Abstract
Obstructive sleep apnea (OSA) is a common but underdiagnosed sleep disorder, which is associated with systemic consequences such as hypertension, stroke, metabolic syndrome, and ischemic heart disease. Nocturnal laboratory-based polysomnography (PSG) is the gold standard test for diagnosis of OSA. PSG consists of a simultaneous recording of multiple physiologic parameters related to sleep and wakefulness including electroencephalography (EEG), electrooculography (EOG), surface electromyography (EMG), airflow measurement using thermistor and nasal pressure transducer, pulse oximetry and respiratory effort (thoracic and abdominal). Multiple alternative and simpler methods that record respiratory parameters alone for diagnosing OSA have been developed in the past two decades. These devices are called portable monitors (PMs) and enable performing sleep studies at a lower cost with shorter waiting times. It has been observed and reported that comprehensive sleep evaluation coupled with the use of PMs can fulfill the unmet need for diagnostic testing in various out-of-hospital settings in patients with suspected OSA. This article reviews the available medical literature on PMs in order to justify the utility of PMs in the diagnosis of OSA, especially in resource-poor, high-disease burden settings. The published practice parameters for the use of these devices have also been reviewed with respect to their relevance in the Indian setting.
KEY WORDS: Obstructive sleep apnea (OSA), polysomnography (PSG), portable monitors (PMs), sleep
Introduction
Obstructive sleep apnea (OSA) is an important, yet inadequately diagnosed public health problem that is characterized by repetitive collapse of the upper airway during sleep.[1] It has been reported that OSA affects up to 5% of the world's population[2] with a prevalence of prevalence of 4% in males and 2% in females[3] aged between 30 years and 60 years of age. The available literature on the prevalence of this disorder in the Indian population is limited. A study performed by Sharma et al.[4] in an urban setting estimated the prevalence of OSA to be 13.7%.
OSA is a predisposing factor for multiple comorbidities such as hypertension, cardiovascular consequences (including sudden death),[5,6] ischemic heart disease, stroke[7,8,9,10,11,12], and uncontrolled diabetes. An increased risk of road traffic accidents has also been reported in patients suffering from OSA.[13] The awareness about this disorder and its associated outcomes is on the rise among patients and clinicians. This has resulted in ever-increasing referrals to sleep clinics for diagnostic evaluation but the infrastructure to support the demand for rising sleep studies has not escalated commensurately.[14] The “gold standard” test for diagnosis of sleep disorders is a conventional, fully supervised, in-laboratory polysomnography (PSG).[15] With the limited availability of accredited sleep laboratories, certified sleep technologists, and complete polysomnography (CPSG) equipment in many countries, long waiting times and high costs of testing are inevitable.[14]
In view of the above mentioned reasons, several alternative diagnostic tools such as sleepiness questionnaires, clinical decision rules,[16] portable monitors (PMs), actigraphs, and peripheral arterial tone (PAT) monitors[16,17] have been developed for the evaluation of OSA. Of these, portable monitors have been evaluated and accepted for selected patient groups in clinical guidelines in various countries.[18,19]
This article reviews recent guidelines and published literature on the clinical use of portable monitoring devices to diagnose OSA and attempts to define the role of portable monitors, especially in high-disease burden, resource poor settings.
Materials and Methods
A search of the available literature was done using the keywords “portable monitoring in obstructive sleep apnea” with filters over last 10 years in humans. The Medline search generated 125 results over the last 10 years. This included 2 systematic reviews and meta-analysis, 3 guideline statements, 16 review articles, 16 randomized controlled trials, and 24 validation studies. Sixty-four articles included expert opinions and cross-sectional studies. In addition, the search was supplemented with data from cross references cited in the bibliography of relevant articles.
The present review has been organized into the following sections:
Introduction and modes of portable monitoring.
PMs: Review of the literature.
Adaptability of available guidelines for PM in an Indian setting.
Introduction and Modes of Portable Monitoring
Sleep testing devices were initially classified by the American Academy of Sleep Medicine into level 1 to level 4 devices based on the type of leads and setting of the device used[20] [Table 1]. Among these, portable monitoring devices were categorized as level 2, level 3, and level 4 sleep monitoring devices. These monitors have limited number of channels and are used for the diagnosis of certain sleep disorders such as OSA, insomnia, and restless legs syndrome. Recent technical advances have resulted in the design of a variety of compact PM devices that are battery-operated and easy to hook up (wearable). Many of these devices also have wireless monitoring capability. These devices are being proposed as feasible options for out-of-lab diagnosis of OSA. The various modes for portable sleep monitoring based on location, type of sleep study, and technician attendance have been depicted in Table 2. However, there are various models of such out of center (OOC) devices, incorporating different combinations of sensors for monitoring. Hence, a blanket acceptance or rejection of such devices has been a problem faced by sleep societies around the world. Recently, a system has been introduced categorizing these devices based on measurements of sleep, cardiovascular, oximetry, position, effort, and respiratory (SCOPER) parameters.[21] Using the SCOPER categorization, available PM devices with appropriate literature and validation studies have been categorized into a common scheme and devices are judged on whether they can produce a likelihood ratio (LR+) of at least 5 and a sensitivity of at least 0.825 at an in-laboratory apnea-hypopnea index (AHI) of 5.[21]
Table 1.
AASM classification of sleep study equipment[20]
| Level 1 | Full-attended polysomnography (≥7 channels) in a laboratory setting |
| Level 2 | Full-unattended polysomnography (≥7 channels) |
| Level 3 | Limited channel devices (usually using 4-7 channels) |
| Level 4 | 1 or 2 channels usually using oximetry as one of the parameters |
Table 2.
Modes of portable sleep monitoring
| Type of sleep monitoring | Study site | Hookup |
|---|---|---|
| Attended | Hospital/sleep laboratory | Technician |
| Home | Technician | |
| Unattended | Hospital/sleep laboratory | Technician |
| Home | Patient/technician |
Portable Monitors: Review of Literature
PMs have been used as diagnostic tools for OSA for over two decades.[22] Several of these devices have been compared with the gold standard PSG in patients with suspected OSA. However, there is a paucity of data comparing the available PM devices with one other. Some of the PMs that have been validated against PSG include ApneaLink,[23] Edentec,[24,25,26] PolyG,[27] AutoSet,[28,29,30,31,32] Embletta,[33] Sibel home,[34] Bedbugg,[35] NovaSom,[36] WatchPAT,[37,38,39,40] SNAP,[41] SOMNOcheck,[42] Stardust II,[43] Apnomonitor,[44] and Apnea Risk Evaluation System (ARES)[45] [Table 3]. The results of the studies conducted with some of these devices are discussed in detail below.
Table 3.
Studies conducted for validation of various PM devices
| PM device | Studies (authors, year) | No. of patients | Salient results |
|---|---|---|---|
| Apnea Link™ | Chen et al.[23] (2008) | 50 | ApneaLink demonstrated the best agreement with laboratory PSG data at cutoff of AHI ≥10. No significant difference between PSG and ApneaLink AHI. |
| Milton[46] (2007) | 59 | ApneaLink AHI compared with AHI from simultaneous PSG at all AHI levels, with the best results at AHI of ≥15/h (sensitivity 91%, specificity 95%). | |
| Edentec | Redline et al.[24] (1991) | 31 | High level of agreement between PM and PSG – AHI, RDI (r=0.96) using RDI cutoff of >10 |
| Whittle et al.[25] (1997) | 23 | Faster time to diagnosis, lesser cost using PM | |
| Oxyflow™ | Jimenez Gomez et al.[26] (2000) | 62 | RDI obtained by level 1 PSG and PM had comparable areas under ROC* curve (0.90 for AHI ≥10; 0.94 for AHI ≥15 and 0.96 for AHI > or 30) |
| PolyG™ | Man[27] (1995) | 104 | Apnea index (AI) correlation – 0.94; AHI correlation: 0.97; sensitivity – 82.6%, specificity – 91.4%. |
| Embletta | Dingli et al.[33] (2003) | 101(40 synchronous, 61 home studies compared with PSG) | In the home study, the mean difference in AHI between PSG AHI and Embletta AHI was 3/h 29/ 61 (47.5%) required further investigation. 42% saving in diagnostic costs over polysomnography with a screening study with Embletta |
| Sibel home 300™ | Ballester[34] (2000) | 116 | At AHI cutoff of 10 on full PSG, sensitivity of the PM was 95% and specificity was 92% At AHI cutoff of 30/h, sensitivity was 100% and specificity was 97% |
| Apnea Risk Evaluation System (ARES) | Westbrook[45] (2005) | 299 | A diagnostic AHI cutoff of >10, in-laboratory PSG yielded a sensitivity of 97.4%, specificity of 85.6%, PPV** of 93.6%, and NPV*** of 93.9% while with the ARES the sensitivity, specificity, PPV and NPV were 91.5%, 85.7%, 91.5%, and 85.7%, respectively |
| Somnocheck | OAC tonelli[42] (2009) | 157 | Compared to PSG for detecting AHI >5, the laboratory-PM demonstrated sensitivity of 95.3% and specificity of 75% while the home-PM exhibited sensitivity of 96% and specificity of 64%. |
| Stardust II | Santos Silva[43] (2009) | 80 | Strong correlation between AHI from Stardust II and PSG recordings (r>0.87) |
| Watch PAT 100 (PAT signal Device) | Bar et al.[37] (2003) | 102 | PAT-RDI highly correlated with the PSG-RDI (r=0.88, P<0.0001). RDI scores were highly reproducible with high correlation between home and in-laboratory sleep studies (r=0.89, P<0.001). |
| Zou[40] (2006) | 98 | RDI, AHI, and ODI with Watch PAT 100 correlated closely (0.88, 0.90, and 0.92; P<.0001, respectively) with the corresponding indices obtained by PSG. The agreement of sleep-wake assessment based on 30-s bins between the 2 systems was 82 +/– 7% | |
| AutoSet CPAP (ResMed) | Fleury et al.[29] (1996) | 44 | AutoSet overestimated the number of apneas. Correlation between the AI assessed by PSG and AutoSet (r=0.98). For an AI of 20/h the AutoSet was 100% sensitive and 88% specific. AutoSet AHI and the AHI – PSG: r=0.92; P<0.001. AutoSet PPV – 86% |
| Kiely et al.[30] (1996) | 36 | AutoSet detection of AHI-PSG >20: Sensitivity 97%, specificity of 77%. | |
| Gugger[31] (1997) | 67 | Mean difference between the AHI-AutoSet minus the AHI-PSG was 4.2 /h(SD 7.2) (P<0.001) | |
| Mayer et al.[32] (1998) | 95 | AHI assessed by AutoSet and PSG: r–0.87, P<0.0001, sensitivity – 92%, specificity – 79%, PPV – 93%, NPV – 76%. |
*ROC: Receiver operating characteristic, **PPV: Positive predictive value, ***NPV: Negative predictive value
Studies conducted to validate PM
Level 4 devices are the simplest forms of PMs and consist of a nasal cannula to monitor airflow and a finger probe to record oxygen saturation. Milton et al. evaluated the sensitivity and specificity of a Level 4 device, the ApneaLink™[46] (ResMed Corporation) as a screening tool for sleep apnea. The AHI obtained using this device in the laboratory and at home was compared to attended sleep-laboratory PSG in fifty-nine patients with type 2 diabetes mellitus. The study demonstrated a high sensitivity and specificity (91% and 95%, respectively) of the at-home ApneaLink AHI with the simultaneous PSG at all AHI levels, the best results being at an AHI of ≥15 events/h. The AHI comparison from the home and laboratory PM studies also demonstrated good sensitivity and specificity at AHI levels of ≥15 and ≥20 events/h (sensitivity 76% and specificity 94% for both).
The same device was also compared with a level 1 device (Alice 4) for diagnostic accuracy by Ng et al.[47] in 50 patients with suspected OSA. They observed a close correlation (r = 0.978) between AHI obtained by the two devices at AHI >10/h as well as >20/h. Further, the correlation between PSG AHI and oxygen desaturation index by ApneaLink was strong (r = 0.895) thereby drawing the conclusion that the ApneaLink device was highly sensitive and specific in quantifying AHI for screening and diagnostic purposes when access to PSG was limited.
Another level 3 sleep monitor, Apnoeascreen II (hooked up by a technician) was compared with in-laboratory PSG by Quintana-Gallego et al.[48] in 75 patients with stable heart failure. Using AHI cutoff points of >5, >10, and >15, the authors generated diagnostic accuracies of 78.6%, 84%, and 80%, respectively. However, they reported an overall diagnostic failure rate of 8% in home studies.
While validation studies of several such monitors have been conducted, most studies comprised a small number of subjects. The details of studies performed with such devices are summarized in Table 3.
In-Laboratory Versus Home Sleep Studies
Home sleep studies have been evaluated with simultaneous in-laboratory PSG by many researchers. Iber et al.[48] compared in-laboratory and home monitoring in 76 participants using a level 2 device (Compumedics, Australia) using the Sleep Heart Health Study (SHHS) methodology. They reported that the median sleep time and sleep efficiency were better in home sleep studies. Further, median RDI values were comparable between at-home and in-laboratory measurements. They suggested that patients with an RDI <20 were more likely to be diagnosed in the laboratory when compared to those with RDI >20. The misclassification rate in home studies was 22% and was hypothesized as being due to night-to-night variability of OSA. Other studies[48,49,50,51,52] have also highlighted the night-to-night variability ranging from 10% to 20% in attended as well as unattended studies.
Dingli et al.[33] evaluated synchronous recording with PM and traditional in-laboratory PSG followed by a home sleep study with the Embletta device (Flaga, Iceland), a level 3 monitor. The home-based studies showed an overall good agreement with laboratory-based PSG outcomes (kappa coefficient: 0.54; P – 0.001). Apart from this, home screening resulted in 42% saving in diagnostic costs. Thus, home monitoring was suggested as a reliable and cost-effective method for screening in patients with suspected OSA.
The AASM guideline published in 2007 defines indications for unattended portable monitoring in specific patient populations with high suspicion of OSA.[53] According to the Indian Initiative on Obstructive Sleep Apnea (INOSA) guidelines, a laboratory-attended PSG is not necessary in all patients suspected to have OSA. Portable monitoring or out of center testing (OCST) with type 3 or type 4 devices (that should at least include airflow, oxygen saturation, and respiratory effort) is adequate for diagnosis. This is acceptable in conjunction with comprehensive sleep evaluation and in patients with high pretest probability of moderate to severe OSA without comorbid sleep disorders or medical disorders such as pulmonary disease, neuromuscular disease, or congestive heart failure.[18]
According to INOSA guidelines, comprehensive evaluation and assessment of pretest probability can be performed using questionnaires such as Epworth Sleepiness Scale (ESS), Berlin questionnaire, modified Berlin questionnaire and STOP-BANG questionnaire. Out of these, STOP-BANG is the most recommended screening tool before portable monitoring due to the ease of administration and high sensitivity.
PM and CPAP Prescription
Although several comparative studies between PM and level 1 sleep studies have demonstrated a high sensitivity for PM, it is noteworthy that these studies predominantly concentrate on diagnostic accuracy and reliability. Translation of results derived from PM into continuous positive airway pressure (CPAP) prescription is an important consideration. This is especially important in settings where low cost home testing is a preferable alternative to in-laboratory PSG. Some prospective studies pertaining to this aspect of portable monitoring have been discussed below.
Rafael and coworkers[54] studied 55 patients wherein a therapeutic decision (use of CPAP) made with unattended PM device was compared with that made after a subsequent PSG. Patients were equally subdivided into the technician hookup (group 1) and self-hookup groups (group 2) at the time of initial PM monitoring. The authors reported that 16% of the recordings among the home study group were inconclusive. In 75% of the interpretable home studies, the diagnosis was seen to correlate with in laboratory PSG; this figure rose to 89% on exclusion of inconclusive home studies. Among the two subsets of home study, data were not interpretable due to artifacts in 7% of the patients in group 1 and 33% in group 2 (P < 0.05). CPAP decision disagreement between home and lab studies occurred in about one third of patients. Cost effectiveness analysis showed reduction in study costs at home compared to in lab study. Thus, this study projected home sleep studies with technician hook-up as a viable diagnostic modality to decide about CPAP prescription.
In a similar study, Parra et al.[55] tested 89 patients with suspected OSA in two settings, in the sleep laboratory using level 1 sleep study and at the patient's home using a portable monitor. In the latter setting, 50 patients were assisted by a technician and 39 patients set up the equipment themselves. An acceptable agreement was obtained between the AHI measured by full-PSG and PM. The clinical therapeutic decision taken after PM agreed with that taken with full-PSG in 89% of patients. Apart from this, the domiciliary studies were about three times less costly than level 1 PSG. They concluded that patients with suspected OSA should initially be studied in a home setting with a PM. Parra et al.[55] also noted that patients who were located far away from the hospital frequently preferred to be studied in the laboratory than at home. These findings were further validated by other studies.[56] However, in contrast there are two studies with a similar design that have found a higher frequency of unacceptable studies with PMs.[25,57] The discrepancy with respect to error rates and acceptability of PMs among the various studies cited above can be explained in relation to the differences in technical specifications, data analyzed, sensors used in various PM devices, diverse patient groups and preferences, and study setting and availability of technicians for hookup at home.
Since continuous positive airway pressure can be titrated most effectively with monitoring in a sleep laboratory,[58] an attended in-laboratory CPAP titration study/split night study was also necessary conventionally after a diagnosis of OSA was made. However, nowadays the validation and clinical use of the auto-titrating PAP device minimizes the need for an in-laboratory titration study for patients with moderate to severe OSA. This is feasible since these devices can be used in an unattended way to determine fixed CPAP treatment pressure for such patients who do not have significant co morbidities such as congestive cardiac failure, chronic obstructive pulmonary disease, central sleep apnea syndrome, and hypoventilation syndrome.[59]
Further, in patients with more than one sleep disorder such as OSA and insomnia, in patients with problems such as overlap syndrome (where suboptimal levels of CPAP can actually worsen hypoxia) as well as patients underdiagnosed by PM, home studies may be followed by repeat type I test. Apart from this, knowledge of autotitrating CPAP devices may not be uniform, especially with respect to known contraindications in certain special populations (heart failure), limitations (nasal obstruction), and variability in device performance, and air leak between manufacturers.[60]
Portable Monitoring: The Indian Scenario
As opposed to the global data described above, awareness about sleep disorders and scientific research in sleep medicine in the Indian subcontinent has demonstrated a gradual upward trend only since the past two decades. The paucity of trained sleep technologists and physicians, financial considerations, and an unknown disease burden has led to a widespread use of PMs in both clinical and research settings. In a study by Udwadia et al.,[61] the prevalence of OSA was estimated using technician hookup-based PM (Compumedics P series). OSA prevalence in middle-aged urban Indian men was estimated to be 19.5%.
Portable Monitors: Summary of Available Literature
It is evident from the literature reviewed in the preceding paragraphs that there is considerable heterogeneity in the applicability and modes of portable monitoring for the diagnosis of OSA.
However, there is compelling evidence to suggest that home sleep studies have evolved as a viable and more economical mode of diagnosing OSA worldwide. When equipment with multiple channels are used, the involvement of a sleep technician for hookup in the patient's home has been shown to be a good intervention to improve the accuracy and reliability of testing. The inferred merits and demerits of PM are summarized in Table 4.
Table 4.
Merits and demerits of portable sleep monitoring
| Merits of portable monitors |
| Can resolve problems related to accessing sleep clinics and laboratories |
| Reduction in costs and waiting times for level 1 and level 2 sleep studies |
| May obviate the need for exhaustive testing when OSA is strongly suspected |
| Comfort and convenience of sleeping at home ensures less disruption of sleep |
| Feasible option for patients with limited mobility |
| Demerits of portable monitors |
| Patient self-hookup/unattended study may affect data quality |
| Variability owing to diversity in devices and scoring schemes |
| Non-OSA sleep disorders, occult seizures and cardiac arrhythmias may be missed |
| Lack of EEG channels precludes sleep staging, thus affecting accuracy of grading severity if it is OSA |
*EEG: Electroencephalography
Adaptablity of Available Guidelines for PM in Indian Setting
Practice parameters for PSG were first published by the American Sleep Disorders Association in 1994.[20] On the basis of the then available literature on PM, these guidelines considered that PM had inadequate sensitivity for routine diagnostic testing. Subsequent to these guidelines, several systematic reviews and meta-analyses regarding the use of portable devices were published.
In 2007, the American Academy of Sleep Medicine (AASM) updated practice parameters for the use of unattended PMs in the diagnosis of OSA in adult patients and since its publication, further studies have already been conducted adding to the pool of evidence. The major recommendations in this statement are hereby reviewed in the context of their adaptability in the Indian setting [Table 5]. Recently published Indian guidelines (INOSA) accept PM (type 3 and 4) as a useful, cost-effective, convenient, and speedy method of diagnosis after careful patient selection and by a trained sleep physician.
Table 5.
Summary of major recommendations by AASM with adaptability in India
| AASM recommendation | Adaptability in India |
|---|---|
| Unattended portable monitoring should be performed only in conjunction with comprehensive sleep evaluation | In view of the unexplored OSA burden, complete sleep evaluation with portable monitoring may be acceptable |
| Sleep evaluations using PM must be supervised by a board certified practitioner | Number of trained sleep specialists needs to be increased |
| PM may be used as an alternative for diagnosis of OSA in patients with high pretest probability of moderate-to-severe OSA | As lack of awareness has led to patients being diagnosed at the severe end of the OSA spectrum, pretest probability is naturally high, favoring portable monitoring |
| PM is not appropriate for patients with significant comorbid medical conditions | Use of level 1 PSG could be reserved for patients suspected of having non-OSA/multiple sleep disorders |
| PM is not appropriate for a general screening of asymptomatic populations | PM can be used in high-risk populations/to determine the prevalence of OSA |
| PM may be indicated when in-laboratory PSG is not possible due to immobility/critical illness/monitoring response to non-CPAP treatments for OSA | Due to paucity of sleep laboratories and trained personnel, PM may be undertaken in a hospital ward or at home |
| PM must record at least the airflow, respiratory effort, and blood oxygenation using conventional biosensors | Use of standardized equipment may facilitate screening of more patients |
| PM must allow for display of raw data with the capability of manual scoring or editing of automated scoring by a qualified sleep technician | With the right device and a trained technician, PM can be used in resource poor settings |
A flowchart to guide selection of patients for portable sleep studies is depicted in Figure 1.
Figure 1.
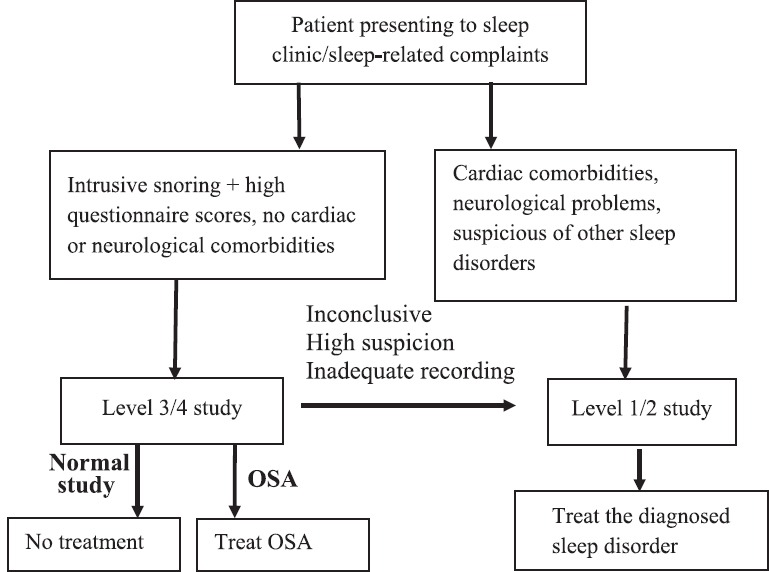
Flowchart to guide PM selection
Conclusion
A detailed evaluation for pretest probability (risk stratification, screening with sleepiness scales, and clinical exam, especially upper airway evaluation) in conjunction with portable monitoring has been recommended to obviate the need for level 1 and level 2 sleep studies in patients with OSA. This holds special importance, especially in resource poor settings. The published practice parameters for PM are relevant and applicable in the Indian setting. In conclusion, a greater adoption of PMs by trained sleep physicians would enable the timely diagnosis of OSA with reduced cost and waiting time.
Financial support and sponsorship
This is to state that none of the authors have received any form of sponsorship, support, or funding in the compilation and drafting of this review article.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lam JC, Sharma SK, Lam B. Obstructive sleep apnoea: Definitions, epidemiology and natural history. Indian J Med Res. 2010;131:165–70. [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130:149–56. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Wessendorf TE, Teschler H, Wang YM, Konietzko N, Thilmann AF. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247:41–7. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 8.Turkington PM, Bamford J, Wanklyn P, Elliott MW. Prevalence and predictors of upper airway obstruction in the first 24 hours after acute stroke. Stroke. 2002;33:2037–42. doi: 10.1161/01.str.0000023576.94311.27. [DOI] [PubMed] [Google Scholar]
- 9.Harbison J, Ford GA, James OF, Gibson GJ. Sleep-disordered breathing following acute stroke. QJM. 2002;95:741–7. doi: 10.1093/qjmed/95.11.741. [DOI] [PubMed] [Google Scholar]
- 10.Iranzo A, Santamaria J, Berenguer J, Sánchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002;58:911–6. doi: 10.1212/wnl.58.6.911. [DOI] [PubMed] [Google Scholar]
- 11.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–7. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 12.Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: Final report on 128 patients. Sleep. 1999;22:217–23. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 13.Masa JF, Rubio M, Findley LJ. Habitually sleepy drivers have a high frequency of automobile crashes associated with respiratory disorders during sleep. Am J Respir Crit Care Med. 2000;162:1407–12. doi: 10.1164/ajrccm.162.4.9907019. [DOI] [PubMed] [Google Scholar]
- 14.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 15.Gay PC, Selecky PA. Are sleep studies appropriately done in the home? Respir Care. 2010;55:66–75. [PubMed] [Google Scholar]
- 16.Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427–32. doi: 10.1164/rccm.200112-110OC. [DOI] [PubMed] [Google Scholar]
- 17.Gurubhagavatula I, Maislin G, Pack AI. An algorithm to stratify sleep apnea risk in a sleep disorders clinic population. Am J Respir Crit Care Med. 2001;164:1904–9. doi: 10.1164/ajrccm.164.10.2103039. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SK, Katoch VM, Mohan A, Kadhiravan T, Elavarasi A, Ragesh R, et al. ; Apnoea INOSA Guidelines Working Group; Indian Initiative on Obstructive Sleep. Consensus & evidence-based INOSA Guidelines 2014 (first edition) Indian J Med Res. 2014;140:451–68. [PMC free article] [PubMed] [Google Scholar]
- 19.Canadian Agency for Drugs and Technologies in Health (CADTH). Portable monitoring devices for diagnosis of obstructive sleep apnea at home: Review of Accuracy, cost-effectiveness, guidelines, and coverage in Canada. CADTH Technol Overv. 2010;1:e0123. [PMC free article] [PubMed] [Google Scholar]
- 20.Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1994;17:372–7. [PubMed] [Google Scholar]
- 21.Collop NA, Tracy SL, Kapur V, Mehra R, Kuhlmann D, Fleishman SA, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: Technology evaluation. J Clin Sleep Med. 2011;7:531–48. doi: 10.5664/JCSM.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M, Patel NP, Rosen I. Portable Monitors in the diagnosis of obstructive sleep apnea. Chest. 2007;132:1672–7. doi: 10.1378/chest.06-2793. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Lowe AA, Bai Y, Hamilton P, Fleetham JA, Almeida FR. Evaluation of a portable recording device (ApneaLink) for case selection of obstructive sleep apnea. Sleep Breath. 2009;13:213–9. doi: 10.1007/s11325-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 24.Redline S, Tosteson T, Boucher MA, Millman RP. Measurement of sleep-related breathing disturbances in epidemiologic studies. Assessment of the validity and reproducibility of a portable monitoring device. Chest. 1991;100:1281–6. doi: 10.1378/chest.100.5.1281. [DOI] [PubMed] [Google Scholar]
- 25.Whittle AT, Finch SP, Mortimore IL, MacKay TW, Douglas NJ. Use of home sleep studies for diagnosis of the sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:1068–73. doi: 10.1136/thx.52.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiménez Gómez A, Golpe Gómez R, Carpizo Alfayate R, de la Roza Fernández C, Fernández Rozas S, García Pérez MM. The validation of a portable 3-channel recording system (Oxyflow, Edentec) for the diagnosis of the sleep apnea syndrome. Arch Bronconeumol. 2000;36:7–12. doi: 10.1016/s0300-2896(15)30226-x. [DOI] [PubMed] [Google Scholar]
- 27.Man GC, Kang BV. Validation of a portable sleep apnea monitoring device. Chest. 1995;108:388–93. doi: 10.1378/chest.108.2.388. [DOI] [PubMed] [Google Scholar]
- 28.Bradley PA, Mortimore IL, Douglas NJ. Comparison of polysomnography with ResCare Autoset in the diagnosis of the sleep apnoea/hypopnoea syndrome. Thorax. 1995;50:1201–3. doi: 10.1136/thx.50.11.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleury B, Rakotonanahary D, Hausser-Hauw C, Lebeau B, Guilleminault C. A laboratory validation study of the diagnostic mode of the Autoset system for sleep-related respiratory disorders. Sleep. 1996;19:502–5. doi: 10.1093/sleep/19.6.502. [DOI] [PubMed] [Google Scholar]
- 30.Kiely JL, Delahunty C, Matthews S, McNicholas WT. Comparison of a limited computerized diagnostic system (ResCare Autoset) with polysomnography in the diagnosis of obstructive sleep apnoea syndrome. Eur Respir J. 1996;9:2360–4. doi: 10.1183/09031936.96.09112360. [DOI] [PubMed] [Google Scholar]
- 31.Gugger M. Comparison of ResMed AutoSet (version 3.03) with polysomnography in the diagnosis of the sleep apnoea/hypopnoea syndrome. Eur Respir J. 1997;10:587–91. [PubMed] [Google Scholar]
- 32.Mayer P, Meurice JC, Philip-Joet F, Cornette A, Rakotonanahary D, Meslier N, et al. Simultaneous laboratory-based comparison of ResMed Autoset with polysomnography in the diagnosis of sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12:770–5. doi: 10.1183/09031936.98.12040770. [DOI] [PubMed] [Google Scholar]
- 33.Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 34.Ballester E, Solans M, Vila X, Hernandez L, Quintó L, Bolivar I, et al. Evaluation of a portable respiratory recording device for detecting apnoeas and hypopnoeas in subjects from a general population. Eur Respir J. 2000;16:123–7. doi: 10.1034/j.1399-3003.2000.16a22.x. [DOI] [PubMed] [Google Scholar]
- 35.Claman D, Murr A, Trotter K. Clinical validation of the Bedbugg in detection of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2001;125:227–30. doi: 10.1067/mhn.2001.118126. [DOI] [PubMed] [Google Scholar]
- 36.Reichert JA, Bloch DA, Cundiff E, Votteri BA. Comparison of the NovaSom QSG, a new sleep apnea home-diagnostic system, and polysomnography. Sleep Med. 2003;4:213–8. doi: 10.1016/s1389-9457(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 37.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123:695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 38.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: In-laboratory and ambulatory validation. Sleep. 2004;27:923–33. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayas NT, Pittman S, MacDonald M, White DP. Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med. 2003;4:435–42. doi: 10.1016/s1389-9457(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 40.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29:367–74. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 41.Su S, Baroody FM, Kohrman M, Suskind D. A comparison of polysomnography and a portable home sleep study in the diagnosis of obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2004;131:844–50. doi: 10.1016/j.otohns.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Tonelli de Oliveira AC, Martinez D, Vasconcelos LF, Gonçalves SC, Lenz MC, Fuchs SC, et al. Diagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoring. Chest. 2009;135:330–6. doi: 10.1378/chest.08-1859. [DOI] [PubMed] [Google Scholar]
- 43.Santos-Silva R, Sartori DE, Truksinas V, Truksinas E, Alonso FF, Tufik S, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629–36. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yagi H, Nakata S, Tsuge H, Yasuma F, Noda A, Morinaga M, et al. Significance of a screening device (Apnomonitor 5) for sleep apnea syndrome. Auris Nasus Larynx. 2009;36:176–80. doi: 10.1016/j.anl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Westbrook PR, Levendowski DJ, Cvetinovic M, Zavora T, Velimirovic V, Henninger D, et al. Description and validation of the apnea risk evaluation system: A novel method to diagnose sleep apnea-hypopnea in the home. Chest. 2005;128:2166–75. doi: 10.1378/chest.128.4.2166. [DOI] [PubMed] [Google Scholar]
- 46.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: A novel and simple single-channel recording device. J Clin Sleep Med. 2007;14:387–92. [PMC free article] [PubMed] [Google Scholar]
- 47.Ng SS, Chan TO, To KW, Ngai J, Tung A, Ko FW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern Med J. 2009;39:757–62. doi: 10.1111/j.1445-5994.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 48.Iber C, Redline S, Kaplan Gilpin AM, Quan SF, Zhang L, Gottlieb DJ, et al. Polysomnography performed in the unattended home versus the attended laboratory setting--Sleep Heart Health Study methodology. Sleep. 2004;27:536–40. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 49.Stepnowsky CJ, Jr, Orr WC, Davidson TM. Nightly variability of sleep-disordered breathing measured over 3 nights. Otolaryngol Head Neck Surg. 2004;131:837–43. doi: 10.1016/j.otohns.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Quan SF, O’Connor GT, Quan JS, Redline S, Resnick HE, Shahar E, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11:149–57. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 51.Meyer TJ, Eveloff SE, Kline LR, Millman RP. One negative polysomnogram does not exclude obstructive sleep apnea. Chest. 1993;103:756–60. doi: 10.1378/chest.103.3.756. [DOI] [PubMed] [Google Scholar]
- 52.Wittig RM, Romaker A, Zorick FJ, Roehrs TA, Conway WA, Roth T. Night-to-night consistency of apneas during sleep. Am Rev Respir Dis. 1984;129:244–6. [PubMed] [Google Scholar]
- 53.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 54.Golpe R, Jiménez A, Carpizo R. Home sleep studies in the assessment of sleep apnea/hypopnea syndrome. Chest. 2002;122:1156–61. doi: 10.1378/chest.122.4.1156. [DOI] [PubMed] [Google Scholar]
- 55.Parra O, García-Esclasans N, Montserrat JM, García Eroles L, Ruíz J, López JA, et al. Should patients with sleep apnoea/hypopnoea syndrome be diagnosed and managed on the basis of home sleep studies? Eur Respir J. 1997;10:1720–4. doi: 10.1183/09031936.97.10081720. [DOI] [PubMed] [Google Scholar]
- 56.Parthasarathy S, Haynes PL, Budhiraja R, Habib MP, Quan SF. A national survey of the effect of sleep medicine specialists and American Academy of Sleep Medicine Accreditation on management of obstructive sleep apnea. J Clin Sleep Med. 2006;2:133–42. [PubMed] [Google Scholar]
- 57.Portier F, Portmann A, Czernichow P, Vascaut L, Devin E, Benhamou D, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162:814–8. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 58.Skjodt NM. Approach to outpatient management of adult sleep apnea. Can Fam Physician. 2008;54:1408–12. [PMC free article] [PubMed] [Google Scholar]
- 59.Kirsch DB. PRO: Sliding into home: Portable sleep testing is effective for diagnosis of obstructive sleep apnea. J Clin Sleep Med. 2013;9:5–7. doi: 10.5664/jcsm.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parthasarathy S. CON: Thoughtful steps informed by more comparative effectiveness research is needed in home testing. J Clin Sleep Med. 2013;9:9–12. doi: 10.5664/jcsm.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–73. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]


