of these cells is different from the more widely used bone marrow MSCs, making them unsuitable as standard MSCs. hESC derived MSCs have impaired chondrogenic capability [34]. Recently, such hESC derived MSCs (satisfying the ISCT minimal criteria) shown to engraft in immunocompromised mice and supported hematopoiesis in vivo [35]. However it is unclear if these cells are similar to adult MSCs if a more comprehensive ruler (like transcriptome analysis, miRNA analysis or metabolite analysis) would be used. The colony forming ability of these hESC derived MSCs was also lower than bone marrow derived MSCs highlighting important functional differences between the two cell types.
Therefore, as suggested by Rao et al - use of iMSCs or MSCs derived from iPSCs would be advantageous because of the defined nature of the cells, financially more attractive due to low costs of production. iMSCs can now be derived by exposing iPSCs to a single factor like the TGF-b signaling inhibitor SB431542 [36]. Such single factor conversion would make it easier to manufacture iMSCs on a massive scale. These iMSCs were functionally similar to bone marrow MSCs in vitro and in vivo [37, 38]. However important differences remain between iMSCs and primary tissue derived MSCs. iMSCs yield fewer Colony Forming Unit-Fibroblast (CFU-F) colonies compared to bone marrow MSCs [39]. The epigenetic profiles of iMSCs may also be different compared to bone marrow MSCs. Furthermore, irrespective of speculative interpretation of current knowledge on various cell types, developing a reference cell type for clinical therapeutics would require further feasibility studies.
Preliminary stage data analysis using published gene expression profiles of MSCs and iMSCs demonstrate that the iMSCs show an excellent Pearson correlation coefficient with tissue specific MSCs (R2>0.93) [40]. This correlation was similar to that observed between marrow derived MSCs, adipose derived MSCs and umbilical cord derived MSCs (Figure 1). Thus based on gene expression data alone, it appears that iMSCs are comparable to adult tissue derived MSCs. Taken together it appears that variation in gene expression of iMSCs is similar to variation in gene expression between adult MSCs derived from tissues such as bone marrow, adipose tissue and umbilical cord.
Figure 1.

iMSCs show a similar gene expression paCern to MSCs derived from bone marrow, adipose Hssue and umbilical cord. Gene expression profiles of MSCs from different sources were compared and the Pearson correlaHon coefficient was calculated. The top panel shows comparison of iMSCs to marrow, adipose Hssue and umbilical cord derived MSCs. Excellent correlaHon in gene expression was observed in these cells (R2>0.93). This was similar to correlaHon observed between marrow and adipose derived MSC and umbilical cord derived MSCs.
Establishment of a virtual database for gene expression data of reference iMSC on a webserver
It will be important to share in a timely manner expression data (including transcriptome, miRnome and epigenome data) generated from these reference lines among the different stakeholders in the community. Such data will enable access to a larger number of samples than what an individual researcher can perform and this will improve the statistical power of such studies. Many journals require such datasets be publicly available, so this idea should be palatable to most MSC researchers. For this we propose two modes that we think are workable:
Establishment of a MSC reference gene expression data repository for reference MSC lines. This would work if it were mandatory for researchers using the reference lines to submit their data to such a repository. However, this would require dedicated computing infrastructure for storage, organization and retrieval of such data at the host institution. Unless the institution is publicly funded (like the NIH), many researchers may also be unwilling to submit their data.
The second alternative is to let researchers submit their data to either Gene Expression Omnibus (GEO) hosted by NCBI or ArrayExpress hosted by the European Bioinformatics Institute. The advantage of this approach is that there is no requirement to host the data on proprietary servers and the structure and retrievability of the data is already well developed over a number of years. Moreover researchers using the reference lines would already be familiar with these agencies. One problem with such submissions would be that the datasets concerning MSC reference lines would be lost amongst the thousands of other datasets in these repositories including other MSC datasets. However this problem can be overcome by designing diligent curating protocols for these repositories. This could be enabled by having a standardized set of keywords used for submission of MSC reference line data. This would make it easier to search data repositories using these standardized keywords. In addition we also propose a webserver that will link to such carefully curated datasets making it easier for researchers to find and access this data. The webserver could also house some tools for making preliminary comparisons of the data making it easier for researchers accessing this server to compare their data with that of the reference MSC lines.
A good example of such a webserver is the fluserver (http://flusurver.bii.a-star.edu.sg/) developed by the Bioinformatics Institute that allows researchers with influenza viral sequence information to quickly identify the strain, check its prevalence in different parts of the world, map mutations and determine its resistance to commonly used antivirals like Relenza & Tamiflu. This server is now part of the Global Initiative on Sharing Avian Influenza Data (GISAID), an international consortium of influenza researchers and is available to all its members.
Challenges associated with reference panel
Challenges associated with one reference panel for MSCs in the form of cell repository for both clinical and basic science research: the needs of basic science community and clinical trials are different. Enforcing a cell type on basic scientists could be too restrictive for reasons described above. However, in light of recent data from clinical trials, having such a cell type has become a necessity for clinical trials.
That said there are numerous challenges associated with such an endeavor of generating a reference cell type and it is important to achieve at least some degree of harmonization within the MSC community for such an effort to be effective. The major challenges are:
Sources of MSCs. As mentioned earlier, reference panels using both pure and mixed cells have their advantages and disadvantages. If a pure source of MSCs were to be used, it may be necessary to have multiple reference panels for MSCs from different sources. This would be necessary since MSCs from different sources may have different marker expression profiles, growth and differentiation kinetics and ability to differentiate into specific lineages. Thus a reference panel developed for one source of MSCs (e.g. bone marrow) may not be applicable to other sources (e.g. dental pulp MSCs). Even in case of a mixed reference panel, the sources of MSCs that need to be included in this panel and their relative proportions would need to be addressed.
Protocols for generating and characterizing a cell based reference panel. To ensure consistency in culture protocols, characterization of the reference panel etc., it will be important that this will be carried out by a single entity or a collection of few entities. These entities could be an academic center such as the NIH- Center for Regenerative Medicine (NIH-CRM) or a company with significant expertise in the manufacture of cell based therapeutics. Such an entity would have the necessary expertise to manufacture these MSCs, characterize them and distribute them globally to interested parties. The NIH-CRM started a similar effort for induced pluripotent stem cells (iPSCs) recently and this experience would be invaluable for a MSC reference panel as well.
Cost of acquiring the reference panel. Cell based reference panels are usually very expensive and thus the cell therapy industry would be unwilling to use such panels, since it adds to the already high cost of manufacturing such therapeutics. A possible solution would be government subsidies towards manufacturing the panel since this would improve the safety and reliability of this therapeutics. Another possible solution (as implemented by NIH-CRM for iPSCs) would be to manufacture large volumes of such panels and distribute these so that the cost of manufacture and characterization gets distributed.
Distribution of the reference panel. If a single or few entities are involved, it will be essential to ensure timely and reliable distribution of these cells. Delay in shipping and availability of such panels would lead to poor uptake by the cell therapy industry.
Other limitations
Towards this end of a realistic repository of a standard or reference line generation, the factors that could be limiting are: (a) funding sources (b) researchers in terms of their effort and time contribution.
Funding sources
Recent NIH common fund initiatives to develop normal population databases; such as exRNA, genomic and epigenomic profiles have been trailblazers in setting an example to develop reference materials. Extension of such initiatives to study MSCs for cell therapy would alleviate the conflict of interests that arise from private funding. Second best sources of funding would be large biotech and pharma companies that are interested in the space of cell therapy. Most attractive cells to secure funding at this time are iMSCs from bone marrow. Apart from the obvious reasons of having survived several clinical trials, the ease of involving several labs that are interested in developing the reference material would expand the available intellectual and research resources.
An important consideration in addition to developing the reference cell type is developing an entity to regulate the appropriate usage of such reference material. An important drawback for any reference material be it a database or a cell type, is that the interpretation of such reference would depend on the user. Therefore validated guidelines to help the user interpret results obtained from such reference material would be important. Developing a reference cell type would be just the starting point for developing a successful and consistent cell therapy entity.
Proposed minimal criteria
We believe that redefining the minimal criteria for MSCs, as in a virtual ruler, would be more beneficial and have a potential to be adapted by many laboratories across the globe. While the argument used to set a ruler is convincing, proposing a live cell type comes with several shortcomings. In order to obtain such a ruler, we propose performing rigorous studies to develop a ruler to redefine the minimal criteria akin to the one described below:
-
Growth characteristics: proliferation, survival in serum deprivation, hypoxia, single cell cloning
Mean doubling time less than 24 hours in passage 1
>75% survival in serum deprived media after 10 days
>75% survival in hypoxia for 5 days
Over 50% colony forming unit formation ability using a single cell colony forming assays.
-
Marker expression:
Cell surface antigens, CD13+, CD29+, CD31−, CD34−, CD44+, CD49e+, CD45−, CD73+, CD90+, CD105+, CD146+, CD166+, CD271−, c-Kit (CD117)−, Sca-1−, SSEA4+, Stro-1+, W8-B2/MSCA-1+, podXL+, CD49f+, CD11−, MHC I, MHC II,
-
Immunocytochemistry.
Vimentin positive, Laminin positive, Fibronectin positive
Gene expression (data compiled from microarrays). A validated reference gene expression panel compiled from publicly available datasets from Gene Expression Omnibus (GEO) curated by National Centre for Biotechnology Information (NCBI) and Array express curated by European Bioinformatics Institute (EBI). Such a validated gene expression panel does not currently exist, but could be developed by an international consortium of interested parties. Such a reference panel would also allow virtual comparison of different MSC lines (as well as fresh isolates) similar to the recently described CellNet platform (Collins 2014) that uses gene regulatory networks to assess reprogramming fate and efficiency of engineered cells.
-
Secretome assays that include the cytokine expression and characterization of extracellular vesicles
Extracellular vesicles with a defined microRNA profile.
Cytokine/growth factor expression in the secretome of the cells
-
Metabolite expression.
Metabolites are increasingly being used to characterize stem cells functionally. MSCs from different tissues may exhibit differences in their metabolome. Metabolomic characterization of reference panel would enable using this information for identifying tissue specific MSCs as well as their ability to differentiate into a particular progeny. One commonly used metabolism assay is a specialized HALO assay to predict the activity through ATP levels [41]. A modification of this assay is to determine other metabolite products such as NAD and FAD.
-
miRNA and lncRNA expression
Intracellular expression of non-coding RNA families. Since miRNAs are part of gene regulatory networks and determine cell fate, these could also be good markers for defining MSCs.
Differentiation assays – Mineralizing cells, adipocytes, chondrocytes and immune activity such as IDO expression. Trans differentiation assays such as angiogenesis.
Engraftment assays such as bone fracture model, ischemia healing model, tumor engraftment xenograft model.
Studies on effect of cryopreservation on MSCs. Effects of cryopreservation on MSCs have been a focus of several studies recently. Therefore, there is a need for risk verses benefit analysis between costs associated with generating enough cells for cell therapy before cryopreservation and the benefit of using preserved cells. Ideally we would have a reference material with a consistent phenotype before and after cryopreservation.
Furthermore, as proposed by Reger and Prockop, there is a need for in process data generation. A typical manuscript using MSCs would include information on the number of cells in the frozen vial, the cell viability and yields on cells from thawed vial along with a table in supplementary data alike the following
|
| ||
| Characteristic | Reference material | Our cell type |
|
| ||
| Source | Mixed cell population | B-MSCs, A-MSCs etc |
|
| ||
| Cell surface markers | CD13+, CD29+, CD31−, CD34−, CD44+, CD49e+, CD45−, CD73+, CD90+, CD105+, CD146+, CD166+, CD271−, c-Kit (CD117)−, Sca-1−, SSEA4+, Stro-1+, W8-B2/MSCA-1+, podXL+, CD49f+, CD11−, MHC I, MHC II, different types | CD90+, CD105+, CD11 others not tested |
|
| ||
| Differentiation assays | Osteo, adipo, chondro, IDO expression, | Osteo+, Adipo+, others not tested |
|
| ||
| Gene expression assays | A panel of over 1000 genes | Tested 96 gene microarray kit for stem cell markers. |
|
| ||
| Secretome assays | Cytokine panel and EV panel | Cytokine panel shows TGFb+, IGF etc. EV panel not tested |
|
| ||
| Metabolite assays | ATP/ADP, NAD/NADH, FAD/FADH | ATP/ADP tested and others not tested |
|
| ||
| Freeze thaw assays | The above phenotype before and after thaw | Not tested |
|
| ||
In addition to the cell surface markers previously listed, any quality metric toward choosing a reference material should give careful consideration of potential immunomodulatory effects associated with BMSC clones. For example, Genever et al. have recently generated immortalized BM-MSC clones without or without osteogenic, adipogenic, or chondrogenic capacity. While the multipotent clones overexpressed genes related to vascular growth, the nullipotent cells showed a high basal expression level of pro-inflammatory and anti-viral factors [42]. These colonies also displayed a biomolecular similarity to CD317+ BM-MSCs, which comprise 1–3% of BM-MSCs and express high levels of IL-7, a growth factor known to induce the differentiation and proliferation of some hematological malignancies and is aberrantly expressed in invasive breast cancers [43–45] Therefore, while a clonal strategy is a powerful means of by-passing the heterogeneity of stem cell populations it does not guarantee a safe medicinal product.
In summary, we corroborate the argument for the need of a reference material to demonstrate the quality of cells used. Supporting the three reasons given in the solicitation, we believe that such reference material will bring in a much needed a unification theme in the cell therapy community. It is unclear at this stage if the ruler will be cell based or virtual and if a single ruler will be sufficient to ‘measure’ all MSCs or multiple rulers will be needed. However having such a material would allow for a standardized and peer review both at the publications and grants, leading to fewer retractions. The downside of such a reference material (although the authors in the original paper clearly state that its use should not be mandatory) could be the unintended consequence forcing new discoveries to conform to the reference material. Therefore, a “user manual” should be generated in addition to “product usage insert” when generating such a reference material. Such a user manual will clearly outline the intended and appropriate use of such a reference and avoid it from being used as a gold standard as mentioned by Viswanathan et al. An MSC ruler(s) would thus remove one of the major hurdles to successful translation of MSC based therapeutics.
Supplementary Material
Figure 2.
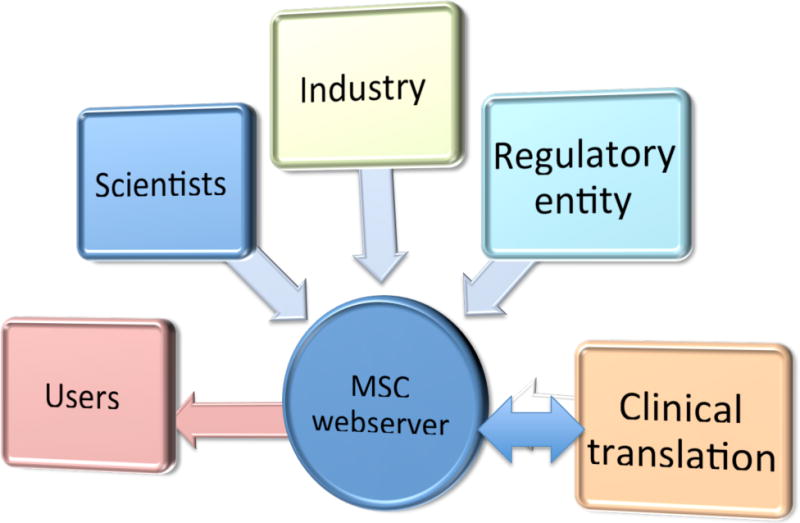
Schematic to demonstrate the information flow to the webserver.
Acknowledgments
The opinion reflected in this article is the consensus opinion of the authors does not represent the official view of their employers or societies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: RP, VT and MV declare no conflict of interests
References
- 1.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Casaroli-Marano RP, et al. Regulatory issues in cell-based therapy for clinical purposes. Dev Ophthalmol. 2014;53:189–200. doi: 10.1159/000357766. [DOI] [PubMed] [Google Scholar]
- 3.Rosner M, Schipany K, Hengstschlager M. The decision on the “optimal” human pluripotent stem cell. Stem Cells Transl Med. 2014;3(5):553–9. doi: 10.5966/sctm.2013-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reger RL, Prockop DJ. Should publications on mesenchymal stem/progenitor cells include in-process data on the preparation of the cells? Stem Cells Transl Med. 2014;3(5):632–5. doi: 10.5966/sctm.2013-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viswanathan S, et al. Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev. 2014;23(11):1157–67. doi: 10.1089/scd.2013.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucia M, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857–69. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 7.Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci. 2001;938:231–3. doi: 10.1111/j.1749-6632.2001.tb03593.x. discussion 233–5. [DOI] [PubMed] [Google Scholar]
- 8.Lu LL, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–26. [PubMed] [Google Scholar]
- 9.Wang HS, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–7. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 10.Bunnell BA, et al. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–20. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47(2):126–31. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 13.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–16. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. Minimal Manipulation of Human Cells, Tissues, and Cellular and Tissue-Based Products: Draft Guidance for Industry and Food and Drug Administration Staff. 2014 Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM427746.pdf.
- 15.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 16.Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11(10):1255–65. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, et al. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology (Carlton) 2013;18(3):201–8. doi: 10.1111/nep.12018. [DOI] [PubMed] [Google Scholar]
- 18.Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine? Bone Marrow Transplant. 2012;47(2):164–71. doi: 10.1038/bmt.2011.81. [DOI] [PubMed] [Google Scholar]
- 19.Barzilay R, Melamed E, Offen D. Introducing transcription factors to multipotent mesenchymal stem cells: making transdifferentiation possible. Stem Cells. 2009;27(10):2509–15. doi: 10.1002/stem.172. [DOI] [PubMed] [Google Scholar]
- 20.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25(11):2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki M, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–7. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 22.Evans CH. Gene delivery to bone. Adv Drug Deliv Rev. 2012;64(12):1331–40. doi: 10.1016/j.addr.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pochampally RR, et al. Correction of a mineralization defect by overexpression of a wild-type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: a strategy for rescuing mutations that produce dominant-negative protein defects. Gene Ther. 2005;12(14):1119–25. doi: 10.1038/sj.gt.3302514. [DOI] [PubMed] [Google Scholar]
- 24.Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35(2):213–21. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 25.Selmani Z, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dittmer J, Leyh B. Paracrine effects of stem cells in wound healing and cancer progression (Review) Int J Oncol. 2014;44(6):1789–98. doi: 10.3892/ijo.2014.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JW, et al. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913–9. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95(12):2196–211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Penfornis P, et al. Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment. Int J Cancer. 2015 doi: 10.1002/ijc.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallabhaneni KC, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953–4967. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13(1):49. doi: 10.1186/s12967-015-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5(1):79–88. doi: 10.1089/153623003321512193. [DOI] [PubMed] [Google Scholar]
- 34.Brown PT, Squire MW, Li WJ. Characterization and evaluation of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Cell Tissue Res. 2014;358(1):149–64. doi: 10.1007/s00441-014-1926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li O, et al. Human embryonic stem cell-derived mesenchymal stroma cells (hES-MSCs) engraft in vivo and support hematopoiesis without suppressing immune function: implications for off-the shelf ES-MSC therapies. PLoS One. 2013;8(1):e55319. doi: 10.1371/journal.pone.0055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong J, et al. Patient-tailored application for Duchene muscular dystrophy on mdx mice based induced mesenchymal stem cells. Exp Mol Pathol. 2014;97(2):253–8. doi: 10.1016/j.yexmp.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Chen YS, et al. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med. 2012;1(2):83–95. doi: 10.5966/sctm.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao Q, et al. iPSC-derived human mesenchymal stem cells improve myocardial strain of infarcted myocardium. J Cell Mol Med. 2014;18(8):1644–54. doi: 10.1111/jcmm.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frobel J, et al. Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Reports. 2014;3(3):414–22. doi: 10.1016/j.stemcr.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheyn D, Shiran B-D, Sandra Ann De Mel, Loren Ornelas, Anais Sahabian, Dhruv Sareen, et al. Ips Cells Can Be Efficiently Differentiated Back To MSCs Using Shorty Exposure To Tgf?; Orthopaedic Research Society 2015 Annual Meeting; 2015; Las Vegas, Nevada. [Google Scholar]
- 41.Junker BH. Flux analysis in plant metabolic networks: increasing throughput and coverage. Curr Opin Biotechnol. 2014;26:183–8. doi: 10.1016/j.copbio.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 42.James S, et al. Multiparameter Analysis of Human Bone Marrow Stromal Cells Identifies Distinct Immunomodulatory and Differentiation-Competent Subtypes. Stem Cell Reports. 2015;4(6):1004–15. doi: 10.1016/j.stemcr.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barata JT, et al. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1) Blood. 2001;98(5):1524–31. doi: 10.1182/blood.v98.5.1524. [DOI] [PubMed] [Google Scholar]
- 44.Sexl V, et al. Stat5a/b contribute to interleukin 7-induced B-cell precursor expansion, but abl- and bcr/abl-induced transformation are independent of stat5. Blood. 2000;96(6):2277–83. [PubMed] [Google Scholar]
- 45.Al-Rawi MA, et al. Aberrant expression of interleukin-7 (IL-7) and its signalling complex in human breast cancer. Eur J Cancer. 2004;40(4):494–502. doi: 10.1016/j.ejca.2003.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


