This study evaluated the Cervista human papillomavirus (HPV), Hybrid Capture 2, and careHPV tests in diagnosing cervical intraepithelial neoplasia 2 or worse lesions in Xinjiang Uyghur women. The careHPV test was comparatively economical and efficient and may be more suitable for such resource-limited regions as Xinjiang.
Keywords: Cervista HPV test, careHPV, Hybrid Capture 2, High-risk human papillomavirus, Cervical cancer screening
Abstract
Objective.
The study aimed to evaluate the value of the Cervista human papillomavirus (HPV), Hybrid Capture 2 (HC-2), and careHPV tests in diagnosing cervical intraepithelial neoplasia grade 2 (CIN2) or worse in Xinjiang Uyghur women.
Methods.
Three high-risk human papillomavirus (HR-HPV) detection methods were studied on two different populations by different combination modes; a cytology specimen was obtained at the same time. An abnormal result of any test resulted in referral to colposcopy. Cervical biopsy was also performed.
Results.
In population 1, HR-HPV-positive rates were 57.6% and 54.3% as detected by HC-2 and Cervista, respectively; κ = 0.892 for consistency check of HC-2 and Cervista (p < .001). Area under the receiver operating characteristic curve (AUC) of HC-2 and Cervista was 0.744 (95% confidence interval [CI]: 0.664∼0.824, p < .001) and 0.786 (95% CI: 0.715∼0.858, p < .001), respectively, for diagnosing CIN2+. The A9 probe can detect six subtypes of HPV, including HPV16, HPV31, HPV33, HPV35, HPV52, and HPV58. If one or more of these subtypes are postitive, then A9 will be positive. A diagnosis of class A9 by the Cerevista test correlated with pathological interpretations (chi-square = 43.063, p < .001). In population 2, HR-HPV-positive rates were 40.1% and 34.4%, respectively, by HC-2 and careHPV; κ value was 0.779 for the two tests (p < .001). AUC of HC-2 was 0.895 (95% CI: 0.849∼0.940, p < .001), and careHPV was 0.841 (95% CI: 0.770∼0.899, p < .001) for diagnosing CIN2+.
Conclusion.
Good consistency was shown between HC-2 and Cervista tests and also between the HC-2 and careHPV tests. In the detection of CIN2+, Cervista showed better specificity than HC-2, and interpretation of the A9 subgroup showed high predicted value. The HC-2 test demonstrated better sensitivity than careHPV in detection of CIN2+. HC-2, Cervista, and careHPV may be applied as a triage test for visual inspection with acetic acid/Lugol’s iodine-positive or ThinPrep cytologic test-positive women. The careHPV test was comparatively economical and efficient and may be more suitable for resource-limited regions, such as Xinjiang.
Implications for Practice:
This study was designed to evaluate the value of the Cervista human papillomavirus (HPV), Hybrid Capture 2 (HC-2), and careHPV tests in diagnosing cervical intraepithelial neoplasia grade 2 (CIN2) or worse (CIN2+) lesions in Xinjiang Uyghur women. Results showed that there was good consistency between the HC-2 and Cervista tests, as well as between the HC-2 and careHPV tests. In detecting CIN2+, Cervista had higher specificity than HC-2, whereas analysis of the A9 subgroup had high predictive value. (The A9 probe can detect six subtypes of HPV, including HPV16, HPV31, HPV33, HPV35, HPV52, and HPV58. If one or more of these subtypes are postitive, then A9 will be positive.) The HC-2 test demonstrated better sensitivity than careHPV in detecting CIN2+. HC-2, Cervista, and careHPV could be applied as a triage test for visual inspection with acetic acid/Lugol’s iodine-positive or ThinPrep cytologic test-positive women. The careHPV test was comparatively economical and efficient and may be more suitable for resource-limited regions, such as Xinjiang.
Abstract
摘要
目的. 本研究旨在评价Cervista人乳头瘤病毒 (HPV) 检测、第二代杂交捕获技术 (HC-2) 和careHPV检测在诊断新疆维吾尔族妇女宫颈内瘤变2级 (CIN2) 或以上级别病变中的价值。
方法. 使用三种高危人乳头瘤病毒 (HR-HPV) 检测方法, 采用不同组合模式在两个不同人群中开展研究, 同时留取细胞学样本。发现任何检验异常结果即进行阴道镜检查。同时进行宫颈活检。
结果. 在人群1中, HC-2和Cervista检测的HR-HPV阳性率分别为57.6%和54.3% ; HC-2和Cervista的一致性检验κ=0.892 (P<0.001)。HC-2和Cervista诊断CIN2+的接受者操作特征曲线下面积 (AUC) 分别为0.744[95%置信区间 (CI) : 0.664∼0.824, P<0.001]和0.786 (95%CI : 0.715∼0.858, P<0.001)。A9探针能检测出6种亚型的 HPV, 包括HPV16、HPV31、HPV33、HPV35、HPV52和HPV58。如果其中≥1种亚型检测结果为阳性, 则A9检测结果为阳性。使用Cervista检测的A9组诊断与病理学解读具有相关性 (x2=43.063, P<0.001)。在人群2中, HC-2和careHPV检测的HR-HPV阳性率分别为40.1%和34.4%, 两种检测的κ=0.779 (P<0.001)。诊断CIN2+的AUC分别为HC-2 : 0.895 (95%CI : 0.849∼0.940, P<0.001), careHPV为0.841 (95%CI : 0.770∼0.899, P<0.001)。
结论. HC-2与Cervista检测以及HC-2与careHPV检测之间均具有良好的一致性。Cervista诊断CIN2+的特异性优于HC-2, 在A9亚组的解读中也显示出高预测值。HC-2检测CIN2+的敏感性高于careHPV。HC-2、Cervista和careHPV也许可以用于醋酸染色/Lugol碘着色肉眼观察法阳性或ThinPrep细胞学检测阳性妇女的分类检验。CareHPV检测相对较为经济高效, 可能更适用于新疆等资源有限的地区。The Oncologist 2016;21:825–831
对临床实践的提示: 本研究旨在评价Cervista人乳头瘤病毒 (HPV) 检测、第二代杂交捕获技术 (HC-2) 和careHPV检测在诊断新疆维吾尔族妇女宫颈内瘤样变2级 (CIN2) 或以上级别 (CIN2+) 病变中的应用。结果显示HC-2与Cervista检测之间以及HC-2与careHPV检测之间均有较好的一致性。Cervista检测CIN2+的特异性高于HC-2, 而在A9亚组分析中具有高预测值。(A9探针能检测出6种HPV亚型, 包括HPV16、HPV31、HPV33、HPV35、HPV52和HPV58。如其中≥1种亚型检测结果为阳性, 则A9检测结果为阳性。) HC-2检测CIN2+的病变的敏感性高于careHPV。HC-2、Cervista和careHPV也许可以作为醋酸染色/Lugol碘着色肉眼观察法阳性或ThinPrep细胞学检测阳性妇女的分类检验。CareHPV检测相对较为经济高效, 可能更适用于新疆等资源有限地区。
Introduction
Cervical cancer has rapidly gained global attention as a worldwide health concern [1]. The incidence and mortality rates of cervical cancer are highest in developing countries [2]. Remarkably, this disease has declined over the past 50 years because of wider implementation of cytological screening. However, both false-positive and false-negative interpretations have given rise to severe medical, financial, and mental issues, and even some legal actions. The reproducibility of cytology diagnoses has been questioned, because varying cytopathologists interpret cervical samples differently based on their respective levels of expertise and experience, often leading to considerable discrepancies in diagnosis [3]. A plethora of evidence indicates that high-risk human papillomaviruses (HR-HPVs) are important factors in cervical cancer [4–6]. In comparison with traditional cytology tests, the HR-HPV test has demonstrated enhanced clinical sensitivity, indicating that the HR-HPV test has improved efficacy in cervical cancer screening [7–9].
Currently, second-generation Hybrid Capture 2 (HC-2; Qiagen Gaithersburg, Inc., Gaithersburg, MD, https://www.qiagen.com) is the most widely used clinical HR-HPV detection method. The HC-2 test is the first diagnostic kit certified for mass screening of HPV DNA by the U.S. Food and Drug Administration (FDA). It has also been approved for use by China’s State Food and Drug Administration (SFDA) as a test with high sensitivity and specificity. When detecting HR-HPV infections, HC-2 tests have been the gold standard for a variety of experiments. However, there are several defects with this method—including experimental expenses and the presence of cross-reactions—and recent literature details the advantages and disadvantages of this method [10–12]. In addition to the HC-2 test, the Cervista test was approved by the FDA as a clinical application testing platform in March 2009 and was later approved by the SFDA. Internal quality-control techniques of the Cervista test reduced false-negative results. HPV infection diagnosis was classified into high-risk groups by using the Cervista test, which has improved prognostic significance. The careHPV test was approved by the SFDA in 2012 as a convenient, rapid HR-HPV detection method in comparison with the HC-2 test. The careHPV diagnosis has low environmental impact and convenient procedural training, takes little time, and is low-cost. The careHPV test can be implemented in low-resource settings as an economical method for screening for cervical cancer.
This study examines these three different HPV detection methods. The experimental data detail the consistency between HPV test results, the consistency of HPV interpretation and corresponding pathology results, and the sensitivity/specificity of the HPV test when predicting cervical lesions. This study aimed to make better use of HPV tests in cervical cancer screening by analyzing the statistical indicators and characteristics of different HPV detection methods.
Materials and Methods
Study Population
The study population was divided into two groups. Population 1 was composed of 151 cases from field cervical cancer screenings conducted from March 2014 to September 2014 in a county in Kashgar, Xinjiang. Cluster sampling was adopted to filter the screened population. Only women who were sexually active, between 21 and 60 years old, had no clinical symptoms of pregnancy, had an intact cervix, had no history of cervical cancer, and had no history of chemotherapy or radiotherapy were considered eligible for the research. Screening methods included visual inspection with acetic acid/Lugol’s iodine (VIA/VILI) and ThinPrep cytologic test (TCT), and women with positive results for either test were recruited to population 1. The Cervista HPV and HC-2 tests were performed while colposcopy was conducted.
Population 2 was composed of 212 cases randomly selected from opportunistic screenings for cervical cancer in the outpatient department of the Affiliated Tumor Hospital of Xinjiang Medical University from May 2014 to September 2014. Similar to population 1, VIA/VILI and TCT were utilized for the first visit. Women with positive results for either test received careHPV and HC-2 tests while colposcopy was performed. Inclusion criteria were the same as in population 1.
Methods
VIA/VILI procedure and specimen collection for both the field and hospital surveys were performed by two professional doctors from the Affiliated Tumor Hospital of Xinjiang Medical University. Colposcopy and three types of HPV test specimen collection were performed by an experienced doctor as detailed for each test. Qiagen specimen collection brushes were used for the careHPV and HC-2 tests; the Cervista test had its own brushes. Each HPV test utilized different collection media. After the cervix was fully exposed, the doctor wiped secretions from the surface and probed with specimen brush to a 1-cm depth into the cervical canal. The specimen brush was rotated 6–8 turns clockwise before being removed. Samples were preserved in liquid and kept at room temperature, and each vial was labeled with corresponding identification numbers. All HR-HPV DNA testing was performed according to the manufacturers’ instructions. The specimens were analyzed within 14 days of collection.
The second-generation Hybrid Capture test is able to detect 13 HPV types: types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. The HC-2 platform detects DNA through hybridization of an RNA probe cocktail with the target DNA. The DNA:RNA hybrid is captured by an anti-DNA:RNA antibody and detected by chemiluminescence. Some amount of cross-reactivity occurs between the probes with other HPV types (including type 66). HPV-positive hits were measured by the relative light unit (RLU)/cutoff (CO) ratio, which was derived by comparing the RLU emitted by the microplate reader to the CO value that was established as the minimum positive control value. An RLU/CO ratio ≥1.0 indicates a high probability of a positive HPV result, whereas an RLU/CO ratio <1.0 indicates a low probability.
The careHPV test is an accurate, rapid HR-HPV DNA detection method. This test comprises an in vitro nucleic acid hybridization assay coupled to signal amplification that uses microplate chemiluminescence for the qualitative detection of 14 kinds of HR-HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) in cervical specimens. Improving upon HC-2 technology, careHPV testing replaces microplates with monoclonal antibody-coated magnetic beads, which have a high affinity for RNA-DNA hybrids. The careHPV test shares the same HPV positive control with the HC-2 test.
The Cervista HPV-HR test (Hologic Inc., Madison, WI, http://www.hologic.com) is a qualitative test for the detection of DNA from 14 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). The test uses Invader chemistry, which is a signal amplification method for detecting specific nucleic acid sequences [13]. The Invader technology comprises two isothermal reactions: a primary reaction that occurs on the targeted DNA sequence and a secondary reaction that produces a fluorescent signal. The reagents for this test are provided as three oligonucleotide mixtures, which detect the 14 high-risk types of HPV as grouped based on phylogenetic relationships. Viral types that shared the most similar DNA sequences were detected from the same reaction. Oligonucleotides that bind to the human histone 2 gene (HIST2H2BE) were present in all three oligonucleotide mixtures. HIST2H2BE serves as an internal control that produces a semiquantitative signal from cellular DNA present in the sample. A positive result indicates that at least one of the 14 high-risk types is present in the DNA sample. The results of the Cervista test are classified as A9, A7, or A5/A6. The A9 probe can detect six subtypes of HPV, including HPV16, HPV31, HPV33, HPV35, HPV52, and HPV58. If one or more of these subtypes are postitive, then A9 will be positive. Similarly, A5/A6 includes HPV51, HPV56, and HPV61, and A7 includes HPV18, HPV39, HPV45, HPV59, and HPV68.
Cervical cell specimens were stored in ThinPrep collection medium, and the specimen vials were transported to the Third Affiliate Hospital of Xinjiang Medical University. Cytology slides were produced automatically and diagnosed by two cytology experts using the Bethesda diagnostic system. Atypical squamous cells of undetermined signification (ASC-US) and higher interpretations were regarded as positive. After collecting cervical cell specimens, colposcopy was performed to determine positive results from VIA/VILI or TCT, and direct biopsy or four-quadrant biopsies were taken. Endocervical curettage was performed as necessary.
In this study, histological diagnosis was used as the gold standard, and the interpretations were denoted as the cervical intraepithelial neoplasia (CIN) diagnostic system. Cervical biopsy slides were diagnosed by two fixed histopathological experts. Cervical intraepithelial neoplasia grade 2 (CIN2) or worse (CIN2+) was used as a reference standard.
Statistical Analysis
The consistency checks of careHPV, Cervista, and HC-2 tests were evaluated by κ value. Using CIN2+ as a reference, sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve (AUC) were calculated. Analyses were performed by using SPSS software (version 19.0; IBM, Armonk, NY, http://www.ibm.com). All p values <.05 (two-tailed) were considered to be statistically significant. The study design was approved by the Medical Ethics Committee of the Affiliated Tumor Hospital of Xinjiang Medical University.
Results
Analysis of Population 1
The positive rates for high-risk HPV using the Cervista and HC-2 tests were 54.3% (85 of 151) and 57.6% (87 of 151), respectively. Of the 151 cases, 90 were normal in pathology, whereas the number of cases with CIN1, CIN2, CIN3, and cervical cancer was 19, 16, 20, and 6, respectively. Positive results for HPV were determined by two tests in different pathological grades (Table 1 and Fig. 1).
Table 1.
HC-2 and Cervista test results in detecting CIN2+ and consistency test results using two methods
Figure 1.
Distribution of positive results of the HC-2 and Cervista tests with different pathological interpretation.
Abbreviations: CIN1, cervical intraepithelial neoplasia grade 1; CIN2, cervical intraepithelial neoplasia grade 2; CIN3, cervical intraepithelial neoplasia grade 3; HC-2, Hybrid Capture 2.
After analysis, the κ value of the Cervista versus HC-2 consistency test was 0.892 (p < .001). With CIN2+ as the reference standard, the sensitivity of the HC-2 and Cervista tests was 92.9% and 97.6%, respectively, whereas the specificity of the HC-2 and Cervista tests was 56.0% and 60.0%, respectively. The AUC of the Cervista and HC-2 methods were 0.786 (95% confidence interval [CI]: 0.715∼0.858, p < .001) and 0.744 (95% CI: 0.664∼0.824, p < .001), respectively. The larger AUC indicated a higher predictive value from the laboratory diagnosis (Fig. 2).
Figure 2.
ROC curve of the HC-2 and Cervista tests to detect women with cervical intraepithelial neoplasia grade 2+.
Abbreviations: HC-2, Hybrid Capture 2; HPV, human papillomavirus; ROC, receiver operating characteristic.
The positive results of the Cervista test are presented with the name of the HPV subgroups. The cases of each subgroup were counted among the various pathological interpretation groups, and the positive and negative rates were calculated. Positive rates from the A9 group featuring normal results yielded CIN1, CIN2, CIN3, and cervical cancer at 50.9%, 87.5%, 90.0%, and 83.3%, respectively. There was a statistically significant correlation based on the trend test (chi-square = 43.063, p < .001). Negative results in normal pathological results, CIN1, CIN2, CIN3, and cervical cancer were 67.8%, 21.1%, 0%, 0%, and 16.7%, respectively, and there was a statistically significant correlation based on the trend test (chi-square = 44.078, p < .001). There were no significant trends in the A5/A6 or A7 groups (Table 2).
Table 2.
Comparison of various proportions of HPV types based on different pathology interpretation classes
Analysis of Population 2
The positive rates of high-risk HPV using the careHPV and HC-2 tests were 34.4% (73 of 212) and 40.1% (85 of 212), respectively. After calculation, the κ value of the careHPV and HC-2 consistency tests was 0.779 (p < .001). A total of 120 cases were normal in pathology, and the number of cases of CIN1, CIN2, CIN3, and cervical cancer was 32, 21, 19, and 20, respectively. Positive results for HPV were determined by two tests in different pathological grades (Table 3 and Fig. 3).
Table 3.
HC-2 and careHPV test results in detecting CIN2+
Figure 3.
Distribution of positive results of the HC-2 and careHPV tests with different pathological interpretations.
Abbreviations: CIN1, cervical intraepithelial neoplasia grade 1; CIN2, cervical intraepithelial neoplasia grade 2; CIN3, cervical intraepithelial neoplasia grade 3; HC-2, Hybrid Capture 2.
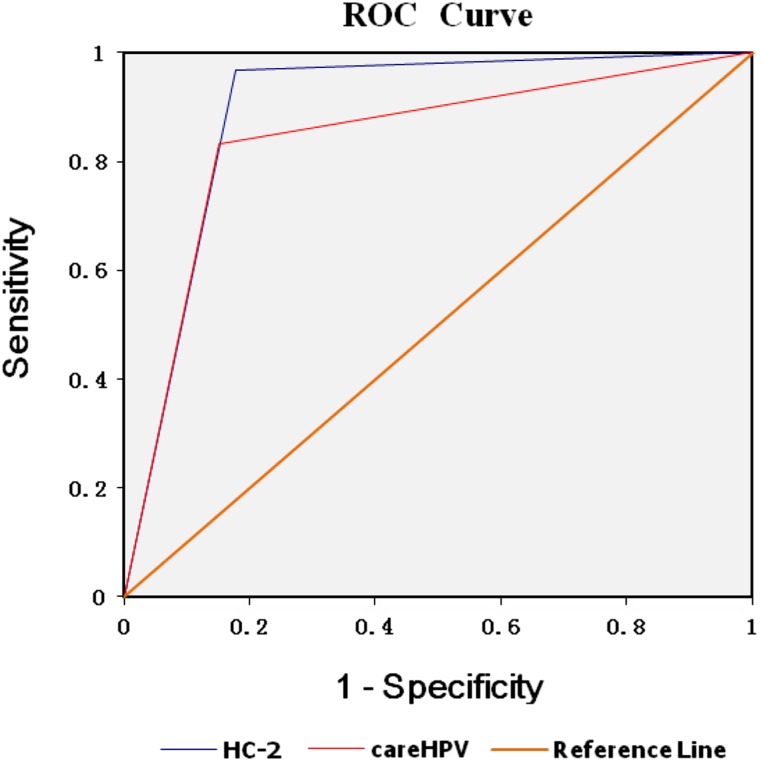
With CIN2+ as the reference standard, the sensitivity of the careHPV and HC-2 tests was 83.3% and 96.7%, respectively, whereas the specificity of the careHPV and HC-2 tests was 84.9% and 82.2%, respectively. The AUC of the careHPV and HC-2 methods were 0.841 (95% CI: 0.770∼0.899, p < .001) and 0.895 (95% CI: 0.849∼0.940, p < .001), respectively (Fig. 4).
Figure 4.
ROC curve of HC-2 and careHPV test to detect women with cervical intraepithelial neoplasia grade 2+.
Abbreviations: HC-2, Hybrid Capture 2; ROC, receiver operating characteristic.
Discussion
Significance of the HR-HPV Test in Cervical Cancer Screening
HR-HPV DNA testing is currently recommended by the American Society for Colposcopy and Cervical Pathology screening guidelines for two principal clinical situations. HR-HPV DNA testing can be beneficial when cytological findings are ambiguous or equivocal. HR-HPV DNA testing for evaluating patients with ASC-US dramatically decreases colposcopic referrals (∼1/2) without sacrificing screening sensitivity [14]. HR-HPV testing is useful for primary screening (“cotesting,” along with cytology) in patients aged 30 years or older. Women in this group with both normal cytological interpretations (i.e., negative for intraepithelial lesion or malignancy) and negative HR-HPV test results are at extremely low risk for developing cervical cancer over several years [15].
In the clinical setting, most HPV testing in the United States before 2009 was performed by using the HC-2 test, which was the only test approved by the FDA. As the first approved diagnostic kit with ideal sensitivity, specificity, and stability, HC-2 was the gold standard for diagnosing HPV infections in many experimental designs [15, 16].
In this study, HC-2 was also used as the gold standard for HPV infection. By using CIN2+ as the reference standard, the sensitivity of HC-2 was high (92.9% in population 1 and 96.7% in population 2), and the specificity was ideal (56.0% in population 1 and 82.2% in population 2). The AUC of the HC-2 method was 0.744 (95% CI: 0.664∼0.824, p < .001) in population 1 and 0.895 (95% CI: 0.857∼0.946, p < .001) in population 2. In conclusion, the HC-2 test demonstrates enhanced sensitivity in comparison with specificity when detecting CIN2+ lesions. This matches well with other reports [17], indicating an optimal sensitivity with the HC-2 test (96.3%; 95% CI: 94.9%–97.4%), together with a high specificity (86.4%; 95% CI: 83.8%–89.0%).
The positive rate for HPV was different in two populations (56.7% in population 1 and 40.1% in population 2) because of the different source of patients. Xinjiang Uyghur Autonomous Region is a less developed area located in west China, whereas Kashgar city (where the spot field screening was conducted) is a high cervical cancer incidence region in South Xinjiang [18]. Previous results demonstrated that the detection rate for cervical cancer in Kashgar was 340 of 100,000 (unpublished data), exceeding reports regarding other regions in mainland China [19]. Population 2 was collected from an outpatient department, and the patients were from many locations, including South Xinjiang, North Xinjiang, and East Xinjiang, whereas only South Xinjiang is considered to be a high incidence region. In addition, South Xinjiang is the farthest region from Urumqi City, where the research hospital is located, and the outpatient population thus mainly constituted women from the North and East. These data may indicate higher HPV infection rates in high-incidence regions than elsewhere. Thus far, few studies have examined HPV prevalence in different region of Xinjiang. Some reports have indicated that the interpretation of HC-2 tests varies between different populations, which may result from different HPV infection rates and cross-reaction [20, 21].
Comparison of the Cervista and HC-2 Tests
One limitation of the current FDA-approved HC-2 test is that it lacks internal standards to evaluate specimen adequacy or the presence of compounds that could interfere with the test. Without an internal control or standard, the laboratory and clinician cannot confirm that the negative HPV results indicate a lack of HR-HPV DNA, as opposed to hypocellularity of the samples, the presence of an inhibitor that halts the signal amplification reaction, or incorrect processing [22]. The 2006 American Society for Colposcopy and Cervical Pathology guidelines state that it is “unacceptable” for an HPV assay to test for nononcogenic HPV types in cervical cancer screening, because the false-positive HR-HPV rates (due to cross-reactivity with a nononcogenic HPV) may result in unnecessary colposcopy procedures [23]. A test that minimizes potential cross-reactivity would not only reduce unnecessary invasive procedures from being performed, but could also potentially reduce health care costs. The Cervista HPV-HR test did not cross-react with common low-risk HPV types and only nonspecifically interacted with two nononcogenic types (HPV 67 and 70), the clinical significance of which is currently unknown. Multiple studies have documented that the HC-2 test cross-reacts with at least 15 HPV types not specifically targeted by its probes, most of which are nononcogenic, resulting in a 10% false-positive rate [10, 24]. Another recent study demonstrated that the HC-2 test also has an additional 5% false-positive rate due to positive HR-HPV hits when no HPV DNA is present, based on an independent polymerase chain reaction test with DNA sequencing of the generated amplicons [11]. In each of these cases, if the false-positive HPV result occurred in conjunction with ASC-US cytology, the patient would be referred for an unnecessary colposcopy, as indicated by current standard of care guidelines. In detecting HPV infection in this study, there was consistency between the Cervista and HC-2 tests (κ = 0.892, p < .001). With CIN2+ as the reference standard, the sensitivity of the Cervista test (97.6%) was higher than the HC-2 test sensitivity (92.9%), and the specificity of the Cervista test (60.0%) was also higher than that of the HC-2 test (56%), whereas the AUC of the Cervista test (0.786; 95% CI: 0.715∼0.858, p < .001) also exceeds that of the HC-2 test (0.744 [95% CI: 0.664∼0.824, p < .001]). Therefore, there was reasonable consistency between the HC-2 and Cervista tests, whereas the Cervista test had greater sensitivity and better specificity than HC-2 test when detecting CIN2+.
When divided into pathological groups, the positive rates of the A9 group progressively increased with pathological grading, and the trend was statistically significant (chi-square = 43.063, p < .001). Negative rates progressively decreased with pathological grading, and the trend was statistically significant (chi-square = 44.078, p < .001). There was no significant trend test in the A5/A6 or A7 groups. These findings are in agreement with a previous study [25], which found that the sensitivity and specificity of the A9 group was 90.91% and 70.62%, respectively. The negative predictive value of the A9 group was 35.5%, whereas the positive predictive value was 97.67%. A positive result in the A9 group indicates infection with one or more types of HPV, including HPV 16, 31, or 33. One study in northeast Thailand [26] demonstrated that the prevalence of HPV 16 increased significantly with histological grade. The most common variant found was the Asian lineage (58.7%), followed by the European lineage (41.3%). The HPV 16 as lineages had a higher risk association in 73.9% of squamous cervical cancers and 57.1% of CIN2-3 lesions. Another study [27] found that the specificity of HPV 16/18 genotyping for predicting CIN2+ in women after a HPV+/Pap result was high, although the difference in CIN2+ risk in women with or without a positive HPV 16/18 result was insignificant. Keegan et al. [28] discovered that the most common HPV genotypes were HPV 16, 31, 33, 58, 42, 61, and 53, and that the most common high-risk HPV genotypes were HPV 16, 31, 33, 58, 18, 45, 59, 51, 56, and 39, with detection of multiple infections in 57.7% of all cases. HPV 16 was found to be the most common genotype across all grades of cytology, whereas HPV 18 was the eighth most common HPV genotype overall and the sixth most common HPV genotype (6.1%) in cytology specimens with confirmed CIN2+. The results from this study closely match the data from this previous work. Because the analysis of the A9 subgroup has good predictive value, the women who were diagnosed as positive in the A9 subgroup should be referred to colposcopy immediately and closely monitored.
careHPV Test Is an Acceptable Cervical Cancer Screening Method in Developing Countries
The careHPV test shows acceptable consistency with the HC-2 test in HPV infection diagnosis (κ = 0.779, p < .001). The sensitivity of the careHPV test (83.3%) was slightly lower than the HC-2 test (96.7%), whereas the specificity of the careHPV test (84.9%) was similar to the HC-2 test (82.2%), and the AUC of the careHPV test (0.841, 95% CI: 0.777∼0.905, p < .001) was less than the HC-2 test (0.895, 95% CI: 0.849∼0.940, p < .001). In this study, the careHPV test performed with enhanced specificity in detecting CIN2+ lesions. Reports from undeveloped countries [29–31] have demonstrated that the careHPV test is a feasible cervical cancer screening method, demonstrating good consistency with the HC-2 test and matching the results of this study. Another study from rural China indicated that the careHPV test reduced the mortality of tested patients when compared with the VIA/VILI method. A single careHPV test in a lifetime could reduce the incidence and mortality of cervical cancer (10% and 12%, respectively). Tests every 3, 5, or 10 years could reduce incidence of cervical cancer by 24%–28% and the mortality rate by 28%–54% [32].
The careHPV test is an easy, rapid, accurate, safe, and inexpensive HPV detection method that was developed based on the HC-2 test. Although careHPV and HC-2 HPV DNA testing share many of the same procedures—including setup, denaturation, hybridization, hybrid capture, hybrid detection, washing, signal amplification, and reading—some differences do exist. The careHPV test has low environmental impact, nontoxic reagents, increased safety, simple laboratory operator training, and relatively inexpensive cost in comparison with the HC-2 test [33]. The assay time for careHPV is less than 2.5 hours, compared with 5–6 hours for HC-2. The patients could be given their results in less than 1 day, shortening times for follow-up and treatment. Although the sensitivity and specificity were slightly lower than the HC-2 test, the careHPV has the many advantages detailed above, which makes it optimal for utilization in resource-poor areas. Because the SFDA has approved HPV detection reagents, the careHPV test is recommended as a second form of prevention of cervical cancer in undeveloped countries, which lack health care professionals and rigorous medical testing facilities.
Conclusion
The HC-2 and Cervista tests resulted in fairly consistent diagnoses, as did the HC-2 and careHPV tests. In detecting CIN2+ lesions, the Cervista test was both more sensitive and more specific than the HC-2 test, and an analysis of the A9 subgroup demonstrated good predicted values. The HC-2 test was more sensitive than the careHPV test when detecting CIN2+ lesions. HC-2, Cervista, and careHPV may be applied as a triage test for VIA/VILI- or TCT-positive women in Xinjiang for cervical cancer screening. Among the three tested methods, the careHPV test was the most economical and suitable for resource-limited regions, such as Xinjiang. Studies that examine a larger sample size are necessary to evaluate fully the value of different HPV methods in cervical cancer.
Acknowledgment
This study was supported by the National Natural Science Foundation of China Project Approval No. 81272335.
Author Contributions
Conception/Design: Gulixian Tuerxun, Guzhalinuer Abulizi
Provision of study material or patients: Ling Lu, Hua Li
Collection and/or assembly of data: Gulixian Tuerxun, Kailibinuer Aierken, Yujie Jiang, Axianguli Abulizi, Guzhanuer Abuduxikuer, Hua Li
Data analysis and interpretation: Awaguli Yukesaier, Kailibinuer Aierken, Patiman Mijiti, Yuanyuan Zhang
Manuscript writing: Awaguli Yukesaier, Ling Lu, Patiman Mijiti
Final approval of manuscript: Guzhalinuer Abulizi
Disclosures
The authors indicated no financial relationships.
References
- 1.Arbyn M, Raifu AO, Weiderpass E, et al. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer. 2009;45:2640–2648. doi: 10.1016/j.ejca.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Stoler MH, Schiffman M, Atypical Squamous Cells of Undetermined Significance-Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group Interobserver reproducibility of cervical cytologic and histologic interpretations: Realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–1505. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization and IARC Human PapillomavirusIARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Geneva, Switzerland: WHO Press, 200590. [Google Scholar]
- 6.Meijer CJ, Snijders PJ, Castle PE. Clinical utility of HPV genotyping. Gynecol Oncol. 2006;103:12–17. doi: 10.1016/j.ygyno.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 8.Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: Design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94–101. doi: 10.1002/ijc.20076. [DOI] [PubMed] [Google Scholar]
- 9.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: A randomised controlled trial. Lancet Oncol. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 10.Poljak M, Marin IJ, Seme K, et al. Hybrid Capture II HPV Test detects at least 15 human papillomavirus genotypes not included in its current high-risk probe cocktail. J Clin Virol. 2002;25(suppl 3):S89–S97. doi: 10.1016/s1386-6532(02)00187-7. [DOI] [PubMed] [Google Scholar]
- 11.Schutzbank TE, Jarvis C, Kahmann N, et al. Detection of high-risk papillomavirus DNA with commercial invader-technology-based analyte-specific reagents following automated extraction of DNA from cervical brushings in ThinPrep media. J Clin Microbiol. 2007;45:4067–4069. doi: 10.1128/JCM.01833-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle PE, Solomon D, Wheeler CM, et al. Human papillomavirus genotype specificity of hybrid capture 2. J Clin Microbiol. 2008;46:2595–2604. doi: 10.1128/JCM.00824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youens KE, Hosler GA, Washington PJ, et al. Clinical experience with the Cervista HPV HR assay correlation of cytology and HPV status from 56,501 specimens. J Mol Diagn. 2011;13:160–166. doi: 10.1016/j.jmoldx.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ASCUS-LSIL Traige Study (ALTS) Group Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383–1392. doi: 10.1067/mob.2003.457. [DOI] [PubMed] [Google Scholar]
- 15.Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: A 10-year cohort analysis. J Natl Cancer Inst. 2003;95:46–52. doi: 10.1093/jnci/95.1.46. [DOI] [PubMed] [Google Scholar]
- 16.Ronco G, Cuzick J, Segnan N, et al. HPV triage for low grade (L-SIL) cytology is appropriate for women over 35 in mass cervical cancer screening using liquid based cytology. Eur J Cancer. 2007;43:476–480. doi: 10.1016/j.ejca.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Sherman ME, Schiffman M, Cox JT. Effects of age and human papilloma viral load on colposcopy triage: Data from the randomized Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS) J Natl Cancer Inst. 2002;94:102–107. doi: 10.1093/jnci/94.2.102. [DOI] [PubMed] [Google Scholar]
- 18.Jiang S, Deng X, Ran Q. Experience on screening of women’s disease. Maternal Child Health Care China. 2006;21:2647–2648. [Google Scholar]
- Li M, Gu X, Zhao F. The analysis of cervical cancer screening and the awareness of early detection and treatment of cervical cancer among women in Shenzhen [in Chinese]. Mod Prevent Med 2013;40.
- 20.Giorgi Rossi P, Chini F, Bisanzi S, et al. Distribution of high and low risk HPV types by cytological status: A population based study from Italy. Infect Agent Cancer. 2011;6:2. doi: 10.1186/1750-9378-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitchener HC, Almonte M, Gilham C, et al. ARTISTIC: A randomised trial of human papillomavirus (HPV) testing in primary cervical screening. Health Technol Assess. 2009;13:1–150, iii–iv. doi: 10.3310/hta13510. [DOI] [PubMed] [Google Scholar]
- 22.Stoler MH, Castle PE, Solomon D, et al. The expanded use of HPV testing in gynecologic practice per ASCCP-guided management requires the use of well-validated assays. Am J Clin Pathol. 2007;127:335–337. doi: 10.1309/RNF3C01JKADQCLKP. [DOI] [PubMed] [Google Scholar]
- 23.Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Kwon MJ, Roh KH, Park H, et al. Comparison of the AnyplexII HPV 28 assay with the Hybrid Capture 2 assay for the detection of HPV infection. J Clin Virol. 2014;59:246–249. doi: 10.1016/j.jcv.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J, Zhang XG, Chen R, et al. High risk human papilloma-virus DNA detection kit (Cervista HPV HR) should be highly validated clinically in cervical cancer screening programs [in Chinese] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2011;25:149–151. [PubMed] [Google Scholar]
- 26.Chopjitt P, Ekalaksananan T, Pientong C, et al. Prevalence of human papillomavirus type 16 and its variants in abnormal squamous cervical cells in Northeast Thailand. Int J Infect Dis. 2009;13:212–219. doi: 10.1016/j.ijid.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Khanna A, Patel S, Gong Y, et al. The efficacy of HPV16/18 genotyping in predicting CIN2+ in women with HPV+/Pap results. J Am Soc Cytopathol. 2014;3:S1–S2. [Google Scholar]
- 28.Keegan H, Pilkington L, McInerney J, et al. Human papillomavirus detection and genotyping, by HC2, full-spectrum HPV and molecular beacon real-time HPV assay in an Irish colposcopy clinic. J Virol Methods. 2014;201:93–100. doi: 10.1016/j.jviromet.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Young G, Badman B, Lazenby G, et al. Rapid high-risk human papillomavirus test shows excellent agreement with standard Hybrid Capture 2 when used onsite in rural northern Tanzania. Gynecol Oncol. 2011;120(suppl 1):S123. [Google Scholar]
- 30.Rosenbaum AJ, Gage JC, Alfaro KM, et al. Acceptability of self-collected versus provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int J Gynaecol Obstet. 2014;126:156–160. doi: 10.1016/j.ijgo.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzi AT, Fregnani JH, Possati-Resende JC, et al. Self-collection for high-risk HPV detection in Brazilian women using the careHPV™ test. Gynecol Oncol. 2013;131:131–134. doi: 10.1016/j.ygyno.2013.07.092. [DOI] [PubMed] [Google Scholar]
- 32.Shi JF, Canfell K, Lew JB, et al. Evaluation of primary HPV-DNA testing in relation to visual inspection methods for cervical cancer screening in rural China: An epidemiologic and cost-effectiveness modelling study. BMC Cancer. 2011;11:239. doi: 10.1186/1471-2407-11-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: A cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]