This review summarizes the development of vincristine sulfate liposome injection, a new formulation of vincristine. The pharmacokinetics of liposomal drug delivery are examined, the limitations and advantages of conventional and liposomal vincristine are compared, and the use of vincristine sulfate liposome injection in clinical trials and case studies is included.
Keywords: Liposome, Marqibo, Pharmacokinetics, Vincristine, Vincristine sulfate liposome injection
Abstract
Acute lymphoblastic leukemia (ALL) is a heterogeneous group of hematologic malignancies that arise from clonal proliferation of immature lymphoid cells in the bone marrow, peripheral blood, and other organs. The vinca alkaloid vincristine is a standard component of chemotherapy regimens used to treat ALL, because of its well-defined mechanism of action, demonstrated anticancer activity, and ability to be combined with other agents. However, the dosage of vincristine is frequently capped because of neurotoxicity concerns, and patients with large body surface areas are, therefore, almost always underdosed. Liposomal formulations have the ability to “passively” accumulate at sites of increased vasculature permeability and reduce the adverse effects of encapsulated relative to free drug. Vincristine sulfate liposome injection (VSLI) is a sphingomyelin/cholesterol-based liposome-encapsulated formulation that is delivered weekly in a 1-hour infusion. Based on the pharmacokinetics of the liposomal delivery system, vincristine is slowly released from the liposome and delivered into the tissues more efficiently than with the standard preparation, allowing a higher dose. This increase in therapeutic index from reduced toxicity is a valuable difference between the two formulations. VSLI is indicated for the treatment of adults with second or greater relapse and clinically advanced Philadelphia chromosome-negative ALL. For the first time, studies will be able to exploit the delivery of higher and uncapped doses of vincristine in randomized studies comparing first-line chemotherapy with standard vincristine versus VSLI in both ALL and lymphoma to determine whether VSLI is superior to conventional vincristine.
Implications for Practice:
This review summarizes the development of vincristine sulfate liposome injection, a new formulation of vincristine. The pharmacokinetics of liposomal drug delivery are examined, the limitations and advantages of conventional and liposomal vincristine are compared, and the use of vincristine sulfate liposome injection in clinical trials and case studies is included. Clinicians will be informed of a new chemotherapy agent that is indicated for the treatment of adults with Philadelphia chromosome-negative acute lymphoblastic leukemia, whose disease has relapsed two or more times or whose leukemia has progressed after two or more regimens of antileukemia therapy.
Introduction: The Treatment of Relapsed ALL
Adult acute lymphoblastic leukemia (ALL) is an uncommon hematological malignancy, accounting in adults for approximately 20% of new acute leukemias [1]. The proliferation and accumulation of blast cells in the marrow result in suppression of normal hematopoiesis and subsequent anemia, thrombocytopenia, and neutropenia. Extramedullary accumulations of lymphoblasts may also occur in various other sites, especially the meninges, gonads, thymus, liver, spleen, or lymph nodes [2]. Major cytogenetic and molecular genetic abnormalities seen in ALL include gene mutations, hyperdiploidy (>50 chromosomes), hypodiploidy (<44 chromosomes), and chromosomal translocations, of which t(12;21)(p13;q22) encoding ETV6-RUNX1 fusion gene is the most common in children, but very rarely seen in adults [3]. The most common chromosomal translocation in adults is the Philadelphia (Ph) chromosome—t(9;22) (Ph+ ALL)—resulting in the BCR-ABL1 fusion gene. Chromosomal rearrangements are significantly associated with response to chemotherapy (CT) and are used in the classification and risk stratification of patients with ALL [2, 4, 5]. Ph+ ALL has a poorer prognosis than Ph−, and the t(12;21) subtype of ALL has a very favorable prognosis, resulting in a higher rate of biologically favorable forms of ALL in children [1, 6–9].
Approximately 6,250 new cases of ALL were estimated to occur in the U.S. in 2015, accounting for 1,450 deaths [10]. Despite a high complete remission (CR) rate in adults, relapses are common [11]. In contrast to children with ALL, for whom cure approaches 90%, fewer than 50% of adults remain in remission, even with hematopoietic stem cell transplantation (HSCT) [1], mostly because of relapse. For older adults aged 40–59 years or 60–69 years with ALL, 5-year overall survival (OS) rates in the U.S. are 24% and 18%, respectively, despite aggressive first-line treatment [11, 12].
Currently, there is no uniformly accepted standard salvage treatment for relapsed ALL. The only potential curative approach is allogeneic HSTC after achieving a CR [13]. In a large retrospective summary of patients in first relapse treated by a variety of regimens, the CR rate was 31%, and overall survival was 5 months [13]. In a more recent study, the Programa Espanol de Tratamiento en Hematologia study, the median survival after relapse was 4.5 months [14], and in the Medical Research Council UKALL12/Eastern Cooperative Oncology Group 2993, the median overall survival after relapse was 6 months [15].
The most reliable predictor of treatment outcome after relapse is the duration of first remission (CR1), with better OS in patients with longer CR1 (>1 year), compared with patients with shorter CR1 [13–20]. A retrospective study performed in Japan of 332 patients with relapsed Ph− ALL aged 16–65 years showed that Allo-HSCT in CR1 did not influence the OS after the first relapse; at 5 years, for patients who received chemotherapy alone, OS was 16.3%, and OS was 10.6% in patients who received Allo-HSCT [19]. Among 270 patients who relapsed after CT alone in CR1, 52.5% achieved a second complete remission (CR2) after salvage CT, of whom 62 subsequently underwent Allo-HSCT. OS from CR2 was significantly better in patients who underwent Allo-HSCT in CR2 than in those who did not (74% vs. 50% at 1 year and 44% vs. 11% at 5 years, respectively) [19].
In the U.S., approximately 1,600 patients can be categorized as Ph− ALL in second or later relapse [20], and their outcome is worse. A retrospective analysis study reported a CR rate of 18% and median survival of 3 months in patients in second or subsequent relapse [21]; for patients receiving a single agent in second relapse, the CR rate was 2%, and median survival was 1.9 months [22].
Given the current unfavorable treatment landscape for relapsed Ph− ALL, for which no standard of care exists [15], novel, more effective agents are obviously needed [23]. Vincristine encased within a liposome—a drug delivery vehicle composed of material similar to that of cell membranes—is one such new approach that holds promise for improving the outcome of ALL [3]. Vincristine sulfate liposome injection (VSLI; Marqibo, Spectrum Pharmaceuticals, Inc., Henderson, NV, http://www.sppirx.com)—as a single agent—was approved in the U.S. under the Food and Drug Administration’s accelerated approval program in August 2012 for the treatment of Ph− ALL patients in second or greater relapse or whose disease progressed after two or more antileukemia therapies [24]. Vincristine sulfate liposome injection, vincristine administered in a liposomal package, allows for a safer delivery of a higher dose. The well-known toxicity profile of vincristine from decades of clinical use prompted the approval of the drug, based on the results from a relatively small phase II clinical trial.
Vincristine in the Treatment of ALL
Vincristine (VCR), an alkaloid obtained from the periwinkle plant, has been in clinical use as an anticancer agent for approximately 50 years and is a standard component in every combination CT regimen for ALL and other lymphoid malignancies. There is no standard front-line treatment for adult ALL patients. The two most commonly used are the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) and different variants of the Berlin–Frankfurt–Münster model, and although they are totally different regimens, their long-term survival rates are 35%–40% [25–29]. More recently, pediatric approaches are being adopted in young adults with improved outcomes. However, the importance of VCR in ALL is that, regardless of the chemotherapy combination, all regimens in all ages always include multiple doses of VCR.
The mechanism of action of VCR is related to the inhibition of microtubule formation in mitotic spindle, resulting in an arrest of dividing cells at the metaphase stage. VCR-induced abnormal microtubule formation inhibits cellular replication, ultimately causing cell death [29]. The efficacy of VCR increases with time of exposure and fraction of cells in mitosis [30]. Although VCR is a potent antineoplastic, its clinical use is limited by unpredictable pharmacologic characteristics (e.g., wide interpatient variation in half-life, volume of distribution, and clearance), a narrow therapeutic index, and, particularly, neurotoxicity [31–34]. Vincristine-induced peripheral neuropathy (VIPN) is characterized by progressive sensory, motor, and autonomic nerve damage, commonly manifesting as reduced motility of the intestines, resulting in constipation. Peripheral neuropathy is predominantly sensory in nature. Early symptoms include numbness and tingling of the hands and feet, accompanied by loss of deep tendon reflexes. In its more severe form, muscle weakness develops, which is more marked in distal muscles of the hands and feet. Other manifestations of neurotoxicity include muscle cramps, ocular palsies, hoarseness, and autonomic neuropathy in the form of postural hypotension and atony of the urinary bladder. The neurotoxicity of VCR is dose-related and cumulative; difficulty walking, paresthesias, and muscle wasting can persist for as long as VCR treatment is continued [35–37].
The neurotoxic effects of VCR significantly impair the use of higher doses in the treatment of ALL [13, 31]. The high affinity of VCR to both mitotic and neuronal microtubules makes it difficult to prevent neurotoxicity without compromising efficacy [33]. The usual dose of VCR for adult ALL patients is 1.4 mg/m2, but the dose is generally capped at 2 mg to prevent severe VIPN. However, VIPN is common, and neuropathic symptoms may appear even with dose capping and only after a few doses [35, 38]. Capping of the VCR dose at 2 mg results in underdosing in ALL patients with body surface areas >1.42 mg/m2. Because the efficacy, dosing, and neurotoxicity related to VCR are linked, patients with a body surface area >1.4 mg/m2 (i.e., almost all adults and some children) receive a suboptimal dose [38, 39]. Of note, studies performed more than 20 years ago reported conflicting results on the impact of capping versus noncapping on outcome [40–42]. Despite this, no recent studies addressed this question, and dose capping remains an almost universally accepted practice to curtail toxicity. Because the half-life of free VCR in serum is a few minutes, continuous infusion has been used as an alternate method to increase drug exposure, but is also complicated by significant neurotoxicity [43].
The high affinity of VCR to both mitotic and neuronal microtubules makes it difficult to prevent neurotoxicity without compromising efficacy.
Rationale for Vincristine Sulfate Liposome Injection
Liposomes are spherical nanoparticles composed of a phospholipid bilayer to encapsulate and deliver hydrophilic and lipophilic molecules. Their similarity to cell membranes and nonimmunogenicity allows liposomes to overcome potential barriers to many drugs and provide effective delivery to target tissues, such as tumors. Liposomes balance stability and time in the systemic circulation with bioavailability of the drug at the target site, permitting higher doses of drug to be administered [31, 44–46]. The physicochemical and pharmacokinetic properties of VCR include low solubility in aqueous solutions at physiologic pH in vitro, rapid initial plasma clearance, and extensive volume of distribution in vivo [32, 47]. These properties make VCR a likely candidate for liposome technology to improve its effectiveness and safety.
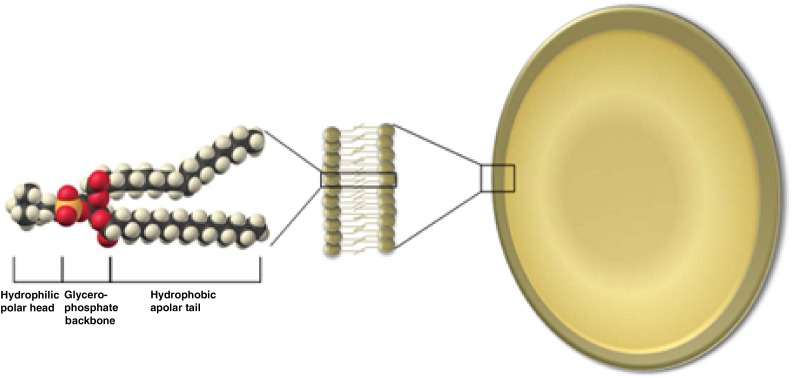
The poor therapeutic index of free VCR prompted the development of liposomal VCR [13, 31, 48, 49]. Vincristine sulfate liposome injection encapsulates VCR in a liposome composed of the phospholipid sphingomyelin and cholesterol (a drug-delivery vehicle similar to that of cell membranes) to improve tumor drug exposure by providing prolonged circulation of VCR in the blood [13, 50]. The phospholipid permits the formation of bilayer membranes, in which proteins, cofactors, or chemical compounds such as vincristine can be encapsulated (Fig. 1). The sphingosome-encapsulated technology of VSLI results in a liposome that is more rigid than conventional liposomes. Active VCR leaks out of the liposome slowly, and drug levels are maintained over prolonged periods. This improved pharmacokinetic profile—mimicking a continuous VCR infusion—may result in greater activity in rapidly dividing cancers [49]. Other factors that improve the therapeutic profile of VSLI compared with conventional VCR include higher doses, altered pharmacokinetics, selective deposition in tumor cells, or a combination of these differences [30].
Figure 1.
Phospholipid: A glycerophosphate backbone covalently bonded to a polar head group and two fatty acyl tails [3].
Vincristine sulfate liposome injection exhibits slower systemic release and better penetration into organs and bone marrow compared with standard VCR [45, 50–53]. At a dose of 2.25 mg/m2 per week, VSLI monotherapy has resulted in an overall response rate (ORR) of 35% and CR rate of 20% in adults with Ph− relapsed or refractory disease, with no new or unexpected toxicities observed [54]. (Of note, vincristine is never used to treat ALL as a single agent, but only as a component of combination CT; see details below.) Vincristine sulfate liposome injection addresses the long-unmet need for an additional option for Ph− ALL patients whose disease is unresponsive to available therapies [39, 55]. Once-per-week dosing makes VSLI a convenient treatment choice in the community setting. The drug does not suppress the bone marrow, and the frequency of adverse events (AEs) with VSLI (e.g., neutropenia, constipation, nausea, or pyrexia) is no higher than that observed with standard VCR, despite the larger dosage. Vincristine sulfate liposome injection is for i.v. use only, and may be fatal if given by other routes (intrathecal administration is fatal) [48].
The hematologic toxicity of VSLI 2.25 mg/m2 given every 7 days or every 14 days was recently assessed in 54 patients with metastatic uveal melanoma, a cancer not known to involve the bone marrow. Patients in the every-7-days cohort received a larger median cumulative exposure (22.6 vs. 17.7 mg) and almost double the median dose density (2.2 vs. 1.2 mg/m2 per week) of patients who received the drug every 14 days. Despite the more frequent exposure and greater dose density, patients in the every-7-days cohort had a lower median decrease from baseline in neutrophil count and a greater increase from baseline in platelet count versus those in the every-14-days cohort. Hematologic AEs were uncommon in this study and were mostly grade 1 or 2. No grade 4 hematologic AEs were reported, suggesting that VSLI could be well suited for use in combination regimens with myelosuppressive drugs [56].
Vincristine sulfate liposome injection exhibits slower systemic release and better penetration into organs and bone marrow compared with standard VCR. At a dose of 2.25 mg/m2 per week, VSLI monotherapy has resulted in an overall response rate of 35% and CR rate of 20% in adults with Ph− relapsed or refractory disease, with no new or unexpected toxicities observed.
Trial Evidence for VSLI in Relapsed ALL
Vincristine sulfate liposome injection demonstrated greater antitumor activity in vitro and in vivo compared with conventional VCR at equivalent milligram-per-kilogram doses in animal models. Vincristine sulfate liposome injection was more likely to be curative in murine systems against L1210 or P388 leukemia cell lines [47, 57, 58]. To optimize dose intensity and discern the tolerance of multiple doses in the salvage setting, a multicenter phase I trial of weekly dose-escalated VSLI (in combination with pulse dexamethasone) was conducted in 36 patients with relapsed/refractory ALL [59]. Doses of VSLI, ranging between 1.5 and 2.4 mg/m2, were infused over 1 hour. The most common toxicities included constipation (67%), fatigue (61%), peripheral neuropathy (55%), anemia (50%), and pyrexia (50%). Grade 3–4 hematologic (28%), neurologic (17%), and endocrine (11%) toxicities were recorded. The overall incidence of treatment-related peripheral neuropathy (55%) was similar across the treatment cohorts, despite dose escalation. The maximal tolerated dose was established at 2.25 mg/m2, based on dose-limiting toxicities of motor neuropathy, seizure, and hepatotoxicity at the 2.4 mg/m2-dose level. Four of seven patients who achieved a CR and one patient who experienced hematologic improvement subsequently underwent potentially curative HSCT [59].
Because VCR is excreted primarily by the liver, a recent study evaluated the pharmacokinetics of VSLI in patients with melanoma and impaired hepatic function [60]. The dose-adjusted maximum plasma concentration and area under the curve in patients with moderate hepatic impairment were comparable with those of patients with normal hepatic function. Vincristine sulfate liposome injection was generally well tolerated in all subjects participating in this trial.
Based on the medical need, the superiority of VSLI over standard VCR in nonclinical models, and encouraging activity attributed to VSLI in the phase I study [59], a multinational, pivotal, phase II, single-arm, open-label trial was conducted [54]. High-dose (2.25 mg/m2 [no dose capping]), once-per-week VSLI monotherapy was given to heavily pretreated adults with advanced, relapsed, and refractory B- or T-cell lineage Ph− ALL. All patients (n = 65) had previously been treated with standard VCR, and 77% had ongoing grade 1–2 neuropathy. Nearly half of patients enrolled in the trial (48%) had prior allogeneic HSCT, and >50% had received ≥3 lines of treatment. The OR rate among the 65 patients treated with VSLI was 35%, with 20% of patients having a CR or a CR with incomplete hematologic recovery (CRi). The median OS was 4.6 months, with 34% of patients living longer than 6 months. There were five long-term survivors (i.e., survival for longer than 1 year) and two patients who remain alive and could potentially represent cures. The median OS in responders (i.e., patients experiencing CR, CRi, partial remission, or bone marrow blast response) was 7.7 months, with 70% of patients alive for longer than 6 months [54].
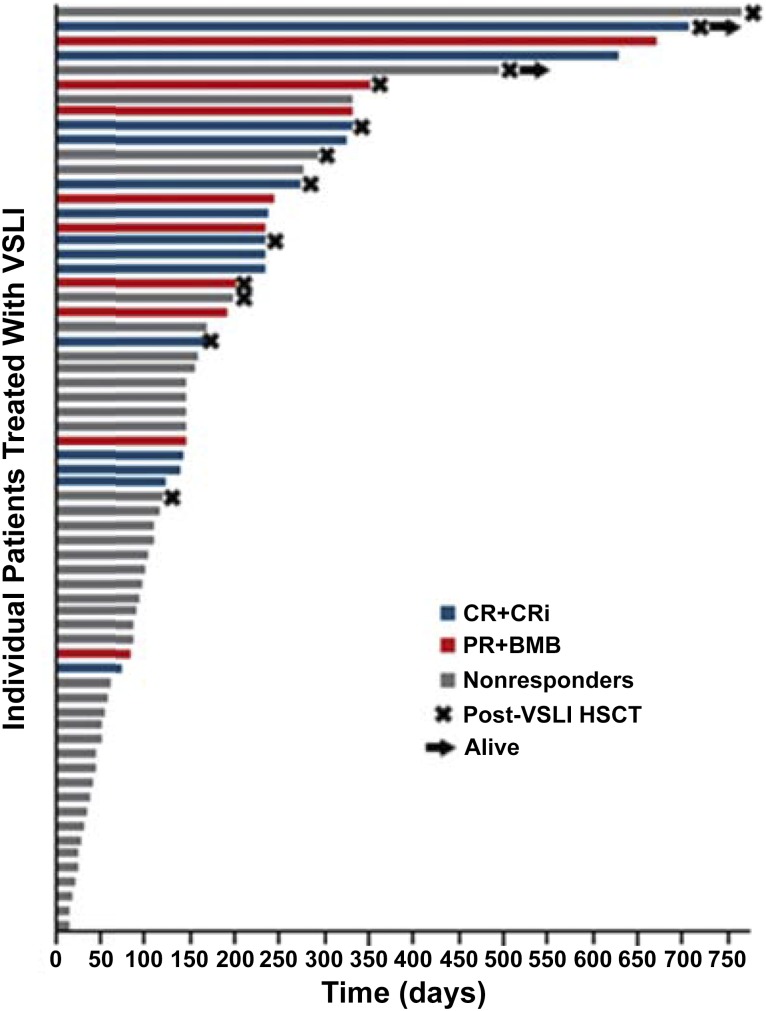
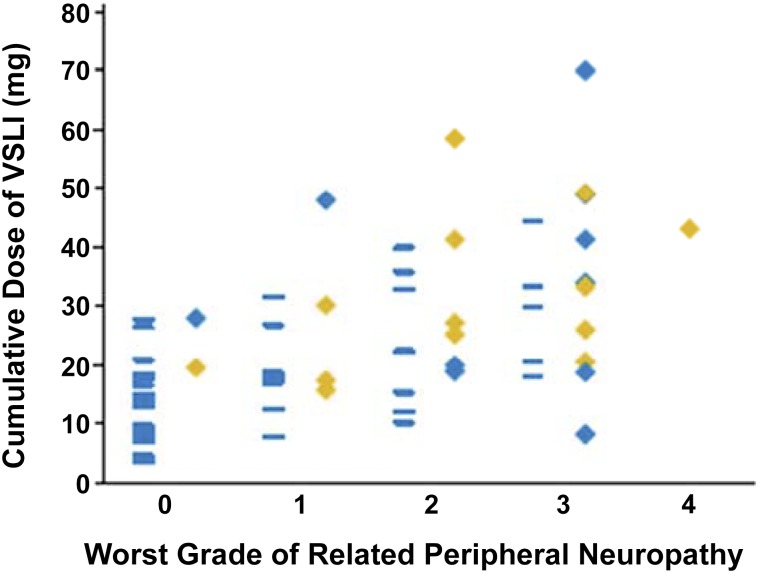
A total of 22% of patients who achieved a CR/CRi had more than five prior lines of therapy, and 26% had undergone allogeneic HSCT. Response to VSLI was seen in both B- and T-cell disease and in patients who did not experience significant neuropathy. Among those with a CR, 19% were successfully bridged to HSCT, with a median OS of 8.9 months (Fig. 2). Toxicity in this study was predictable, manageable, and comparable with that resulting from standard doses of free VCR, despite the delivery of large—normally unachievable—individual and cumulative doses of the drug (Fig. 3).
Figure 2.
Overall survival and HSCT among 65 patients treated with VSLI [54].
Abbreviations: BMB, bone marrow blast; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; HSCT, hematopoietic stem cell transplantation; PR, partial remission; VSLI, vincristine sulfate liposome injection.
Figure 3.
Relationship between cumulative VSLI exposure, the worst grade of related vincristine-induced peripheral neuropathy (VIPN) adverse events, and response [54]. Blue rectangles in the left-hand column above each grade of VIPN represent nonresponders; blue diamonds in the right-hand column represent patients who achieved a partial remission or bone marrow blast response; gold diamonds represent patients who achieved complete remission (CR) or CR with incomplete hematologic recovery (CRi). Responses, including CR or CRi, were achieved at each grade of related VIPN.
Abbreviation: VSLI, vincristine sulfate liposome injection.
The Clinical Impact of VSLI in the Overall Treatment of ALL
This pivotal trial served as the basis for accelerated U.S. approval of VSLI in August 2012 for the treatment of adult patients with Ph− ALL in second or greater relapse. In general—when using any single or combination chemotherapy—the outcome in this setting is dismal, with patients surviving for only a few weeks due to a very low CR rate of very short duration [21, 22]. In many instances, such patients are offered hospice care. However, with an overall response of 35%, relative low toxicity, and ease of administration, VSLI would be a convenient component of an overall palliative plan. In several of these ALL subjects, the pivotal clinical trial showed that VSLI monotherapy resulted in a more meaningful improvement, as well as durable responses, in clinical outcomes when used as a bridge to HSCT [54]. Vincristine sulfate liposome injection—which is nonmyelosuppressive, and, compared with steroids, less immunosuppressive—could also reduce or stabilize tumor burden while chimeric antigen receptor (CAR) T cells were being produced. In addition, it should be pointed out that, in contrast to the emerging immunotherapies (i.e., blinotumomab, CAR T cells, and inotuzomab ozogomycin), which only target B-lineage ALL, VSLI is also active in T-cell ALL.
Vincristine sulfate liposome injection has potential future applications in patients with ALL in which vincristine is used. As mentioned previously, vincristine is always given in combination with other chemotherapy agents. Such studies could determine whether substituting VSLI for standard VCR in combination front-line regimens will be beneficial, by delivering a higher dose of vincristine without the 2-mg cap, without more toxicity, and possibly diminishing the development of drug-resistant ALL [38]. One challenge of such studies is selecting the multiagent CT “backbone’” regimen, because there is no agreed-upon approach for front-line adult ALL, as well as the more the recent preference of using a pediatric regimen in young adults. For example, a phase II study is now recruiting subjects at the MD Anderson Cancer Center in Texas using intensive CT (i.e., hyperfractionated cyclophosphamide/VSLI/ doxorubicin/dexamethasone ± rituximab) to assess the CR duration, toxicity, and OS of newly diagnosed ALL patients versus hyper-CVAD (the same CT with standard vincristine) [61]. However, clinical trials replacing vincristine with VSLI in different widely used or pediatric CT backbones may yield different efficacy and toxicity outcomes. An attractive design for a clinical trial comparing vincristine to VSLI may be during maintenance therapy, in which monthly vincristine is always included for 2–3 years, with endpoints of reducing neuropathy and perhaps improving overall survival. However, a phase III trial will require large cohorts of patients. In the relapse setting, an active phase II study is expanding the clinical utility of VSLI for patients with ALL by testing bortezomib in combination with intensive reinduction CT (including VSLI) for patients 1–31 years old [62].
Case Studies
Successfully treating relapsed ALL is complex and difficult, and it is essential for oncologists to individualize care. Currently, the choice of a treatment for ALL is generally based on prior training and practice preferences, rather than a gold standard stratified by risk factors that are increasingly becoming more distinct. The following case studies illustrate important points in the management of adult patients with relapsed/refractory ALL, having different patient characteristics and at various points along the disease continuum.
Patient With T-Cell ALL in Second Relapse
A 44-year-old male was diagnosed with T-cell ALL. He entered a complete remission with hyper-CVAD CT, but relapsed 3 months after starting treatment. He was treated with the salvage combination of nelarabine/cyclophosphamide/etoposide and achieved a second CR. This was followed by allogeneic stem cell transplantation (SCT) from a matched sibling donor. He relapsed 6 months after the transplant with a white blood cell (WBC) count of 50,000 (90% blast) and bone marrow 50% blasts.
He has mild sensory grade 1 peripheral neuropathy and is transfusion-dependent. It would be prudent to consider one of the novel immunotherapy approaches: the first-in-class bispecific T-cell engager (BiTE) monoclonal antibody (mAb) blinatumomab, an experimental CAR T program (both of which target CD19), or the experimental mAb inotuzumab, which targets CD22. However, these approaches are limited to B-cell-lineage ALL and do not target malignant T cells, and therefore are inactive in this patient’s ALL subtype. Given the absence of standard effective combination CT, a palliative approach was discussed with the patient, which involved using an active agent with limited toxicity and no myelosuppression that may improve his quality of life (QOL) but not cure. Because his baseline neuropathy was mild, VSLI was chosen, which he received weekly at 2.25 mg/m2 (without a dose cap). After 3 weekly doses, his blood counts recovered, he became transfusion-free, and he obtained a CR. Four months later, he relapsed and died from disease progression.
Conclusions From Case 1
In this patient with T-cell ALL (T-ALL), VSLI provided a therapeutic option, given the limitation of promising immunotherapeutic approaches (blinatumomab, CAR T cells, and inotuzumab), which specifically target only B-lineage ALL, but are not active in T-ALL.
Patient Waiting for Treatment With Autologous Chimeric Antigen Receptor T Cells
A 34-year-old man was referred for increasing fatigue, petechiae on the lower extremities, pallor, and a palpable spleen tip. Laboratory studies showed a WBC count of 50,000 × 103 per μL (90% blasts), platelets 15,000 × 103 per μL, hemoglobin 7.1 g/dl, and elevated serum lactate dehydrogenase. Bone marrow examination revealed 70% blasts positive for terminal deoxynucleotidyltransferase, CD79a, CD10, CD19, CD22, CD33, CD34, and human leukocyte antigen-DR. A diagnosis of precursor B-cell ALL was made. Cytogenetic analysis was negative for the Philadelphia chromosome.
Hyper-CVAD CT as given in four cycles (each cycle as A+B) with central nervous system prophylaxis. He achieved a complete remission and continued onto maintenance treatment. Four months after completing hyper-CVAD, he relapsed. He was then treated with fludarabine + cytosine arabinoside + granulocyte colony-stimulating factor CT, and consideration was given to allogeneic SCT. While typing and donor search were being performed, the patient relapsed. His WBC count was 25,000 × 103 per μL (70% blasts). A bone marrow biopsy was performed and showed 70% lymphoid blasts. He was referred for an experimental treatment with autologous CAR T cells to a site that provides this experimental treatment, which is approximately a 4-hour commute. He signed the informed consent and was scheduled for apheresis for T-cell collection 2 weeks later. At that time, the cells would be sent to a laboratory for expansion and genetic modification, a process estimated to take an additional 5 weeks before the cells could be reinfused.
His WBC count was 45,000 × 103 per μL (90% blasts), and he needed to be treated for the next 7 weeks to prevent further disease progression and reduce his tumor burden. Steroids could not be given because they would interfere with the patient’s T-cell functions. The referring physician also would have liked to minimize toxicity and limit complications from myelosuppressive agents before the CAR T procedure, knowing that eligibility for CAR T treatment does not require a CR, but that minimizing tumor burden would be very beneficial. Vincristine sulfate liposome injection was given by his local oncologist, and the patient’s WBC count dropped to 5,000 × 103 per μL with 10% blasts, and the bone marrow showed 10% blasts. He was considered in partial remission and continued with weekly VSLI until he received the CAR T cells on schedule. Five days after CAR T cells were reinfused, he developed cytokine release syndrome with fever, hypotension, and hypoxia, which required intensive care unit support. He recovered, and 30 days later, a CR was documented.
Conclusions From Case 2
Chimeric antigen receptor T-cells have excellent activity in relapsed pre-B-ALL. In this patient, VSLI was a safe, relatively simple, and nonimmunosuppressive way of keeping the tumor from progressing while his CAR T cells were being prepared in the laboratory.
Patient With Severe Disease and Symptoms
A 70-year-old woman presented to the emergency room with fever and abdominal pain. Workup revealed an enlarged spleen and elevated WBC count with lymphoblasts. She was subsequently diagnosed with Philadelphia chromosome-negative ALL. Immunophenotyping was positive for CD10, CD19, and CD20, and the patient had a history of hypertension and mild chronic obstructive pulmonary disease. Left ventricular ejection fraction was 55%.
The patient began treatment with daunorubicin, vincristine, cyclophosphamide, and prednisone and achieved a CR that lasted 1 month. She was admitted to the hospital and started continuous infusion of blinatumumab. On day 2, she developed fever and hypotension, which are controlled with steroids. After 10 days, she was discharged. Two weeks after discharge, the patient experienced dyspnea on mild exertion and pedal edema; her LVEF was 30%, and her Eastern Cooperative Oncology Group performance status (PS) was 2. At the same time, her WBC count increased to 30,000 × 103 per μL (30% blasts). The patient and her family were concerned that she may not be able to tolerate more intensive treatment. Based on this information, palliative treatment was recommended, with weekly doses of VSLI. Together with treatment for her heart failure, her PS improved, and the blasts decreased. She developed grade 2 constipation, relieved with stool softeners. Because the blood count was stable and her QOL was satisfactory, VSLI was continued without performing a bone marrow examination. Five months later, her disease progressed, and she was sent to home hospice care, where she died.
Conclusions From Case 3
Given the dismal outcome of relapsed ALL with all treatment modalities, palliation and quality of life become the treatment goals. Vincristine sulfate liposome injection provided a useful, convenient, and often effective agent when included in an overall palliative treatment plan.
Conclusion
The key therapeutic goal in treating relapsed ALL is to rapidly induce a CR. However, there is no standard induction therapy after relapsed ALL, and most intensive salvage regimens have reached their limits of tolerability [63]. Because responses to single-agent therapy for relapsed Ph− ALL generally have been poor, integrating new agents in combination with established CT platforms is being explored. Although novel therapies are usually developed for use as single agents, many are likely to be used in combination CT regimens. In the absence of a standard ALL regimen, selecting an optimal CT regimen to serve as the backbone of treatment remains problematic [55].
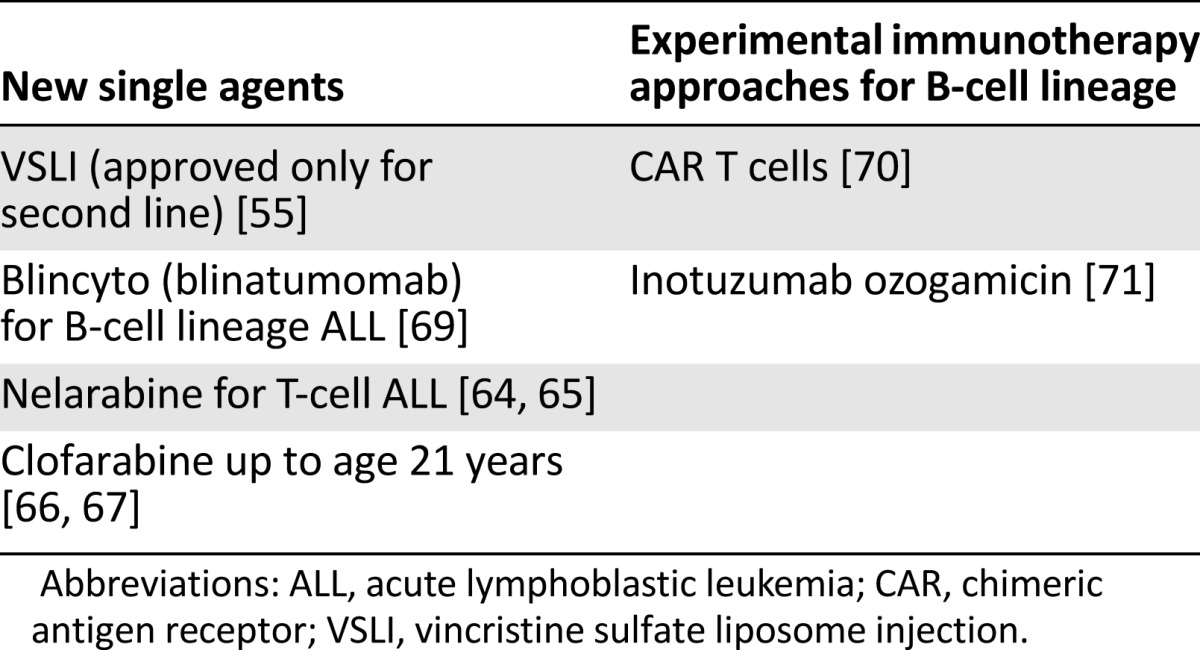
Current strategies focus on identifying new agents tailored to relapsed ALL and the development of these agents for clinical use. Given the toxicity associated with most CT regimens used to treat relapsed ALL, an ideal new agent would have a favorable safety profile. Table 1 shows new agents for relapsed ALL. An alternative to CT is immunotherapy limited to B-cell-lineage ALL, such as the first-in-class BiTE blinatumomab, CAR T cells, or the antibody-drug conjugate inotuzumab ozogamicin. Newer chemotherapeutics include nelarabine for T-cell ALL and clofarabine for patients younger than 21 years, both of which have been studied in combination with other drugs [64–67].
Table 1.
New agents for the treatment of relapsed ALL
Vincristine is a standard component of every ALL CT regimen, but is never used as a single agent. The usual dose of 1.4 mg/m2 is almost always capped at 2 mg because of neurotoxicity concerns. This de facto universal dosage cap has limited evidence to support its use, and many ALL patients receive subtherapeutic dosages [55]. Vincristine sulfate liposome injection—the sphingomyelin- and cholesterol-based nanoparticle formulation of VCR—is a new form designed to overcome dosing and pharmacokinetic limitations of standard VCR. The ability to deliver a higher dose of VCR—for the first time—has led to ongoing studies comparing CT with standard VCR versus CT with VSLI in first-line lymphoma and ALL [61].
Although the targeted therapeutics area (e.g., kinase inhibitors) is a critical avenue of research, liposomal drug delivery has become an established technology platform, gained considerable clinical acceptance, and is improving the therapeutic usefulness of combination CT [53, 68]. Liposomal formulations have the ability to “passively” accumulate at sites of increased vasculature permeability and reduce the AEs of the encapsulated drug relative to the free drug. This overall increase in therapeutic index from reduced toxicity is a welcome and valuable difference.
Acknowledgments
Medical writer Patrick McCarthy provided copyediting, editorial assistance, and production assistance.
Disclosures
Dan Douer: Pfizer, Amgen, Gilead, Sigma Tau, Spectrum (C/A), Gilead, Sigma Tau, Incyte, Bristol-Myers Squibb (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Lazarus HM, Advani AS. When, how, and what cell source for hematopoietic cell transplantation in first complete remission adult acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:382–388. doi: 10.1182/asheducation-2012.1.382. [DOI] [PubMed] [Google Scholar]
- 2.Pui C-H. Acute lymphoblastic leukemia. In: Kaushansky K, Lichtman MA, Seligsohn U, editors. Williams Hematology. 8th ed. New York, NY: McGraw-Hill; 2010. pp. 1409–1430. [Google Scholar]
- 3.Raj TA, Smith AM, Moore AS. Vincristine sulfate liposomal injection for acute lymphoblastic leukemia. Int J Nanomedicine. 2013;8:4361–4369. doi: 10.2147/IJN.S54657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huo FF, Liu X, Sun ZM, et al. Study on cytogenetic changes with relation to FAB classification in 397 patients with acute leukemias [in Chinese] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:6–10. [PubMed] [Google Scholar]
- 6.Chiaretti S, Zini G, Bassan R. Diagnosis and subclassification of acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis. 2014;6:e2014073. doi: 10.4084/MJHID.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raynaud SD, Dastugue N, Zoccola D, et al. Cytogenetic abnormalities associated with the t(12;21): A collaborative study of 169 children with t(12;21)-positive acute lymphoblastic leukemia. Leukemia. 1999;13:1325–1330. doi: 10.1038/sj.leu.2401506. [DOI] [PubMed] [Google Scholar]
- 8.Dombret H, Gabert J, Boiron J-M, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia—results of the prospective multicenter LALA-94 trial. Blood. 2002;100:2357–2366. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- 9.Shurtleff SA, Buijs A, Behm FG, et al. TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis. Leukemia. 1995;9:1985–1989. [PubMed] [Google Scholar]
- 10.American Cancer Society . Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 11.Pulte D, Jansen L, Gondos A, et al. Survival of adults with acute lymphoblastic leukemia in Germany and the United States. PLoS One. 2014;9:e85554. doi: 10.1371/journal.pone.0085554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson RA. Acute lymphoblastic leukemia: Older patients and newer drugs. Hematology Am Soc Hematol Educ Program. 2005:131–136. doi: 10.1182/asheducation-2005.1.131. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: Characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Oriol A, Vives S, Hernández-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95:589–596. doi: 10.3324/haematol.2009.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 16.Gökbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–1876. doi: 10.1182/blood-2011-09-377713. [DOI] [PubMed] [Google Scholar]
- 17.Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: A Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: Results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 19.Kako S, Kanamori H, Kobayashi N, et al. Outcome after first relapse in adult patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia. Br J Haematol. 2013;161:95–103. doi: 10.1111/bjh.12225. [DOI] [PubMed] [Google Scholar]
- Spectrum Pharmaceuticals launches Marqibo (vinCRIStine sulfate LIPOSOME injection) and ships first commercial orders [press release]. Henderson, NV: Spectrum Pharmaceuticals, September 3, 2013.
- 21.O’Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113:3186–3191. doi: 10.1002/cncr.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis T, Farag SS. Treating relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: Liposome-encapsulated vincristine. Int J Nanomedicine. 2013;8:3479–3488. doi: 10.2147/IJN.S47037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitcher OR, O’Brien S, Deitcher SR et al. Single-agent vincristine sulfate liposomes injection (Marqibo) compared to historical single-agent therapy for adults with advanced, relapsed and/or refractory Philadelphia chromosome negative acute lymphoblastic leukemia. Poster presented at the 53rd ASH Annual Meeting and Exposition, December 10–13, 2011; San Diego, CA [2592a]. [Google Scholar]
- FDA approves Marqibo to treat rare type of leukemia [press release]. Silver Spring, MD: U.S. Food and Drug Administration, August 9, 2012.
- 25.Larson RA, Dodge RK, Burns CP, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: Cancer and leukemia group B study 8811. Blood. 1995;85:2025–2037. [PubMed] [Google Scholar]
- 26.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: Results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 27.Stock W, Johnson JL, Stone RM, et al. Dose intensification of daunorubicin and cytarabine during treatment of adult acute lymphoblastic leukemia: Results of Cancer and Leukemia Group B Study 19802 [published correction appears in Cancer 2014;120:2222] Cancer. 2013;119:90–98. doi: 10.1002/cncr.27617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gökbuget N, Arnold R, Buechner T, et al. Intensification of induction and consolidation improves only subgroups of adult ALL: Analysis of 1200 patients in GMALL study 05/93. Blood. 2001;98:802a. [Google Scholar]
- 29.Jordan MA, Himes RH, Wilson L. Comparison of the effects of vinblastine, vincristine, vindesine, and vinepidine on microtubule dynamics and cell proliferation in vitro. Cancer Res. 1985;45:2741–2747. [PubMed] [Google Scholar]
- 30.Sarris AH, Hagemeister F, Romaguera J, et al. Liposomal vincristine in relapsed non-Hodgkin’s lymphomas: Early results of an ongoing phase II trial. Ann Oncol. 2000;11:69–72. doi: 10.1023/a:1008348010437. [DOI] [PubMed] [Google Scholar]
- 31.Pathak P, Hess R, Weiss MA. Liposomal vincristine for relapsed or refractory Ph-negative acute lymphoblastic leukemia: A review of literature. Ther Adv Hematol. 2014;5:18–24. doi: 10.1177/2040620713519016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gidding CE, Kellie SJ, Kamps WA, et al. Vincristine revisited. Crit Rev Oncol Hematol. 1999;29:267–287. doi: 10.1016/s1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- 33.Said R, Tsimberidou AM. Pharmacokinetic evaluation of vincristine for the treatment of lymphoid malignancies. Expert Opin Drug Metab Toxicol. 2014;10:483–494. doi: 10.1517/17425255.2014.885016. [DOI] [PubMed] [Google Scholar]
- 34.Dennison JB, Kulanthaivel P, Barbuch RJ, et al. Selective metabolism of vincristine in vitro by CYP3A5. Drug Metab Dispos. 2006;34:1317–1327. doi: 10.1124/dmd.106.009902. [DOI] [PubMed] [Google Scholar]
- 35.Talebian A, Goudarzi RM, Mohammadzadeh M, et al. Vincristine-induced cranial neuropathy. Iran J Child Neurol. 2014;8:66–68. [PMC free article] [PubMed] [Google Scholar]
- 36.Lash SC, Williams CP, Marsh CS, et al. Acute sixth-nerve palsy after vincristine therapy. J AAPOS. 2004;8:67–68. doi: 10.1016/j.jaapos.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Legha SS. Vincristine neurotoxicity. Pathophysiology and management. Med Toxicol. 1986;1:421–427. doi: 10.1007/BF03259853. [DOI] [PubMed] [Google Scholar]
- 38.Liesveld J, Asselin B. It’s ALL in the liposomes: Vincristine gets a new package. J Clin Oncol. 2013;31:657–659. doi: 10.1200/JCO.2012.46.8165. [editorial] [DOI] [PubMed] [Google Scholar]
- 39.Douer D. Advances in the treatment of relapsed/refractory ALL: Introduction to a case study compendium. Clin Adv Hematol Oncol. 2014;12(suppl 20):8–18. [PubMed] [Google Scholar]
- 40.Longo DL, Young RC, Wesley M, et al. Twenty years of MOPP therapy for Hodgkin’s disease. J Clin Oncol. 1986;4:1295–1306. doi: 10.1200/JCO.1986.4.9.1295. [DOI] [PubMed] [Google Scholar]
- 41.Longo DL, DeVita VT, Jr, Young RC. CHOP versus intensive regimens in non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:580–581; author reply 581–582. doi: 10.1056/NEJM199308193290817. [DOI] [PubMed] [Google Scholar]
- 42.Moore MR, Jones SE, Bull JM, et al. MOPP chemotherapy for advanced Hodgkin’s disease. Prognostic factors in 81 patients. Cancer. 1973;32:52–60. doi: 10.1002/1097-0142(197307)32:1<52::aid-cncr2820320107>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Jackson DV, Jr, Paschold EH, Spurr CL, et al. Treatment of advanced non-Hodgkin’s lymphoma with vincristine infusion. Cancer. 1984;53:2601–2606. doi: 10.1002/1097-0142(19840615)53:12<2601::aid-cncr2820531205>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 44.Markman JL, Rekechenetskiy A, Holler E, et al. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev. 2013;65:1866–1879. doi: 10.1016/j.addr.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverman JA, Deitcher SR. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71:555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mufamadi MS, Pillay V, Choonara YE, et al. A review on composite liposomal technologies for specialized drug delivery. J Drug Deliv. 2011;2011:939851. doi: 10.1155/2011/939851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer LD, Masin D, Nayar R, et al. Pharmacology of liposomal vincristine in mice bearing L1210 ascitic and B16/BL6 solid tumours. Br J Cancer. 1995;71:482–488. doi: 10.1038/bjc.1995.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqibo [package insert]. Henderson, NV: Spectrum Pharmaceuticals, Inc, 2013.
- 49.Liposomal vincristine receives orphan drug designation for treatment of ALL. Psychiatric Times February 1, 2007. Available at: http://www.psychiatrictimes.com/articles/liposomal-vincristine-receives-orphan-drug-designation-treatment-all. Accessed September 13, 2015.
- 50.Krishna R, Webb MS, St Onge G, et al. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J Pharmacol Exp Ther. 2001;298:1206–1212. [PubMed] [Google Scholar]
- 51.Kanter PM, Klaich GM, Bullard GA, et al. Liposome encapsulated vincristine: Preclinical toxicologic and pharmacologic comparison with free vincristine and empty liposomes in mice, rats and dogs. Anticancer Drugs. 1994;5:579–590. doi: 10.1097/00001813-199410000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Webb MS, Logan P, Kanter PM, et al. Preclinical pharmacology, toxicology and efficacy of sphingomyelin/cholesterol liposomal vincristine for therapeutic treatment of cancer. Cancer Chemother Pharmacol. 1998;42:461–470. doi: 10.1007/s002800050846. [DOI] [PubMed] [Google Scholar]
- 53.Ait-Oudhia S, Mager DE, Straubinger RM. Application of pharmacokinetic and pharmacodynamic analysis to the development of liposomal formulations for oncology. Pharmaceutics. 2014;6:137–174. doi: 10.3390/pharmaceutics6010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Brien S, Schiller G, Lister J, et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol. 2013;31:676–683. doi: 10.1200/JCO.2012.46.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Douer D. New developments in acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2014;12(suppl 12):18–22. [PubMed] [Google Scholar]
- 56.Deitcher OR, Glaspy J, Gonzalez R, et al. High-dose vincristine sulfate liposome injection (Marqibo) Is not associated with clinically meaningful hematologic toxicity. Clin Lymphoma Myeloma Leuk. 2014;14:197–202. doi: 10.1016/j.clml.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Mayer LD, Bally MB, Loughrey H, et al. Liposomal vincristine preparations which exhibit decreased drug toxicity and increased activity against murine L1210 and P388 tumors. Cancer Res. 1990;50:575–579. [PubMed] [Google Scholar]
- 58.Boman NL, Masin D, Mayer LD, et al. Liposomal vincristine which exhibits increased drug retention and increased circulation longevity cures mice bearing P388 tumors. Cancer Res. 1994;54:2830–2833. [PubMed] [Google Scholar]
- 59.Thomas DA, Kantarjian HM, Stock W, et al. Phase 1 multicenter study of vincristine sulfate liposomes injection and dexamethasone in adults with relapsed or refractory acute lymphoblastic leukemia. Cancer. 2009;115:5490–5498. doi: 10.1002/cncr.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bedikian AY, Silverman JA, Papadopoulos NE, et al. Pharmacokinetics and safety of Marqibo (vincristine sulfate liposomes injection) in cancer patients with impaired liver function. J Clin Pharmacol. 2011;51:1205–1212. doi: 10.1177/0091270010381499. [DOI] [PubMed] [Google Scholar]
- 61.U.S. National Institutes of Health; MD Anderson Cancer Center. Hyper-CVAD with liposomal vincristine in acute lymphoblastic leukemia. Available at: https://clinicaltrials.gov/ct2/show/NCT01319981. NLM Identifier: NCT01319981
- 62.U.S. National Institutes of Health; National Cancer Institute. Bortezomib and combination chemotherapy in treating young patients with relapsed acute lymphoblastic leukemia or lymphoblastic lymphoma. Available at: https://clinicaltrials.gov/ct2/show/NCT00873093. NLM Identifier: NCT00873093.
- 63.Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129–136. doi: 10.1182/asheducation-2012.1.129. [DOI] [PubMed] [Google Scholar]
- 64.DeAngelo DJ, Yu D, Johnson JL, et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood. 2007;109:5136–5142. doi: 10.1182/blood-2006-11-056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Commander LA, Seif AE, Insogna IG, et al. Salvage therapy with nelarabine, etoposide, and cyclophosphamide in relapsed/refractory paediatric T-cell lymphoblastic leukaemia and lymphoma. Br J Haematol. 2010;150:345–351. doi: 10.1111/j.1365-2141.2010.08236.x. [DOI] [PubMed] [Google Scholar]
- 66.Hijiya N, Thomson B, Isakoff MS, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118:6043–6049. doi: 10.1182/blood-2011-08-374710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shukla N, Kobos R, Renaud T, et al. Phase II trial of clofarabine with topotecan, vinorelbine, and thiotepa in pediatric patients with relapsed or refractory acute leukemia. Pediatr Blood Cancer. 2014;61:431–435. doi: 10.1002/pbc.24789. [DOI] [PubMed] [Google Scholar]
- 68.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 69.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 70.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224–225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohanian M, Kantarjiana H, Guya D, et al. Inotuzumab ozogamicin in B-cell acute lymphoblastic leukemias and non-Hodgkin's lymphomas. Expert Opin Biol Ther. 2015;15:601–611. doi: 10.1517/14712598.2015.1024652. [DOI] [PubMed] [Google Scholar]