Abstract
Adult human mesenchymal stem cells (hMSCs) have been shown to home to sites of breast cancer and integrate into the tumor stroma. We demonstrate here the effect of hMSCs on primary breast tumor growth and the progression of these tumors to hormone independence. Co-injection of bone marrow-derived hMSCs enhances primary tumor growth of the estrogen receptor-positive, hormone-dependent breast carcinoma cell line MCF-7 in the presence or absence of estrogen in SCID/beige mice. We also show hormone-independent growth of MCF-7 cells when co-injected with hMSCs. These effects were found in conjunction with increased immunohistochemical staining of the progesterone receptor in the MCF-7/hMSC tumors as compared to MCF-7 control tumors. This increase in PgR expression indicates a link between MCF-7 cells and MSCs through ER-mediated signaling. Taken together, our data reveal the relationship between tumor microenvironment and tumor growth and the progression to hormone independence. This tumor stroma-cell interaction may provide a novel target for the treatment of estrogen receptor-positive, hormone-independent, and endocrine-resistant breast carcinoma.
Keywords: Mesenchymal stem cells, Breast carcinoma, Progesterone receptor, Stromal-derived factor 1, Tumor microenvironment
Introduction
Breast cancer remains the predominant form of carcinoma affecting American women today. The primary treatment for breast cancer continues to be lumpectomy followed by endocrine therapies such as tamoxifen or aromatase inhibitors. However, many tumors eventually become resistant to these treatments. The mechanism of transition of cells from hormone dependent to hormone independent remains unclear.
Human mesenchymal stem cells (hMSCs) have recently become a topic of great interest in relation to cancer, mostly focusing on the use of stem cells as a vehicle for targeted drug delivery. These hMSCs are pluripotent progenitor cells that contribute to tissue repair and wound healing [1]. Though most hMSCs are present in the bone marrow, small stores of stem cells are distributed throughout various tissues as reserves for wound repair and scar formation [2]. When tissue damage occurs, specific endocrine signals are released from the injury site that mobilizes hMSCs from bone marrow to the location of damage [3]. Furthermore, small amounts of hMSCs are retained in the blood circulation and have the ability to home to injury sites for tissue repair [1, 4].
A tumor mass is composed of malignant cancer cells and nonmalignant benign cells. These benign cells include tumor vasculature, inflammatory cells, and stromal cells, which provide structural support to malignant cells. Stromal fibroblasts within the tumor microenvironment can influence tumor cell activities such as proliferation and aggressiveness [5]. Tumor cells stimulate de novo formation of connective tissue in order to provide stromal support to the growing tumor [6, 7]. The response to the establishment of tumor stroma closely mimics that of wound healing and scar development [8]. It has been proposed that the signals regulating increased turnover and proliferation of stromal cells in the tumor microenvironment may mediate engraftment and proliferation of hMSCs at the tumor site [9]. Others have shown that stromal cells contribute to primary tumor growth in vivo [10].
Human mesenchymal stem cells have the capacity to home to tumor sites endogenously or when injected systemically [2, 9]. It is thought that the tumor microenvironment mimics injury sites as “wound[s] that never heals” [4, 8, 9]. The hMSCs home to tumor cells and surround the tumors but do not infiltrate them, suggesting that the effect hMSCs have on tumor growth kinetics is a result of stromal factors and paracrine signaling [11]. Karnoub et al. [12] proposed that the metastatic traits of breast cancer cells are acquired through exposure of the epithelial cells to mesenchymal cells in the tumor-associated stroma. It was suggested that hMSCs provide paracrine signals to the tumor cells to promote metastasis.
Here we set out to explore the effect of hMSC contact with breast tumor cells and the possibility that hMSCs supply a stromal component to breast tumor cells that provide a proliferative/survival advantage. We previously observed that MCF-7 breast carcinoma cells, which normally only form tumors in the presence of estrogen or exogenous matrigel, were able to form tumors in the absence of these substances when mixed with hMSCs. Therefore, we also propose that hMSCs have the ability to induce a hormone-independent phenotype in breast carcinomas through paracrine signaling.
Materials and methods
Cells and reagents
MCF-7 human breast cancer cell line was acquired from American Type Culture Collection (Manassas, VA) and was maintained in Dulbecco’s modified Eagle’s medium (DMEM; pH 7.4; Invitrogen Corp., Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Salt Lake City, UT), 1% nonessential amino acids, minimal essential amino acids, sodium pyruvate, penicillin/streptomycin, and insulin under mycoplasma-free conditions at 37°C in humidified 5% CO2 and 95% air.
Isolation and culture of hMSCs
Human mesenchymal stem cells from bone marrow aspirates were obtained from the NIH-funded National Center for Research Resources (NCRR) Tulane Center for the Preparation and Distribution of Adult Stem Cells and Distribution of Adult Stem Cells (http://www.som.tulane.edu/gene_therapy/distribute.shtml) and were prepared as described previously [13].
Animals
SCID/beige immunocompromised female ovariectomized mice (4–6 weeks old) were obtained from Charles River Laboratories (Wilmington, MA). The animals were allowed a period of adaptation in a sterile and pathogen-free environment with phytoestrogen-free food and water ad libitum. Mice were divided into treatment groups of five mice each: MCF-7 only, MCF-7 plus estrogen, MCF-7 plus MSC, and MCF-7 plus MSC plus estrogen. Placebo or estradiol pellets (0.72 mg, 60-day release; Innovative Research of America, Sarasota, FL) were implanted subcutaneously in the lateral area of the neck using a precision trochar (10 gauge). MCF-7 and MSC cells were harvested in the exponential growth phase using a PBS/EDTA solution and washed. Viable cells (5 × 106) in 50 μl of sterile PBS suspension were mixed with 100 μl reduced growth factor Matrigel (BD Biosciences, Bedford, MA) or injected alone. Injections were administered subcutaneously into both flanks using 27½-gauge sterile syringes. All procedures in animals were carried out under anesthesia using a mixture of isofluorane and oxygen delivered by mask.
Tumor size was measured every 2–3 days using digital calipers. The volume of the tumor was calculated using the following formula: 4/3π LS2 (L = larger radius; S = shorter radius). At necroscopy, animals were euthanized by cervical dislocation after exposure to CO2. Tumors, uteri, livers, and lungs were removed and frozen in liquid nitrogen or fixed in 10% formalin for further analysis. All procedures involving these animals were conducted in compliance with State and Federal laws, standards of the US Department of Health and Human Services, and guidelines established by Tulane University Animal Care and Use Committee. The facilities and laboratory animals program of Tulane University are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Immunohistochemistry
Expression of PgR is upregulated through ER-mediated pathways and thus serves as a marker of estrogen exposure within larger target tissues [14]. Tumor explants were collected at necropsy, fixed in 10% buffered formalin phosphate. FFPE 4-μM-thick tumor sections were analyzed by immunohistochemistry using primary monoclonal antibodies against human PgR (DAKO North America, Inc., Carpinteria, CA) as published previously [15]. The mouse antibodies on mouse tissue polymer detection kit (Biocare medical, LLC, Concord, CA) were used to perform IHC. Briefly, FFPE sections were deparaffinized and hydrated in a graded series of ethanol solutions followed by 3% H2O2 for 5 min to inactivate endogenous peroxides then rinsed. Slides were subjected to 10 min incubation in Avidin followed by 10 min incubation in Biotin. For antigen retrieval, sections were exposed to Rodent decloaker at 95°C for 25 min, rinsed, and allowed to cool to room temperature for 20 min. Slides were incubated with Rodent block for 30 min and then with primary antibodies (PgR; DAKO) or serum alone (negative control) for 75 min. Mouse-on-mouse HRP-polymer secondary antibody was added to the sections and incubated for 15 min. After rinsing, DAB solution (Biocare medical, LLC, Concord, CA) was applied and incubated for 1 min, and sections were counterstained with hematoxylin (Biocare medical, LLC, Concord, CA) followed by Tacha Blueing reagent (Biocare medical, LLC, Concord, CA) for 30 s each. Slides were then allowed to air dry and then cover slipped using Acrymount (Fisher Scientific Inc., Waltham, MA).
Sections were viewed and photographed using the Leica DM IRB Inverted Research microscope and SPOT RT color camera. Five images at 400× were taken of each tumor with care to avoid areas of necrosis. For PgR staining quantification, numbers of positively stained cells were expressed as a percentage of the total number of cells per field of view/image.
Microscopy
The Leica DM IRB Inverted Research Microscope and SPOT RT color camera with SPOT 4.5.9 software (Diagnostic Instruments, Inc.) were used in the acquisition of H&E and PgR slide images.
Statistics
A mixed-effects model was fit to the tumor growth data using the nlme library in R 2.7.0 [16, 17]. The model predicted tumor volume measurements taken between days 13 and 43 with fixed effects for each of the four treatment groups and random effects associated with the five mice in each group and the two tumors within each mouse. A group-wise variance function was added to account for observed heteroscedasticity among the treatment groups.
The immunohistochemical data of PgR were expressed as the arithmetic mean ± SE and evaluated with an unpaired t-test. A value of P < 0.05 was considered statistically significant.
Results
MSCs promote estrogen sensitivity in breast cancer
To test the effect of hMSCs on human breast cancer, a xenograft model was established whereby the human breast cancer cell line MCF-7 was subcutaneously co-injected with human bone marrow-derived MSCs into the flanks of immunocompromised SCID/beige ovariectomized female mice. Mice in both groups were implanted with 60-day time-release 17β-estradiol pellets, and all injections were mixed with reduced growth factor matrigel to ensure tumor formation. Our results show that MCF-7 cells form tumors, as expected, in the presence of estrogen and matrigel by day 6 post injection and continue to gradually increase in volume over time. However, addition of hMSC increased the estrogen response by ~fourfold over that of control tumors (Fig. 1). Our mixed-effects model estimated a growth rate for the hMSC group that was approximately 5 times greater than that of the MCF-7+ estrogen tumors, statistically significant beyond P = 0.001. It is also important to note that hMSCs are not thought to contribute to the tumor mass themselves as they are nontumorigenic [18].
Fig. 1.
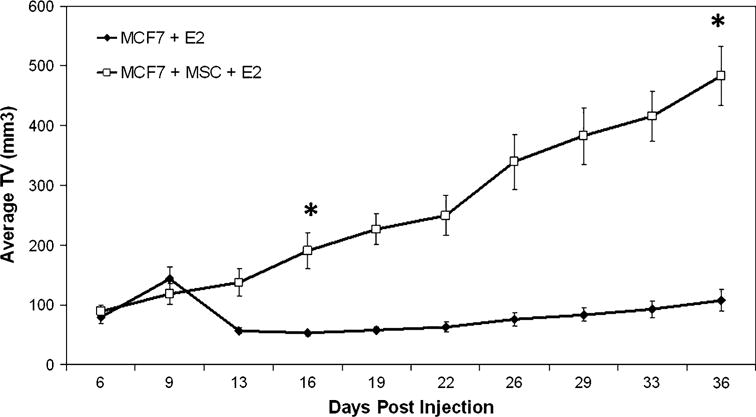
MSCs promote tumor growth and estrogen sensitivity of breast tumors in vivo. Tumor volume in mm3 (mean ± SEM). About 4–6-week-old female ovariectomized SCID/beige mice were injected subcutaneously with 1 × 106 MCF-7 ± 1 × 106 hMSC in 50 μl of sterile PBS with 100 μl reduced growth factor matrigel (BD biosystems), n = 5 mice per group. All mice were implanted with a 0.72 mg 60-day time-release estrogen pellet (Innovative Research of America) subcutaneously in neck. Tumors were measured every 3 days, (*, P < 0.001)
The estrogen response observed in the MCF-7 control tumors was not as robust as previously published [15, 19–21]. Using MCF-7 cells in the same xenograft model under the same conditions reported here demonstrated the estrogen response commonly expected (Supplemental Fig. 1). However, this response is delayed (beginning around day 45 post injection). Other studies have indicated that this delay in estrogen response is due to (1) the site of injection [21, 22], as subcutaneous tissue has a lower relative estradiol-concentrating ability (RCA) as compared to the mammary fat pad as well as (2) the low inoculum size (1 × 106) [19, 21].
MSCs promote enhanced proliferation of breast carcinoma
MCF-7 cells were injected subcutaneously into SCID/beige female mice on each flank with either MCF-7 cells alone or mixed with hMSC in the presence of reduced growth factor matrigel. Mice were ovariectomized to reduce endogenous levels of estrogen. Measurable tumors were observed at day 6 and followed through day 43. There was no measurable difference in tumor volume between control MCF-7 tumors and those tumors containing hMSC (Fig. 2a).
Fig. 2.
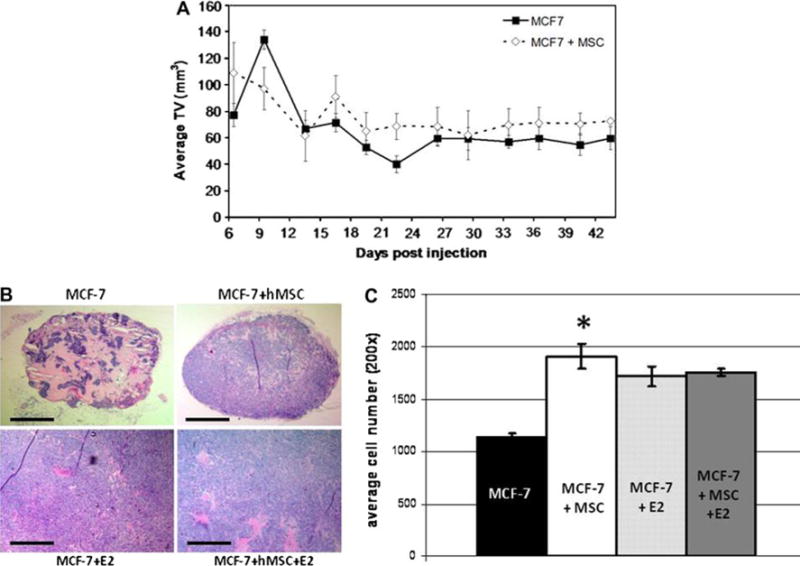
hMSCs promote tumor cell proliferation in the absence of estrogen. a Tumor volume in mm3 (mean ± SEM). About 4–6-week-old female ovariectomized SCID/beige mice injected subcutaneously with 1 × 106 MCF-7 ± 1 × 106 hMSC in 50 μl of sterile PBS with 100 μl reduced growth factor matrigel (BD biosystems), n = 5 mice per group. Tumors were measured every 3 days. b H&E sections at 509 showing differences in tumor cellularity. Scale bar equal to 500 μm. c Cell count averages per field of view at 200×. Bars represent average cell number ± SE, (*, P < 0.001)
Having observed such a large difference in tumor volume between MCF-7 cells alone and those mixed with hMSCs in mice implanted with estrogen pellets, we examined the cellularity of the nonestrogen tumors at endpoint. Formalin-fixed, paraffin-embedded tumors from each group were sectioned and stained with hematoxylin and eosin (H&E). Figure 2b shows that control tumors consist primarily of matrigel with only small clusters of MCF-7 cells throughout. Comparably, tumors containing hMSCs have greater cellularity. Cells were counted in five randomly selected fields of view in three tumor samples from each experimental arm. Figure 2c shows that the cell number per tissue section for MCF-7–hMSC tumors are almost twice that of control tumors even with similar overall tumor volume. This increase in cell number indicates hMSC-mediated, enhanced proliferation of MCF-7 cells within the tumor mass. This method is limited to the field of view and therefore does not demonstrate the differences in cell number over the whole tumor section. Once the tumor density has reached a maximum level, cell number per field of view will not change. This explains why there is no difference between the two estrogen groups (MCF-7 + E2, MCF-7 + MSC + E2) in this graph.
MSCs promote hormone-independent tumor growth of breast carcinoma
Using the same xenograft model, we injected immunocompromised SCID/beige female mice subcutaneously on each flank with a mixture of MCF-7 cells and hMSCs or MCF-7 cells alone in the absence of matrigel. MCF-7 cells are hormone dependent, and previous studies have shown that MCF-7 cells do not form tumors in the absence of estrogen or matrigel when 5 million cells were injected into the mammary fat pad (Fig. 3a). However, as Fig. 3b shows that when hMSCs are co-injected with MCF-7 cells, tumors form with an average tumor volume of 45 mm3 despite the lack of exogenous matrigel or estrogen. MCF-7-hMSC tumor take at day 35 was also much higher (80%) than that of the control MCF-7 tumors where no tumors were observed (Fig. 3c). This indicates a shift to hormone independence and is of importance because the majority of primary hormone-dependent breast tumors progress to a hormone-independent phenotype over the course of the disease; yet, no clear mechanism has been discovered. Understanding the mechanism behind this progression could lead to improved treatment options in the future.
Fig. 3.
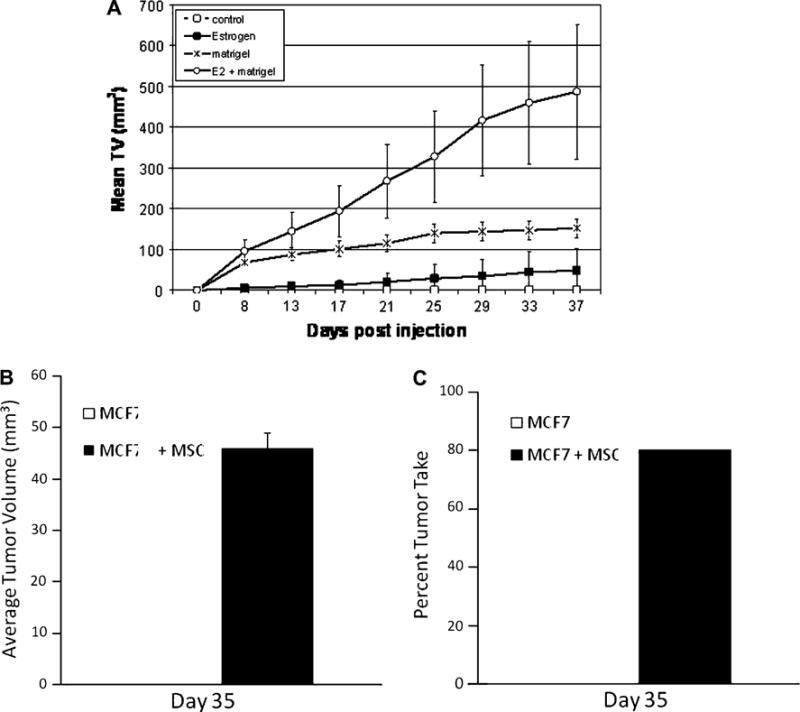
hMSCs promote hormone-independent tumor growth of breast carcinoma. a Tumor volume in mm3 (mean ± SEM). About 4–6-week-old female ovariectomized SCID/beige mice were injected in the mammary fat pad with 5 × 106 MCF7 in 50 μl of sterile PBS ± 100 μl reduced growth factor matrigel, ±estrogen (0.72 mg, 60-day time release) pellets; n = 5 mice per group. Tumors were measured twice weekly. b–c About 4–6-week-old female ovariectomized SCID/beige mice were injected subcutaneously with 1 × 106 MCF7 ± 1 × 106 MSC in 50 μl of sterile PBS. b hMSCs induce hormone-independent tumor growth of MCF-7 cells. Tumor volume in mm3 (mean ± SEM) for day 35 post injection. c hMSCs increase the likelihood of tumor burden. Percent tumor take at day 35 post cell injection. Each group had a total of 10 injection sites (n = 5/group; 2 injections/animal)
hMSCs alter progesterone receptor expression of MCF-7 tumors
Observation of the hormone-independent effect in the MCF-7/MSC tumors led us to explore the possibility of ER-mediated signaling. Endpoint matrigel tumors were harvested at necropsy and either snap frozen in liquid nitrogen for real-time PCR analysis or fixed in formalin and sectioned for immunohistochemistry. We initially ran real-time PCR on endpoint tumor samples. Tumors grown in the presence of MSCs and estrogen pellets showed an increase in progesterone receptor (PgR) gene expression, as well as Stromal-derived factor 1 (SDF-1) (2.5- and 5.5-fold, respectively, P < 0.05) when normalized to beta-actin and compared to MCF-7 estrogen control (data not shown). Both PgR and SDF-1 are well-known ER-mediated genes and increased expression correlates with ER activation [14]. To validate the real-time PCR results, FFPE tumor sections were stained for human progesterone receptor. These results revealed over a 10% increase in positive staining in MCF-7/MSC+ estrogen tumors compared to MCF-7+ estrogen control tumors (Fig. 4). Student’s t-test confirmed statistical significance beyond P < 0.0002. This increase in estrogen-responsive gene expression indicates ER activation in breast cancer cells in the presence of MSCs.
Fig. 4.
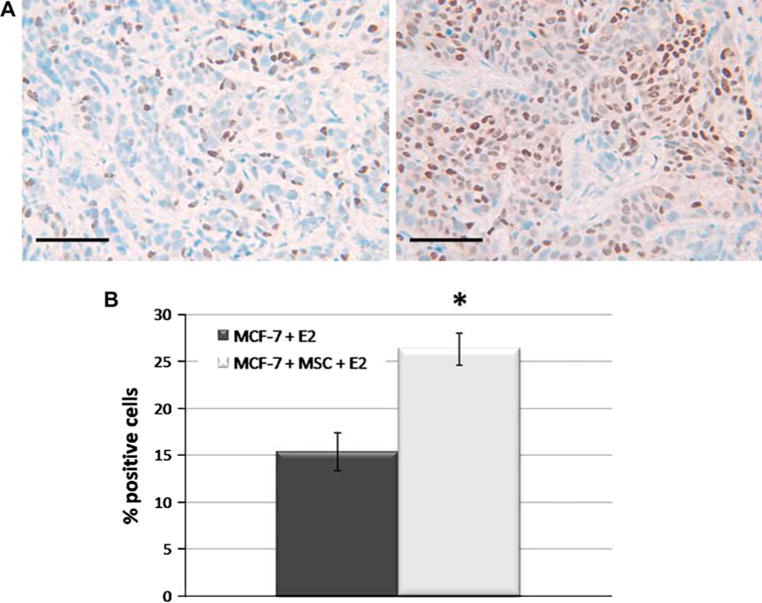
hMSCs alter progesterone receptor expression of MCF-7 tumors. Four tumors per treatment group (n = 4) were harvested at endpoint and 4 μM-thick FFPE sections were utilized for immunohistochemistry. a Representative images of human PgR staining in tumor sections from MCF-7 + E2 (left) and MCF-7/MSC + E2 (right) tumors at 400×. Scale bars equal to 50 μm. b Quantitation of PgR staining expressed as percent positive of total number of cells per image, (*, P < 0.0002)
Discussion
Although hMSCs have the ability to home to tumor sites and have the potential to confer a metastatic phenotype to breast carcinoma cells [12], their effects on primary breast tumors have not previously been examined. Here, we show that hMSCs have the following effects on breast primary tumors: (1) breast carcinoma cells in contact with hMSCs display an increased sensitivity to estradiol; (2) hMSCs induce proliferation of breast carcinoma cells in the absence of estrogen; (3) MCF-7 cells in the absence of both matrigel and estrogen were, with the addition of hMSCs, able to form palpable tumors, indicating a hormone-independent phenotype; and (4) hMSC-mixed tumors exhibit increased staining of progesterone receptor, indicating enhanced estrogen receptor signaling.
It is known that MSCs secrete high levels of multiple growth factors, cytokines, and chemokines including SDF-1 [12, 23–28]. Therefore, the possibility exists that MSCs co-injected with MCF-7 cells are acting primarily as feeder cells supplying the tumor cells with increased levels of these factors. This would in turn give the MCF-7 cells a survival and/or proliferative advantage [19]. One school of thought in this area is that the MSCs are priming the site to make it a suitable environment for tumor cell growth [25, 29].
Mechanistically, hMSCs could be affecting MCF-7 tumor growth via either ER-dependent or ER-independent mechanisms, or both. Future experiments specifically targeting the ER must be performed to determine the details of these pathways and their role in the MCF-7–hMSC interaction. As the hMSCs used in this study were from a male donor, we do not postulate estrogen being secreted from these cells.
Conclusion
As this study shows, hMSCs isolated from the bone marrow have the ability to increase tumor volume, enhance estrogen sensitivity, promote hormone-independent tumor growth, and alter progesterone receptor expression. From a clinical standpoint, hMSC-mediated transition of MCF-7 cells to a hormone-independent phenotype is of great importance since the majority of breast carcinoma cases progress from hormone-dependent to hormone-independent tumors over the course of the disease. By understanding all the cell types that are present in the tumor microenvironment and the factors that contribute to tumor biology, we are closer to developing better treatment options for hormone-independent breast tumors.
Supplementary Material
Acknowledgments
We thank Melyssa Bratton, Ph.D. and Erica Nierth-Simpson, Ph.D. for technical advice and manuscript revision. We also thank the Histology and Pathology laboratory, Tulane University School of Medicine for their IHC expertise. This work was supported in part by the Department of Defense Breast Cancer Research Program (54551G1).
Abbreviations
- hMSC(s)
Human mesenchymal stem cell(s)
- FFPE
Formalin-fixed paraffin-embedded
- PgR
Progesterone receptor
- ER
Estrogen receptor
- DMEM
Dulbecco’s modified eagle medium
- PBS
Phosphate-buffered saline
- EDTA
Ethylenediaminetetraacetic acid
- H&E
Hematoxylin and eosin
- SCID
Severe combined immunodeficiency
- SDF-1
Stromal-derived factor 1
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-009-0458-2) contains supplementary material, which is available to authorized users.
Contributor Information
Lyndsay V. Rhodes, Department of Medicine, Section of Hematology & Medical Oncology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
Shannon E. Muir, Department of Medicine, Section of Hematology & Medical Oncology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
Steven Elliott, Department of Medicine, Section of Hematology & Medical Oncology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA.
Lori M. Guillot, Department of Medicine, Section of Hematology & Medical Oncology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
James W. Antoon, Department of Pharmacology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
Patrice Penfornis, The Center for Gene Therapy, Tulane University Health Sciences Center, New Orleans, LA 70112, USA.
Syreeta L. Tilghman, Department of Pulmonary Diseases Critical Care and Environmental Medicine, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
Virgilio A. Salvo, Department of Medicine, Section of Hematology & Medical Oncology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
Juan P. Fonseca, Department of Medicine, Section of Hematology & Medical Oncology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
Michelle R. Lacey, Department of Mathematics, Tulane University, New Orleans, LA 70118, USA
Barbara S. Beckman, Department of Pharmacology, The Center for Bioenvironmental Research, The Tulane Cancer Center, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
John A. McLachlan, Department of Pharmacology, The Center for Bioenvironmental Research, The Tulane Cancer Center, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
Brian G. Rowan, Department of Structural and Cellular Biology, The Tulane Cancer Center, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
Radhika Pochampally, The Center for Gene Therapy, Department of Pharmacology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA.
Matthew E. Burow, Email: mburow@tulane.edu, Department of Medicine, Section of Hematology & Medical Oncology, The Center for Bioenvironmental Research, The Tulane Cancer Center, Tulane University Health Sciences Center, 1430 Tulane Ave. SL-78, New Orleans, LA 70112, USA.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 3.Fox JM, Chamberlain G, Ashton BA, et al. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 4.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Hasebe T, Sasaki S, Sugitoh M, et al. Highly proliferative intratumoral fibroblasts and a high proliferative microvessel index are significant predictors of tumor metastasis in T3 ulcerative-type colorectal cancer. Hum Pathol. 2001;32:401–409. doi: 10.1053/hupa.2001.23915. [DOI] [PubMed] [Google Scholar]
- 7.Kuniyasu H, Abbruzzese JL, Cleary KR, et al. Induction of ductal and stromal hyperplasia by basic fibroblast growth factor produced by human pancreatic carcinoma. Int J Oncol. 2001;19:681–685. doi: 10.3892/ijo.19.4.681. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 9.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 10.Hall B, Andreeff M, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007;180:263–283. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- 11.Djouad F, Bony C, Apparailly F, et al. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation. 2006;82:1060–1066. doi: 10.1097/01.tp.0000236098.13804.0b. [DOI] [PubMed] [Google Scholar]
- 12.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 13.Sekiya I, Larson BL, Smith JR, et al. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 14.Petz LN, Ziegler YS, Schultz JR, et al. Fos and Jun inhibit estrogen-induced transcription of the human progesterone receptor gene through an activator protein-1 site. Mol Endocrinol. 2004;18:521–532. doi: 10.1210/me.2003-0105. [DOI] [PubMed] [Google Scholar]
- 15.Salvo VA, Boue SM, Fonseca JP, et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006;12:7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 16.Team RDC. A language and environment for statistical computing. Foundation for statistical computing; Vienna, Austria: 2008. [Google Scholar]
- 17.Pinheiro J, Bates DM. Mixed-effects models in S and S-PLUS. Springer Verlag; New York: 2000. [Google Scholar]
- 18.Kagiwada H, Yashiki T, Ohshima A, et al. Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med. 2008;2:184–189. doi: 10.1002/term.79. [DOI] [PubMed] [Google Scholar]
- 19.Hill RP. Identifying cancer stem cells in solid tumors: case not proven. Cancer Res. 2006;66:1891–1895. doi: 10.1158/0008-5472.CAN-05-3450. (discussion 1890) [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Carrier L, Belame A, et al. Combination of methylselenocysteine with tamoxifen inhibits MCF-7 breast cancer xenografts in nude mice through elevated apoptosis and reduced angiogenesis. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0216-x. [DOI] [PubMed] [Google Scholar]
- 21.White AC, Levy JA, McGrath CM. Site-selective growth of a hormone-responsive human breast carcinoma in athymic mice. Cancer Res. 1982;42:906–912. [PubMed] [Google Scholar]
- 22.Levy JA, White AC, McGrath CM. Growth and histology of a human mammary-carcinoma cell line at different sites in the athymic mouse. Br J Cancer. 1982;45:375–383. doi: 10.1038/bjc.1982.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiedler J, Leucht F, Waltenberger J, et al. VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem Biophys Res Commun. 2005;334:561–568. doi: 10.1016/j.bbrc.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 24.Fierro FA, Sierralta WD, Epunan MJ, et al. Marrow-derived mesenchymal stem cells: role in epithelial tumor cell determination. Clin Exp Metastasis. 2004;21:313–319. doi: 10.1023/b:clin.0000046130.79363.33. [DOI] [PubMed] [Google Scholar]
- 25.Hombauer H, Minguell JJ. Selective interactions between epithelial tumour cells and bone marrow mesenchymal stem cells. Br J Cancer. 2000;82:1290–1296. doi: 10.1054/bjoc.1999.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 27.Sasser AK, Mundy BL, Smith KM, et al. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett. 2007;254:255–264. doi: 10.1016/j.canlet.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Yang SH, Wu CC, Shih TT, et al. In vitro study on interaction between human nucleus pulposus cells and mesenchymal stem cells through paracrine stimulation. Spine. 2008;33:1951–1957. doi: 10.1097/BRS.0b013e31817e6974. [DOI] [PubMed] [Google Scholar]
- 29.Nicola MH, Bizon R, Machado JJ, et al. Breast cancer micrometastases: different interactions of carcinoma cells with normal and cancer patients’ bone marrow stromata. Clin Exp Metastasis. 2003;20:471–479. doi: 10.1023/a:1025462417256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


