Abstract
Antibiotic multi-resistant pneumonia is a risk associated with long term mechanical ventilation. Vancomycin is commonly prescribed for methicillin-resistant staphylococcus aureus infections; however, current formulations of vancomycin are only given intravenously. High doses of vancomycin have been associated with severe renal toxicity. In this study we characterized dry powder vancomyin as a potential inhaled therapeutic aerosol and compared pharmacokinetic profiles of i.v. and pulmonary administered vancomycin in intubated rabbits using a novel endotracheal tube catheter system. Cascade Impaction studies indicated that using an endotracheal tube, which bypasses deposition the mouth and throat, increased the amount of drug entering the lung. Drug deposition in the lung was further enhanced by using an endotracheal tube catheter, which did not alter the aerosol fine particle fraction. Interestingly, intubated rabbits administered 1 mg/kg vancomycin via inhalation had similar AUC to rabbits that were administered 1 mg/kg vancomycin via a single bolus i.v. infusion; however, inhalation of vancomycin reduced Cmax and increased Tmax, suggesting that inhaled vancomycin resulted in more sustained pulmonary levels of vancomycin. Collectively, these results suggested that dry powder vancomycin can successfully be delivered by pulmonary inhalation in intubated patients. Furthermore, as inhaled vancomycin is delivered locally to the site of pulmonary infection, this delivery route could reduce the total dose required for therapeutic efficacy and simultaneously reduce the risk of renal toxicity by eliminating the high levels of systemic drug exposure required to push the pulmonary dose to therapeutic thresholds during i.v. administration.
Keywords: Vancomycin, pulmonary delivery, pharmacokinetics, modified insufflator, endotracheal tube, ventilated patients
Introduction
Ventilator associated pneumonia (VAP) is a common cause of hospital acquired-infections1, 2, especially in susceptible populations such as patients with cystic fibrosis and in immunocompromised patients.3, 4 Importantly, VAP is associated with increased intensive care unit days, associated health care costs, and increased mortality in ventilated patients5, 6, with several of these pathogens having multi-drug resistance to antibiotic pharmacotherapy.1 Vancomycin is the drug of choice for the treatment of bronchopneumonia due to methicillin-resistant staphylococcus aureus (MRSA)-positive infections,7 but it is only available in oral and intravenous formulations; the latter being utilized to treat pneumonia due to the extremely poor bioavailability of oral vancomycin.8, 9, 10 Unfortunately, vancomycin is associated with renal toxicity and ototoxicity.11 Since i.v. administration of vancomycin runs a potential risk for renal toxicity, vancomycin use is generally limited to hospital settings. While risk of adverse drug reactions to vancomycin can be minimized with careful administration and patient monitoring, novel delivery methods and/or formulations of vancomycin that could increase the therapeutic index would be of substantial benefit.
Several experimental ‘off-label’ therapies have aimed at reducing overall systemic drug exposure in patients with MRSA-induced pneumonia by delivering vancomycin directly into the lungs via inhalation.12, 13 This route of administration delivers a high concentration of drug to the lungs, while simultaneously bypassing the high systemic load needed to push drug concentrations to therapeutic levels in pulmonary tissues. Currently, this delivery method is accomplished by wet nebulization, which forms fine aerosol droplets of drug in-line with the ventilator for forced ventilator inhalation; however, the only option for pulmonary delivery of vancomycin is to administer a nebulized vancomycin that is only formulated and approved for IV use. The off-label administration of IV vancomycin has proven to be encouraging in clinical settings, although efficacy remains to be determined in ventilated patients. The lack of a pulmonary specific vancomycin formulation can increase the risk of dosing variability, drug stability, and/or lung irritation; by default, intravenously administered vancomycin is remains the standard of care.
Though various kinds of inhalers have been developed for delivering drug aerosols into ambulatory patients, only nebulizers and pressurized metered dose inhalers (pMDIs) have been routinely used for clinical therapy during mechanical ventilation.14, 15 These devices have been fitted to the ventilator circuit by specific in-line adapters. It is well-known that pMDI and nebulized formulations suffer from ‘rain-out’, which is the formation of condensation of the aerosol drug droplets onto the ventilator circuit and endotracheal tubing.16 Drug particles can also get trapped in the existing condensate inside ventilator lines and can escape into the exhalation line between breaths resulting in diminished pulmonary delivery. Several researchers have attempted to overcome some of these pulmonary drug delivery challenges by creating inline adapters that fit dry powder inhaler devices (DPI) to deliver a concentrated drug aerosol more accurately.17, 18 An experimental dry powder inhaler device was designed and provided the convenience of connecting the DPI with the ventilator and endotracheal tubing, while maintaining efficient aerosol delivery compared to the direct-to-mouth Monodose® inhaler.18 Unfortunately, fine particle aerosol formations are highly sensitive to elevated humidity levels and other variable environmental conditions commonly found in ventilator lines and endotracheal tubes.19 Placing a catheter inside of an endotracheal tube integrated into a ventilation line can allow for the delivery of a small bolus of non-humidified air containing fine drug powder aerosol for pulmonary delivery.20 A fresh catheter could be placed into the endotracheal tube prior to dry powder drug delivery and would be minimally affected by humidity within the ventilator line.21 Combining vancomycin dry powder aerosol with a ventilator endotracheal tube catheter could potentially increase the ease, speed, safety, and consistency of vancomycin dosing to ventilated patients with MSRA-dependent pneumonia.
In this study, we characterized micronized vancomycin dry powder and identified aerosol properties using a Monodose® inhaler coupled to an Anderson Cascade Impactor. We further tested the hypothesis that dry powder vancomycin could be delivered through a ‘catheter’ inserted in a pediatric-sized endotracheal tube and evaluated aerosol performance of this system by Anderson Cascade Impaction. Moreover, using this pediatric endotracheal set up, we compared pharmacokinetic profiles of rabbits administered inhaled vancomycin dry powder or intravenous vancomycin solution.
Materials and methods
Materials
Vancomycin hydrochloride (Vanc HCl, Lot No. 14411001) was donated from Savara Pharmaceuticals. Vancomycin HCl reference standard powder (RS) and acetonitrile were purchased from Sigma Chemicals Co., St Louis, MO. Sodium dihydrogen phosphate, phosphoric acid (85%), triethylamine (TEA), tetrahydrofuran (THF) were purchased through Fisher Scientific, Fair Lawn, NJ. Double-distilled water used throughout the study was provided by an EASYpure® RODI (Model# D13321; Barnstead International), Dubuque, IA. A dry powder insufflator was purchased from Penn Century, Inc., Wyndmoor, PA. Portex® Un-cuffed oral/nasal pediatric endotracheal tubes (PVC; 2.5 mm I.D. X 3.7 mm O.D. X 155 mm length) were purchased from Smiths Medical, Dublin, OH. HPLC tubing used for endotracheal catheter (PEEK; 0.6 mm I.D. X 1.2 mm O.D. X 153 mm length) was purchased from Cobert Association, St Louis, MO.
Chemical characterization of vancomycin dry powder
Purity test
The purity of vancomycin HCL dry powder was determined as defined in the U.S. Pharmacopoeia and National Formulary 2011 using HPLC, with slight modifications.22, 23 The HPLC system consisted of a Shimadzu CBM-20A system controller, LC-10AT solvent delivery pump, SPD-10A UV detector, and SIL-10AxL auto-injector (Shimadzu Scientific Instrument Inc., Columbia, Maryland). A 4.6 x 250 mm Phenomenex C18 Luna (2) column with a particle diameter of 5 μm and a security guard Column (Phenomenex C-18 Luna (2) 3.0 x 4.6 mm, 5 μm) were used for separation (Phenomenex, Torrance, CA). A gradient elution system was used at a flow rate of 2 mL/min with a mobile phase consisting of a mixture of two solutions (Supplementary Table 1). Solution A was TEA buffer pH 3.2 (adjusted with phosphoric acid); acetonitrile; and THF (93:6:1) and Solution B was composed of TEA buffer pH 3.2; acetonitrile; and THF (70:29:1). Detection was performed at 280 nm with an injection volume of 20 μL. Chromatograms were acquired and analyzed using Shimadzu Class vp 7.4 software. The run time for the test was 35 minutes, and the retention time for vancomycin B (active component of vancomycin) was 9 ± 0.5 min. For the dry powder tested, a sequence of injections was performed (Supplementary Table 2). The percentage purity of vancomycin B was calculated according to USP as follows22:
Where rB is the area response of the main peak in the chromatogram of Test preparation B; and rA is the sum of area response of all peaks except the main peak in the chromatogram of Test preparation A.
Where rAi is the area response of any peak except the main peak in the chromatogram of Test preparation A.22
Water content
The water content of the tested powder was determined by Karl Fisher (756 KF Coulometer, Metrohm Ion Analysis, Herisau, Switzerland) and were included in the potency calculations.
Potency assay
For the determination of the drug potency and the quantification of vancomycin, another analytical method was used as reported by FDA.24 The same HPLC system was used as mentioned in the purity test. Here, an isocratic system was used with a mobile phase of acetonitrile and 25 mM phosphate buffer pH 3.2 (9:91) at a flow rate of 1 mL/min and UV detection at 230 nm. The injection volume of the sample was 50 μL. The run time for the assay was 30 minutes, and the retention time for vancomycin was 15 ± 0.3 minutes. The assay was done in triplicate. Vancomycin powder was prepared in a drug concentration of 100 μg/mL in 25 mM phosphate buffer pH 3.2 and analyzed for initial drug content using a reverse phase HPLC method.25 For RS solution, the peak areas were normalized at a concentration of 100 μg/mL and calculated as follows:
Where AN is the normalized peak area, Ari is the peak area in the chromatogram of RS, mri is the mass (mg) of RS. The potency of the vancomycin was calculated using the average of normalized peak areas of RS at 100 μg/mL as follows:
Where Ai is the peak areas in the chromatogram of the sample to be examined, AN* is the average of normalized peak areas of RS at 100 μg/mL and mi is the mass (mg) of the sample to be examined.
Physical characterization of vancomycin dry powder
Particle size and morphology by scanning electron microscopy (SEM)
The size and morphology of vancomycin dry powder was assessed using a LEO 1550 field emission scanning electron microscope (Carl Zeiss NTSC, LLC, Peabody, Maine). Prior to imaging, the samples were sputter-coated with gold for 3 min.
Powder flow characteristics
The bulk and tap densities of vancomycin dry powder were estimated by a micro-tap test approach.26, 27 The powder was placed into pre-weighed micro-centrifuge tubes, and the tubes were weighed again to determine the mass of powder. The tube was then tapped twenty times on the lab bench to compress the powder. The volume of the powder was approximated by comparing the height of the compressed powder to that of the volume of water in an identical pre-weighed micro-centrifuge tube. The tube containing the water was then weighed to determine the volume of water (assuming a density of 1 g/cm3). The powder density was calculated by dividing the mass of powder by the volume of water.
Surface area measurement by BET
The surface area of the powder is determined by the physical adsorption of a gas (Nitrogen) onto the surface of the sample particles. The BET (Brunauer, Emmett and Teller) Theory is commonly used to evaluate the gas adsorption data and generate a specific surface area result expressed in units of area per mass of sample (m2/g). To perform this test, a known mass of vancomycin dry powder (100 ± 10 mg) was placed in a sample tube and the tube was connected to a Surface Area Analyzer (Nova® 2200e, Quantachrome Instruments). Liquid nitrogen was used to keep the powder samples at a low temperature to allow for the formation of a gas monolayer during the measurements, leading to an accurate determination of specific surface area. Adsorption data of N2 in the relative pressure region from 0.05 to 0.25 was used to fit the BET equation. A BET plot was generated, and the slope and y-intercept were used to calculate the specific surface area using NovaWin software (version 11.0) that was provided by Quantachrome Instruments.
Differential scanning calorimetry (DSC)
Vancomycin dry powder was investigated by differential scanning calorimetry (DSC, Q100 Universal V4.3A TA instruments, New Castle, DE) using 3–5 mg of dry vancomycin powder. Briefly, dry powder vancomycin HCl was sealed and placed in a hermetic aluminum pan and heated at a scan rate of 10 °C/min over a temperature range of 25–350 °C. An inert atmosphere was maintained by purging with nitrogen at 50 mL/min. Data analysis was completed using Universal Analysis 2000 (Version 4.3A) software that was provided by TA Instruments.
Thermogravimetric analysis (TGA)
TGA was also performed using a Q50 TGA from TA Instruments. Samples weighing 5 mg ± 0.5 mg were loaded on a platinum sample pan and heated from 25 °C to 350 °C at a rate of 10 °C/min under dry nitrogen at a flow rate of 40 mL/min. Data analysis was completed using Universal Analysis 2000 (Version 4.3A) software that was provided by TA Instruments.
Evaluation of aerosol performance of vancomycin
Aerosizer LD
The aerodynamic diameter and size distributions of the dry powder were determined by time-of-flight measurement (TOF) using an Aerosizer LD (Amherst Instruments, Hadely, MA) equipped with a 700 μm aperture operating at 6 psi. Approximately 1 mg of the powder was added to the instrument disperser and data was collected for ~60 s under high shear (~3.4 kPa). The instrument size limits were 0.10–200 μm and particle counts were above 100,000 for all measurements.
Fast Screening Impaction (FSI)
Vancomycin dispersion performance was investigated using a Fast Screening Impactor (FSI; MSP Corporation, Shoreview, MN) at a flow rate of 90 L/min for 2.6 s (4 L inhalation volume). Capsules (HPMC type, size 3, generously provided from Capsugel®, NJ, USA) containing 30 mg ± 0.5 mg of vancomycin powder was inserted into a Plastiape Monodose Inhaler® RS01 Model 7, the capsules punctured, and the powder was drawn through the FSI. The cut-off aerodynamic diameter for the pre-separator was 5 μm. After actuation, the capsule and device along with components of the FSI were washed with predetermined volumes of phosphate buffer pH 3.2. Appropriate sample dilutions were done prior to testing by UV–Vis spectrophotometer (Agilent, Santa Clara, CA) at 280 nm. All FSI tests were carried out under controlled conditions (21 ± 2 °C, 50 ± 5% relative humidity (RH)) in triplicate.
Andersen Cascade Impaction (ACI)
Aerodynamic characteristics of vancomycin dry powder were analyzed using an eight-stage Mark II Andersen Cascade Impactor (Tisch Environmental, Inc.) with or without an endotracheal tube (see below). ACI experiments were operated at 90 L/min for 2.6 s (4 L inhalation volume). All ACI experiments were performed under controlled conditions (21 ± 2 °C, 50 ± 5% RH) in triplicate. The emitted dose (ED), the emitted fraction, and the delivery efficiency were determined. Delivery efficiency is the fine particle fraction of the total dose (FPFTD < 5 μm). The fine particle fraction of the emitted dose (FPFED) was determined from the cumulative mass distribution curve at 5 μm and 3 μm and was calculated as a function of the emitted dose. Additionally, mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were determined from the cumulative mass distribution curve.
a) ACI using a Monodose Inhaler®
The powder was delivered into the cascade impactor with a standard bent throat or a custom fabricated straight throat (to mimic dorsal recumbency of the intubated rabbits) by placing capsules (HPMC type, size 3, generously provided from Capsugel®, NJ, USA) containing 30 mg ± 0.5 mg of powder into the Monodose® Inhaler. The capsule was punctured and the powder was drawn through the cascade impactor. After actuation, the device, capsule, adapter with the endotracheal tube, throat (or straight throat), all plates, stages and the glass filter were individually extracted into separate volumetric flasks using phosphate buffer pH 3.2. Proper sample dilutions were made before quantifying by UV–Vis spectrophotometer.
b) ACI using a custom throat intubated with an endotracheal tube
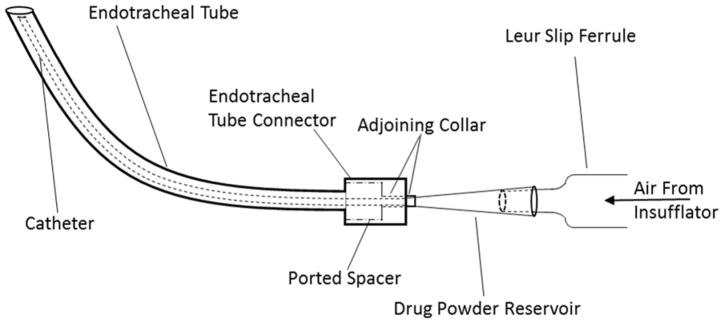
Prior to in vivo work, in vitro ACI experiments were conducted to evaluate initial aerosol particle sizes and deposition following the drug delivery through an endotracheal tube. Briefly a modified insufflator (Supplementary Fig. 1&2) was fabricated to accommodate: 1) a larger drug powder reservoir (standard insufflator reservoir holds ~5mg of vancomycin dry powder), and 2) a catheter that could be introduced through a pediatric endotracheal tube. Both the endotracheal tube with its enclosed catheter tube was inserted into the straight throat with each tip slightly protruding into the O-ring inlet cone (Fig. 1 & Supplementary Fig. 3) to bypass the throat mimicking in vivo dorsal recumbency intubation. Insufflation was actuated at the beginning of the inhalation cycle. After drug delivery, the drug reservoir, adapter along with the endotracheal and catheter tubes, straight throat, all plates, stages and the glass filter were individually extracted using phosphate buffer pH 3.2 and properly diluted before quantifying by UV–Vis spectrophotometer.
Fig. 1.
Schematic of the new insufflator- catheter system passing through an endotracheal tube.
Evaluation of the delivery efficiency of the modified insufflator compared to the standard insufflator
Preliminary studies were carried out before in vivo experiments to test the delivery efficiency of the modified insufflator. The standard insufflator (ISF) or the reservoir attached to the modified insufflator (M-ISF) was loaded with 5–30 mg dry powder vancomycin. The powder was ejected from each device with 5 mL of air via a syringe and was collected into a sealed plastic bag. The amount of vancomycin powder collected in the bag was determined gravimetrically and the Emitted Fraction (%EF) was calculated; expressed at the amount of powder collected as a fraction of the initial delivery mass.
Pulmonary delivery and pharmacokinetic characterization of vancomycin dry powder in intubated rabbits
Female New Zealand White rabbits (2.6–3.5 kg) were purchased from Charles River. Prior to anesthesia induction, rabbits were given a pre-analgesic mix of buprenorphine (0.3 mg/kg) and acepromazine (10 mg/kg) subcutaneously. Thirty minutes later, anesthesia was induced by intramuscular administration of ketamine (40 mg/kg) and xylazine (5 mg/kg). Rabbits were placed under inhaled isoflurane anesthesia (0.5%–2%) for maintenance anesthesia for surgical procedures. An indwelling catheter (22 gauge) was placed in the marginal ear vein and flushed with 0.25 mL 100 U/mL heparin in sterile saline. The indwelling catheter was then glued with tissue glue (Dermabond®) and sutured to the ear for stabilization. Elizabethan collars were placed to prevent animal-induced catheter removal. Rabbits receiving intravenous (i.v.) vancomycin were given a bolus dose of vancomycin (1 mg/kg) in 1 ml/kg sterile saline and the catheter flushed with an additional 0.1 mL 100 U/mL heparin in sterile saline. Rabbits receiving inhaled dry powder vanocomycin (1 or 5 mg/kg) were intubated with a pediatric endotracheal tube (2.5 mm I.D. X 3.7 mm O.D. X 155 mm length, Smiths Medical, Dublin, OH). Vancomycin powder was delivered through an endotracheal catheter attached to a modified insufflator (see Figure 1 and Supplemental Figures 1–2, Penn-Century, Wyndmoor, PA). Prior to dry powder delivery, the animal was momentarily unhooked from the isoflurane anesthesia. The catheter of the modified insufflator (0.6 mm I.D. X 1.2 mm O.D. X 153 mm length) was inserted fully into the endotracheal tube and the powder was delivered during animal inhalation (inhalation was determined with a stethoscope) via 5 mL of air pushed through the insufflator syringe; the modified insufflator and endotracheal tube was then removed. To reverse the anesthesia following drug delivery, animals were given yohimbine (0.2 mg/kg i.v.) through the marginal ear vein catheter and monitored until recovery (~20–40 minutes post yohimbine administration). Blood (0.5 mL) was collected from the marginal ear vein cathether at 0.5, 1, 2, 4, 6, and 24 hours post vancomycin administration for the isolation of plasma and the catheter was kept patent by flushing 0.5 mL 100 U/mL heparin in sterile saline after each blood draw. Animals were housed in an AAALAC accredited facility and all experiments were approved by the University of Kansas IACUC.
Plasma Quantification of Vancomycin
Rabbit plasma samples collected were analyzed by an LC/MS method as describe previously.28, 29 Breifly, standards of vancomycin ranging from 10 ng/mL to 25,000 ng/mL using blank plasma were prepared for quantification, and gentamicin was used as an internal standard. A protein precipitation extraction method was utilized for both plasma samples and spiked vancomycin standards. Briefly, 20 μl of plasma or standards were spiked with 500 ng/ml gentamycin as an internal standard and were then were precipitated with a solution of 500 μl of ACN containing 0.25% trifluoroacetic (TFA) acid. Samples were vortexed then centrifuged for 5 minutes at 13000 rpm. The supernatant was collected and then evaporated to dryness at 1 mm Hg and 40°C. Samples were reconstituted in 100 μl of water containing 0.25% TFA and vortexed. 10 μL of each sample was injected into the LC/MS system, which was comprised of Shimadzu LC20AD Pumps, Shimadzu CT020A Column Oven, and Shimadzu CBM20A System Controller. A Waters X-Bridge 2.1x50 mm, 3 μm column was used with mobile phase A of deionized water and mobile phase B of 95/5/0.1 ACN/deionized water/formic acid with a flow rate of 0.3 L/min. Initial conditions were 10% mobile phase B and increased to 95% B by 4 minutes; the gradient was performed at 40°C. The MS system used was an AB Sciex 3200 Linear Ion Trap mass spectrometer that employed ESI positive mode and quantitated by Analyst 1.6.1 software (AB SCIEX, Ontario, Canada). Vancomycin-144 was detected using Q1 of 725.1 and Q3 of 144.1 with a retention time of 3.75 minutes, while gentamicin standards used Q1 of 479.6 and Q3 of 160.3 with a retention time of 2.50 minutes.
PK analysis
Pharmacokinetic parameters were determined using WinNonLin Pharmacokinetic Software, version 3.1 (Pharsight, Mountain View, CA). Non-compartmental modeling analysis was performed assuming first order input with uniform weighing. Extravascular dosing was chosen for the inhaled administration, while vascular dosing was chosen for the IV group. Plasma type modeling and linear trapezoid interpolation calculation were employed to get the desired PK parameters, which included Cmax, Tmax, half-life, AUC, clearance, volume of distribution, and terminal slope.
Results
Physicochemical characterization of vancomycin dry powder
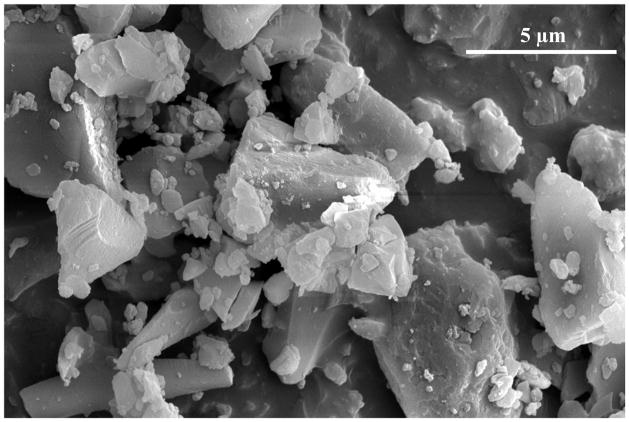
The purity of vancomycin B (active component of vancomycin) was 92.03% ± 0.08% and any individual impurity for dry powder was less than 4%. The water content was measured by Karl Fischer and was equal to 4.8% ± 1.0% for vancomycin dry powder. The potency of vancomycin was chemically analyzed and found to be 94.86% ± 0.68%. Scanning electron microscopy (SEM) of vancomycin dry powder revealed drug particles of various shape and size with an average particle size of ~5 μm (Fig. 2). Bulk and tap densities of micronized vancomycin were determined to be 0.35 ± 0.01 g/cm3 and 0.51 ± 0.01 g/cm3, respectively. The surface area of dry powder was determined to be 1.59 m2/g.
Fig. 2.
Scanning electron micrographs of vancomycin dry powder.
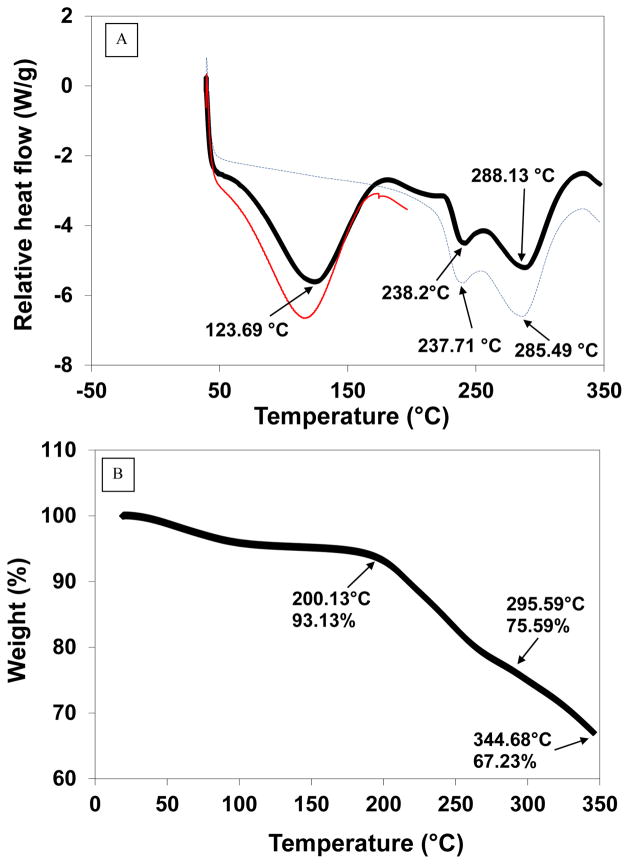
The thermal properties of vancomycin powder were investigated by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The DSC data exhibited a broad endothermic event at 123.7°C and then other two endothermic peaks at 238.2°C and 288.1°C followed by subsequent degradation of the compound (Fig. 3A). Also, TGA was performed on the material and a small step change in TGA was noted at ~50°C, which may possibly be due to the loss of water from the compound, followed by a 7% loss at 200°C, which could be attributed to the first characteristic endotherm of vancomycin (Fig. 3B). A large weight loss of 25% began at 296°C, which may be due to the second characteristic endotherm of the drug (Fig. 3C).
Fig. 3.
A) Differential Scanning Calorimetry (DSC) thermogram for vancomycin dry powder; vancomycin dry powder was heating to 20°C (red line), then cooled to room temperature followed by subsequent reheating to 350°C (blue line) compared to the direct heating of the powder to 350°C (black line). B) Thermogravimetric analysis (TGA) for vancomycin dry powder.
The aerodynamic size distribution of the micronized dry powder was determined on a time-of-flight instrument. The median aerodynamic diameter (MAD) was 2.8 μm with 50% of the powder below 2.7 μm and with 95% of the powder below 7.7 μm (Fig. 4). FSI results were consistent with the Aerosizer data. The aerosol deposition profiles showed that the majority of vancomycin powder was delivered from the device with %EF higher than 90%, with minimal (< 10%) powder remaining in the capsule and device (Table 3 & Fig. 5A). Only 32% was deposited on the filter of the FSI, which corresponds to particles with a diameter less than 5 μm (FPF). Delivery efficiency was found to be ~30%.
Fig. 4.
Aerodynamic size distributions of vancomycin dry powder determined by time-of-flight analysis.
Table 3.
Pharmacokinetic parameters of vancomycin dry powder following i.v. and pulmonary drug administration (values = average ± S.D., n = 3).
| PK parameters | Subject | ||
|---|---|---|---|
| 1 mg/kg I.V. | 1 mg/kg Inhaled | 5 mg/kg Inhaled | |
| Terminal Slope (1/hr) | 0.2073 ± 0.0282 | 0.2244 ± 0.0272 | 0.2369 ± 0.0208 |
| Half-Life (hr) | 3.38 ± 0.43 | 3.12 ± 0.40 | 2.94 ± 0.25 |
| Tmax (hr) | 0.67 ± 0.29 | 2.00 ± 0.00 | 2.67 ± 1.15 |
| Cmax (ng/mL) | 3330 ± 1119 | 1697 ± 337 | 6917 ± 724 |
| C0 (ng/mL) | 5347 ± 4927 | N/A | N/A |
| AUClast (hr*ng/mL) | 12069.85 ± 638.15 | 16066.85 ± 2877.14 | 67100.93 ± 4552.18 |
| AUC(0-∞) (hr*ng/mL) | 12135.34 ± 616.52 | 16135.36 ± 2840.68 | 67304.80 ± 4482.83 |
| Volume of Dist. (mL/kg) | 403.89 ± 64.83 | 289.64 ± 91.54 | 316.87 ± 42.58 |
| Clearance (mL/hr/kg) | 82.55 ± 4.25 | 63.33 ± 11.60 | 74.50 ± 4.93 |
Fig. 5.
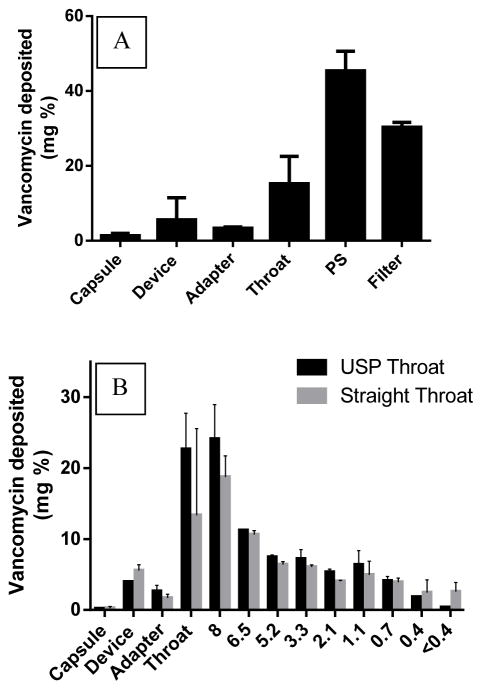
A) FSI deposition profile of vancomycin dry powder at a flow rate of 90 L/min for 2.7 s using Monodose® inhaler (model 7 standard) with the standard USP throat. B) ACI deposition profile of vancomycin dry powder at a flow rate of 90 L/min for 2.67 s using a Monodose® inhaler (model 7 standard) with a standard USP throat (black bars) or a modified straight throat (gray bars) without an endotracheal tube.
Aerosizer and FSI results were corroborated by Andersen Cascade Impaction studies at air flow rates of ~90 L/min. The dry vancomycin powder was delivered into the cascade impactor using the standard USP bent throat and the Monodose® inhaler. A bimodal distribution was observed with peaks at 8 μm (Stage -2) and 1.1 μm (stage 3) (Fig 5B). These results correspond to the particle size variation that was observed in SEM images. The high emitted fraction (~98%) of the dry powder suggested efficient aerosolization of the powder out of the device and capsule. However, the anticipated total lung deposition (i.e. FPF <5 μm) was only about 26%. In addition, the MMAD was ~7 μm with a high geometric standard deviation (GSD) (Table 2).
Table 2.
Cascade impaction results of vancomycin dry powder (values = average ± S.D., n = 3).
| Characteristics of the powder | ACI-USP throat-Monodose® inhaler | ACI-Straight-Monodose®inhaler | ACI-Endotracheal-Monodose® inhaler | ACI-Endotracheal -insufflator | |
|---|---|---|---|---|---|
| % EFa | 98 ± 4 | 79 ± 12 | 86 ± 3 | 89 ± 4 | |
| % FPFb | ≤ 5 | 26 ± 2 | 32 ± 10 | 12 ± 1 | 13 ± 2 |
| ≤ 3 | 18 ± 1 | 23 ± 8 | 10 ± 1 | 10 ± 1 | |
| % Delivery Efficiency < 5μm | 26 ± 3 | 25 ± 4 | 11 ± 1 | 11 ± 2 | |
| MMADc | 7 ± 1 | 6 ± 1 | 7 ± 2 | >10 | |
| GSDd | >10 | >10 | >10 | >10 | |
| EDe (mg) | 29 ± 3 | 22 ± 4 | 25 ± 4 | 26 ± 2 | |
% EF = Percent emitted fraction
FPF = Fine particle fraction.
MMAD = Mass median aerodynamic diameter obtained from cascade impactor.
GSD = Geometric standard deviation.
ED = Emitted Dose
A straight throat was fabricated to mimic dorsal recumbency of the intubated rabbits (Supplemental Figure 3). To validate the efficiency of this throat, the standard USP throat was replaced by a custom straight throat and ACI experiments were performed using the Mondose® inhaler under the same conditions. There was no significant difference in the aerosol performance except with lower EF% (79% vs. 98%) in case of the fabricated throat (Table 4 & Fig. 5B).
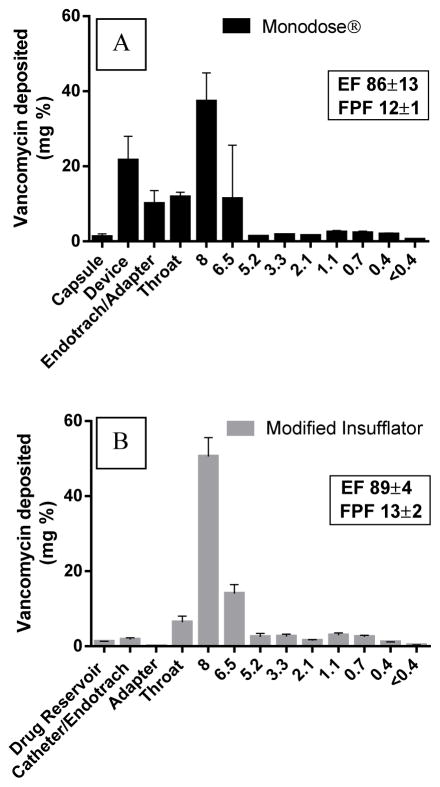
In vitro ACI experiments were conducted using a modified insufflator to assess the deposition efficiency following drug delivery by the new catheter-endotracheal system prior to in vivo delivery to rabbits. The insufflator was connected to a drug reservoir and a catheter placed into a pediatric-sized endotracheal tube that passed through the straight throat to mimic in vivo intubation. To compare the delivery between the modified insufflator and the Monodose®, a Mondose® inhaler was attached directly to the endotracheal tube (without catheter) and was inserted into the straight throat for ACI evaluation. Powder distribution did not show any significant difference following drug delivery by modified insufflator and Mondose® inhaler (Table 2 & Fig. 6). However, substantial powder was retained in the Monodose® device, capsule, and endotracheal tube (32% of filling mass), while the modified insufflator/catheter system exhibited negligible retained powder in the drug reservoir and catheter-endotracheal system (Fig. 6).
Fig. 6.
The ACI deposition profile of vancomycin dry powder at a flow rate of 90 L/min for 2.67 s using; A) Monodose inhaler (model 7 standard) attached to an endotracheal tube; B) Modified insufflator attached to a catheter passing through endotracheal tube.
To further test the delivery efficiency of the modified insufflator-reservoir system compared to the standard insufflator (ISF), 5 mg of vancomycin powder was loaded in both devices and ejected with 5 mL of air. There was no significant difference in the %EF after drug delivery (Fig. 7). Moreover, loading the reservoir attached to the modified insufflator (M-ISF) loaded with 5–30 mg dry powder vancomycin showed an increase in the EF from ~80% (5 mg initial filling mass) to ~90% (15–30 mg initial filling mass) (Fig. 7).
Fig. 7.
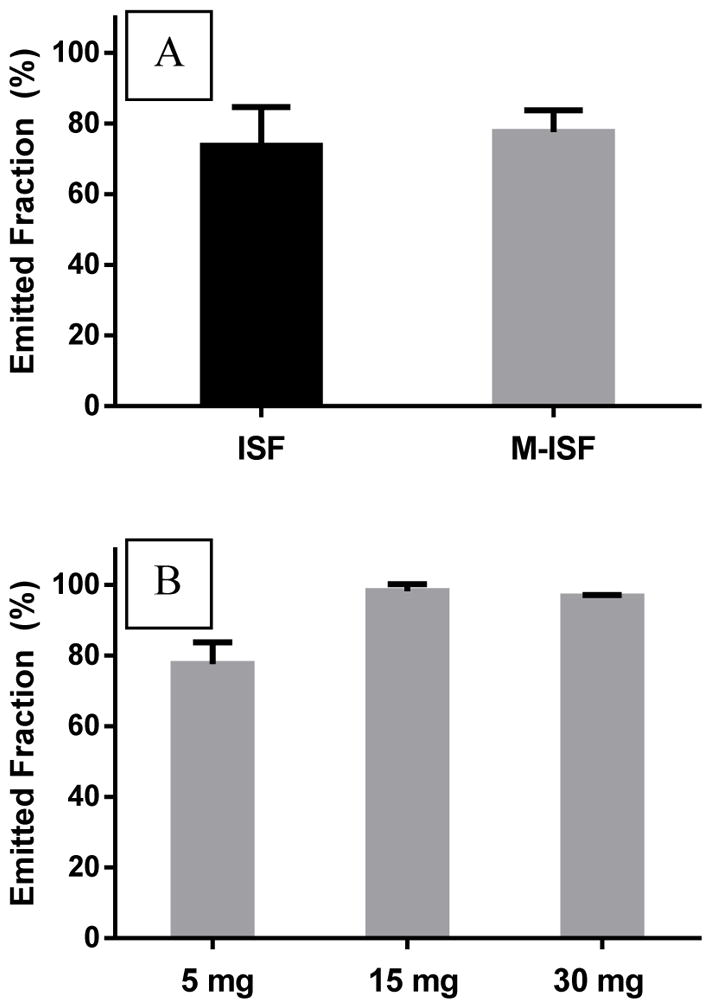
(A) The percentage of powder collected following the delivery of 5 mg loaded vancomycin powder by the modified insufflator (M-ISF, gray) compared to the standard insufflator (ISF, black) using 5 mL of air for. (B) The percentage of powder collected following the delivery of various loaded doses of vancomycin powder by modified insufflator using 5 mL of air.
To evaluate pulmonary delivery as a feasible route of administration for dry powder vancomycin, we compared pharmacokinetic profiles of rabbits given intravenous vancomycin solution or pulmonary insufflated vancomycin dry powder through an endotracheal tube catheter.30 As expected, Tmax in i.v. vancomycin was early (0.67 h), whereas plasma vancomycin concentrations in rabbits administered pulmonary vancomycin dry powder increased until Tmax was achieved at approximately 2 h, which was independent of inhaled dose (Table 3, Fig. 8). Cmax was dose dependent when vancomycin was inhaled as it increased 4.3 fold in rabbits receiving 5 mg/kg compared to 1 mg/kg inhaled vancomycin. Interestingly, the terminal slope was not different between any group receiving vanomycin. Importantly, the AUC in groups receiving 1 mg/kg intravenous or pulmonary vancomycin were similar; as expected the AUC was increased in rabbits receiving 5 mg/kg inhaled vancomycin. The clearance rate in rabbits receiving 1 mg/kg vancomycin tended to be higher in the intravenous group compared to the inhaled group, although this did not achieve the level of statistical significance (p=0.054). The data suggest that inhalation of dry powder vancomycin may be an efficient method to deliver antibiotics to the lungs of intubated patients.
Fig. 8.
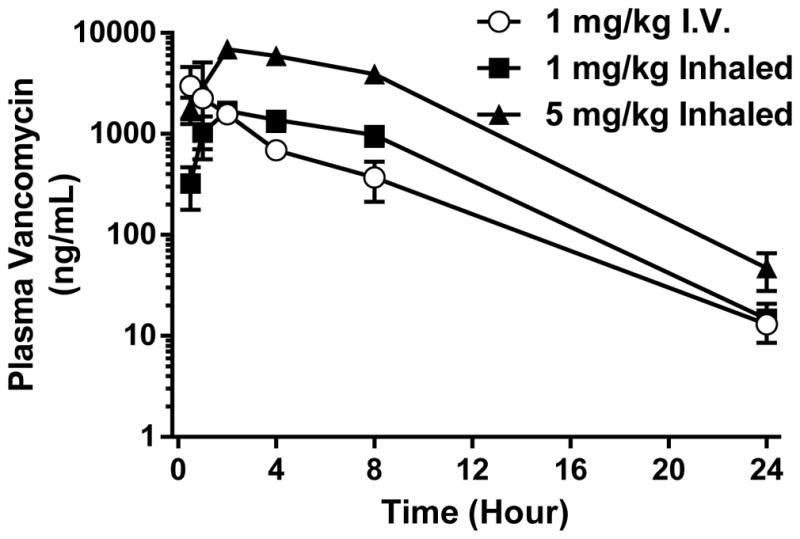
Pharmacokinetic parameters of vancomycin dry powder following i.v. and pulmonary drug administration (values = average ± S.D., n = 3).
Discussion
The acceptance criteria for the % vancomycin B (active component of vancomycin) in the sample should not be less than 85% and the percent of any peak other than the main peak should not be more than 5%, as reported by the USP.22 Here, the purity of the vancomycin was within the acceptable range. It is well-known that vancomycin HCl is a highly hygroscopic drug that can absorb moisture if not packaged appropriately.31 The potency assay showed that the drug content complied with the pharmacopoeial limits for content uniformity with standard deviation <2%.
DSC results indicated that the early endothermic peak may be suggestive of the hydrate form of the compound. The melting endotherm at 238.2°C may be evidence of the characteristic endothermic peak of the anhydrous form of vancomycin. In a similar experiment, the material was also heated from 25 to 200°C (the temperature prior to the characteristic peak of the drug) with a heating rate of 20°C/min under dry nitrogen at 50 mL/min followed by cooling the powder to room temperature using aluminum hermetic pans with holes. Then, the material was re-heated to 350°C at the same rate. This experiment demonstrated the disappearance of the first endothermic peak and only the appearance of the two melting endotherms at 238.2°C and 288.1°C was observed (Fig. 3B). These data support the hypothesis of water loss from the hydrated compound.
The aerodynamic properties of inhaled aerosols are a major contributor to the therapeutic administration of pulmonary delivered therapeutics. Typically, particles with a mean mass aerodynamic diameter (MMAD) of 1–5 μm are considered fine aerosol particles which can deposit in the respiratory airways whereas larger particles tend to deposit in the upper conducting airways and in the mouth and throat. It is important to note that P. aeruginosa colonizes both the alveolar space and the pulmonary sputum32 suggesting that inhaled therapeutics with complete lung coverage (wide particle distribution) may be more efficacious than a uniform fine aerosol typically found in nebulized vapors. Using well defined Anderson Cascade Impaction tests with a Monodose® inhaler, we found that vancomycin dry powder could be aerosolized with a high emitted fraction. Direct inhalation of the dry powder into a dry endotracheal tube resulted in approximately 10% of the dose being deposited on the side of the endotracheal tube after ACI, which is substantially less than what was found in the USP throat after traditional ACI using a Monodose inhaler (22 ± 5 %). Importantly, use of a catheter to deliver the dry powder increased delivery of dry powder to the lung without compromising the fine particle fraction. As humidity droplets tend to build up in endotracheal tubes (and nebulization circuits), we hypothesize that this result underestimates the true benefit of delivering aerosols through a fresh endotracheal tube catheter, which would minimize the amount of drug powder stuck to the side of the endotracheal tube or circuit allowing for more complete and reproducible delivery of the prescribed therapeutic directly into the pulmonary system.21
Unlike current nebulization strategies, our dry powder delivery method is highly efficient at delivering aerosols to the lung in a single 5 mL puff of air with high and consistent emitted fractions (>85%). The single dosing puff delivery scheme presented here eliminates variability accumulated over lengthy nebulization times (10–15 minutes) and ventilator-dependent duty cycling, albeit the dose administration of our method should be closely timed with patient inhalation to facilitate deep lung penetration. Additionally, our novel device aerosolizes the dry powder with minimal external air input (5 mL), suggesting that this scheme could be integrated into both adult and pediatric ventilator circuits without the need to dramatically alter the tidal volume or humidity settings of the ventilator prior to dosing. Furthermore, the use of a valved endotracheal tube suction adapter port placed directly onto the endotracheal tube, into both traditional humidified ventilator circuits and ventilator circuits utilizing heat and moisture exchangers (HMEs) as the primary humidity source to enable a more uniform dosing protocol among different ventilator support systems and hospitals. Taken together, this effective and standardized approach for delivery of an inhaled dry powder antibiotic could dramatically improve the standard of care in ventilated patients suffering from antibiotic pneumonia.
To test this concept, we developed a new device and pulmonary drug delivery method for administration of vancomycin dry powder to intubated rabbits. Utilizing this novel drug delivery system, we compared the pharmacokinetic profile of intravenously administered vancomycin solution and inhaled vancomycin dry powder. Exposures of vancomycin were similar in rabbits receiving 1 mg/kg vancomycin via intravenous solution compared to dry powder insufflation through an endotracheal tube catheter, suggesting that a majority of the pulmonary delivered dry powder was absorbed from the lungs into systemic circulation, although we cannot exclude that some powder deposited in the upper airways may have been cleared via mucociliary clearance mechanisms. Direct delivery into the lungs did not alter the pharmacokinetic half-life or terminal slope of vancomycin, suggesting minimal if any biotransformation of the dry powder in the lung; however, the clearance rate tended to decrease in rabbits receiving 1 mg/kg pulmonary delivered vancomycin and was probably due to the initial ~2 h absorption phase. Importantly, this resulted in reduced peak plasma concentrations (Cmax) of vancomycin when compared to rabbits given an equivalent bolus intravenous dose. Furthermore, as the active drug is delivered straight into the lung, higher local concentrations can be achieved, while simultaneously reducing systemic exposure and mitigating the risk of kidney toxicity. Taken together, this study suggests that inhaled vancomycin dry powder may be a convenient and safe dosing route for patients with pneumonia.
Conclusion
We have characterized both the physical and aerodynamic properties of vancomycin as a pharmaceutical dry powder aerosol. We also designed a novel method of direct pulmonary delivery to rabbits by using a catheter system that minimizes drug deposition in the endotracheal tube. This deliver system completely bypasses ventilator circuit tubing, allowing increased delivery efficiency of the aerosol without the need for substantial ventilator adjustment. Using this new dosing method, we found that dry powder vancomycin can be efficiently delivered to the lungs of intubated rabbits through pediatric-sized endotracheal tubes. This rapid delivery method may serve as a reproducible alternative to intravenous infusion or nebulization of vancomycin solution to ventilated patients. Furthermore, this device may serve as a model to improve local delivery of other dry powder pulmonary therapeutics such as bronchodilators, surfactants, or anti-cancer agents to ventilated patients.
Supplementary Material
Table 1.
FSI results of the vancomycin dry powder at 90 L/min. (n=3; ± S.D.).
| Characteristics of the dry powder | Vancomycin dry powder |
|---|---|
| FPFa (<5 μm) as % | 32 ± 10 |
| FPDb (<5 μm, mg) | 10 ± 2 |
| Delivery efficiency (<5 μm) | 30 ± 5 |
| EFc (%) | 94 ± 6 |
| EDd (mg) | 30 ± 3 |
FPF: Fine Particle Fraction
FPD: Fine Particle Dose
EF: Emitted Fraction
ED: Emitted Dose
Acknowledgments
Funding was provided by Kansas University Center for Technology Commercialization (KUCTC) and Savara Pharmaceuticals. BPS was supported in part by the American Heart Association. CK acknowledges receiving financial support provided by Dynamic Aspects of Chemical Biology Training Grant (T32 GM08545). We also thank The University of Kansas Microscopy Lab for electron microscopy assistance, the Biotechnology Innovation and Optimization Center and Animal Care Unit at The University of Kansas for assistance with analytical support and for technical assistance with surgical procedures, respectively.
Footnotes
The authors declare no competing financial interest
References
- 1.Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive care medicine. 2015;41(1):34–48. doi: 10.1007/s00134-014-3564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charles MP, Kali A, Easow JM, Joseph NM, Ravishankar M, Srinivasan S, Kumar S, Umadevi S. Ventilator-associated pneumonia. The Australasian medical journal. 2014;7(8):334–44. doi: 10.4066/AMJ.2014.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong JK, Ranganathan SC, Hart E Australian Respiratory Early Surveillance Team for Cystic, F. Staphylococcus aureus in early cystic fibrosis lung disease. Pediatric pulmonology. 2013;48(12):1151–9. doi: 10.1002/ppul.22863. [DOI] [PubMed] [Google Scholar]
- 4.Nair GB, Niederman MS. Nosocomial pneumonia: lessons learned. Critical care clinics. 2013;29(3):521–46. doi: 10.1016/j.ccc.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Shorr AF, Wunderink RG. Dollars and sense in the intensive care unit: the costs of ventilator-associated pneumonia. Critical care medicine. 2003;31(5):1582–3. doi: 10.1097/01.CCM.0000063086.33840.54. [DOI] [PubMed] [Google Scholar]
- 6.Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, Cohen MM, Fraser VJ. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Critical care medicine. 2003;31(5):1312–7. doi: 10.1097/01.CCM.0000063087.93157.06. [DOI] [PubMed] [Google Scholar]
- 7.Rybak MJ, Lomaestro BM, Rotscahfer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clinical infectious diseases. 2009;49(3):325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 8.Rodvold KA, McConeghy KW. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(Suppl 1):S20–7. doi: 10.1093/cid/cit614. [DOI] [PubMed] [Google Scholar]
- 9.Shibata N, Ishida M, Prasad YV, Gao W, Yoshikawa Y, Takada K. Highly sensitive quantification of vancomycin in plasma samples using liquid chromatography-tandem mass spectrometry and oral bioavailability in rats. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2003;789(2):211–8. doi: 10.1016/s1570-0232(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 10.Estes KS, Derendorf H. Comparison of the pharmacokinetic properties of vancomycin, linezolid, tigecyclin, and daptomycin. European journal of medical research. 2010;15(12):533–43. doi: 10.1186/2047-783X-15-12-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreno JJ, Kenney RM, Lomaestro B. Vancomycin-associated renal dysfunction: where are we now? Pharmacotherapy. 2014;34(12):1259–68. doi: 10.1002/phar.1488. [DOI] [PubMed] [Google Scholar]
- 12.Solis A, Brown D, Hughes J, Van Saene HK, Heaf DP. Methicillin-resistant Staphylococcus aureus in children with cystic fibrosis: An eradication protocol. Pediatric pulmonology. 2003;36(3):189–95. doi: 10.1002/ppul.10231. [DOI] [PubMed] [Google Scholar]
- 13.Zarogoulidis P, Kioumis I, Lampaki S, Organtzis J, Porpodis K, Spyratos D, Pitsiou G, Petridis D, Pataka A, Huang H, Li Q, Yarmus L, Hohenforst-Schmidt W, Pezirkianidis N, Zarogoulidis K. Optimization of nebulized delivery of linezolid, daptomycin, and vancomycin aerosol. Drug design, development and therapy. 2014;8:1065–72. doi: 10.2147/DDDT.S66576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallet RH. Adjunct therapies during mechanical ventilation: airway clearance techniques, therapeutic aerosols, and gases. Respiratory care. 2013;58(6):1053–73. doi: 10.4187/respcare.02217. [DOI] [PubMed] [Google Scholar]
- 15.Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, Fink JB. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respiratory care. 2010;55(7):845–51. [PubMed] [Google Scholar]
- 16.Dhand R. Inhalation therapy with metered-dose inhalers and dry powder inhalers in mechanically ventilated patients. Respiratory care. 2005;50(10):1331–4. discussion 1344–5. [PubMed] [Google Scholar]
- 17.Ju NY, Gao H, Huang W, Niu FF, Lan WX, Li F, Gao W. Therapeutic effect of inhaled budesonide (Pulmicort(R) Turbuhaler) on the inflammatory response to one-lung ventilation. Anaesthesia. 2014;69(1):14–23. doi: 10.1111/anae.12479. [DOI] [PubMed] [Google Scholar]
- 18.Pornputtapitak W, El-Gendy N, Mermis J, O'Brien-Ladner A, Berkland C. NanoCluster budesonide formulations enable efficient drug delivery driven by mechanical ventilation. International journal of pharmaceutics. 2014;462(1–2):19–28. doi: 10.1016/j.ijpharm.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Lange CF, Finlay WH. Overcoming the adverse effect of humidity in aerosol delivery via pressurized metered-dose inhalers during mechanical ventilation. American journal of respiratory and critical care medicine. 2000;161(5):1614–8. doi: 10.1164/ajrccm.161.5.9909032. [DOI] [PubMed] [Google Scholar]
- 20.Pornputtapitak W, El-Gendy N, Berkland C. Nanocluster budesonide formulations enhance drug delivery through endotracheal tubes. Journal of pharmaceutical sciences. 2012;101(3):1063–72. doi: 10.1002/jps.22818. [DOI] [PubMed] [Google Scholar]
- 21.Berkland CPW, Selvam P. El-Gendy Inhalation device, systems, and methods for administering powdered medicaments to mechanically ventilated subjects. N.US20130319410 A1. 2013
- 22.USP. Vancomycin Hydrochloride. United States Pharmacopeia and National Formulary, vol USP 34/NF 29. Rockville, MD: US Pharmacopeial Convention, Inc; 2011. [Google Scholar]
- 23.Lee J-Y, Lee K-H, Chae H-J, Kim J-H. Systematic evaluation and optimization of crystallization conditions for vancomycin purification. Korean Journal of Chemical Engineering. 2010;27(5):1538–1546. [Google Scholar]
- 24.FDA. Vancomycin Solubility Study. 2008. pp. 1–17. [Google Scholar]
- 25.Nambiar S, Madurawe R, Zuk S, Khan S, Ellison C, Faustino P, Mans D, Trehy M, Hadwiger M, Boyne M., II Product quality of parenteral vancomycin products in the United States. Antimicrobial agents and chemotherapy. 2012;56(6):2819–2823. doi: 10.1128/AAC.05344-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey MM, Gorman EM, Munson EJ, Berkland C. Pure insulin nanoparticle agglomerates for pulmonary delivery. Langmuir. 2008;24(23):13614–13620. doi: 10.1021/la802405p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Gendy N, Desai V, Berkland C. Agglomerates of ciprofloxacin nanoparticles yield fine dry powder aerosols. Journal of Pharmaceutical Innovation. 2010;5(3):79–87. [Google Scholar]
- 28.Ghassempour A, Darbandi MK, Asghari FS. Comparison of pyrolysis-mass spectrometry with high performance liquid chromatography for the analysis of vancomycin in serum. Talanta. 2001;55(3):573–580. doi: 10.1016/s0039-9140(01)00460-x. [DOI] [PubMed] [Google Scholar]
- 29.Ye G, Cai X, Wang B, Zhou Z, Yu X, Wang W, Zhang J, Wang Y, Dong J, Jiang Y. Simultaneous determination of vancomycin and ceftazidime in cerebrospinal fluid in craniotomy patients by high-performance liquid chromatography. Journal of pharmaceutical and biomedical analysis. 2008;48(3):860–865. doi: 10.1016/j.jpba.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Kamaruzaman NA, Kardia E, Kamaldin NA, Latahir AZ, Yahaya BH. The rabbit as a model for studying lung disease and stem cell therapy. BioMed research international. 2013;2013 doi: 10.1155/2013/691830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rautmann G, Daas A. Collaborative Study for the Establishment of the Second International Standard for Vancomycin. Expert Committee on Biological Standardization WHO/BS/10.2151. 2010:1–29. [Google Scholar]
- 32.Bjarnsholt T, Jensen PO, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Hoiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatric pulmonology. 2009;44(6):547–58. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.