Hyperglycemia is common in patients treated with PI3K/AKT/mTOR inhibitors; however, it is manageable with conventional treatment. Predictive factors of age, history of diabetes, and administration of AKT and dual PI3K/mTOR inhibitors warrant prospective validation.
Keywords: PI3K/AKT/mTOR, PI3K inhibitors, AKT inhibitors, Hyperglycemia, Phase I trials
Abstract
Background.
Dysregulation of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway is implicated in human cancer growth and progression. Agents targeting this pathway are associated with hyperglycemia due to interaction with the insulin-glucose regulatory axis. Identifying the predictive factors for hyperglycemia in patients treated with these agents may help direct future management.
Materials and Methods.
Clinical characteristics and outcomes of patients treated consecutively with PI3K, AKT, or mTOR inhibitors in the Drug Development Unit, The Royal Marsden (RM) National Health Service (NHS) Foundation Trust, between 2007 and 2012 were recorded. Baseline variables and their association with grade 3 hyperglycemia (Common Terminology Criteria for Adverse Events, version 3.0) were analyzed by using the chi-square test and Fisher exact test for categorical variables and binary logistic regression for continuous variables.
Results.
A total of 341 patients were treated in 12 phase I trials of PI3K/AKT/mTOR inhibitors, and 298 patients (87.4%) developed hyperglycemia. Hyperglycemia was grade 1 in 217 (72.8%) and grade 2 in 61 (20.5%) patients, respectively. Grade ≥3 hyperglycemia was seen in 6.7% of patients (n = 20). According to the chi-square test, age <65 years (p = .03), history of diabetes (p = .003), and treatment with AKT and dual PI3K/mTOR inhibitors (p < .0005) predicted the occurrence of grade 3 hyperglycemia. Of 24 patients requiring intervention, 20 received metformin, 2 dietary advice, 1 insulin, and 1 both metformin and insulin. One patient required dose reduction. There were no permanent drug discontinuations, and no hyperglycemia-related dose-limiting toxicities were observed; thus, the recommended phase II dose was not affected by the hyperglycemia observed in our cohort.
Conclusion.
Hyperglycemia is common in patients treated with PI3K/AKT/mTOR inhibitors; however, it is manageable with conventional treatment. Predictive factors of age, history of diabetes, and administration of AKT and dual PI3K/mTOR inhibitors warrant prospective validation.
Implications for Practice:
This study reviewed the clinical data of 341 patients treated in 12 phase I trials of agents targeting phosphatidylinositol3-kinase (PI3), protein kinase B (AKT), and mammalian target of rapamycin (mTOR), as well as dual inhibitors. Hyperglycemia was evident in 87.4% of patients but was ≥grade 3 in just 6.7%. Age <65 years, history of diabetes, and treatment with AKT and dual PI3K/mTOR inhibitors were each associated with grade 3 hyperglycemia. Management of patients was uncomplicated, and no permanent drug discontinuations were necessary. Despite the small study size, these findings support continued caution about enrolling patients with a history of diabetes into such trials. However, clinicians may be reassured, pending prospective validation of these results, that significant hyperglycemia is not frequent and, when it occurs, is manageable.
Introduction
The phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling pathway plays a critical role in a range of cell functions, including cell growth, differentiation, proliferation, cellular metabolism, and cytoskeletal reorganization, leading to apoptosis and cell survival [1, 2]. The PI3K/AKT/mTOR pathway also plays a pivotal role in the metabolic and mitogenic actions of insulin and insulin-like growth factor 1 [3, 4]. In the presence of insulin, the insulin receptor phosphorylates insulin receptor substrate proteins that are linked to the activation of two main signaling pathways: the PI3K/AKT/mTOR pathway, which is responsible for most of the metabolic actions of insulin, and the Ras mitogen-activated protein kinase pathway, which regulates expression of some genes and cooperates with the PI3K/AKT/mTOR pathway to control cell growth and differentiation [4]. Therefore, activation of the PI3K pathway is crucial for aspects of insulin-induced glucose and lipid metabolism, such as translocation of glucose transporter type 4 to the cell surface, glucose uptake, glycogen synthesis, and suppression of glucose output, triglyceride synthesis, and insulin-induced mitogenesis [3, 5]. Consequently, it is not surprising that PI3K/AKT/mTOR pathway inhibitors may result in clinically significant and important metabolic effects. This hypothesis is supported by evidence gained from some preclinical models suggesting that the loss of insulin signaling in pancreatic β cells and peripheral tissues through blocking of either of the nodes of the PI3K/AKT/mTOR pathway may result in hyperglycemia and diabetes [6, 7]. Furthermore, germline deletion of AKT2, an AKT isoform that is abundant in muscle and liver, results in a diabetic phenotype in mouse models [7]. The relevance of this model for human disease is supported by the identification of a point mutation in AKT2 in a familial form of severe insulin resistance [8].
Dysregulation of the PI3K/AKT/mTOR pathway has been implicated in most human malignancies [2], and thus several anticancer agents targeting this pathway are in the early phases of clinical development [9]. Some agents, including the mTOR inhibitors temsirolimus and everolimus, are approved by both the European Medicines Agency and U.S. Food and Drug Administration for the treatment of renal cancer, neuroendocrine and pancreatic tumors, and hormone-positive human epidermal growth receptor-2-negative breast cancer [10–13]. Because of the overlapping mechanism of action and interference with the insulin-glucose regulatory axis agents targeting PI3K-AKT-mTOR inhibitors, this may potentially cause on-target effect of hyperglycemia and hyperinsulinemia and thus may hamper the clinical development of these agents. Nevertheless, although hyperglycemia has been observed in various early-phase clinical trials as a class effect of drugs targeting this pathway [14–16], there is a lack of clinical data to inform us about the frequency and severity of hyperglycemia, its clinical implications and management, and its effect on patients. This study aimed to define the frequency, severity, and clinical outcome of hyperglycemia associated with PI3K/AKT/mTOR inhibitors in patients treated with agents targeting this pathway in the phase I setting. We also identified factors that may predict for the occurrence of grade ≥3 hyperglycemia.
Materials and Methods
Baseline Characteristics
This was a retrospective study of patients treated with PI3K, AKT, and mTOR inhibitors in the Drug Development Unit, RM, London, U.K., between 2007 and 2012. Clinical characteristics and outcomes of patients treated consecutively with these agents were recorded in a secure electronic database. Only patients who had received at least one dose of the experimental agent were included in the study.
Baseline demographic and clinical characteristics, including age, sex, height, weight, body mass index, personal history of diabetes mellitus and steroid use, tumor type, type of novel agent used, and laboratory results (including fasting blood glucose at day 1 of cycle [C] 1 and 2, aspartate aminotransferase [AST], γ-glutamyltransferase [GGT], glycosylated hemoglobin A1c [HbA1c], and calculated creatinine clearance [Cockcroft-Gault formula]), were recorded. In addition, the highest grade of hyperglycemia reached during trial participation and the intervention applied was recorded. All study patients had previously provided written informed consent for participation in the relevant phase I trials as approved by the local research ethics committees.
Statistical Analysis
Baseline demographic and clinical characteristics and their association with grade 3 hyperglycemia (Common Terminology Criteria for Adverse Events version 3.0) were analyzed by using the chi-square and Fisher exact test for categorical variables and binary logistic regression analysis for continuous variables. All statistical analyses were performed by using SPSS software, version 21.0 (IBM, Armonk, NY, http://www.ibm.com). Chi-square analysis tested the following parameters: fasting blood glucose at day 1 of C1, age at study entry, sex, body mass index, history of diabetes mellitus, history of steroid use, tumor type, baseline creatinine clearance, class of PI3K pathway inhibitor, maximum blood glucose level in C1, and fasting blood glucose in C2. Binary logistic regression analysis tested AST, GGT, and HbA1c at baseline.
Results
Patients and Characteristics
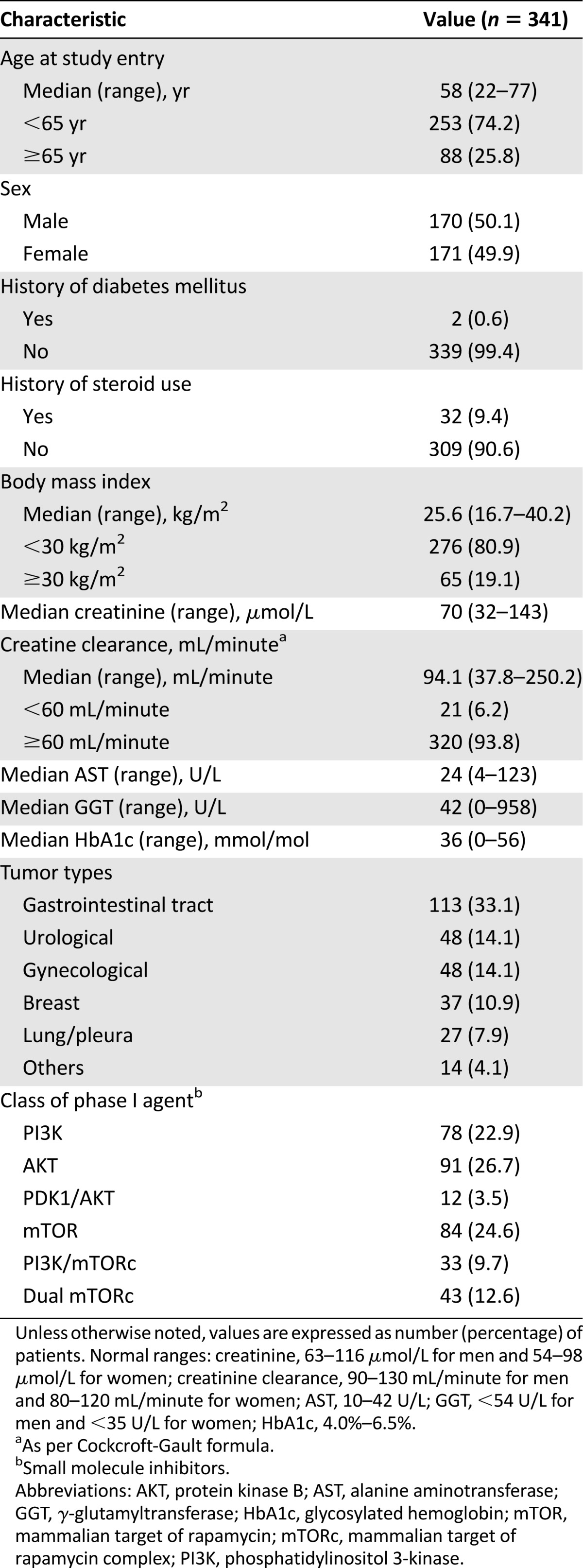
A total of 341 patients were treated in 12 phase I trials of PI3K, AKT, mTOR, dual PI3K/mTOR, and dual mTORC1/mTORC2 inhibitors (Table 1). The female/male ratio was 1.0. Most patients did not have a personal history of diabetes mellitus (99.4%) or history of steroid use (90.6%) because generally these are exclusion criteria for most studies of agents targeting this pathway. The most common cancers were gastrointestinal (33.1%), urological (14.1%), gynecological (14.1%), and breast (10.9%). The median age at study entry was 58 years (range, 22–77 years). Median body mass index was 25.6 kg/m2 (range, 16.7–40.2 kg/m2). Median baseline creatinine was 70 µmol/L (range, 32–143 µmol/L) with median baseline creatinine clearance (Cockcroft-Gault formula) of 94.1 mL/minute (range, 37.8–250.2 mL/minute). Median AST and alanine aminotransferase (ALT) at cycle 1 were 24 U/L (range, 4–123 U/L) and 42 U/L (range, 0–958 U/L), respectively. Median HbA1c at cycle 1 was 36 mmol/mol (range, 0–56 mmol/mol). A total of 223 patients (65.4%) had received at least 2 cycles of dosing, and 54 (15.8%) had received at least 6 cycles at the point of data cutoff.
Table 1.
Baseline patient characteristics
Hyperglycemia
Overall, 298 patients (87.4%) of patients developed any grade of hyperglycemia during treatment (normal range at institution, 3.9–6.0 mmol/L). Of the 298 patients, hyperglycemia was grade 1 in 217 (72.8%) and grade 2 in 61 (20.5%). Hyperglycemia grade ≥3 was seen in 20 patients (6.7%; grade 3, n = 18; grade 4, n = 2). Two hundred sixty-two patients (87.9%) experienced the highest glucose level during cycle 1 and 43 (14.4%) during cycle 2.
Treatment of Hyperglycemia
Most patients did not require medical intervention for hyperglycemia on the basis of the asymptomatic occurrence, close monitoring, and resolution of hyperglycemia without any interventions. Of 24 (8%) patients who required medical intervention, 20 required metformin, 1 insulin, 1 insulin and metformin, and 2 dietary modification. Only one patient’s blood glucose level failed to resolve to grade ≤2 (from grade 3) after pharmacologic intervention and required a dose reduction. This patient, however, had a history of frontal glioblastoma, and he was maintained on low-dose steroids (dexamethasone, 4 mg) until 7 days before starting the trial medication. He developed grade 3 hyperglycemia on day 2 of the trial treatment; in view of his previous issues with steroid-induced hyperglycemia, insulin was commenced after 1 dose of metformin, 500 mg. No other patients in this study required insulin for achieving glycemic control. There were no permanent drug discontinuations in the current study.
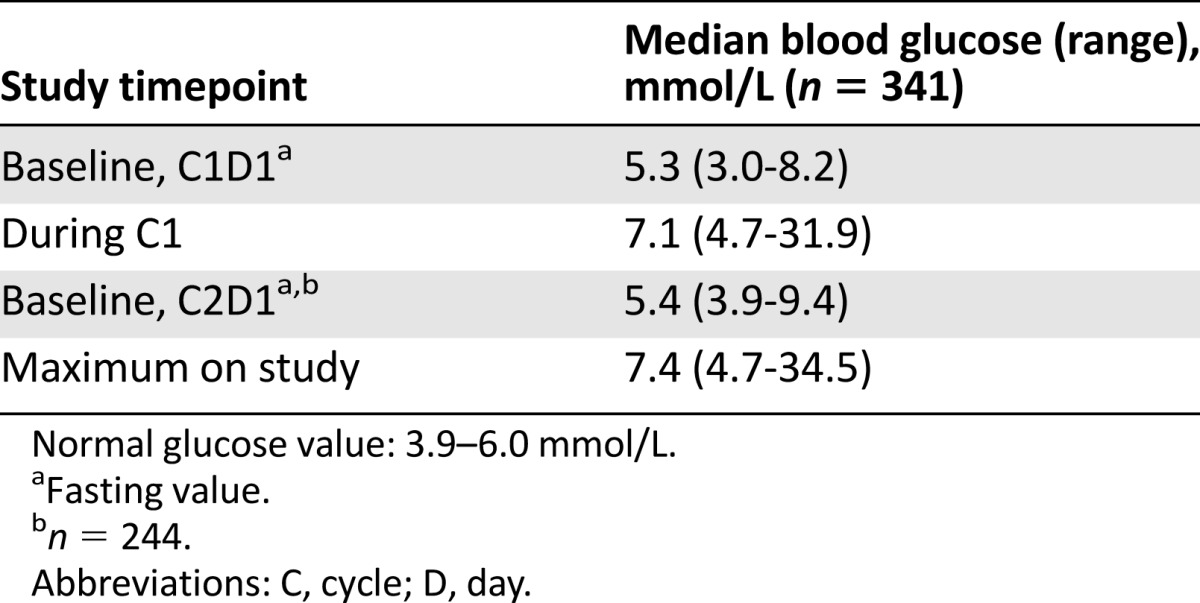
The median fasting glucose at cycle 1 day 1 (baseline, predose) was 5.3 mmol/L (range, 3.0–8.2 mmol/L). The median maximum blood glucose in cycle 1 was 7.1 mmol/L (range, 4.7–31.9 mmol/L). Of the 341 patients, 244 patients went on to receive 2 or more cycles of treatment. The median fasting glucose (predose) at cycle 2 day 1 was 5.4 mmol/L (range, 3.9–9.4 mmol/L), indicating that a rise in blood glucose level, if any, was transient. The median maximum blood glucose in cycle 2 was 7.4 mmol/L (range, 4.7–34.5 mmol/L) (Table 2).
Table 2.
Median blood glucose levels during study
Predictive Factors for Grade ≥3 Hyperglycemia
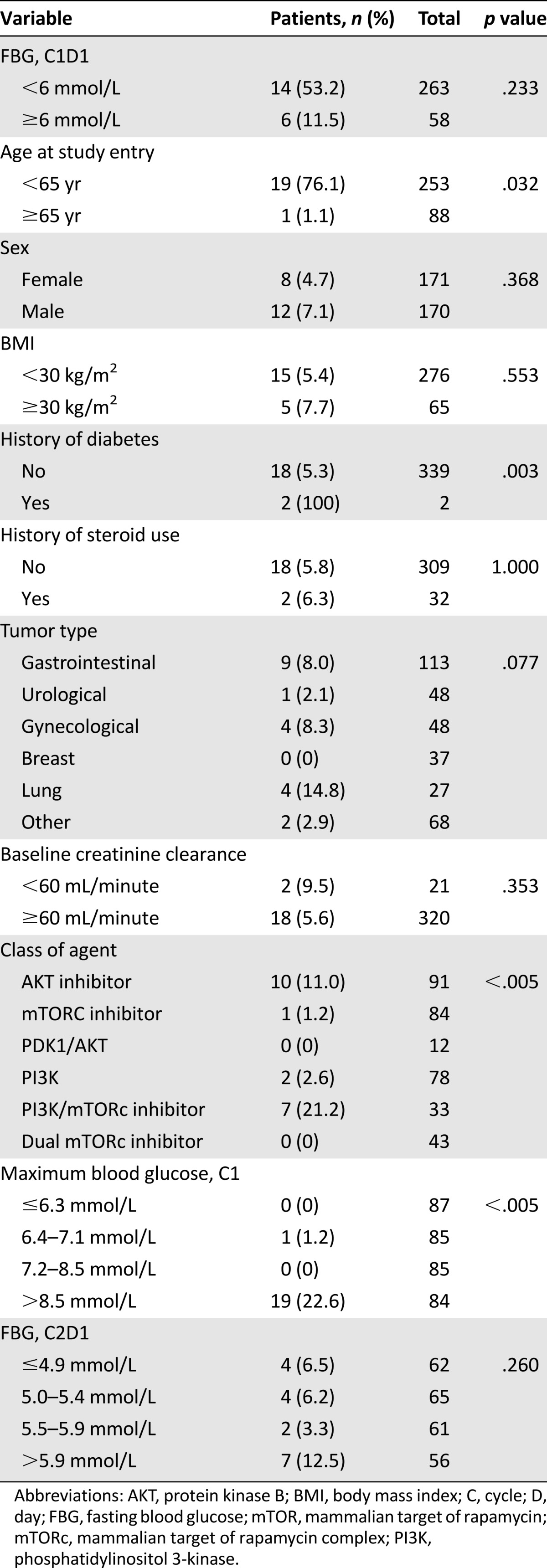
Chi-square analysis indicated that age <65 years (p = .032), history of diabetes mellitus (p = .003), maximum blood glucose >8.5 mmol/L in C1, and treatment with an AKT or dual PI3K/mTOR inhibitor (p < .005) predicted the occurrence of grade 3 hyperglycemia (Table 3). AST, GGT, and HbA1c at baseline did not predict the occurrence of grade ≥3 hyperglycemia (p > .1 for all factors).
Table 3.
Chi-square analysis: predictive factors for grade ≥3 hyperglycemia
Discussion
The PI3K/AKT/mTOR pathway is deregulated in most patients with cancer and is therefore a relevant therapeutic target. Many agents are in the early phase of clinical development, as single agents and in combination. Much is expected of them, and they do hold promise as potential therapeutic agents; however, few have been fully reported on with respect to safety and activity. One of the key toxicities associated with the class is hyperglycemia. This side effect is relevant. If these agents do emerge as meaningful anticancer agents and are approved, their use outside of clinical trials in patients at risk for hyperglycemia may be affected. There are no clinical data to inform us about the actual risk for hyperglycemia in patients being treated with agents targeting this pathway. With the number of agents undergoing clinical testing, this is an opportune time to define the true risk for this side effect of PI3K/AKT/mTOR inhibitors and approach to management.
Our study analyzed data of 341 patients treated with PI3K, AKT, and mTOR inhibitors in 12 phase I clinical trials. Although most patients developed transient early-onset hyperglycemia, the incidence of clinically significant hyperglycemia (grade ≥3) was low at 5.7%. Just 4 cases of grade 2 hyperglycemia and all of those grade ≥3 required treatment. Oral hypoglycemics in the form of metformin was the most commonly used treatment, and insulin was infrequently needed. However, most patients in our series discontinued treatment because of disease progression at a relatively early stage; therefore, the long-term course of hyperglycemia cannot be established from our data. Nevertheless, our experience was that early and regular glucose monitoring and early intervention achieve early and lasting control of hyperglycemia. The intervention threshold for treating hyperglycemia was determined by individual trial protocols, clinical features (such as symptomatic patients), and unit guidelines that included early intervention in patients found to have ≥grade 3 hyperglycemia during the first week of receiving the experimental agent. It is, however, worth noting that different trials had different monitoring requirements; therefore, clinical judgment in conjunction with trial guidelines was taken into account during treatment of these patients. Patients with grade 1 hyperglycemia were provided dietary advice; however, monitoring frequency again varied according to the trial protocols. Interestingly, we demonstrated in a separate series that agents targeting PI3K/AKT/mTOR pathway can be associated with higher risk for infections, which makes it all the more important to achieve good glycemic control to avoid such complications [17].
Diabetes mellitus can be associated with increased morbidity and mortality because of end-organ damage; certain cancers, such as breast and colorectal cancer, are also independently known to be associated with an increased risk for diabetes mellitus [18]. Our study, however, failed to establish a correlation between the development of significant hyperglycemia with a specific tumor type. Insulin resistance and the development of hyperglycemia are hallmarks of metabolic syndrome and are often related to abdominal obesity [19]. Body mass index, however, was not found to be predictive of the risk for grade ≥3 hyperglycemia. Elevated liver enzymes (ALT, AST, and GGT) have been found to negatively predict insulin sensitivity [20, 21]. Likewise, chronic kidney disease has also been known to be associated with insulin resistance and hyperglycemia in nondiabetic patients [22]. We therefore examined these factors in a univariate model to determine whether factors were independently predictive of the risk for grade ≥3 hyperglycemia. We did not find any correlation between grade ≥3 hyperglycemia and deranged liver or renal function. However, most patients in the present study were required to have satisfactory liver and renal function at baseline.
Combined use of fasting plasma glucose and HbA1c is a sensitive and specific screening tool for identifying individuals with diabetes and impaired glucose tolerance at an early stage [23]. However, neither parameter predicted for the risk for development of grade ≥3 hyperglycemia
Although most patients in our study did not have a history of diabetes mellitus because of exclusion criteria of the trials, this was still established to be an independent risk factor for grade ≥3 hyperglycemia. Patients with diabetes mellitus may represent an important proportion of patients with cancers because of an aging population; therefore, it will be inappropriate to exclude patients with diabetes mellitus from receiving these agents [24]. However, these patients will require careful monitoring and early intervention in collaboration with an endocrinologist to manage glucose levels.
Interestingly, the only patient in our study whose hyperglycemia failed to resolve despite pharmacological intervention requiring a dose reduction had received a selective p110α inhibitor. Some studies using PI3K inhibitors have not reported dramatic hyperglycemia, an effect that may be related to inhibition of the p110a isoform of PI3K: a pan class 1A inhibitor may lead to more severe glucose derangement than a selective p110α inhibitor, although the roles of the different p110 isoforms that they play in the metabolic effects of insulin still remains undetermined [24, 25]. The range of metabolic alterations observed with inhibitors of the PI3K/AKT/mTOR pathway differs, suggesting that kinase selectivity among various inhibitors may be responsible for the different levels of glucose and insulin elevation observed with these agents [25–27]. We found that patients receiving AKT or dual PI3K/mTOR inhibitors were at a higher risk for significant hyperglycemia. Inhibition of mTOR in addition to PI3K has been postulated to improve insulin sensitivity because the mTOR/S6 kinase pathway causes serine phosphorylation of insulin receptor substrate-1, which attenuates signaling [28]. Therefore, inhibiting mTOR/S6 kinase activity may reduce some of the insulin resistance caused by PI3K inhibition by relieving the inhibition of serine phosphorylation of insulin receptor substrate-1, allowing tyrosine phosphorylation of insulin receptor substrate-1 and activation of insulin signaling pathway [28]. However, in our series, we found that patients put on dual mTOR/PI3K have a higher risk for significant hyperglycemia. Because our numbers are small, this finding is exploratory and warrants further studies.
Given the limitations of a highly selected patient population treated in a controlled environment of a specialist unit within the context of phase I studies, along with the small numbers of patients in subanalyses involved, our findings may be considered as hypothesis-generating and will need to be validated in larger prospective clinical trials. Targeted therapies are associated with a higher incidence of metabolic toxicities in patients with insulin resistance via a metabolic syndrome phenotype or in patients with a family history of such a phenotype, but these data were not available because of the retrospective nature of this analysis. Thus, this represents one of the limitations of this study; however, all appropriate data points were considered where possible. Moreover, different phase I compounds could be at various stages of their development, and as a result dose-dependent effects of these compounds can’t be determined with accuracy; however, all compounds are tested within a phase I study, which offers homogeneity in the patient population. The data generated from this study will be useful in informing oncologists about the risk factors that may warrant careful monitoring of patients treated with PI3K/AKT/mTOR inhibitors.
Conclusion
Patients aged <65 years who have a history of diabetes mellitus and are receiving an AKT or dual PI3K/mTOR inhibitor are more likely to develop significant hyperglycemia when treated with agents targeting the PI3K/AKT/mTOR pathway. Even given the small number of diabetic patients in our series, such patients may still require careful monitoring, and poorly controlled diabetes could be considered an exclusion criterion. These factors should be carefully considered and specialist consultation should be sought early to aid in clinical trial planning and management of metabolic adverse events that may result from treating patients on this pathway. Finally, our findings are reassuring, pending prospective validation, for physicians and industry researchers working scrupulously on phase I trials to develop these compounds further.
Acknowledgments
The Drug Development Unit of the Royal Marsden National Health Service Foundation Trust and the Institute of Cancer Research is supported by a program grant from Cancer Research U.K. Scientific Executive Board–Centre Award (Grant C347/A15403), a Cancer Research U.K. Quinquennial Award to the Cancer Therapeutics Unit (Grant C309/A11566), and an Experimental Cancer Medicine Centre Network award (joint initiative, Cancer Research U.K. and the U.K. Department of Health) (Program Grant C12540/A15573).
Author Contributions
Conception/Design: Khurum H. Khan, Mabel Wong, Daniel Morganstein, Lulama R. Molife
Provision of study material or patients: Udai Banerji, Lulama R. Molife
Collection and/or assembly of data: Khurum H. Khan, Mabel Wong, Karim Rihawi, Shankar Bodla, Lulama R. Molife
Data analysis and interpretation: Khurum H. Khan, Mabel Wong, Shankar Bodla, Lulama R. Molife
Manuscript writing: Khurum H. Khan, Mabel Wong, Shankar Bodla, Lulama R. Molife
Final approval of manuscript: Khurum H. Khan, Mabel Wong, Karim Rihawi, Shankar Bodla, Daniel Morganstein, Udai Banerji, Lulama R. Molife
Disclosures
Daniel Morganstein: Janssen, Merck, Otsuka, Sanofi (H); Udai Banerji: Astex Pharmaceuticals, Vernalis, Karus Therapeutics (C/A). The other authors indicated no potential conflicts of interest.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 3.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 4.Asano T, Fujishiro M, Kushiyama A, et al. Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol Pharm Bull. 2007;30:1610–1616. doi: 10.1248/bpb.30.1610. [DOI] [PubMed] [Google Scholar]
- 5.Chen XW, Leto D, Xiong T, et al. A Ral GAP complex links PI 3-kinase/Akt signaling to RalA activation in insulin action. Mol Biol Cell. 2011;22:141–152. doi: 10.1091/mbc.E10-08-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biddinger SB, Kahn CR. From mice to men: Insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 7.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 8.George S, Rochford JJ, Wolfrum C, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan KH, Yap TA, Yan L, et al. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J Cancer. 2013;32:253–265. doi: 10.5732/cjc.013.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, Oudard S, et al. RECORD-1 Study Group Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 11.Hudes G, Carducci M, Tomczak P, et al. Global ARCC Trial Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 12.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: A phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 15.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget. 2010;1:530–543. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 17.Rafii S, Roda D, Geuna E, et al. Higher risk of infections with PI3K-AKT-mTOR pathway inhibitors in patients with advanced solid tumors on phase I clinical trials. Clin Cancer Res. 2015;21:1869–1876. doi: 10.1158/1078-0432.CCR-14-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strickler HD, Wylie-Rosett J, Rohan T, et al. The relation of type 2 diabetes and cancer. Diabetes Technol Ther. 2001;3:263–274. doi: 10.1089/152091501300209633. [DOI] [PubMed] [Google Scholar]
- 19.Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br J Dermatol. 2013;169:266–282. doi: 10.1111/bjd.12355. [DOI] [PubMed] [Google Scholar]
- 20.Onitilo AA, Stankowski RV, Berg RL, et al. Type 2 diabetes mellitus, glycemic control, and cancer risk. Eur J Cancer Prev. 2014;23:134–140. doi: 10.1097/CEJ.0b013e3283656394. [DOI] [PubMed] [Google Scholar]
- 21.Gray B, Muhlhausler BS, Davies PS, et al. Liver enzymes but not free fatty acid levels predict markers of insulin sensitivity in overweight and obese, nondiabetic adults. Nutr Res. 2013;33:781–788. doi: 10.1016/j.nutres.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Bagby SP. Obesity-initiated metabolic syndrome and the kidney: A recipe for chronic kidney disease? J Am Soc Nephrol. 2004;15:2775–2791. doi: 10.1097/01.ASN.0000141965.28037.EE. [DOI] [PubMed] [Google Scholar]
- 23.Jarvandi S, Davidson NO, Schootman M. Increased risk of colorectal cancer in type 2 diabetes is independent of diet quality. PLoS One. 2013;8:e74616. doi: 10.1371/journal.pone.0074616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busaidy NL, Farooki A, Dowlati A, et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol. 2012;30:2919–2928. doi: 10.1200/JCO.2011.39.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sopasakis VR, Liu P, Suzuki R, et al. Specific roles of the p110alpha isoform of phosphatidylinsositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab. 2010;11:220–230. doi: 10.1016/j.cmet.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia S, Liu Z, Zhang S, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ihle NT, Paine-Murrieta G, Berggren MI, et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]


