To assess the safety of tamoxifen dose escalation and investigate concentration differences between races, breast cancer patients receiving tamoxifen at 20 mg/day were enrolled for CYP2D6 genotype-guided escalation to 40 mg/day. Endoxifen was measured at baseline and after 4 months, and quality-of-life data were collected. CYP2D6 genotype-guided dose escalation eliminated baseline concentration differences in intermediate, but not poor, metabolizers, without an appreciable effect on quality of life, and endoxifen concentrations were similar in black and white patients.
Keywords: CYP2D6, Genotype, Tamoxifen, Endoxifen, Toxicity, Pharmacogenetics, Quality of life, Race
Abstract
Background.
Polymorphic CYP2D6 is primarily responsible for metabolic activation of tamoxifen to endoxifen. We previously reported that by increasing the daily tamoxifen dose to 40 mg/day in CYP2D6 intermediate metabolizer (IM), but not poor metabolizer (PM), patients achieve endoxifen concentrations similar to those of extensive metabolizer patients on 20 mg/day. We expanded enrollment to assess the safety of CYP2D6 genotype-guided dose escalation and investigate concentration differences between races.
Methods.
PM and IM breast cancer patients currently receiving tamoxifen at 20 mg/day were enrolled for genotype-guided escalation to 40 mg/day. Endoxifen was measured at baseline and after 4 months. Quality-of-life data were collected using the Functional Assessment of Cancer Therapy-Breast (FACT-B) and Breast Cancer Prevention Trial Menopausal Symptom Scale at baseline and after 4 months.
Results.
In 353 newly enrolled patients, genotype-guided dose escalation eliminated baseline concentration differences in IM (p = .08), but not PM (p = .009), patients. Endoxifen concentrations were similar in black and white patients overall (p = .63) and within CYP2D6 phenotype groups (p > .05). In the quality-of-life analysis of 480 patients, dose escalation did not meaningfully diminish quality of life; in fact, improvements were seen in several measures including the FACT Breast Cancer subscale (p = .004) and limitations in range of motion (p < .0001) in IM patients.
Conclusion.
Differences in endoxifen concentration during treatment can be eliminated by doubling the tamoxifen dose in IM patients, without an appreciable effect on quality of life. Validation of the association between endoxifen concentration and efficacy or prospective demonstration of improved efficacy is necessary to warrant clinical uptake of this personalized treatment strategy.
Implications for Practice:
This secondary analysis of a prospective CYP2D6 genotype-guided tamoxifen dose escalation study confirms that escalation to 40 mg/day in patients with low-activity CYP2D6 phenotypes (poor or intermediate metabolizers) increases endoxifen concentrations without any obvious increases in treatment-related toxicity. It remains unknown whether endoxifen concentration is a useful predictor of tamoxifen efficacy, and thus, there is no current role in clinical practice for CYP2D6 genotype-guided tamoxifen dose adjustment. If future studies confirm the importance of endoxifen concentrations for tamoxifen efficacy and report a target concentration, this study provides guidance for a dose-adjustment approach that could maximize efficacy while maintaining patient quality of life.
Introduction
Tamoxifen is a selective estrogen receptor modulator that is highly effective in estrogen receptor-positive breast cancer patients. Use of tamoxifen for 5 years decreases cancer recurrence by approximately 50% in this setting [1], and possibly more if used for 10 years [2, 3]. Tamoxifen is the only approved endocrine agent for premenopausal women (without ovarian suppression), is used in postmenopausal patients who have a contraindication or intolerance to aromatase inhibitors, and may be used in high-risk women for cancer prevention.
Despite the proven efficacy of tamoxifen, some women experience cancer recurrence during or after treatment. Therapeutic failure may be caused by tumor resistance to antiestrogen therapy [4, 5] or inadequate bioactivation of tamoxifen to its active metabolite, endoxifen [6, 7]. Some studies have reported that patients with low endoxifen concentrations have inferior tamoxifen efficacy [8, 9], although this association has not been consistently detected [10].
Activation of tamoxifen to endoxifen occurs through two parallel pathways involving multiple cytochrome P450 (CYP) enzymes [11]. Despite these complementary pathways, the polymorphic CYP2D6 enzyme is responsible for a considerable amount of endoxifen production. CYP2D6 metabolic activity can be predicted from CYP2D6 genotype, enabling classification of patients into poor (PM), intermediate (IM), extensive (EM), or ultra-rapid (UM) metabolizer phenotypes [12]. Patients with low-activity CYP2D6 phenotypes have substantially lower endoxifen steady-state concentrations [8, 13, 14]. It is unclear whether patients with genotypes that confer low CYP2D6 activity have inferior efficacy from tamoxifen treatment [15–18], but if so, preemptive genotyping to guide tamoxifen dose selection could be a viable strategy to improve treatment effectiveness.
We have previously reported results of a prospective clinical study of CYP2D6 genotype-guided tamoxifen dosing that we conducted at the University of North Carolina and several nearby community cancer clinics. Dose escalation in patients with intermediate CYP2D6 activity successfully achieved endoxifen concentrations similar to those seen in patients with normal activity remaining on standard dosing [19]. Building on the success of this pilot study, we expanded enrollment to 500 patients to achieve statistical power to assess whether dose escalation has any adverse effect on treatment-related toxicity or quality of life, and to investigate whether nonwhite patients have similar endoxifen levels during tamoxifen treatment to further refine individualized treatment strategies.
Methods
Patients
A previous publication reported analysis of endoxifen concentrations before and after 4 months of genotype-guided treatment in the original 120-patient cohort of University of North Carolina Lineberger Comprehensive Cancer Center 0801 [19]. Briefly, women 18 years or older who were receiving tamoxifen 20 mg/day for at least 4 months were eligible to enroll in a prospective CYP2D6 genotype-guided dose escalation study. Patients were ineligible if they were taking CYP2D6 moderate or strong inhibitors or had Eastern Cooperative Oncology Group performance status >2 or impaired kidney or liver function. After enrollment, baseline data and serum samples were collected to measure tamoxifen and metabolite concentrations and predict CYP2D6 phenotype. Patients who were EM or UM continued on 20-mg/day treatment. Patients with PM or IM phenotypes had doses increased to 40 mg/day. Patients remained on this genotype-adjusted dose for 4 months, after which additional data and samples were collected for analysis. This study was approved by the institutional review boards of the University of North Carolina at Chapel Hill and all other participating institutions. All patients signed informed consent before participation. This report describes endoxifen concentrations in the 380-patient expansion cohort and combines the 2 cohorts for a joint analysis of toxicity before and 4 months after dose adjustment. A detailed description of the study methods can be found in the original publication.
CYP2D6 Genotype and Phenotype
Whole blood collected at enrollment was used to isolate genomic DNA for CYP2D6 genotyping using the AmpliChip CYP450 test (Roche Diagnostics, Indianapolis, IN, https://usdiagnostics.roche.com) in a Clinical Laboratory Improvement Amendments-certified laboratory. The AmpliChip can identify 20 distinct alleles and 7 known duplications, which can then be combined to predict a CYP2D6 phenotype (PM, IM, EM, or UM). A detailed description of the allelic coverage and the translation system used can be found in our recent publication describing the metabolic activity of individual alleles and diplotypes [13]. Because of the small number of UM patients, the similarity in tamoxifen dosing with EM patients, and the marginal difference in endoxifen concentrations between these groups, patients with UM and EM phenotypes were combined for the toxicity analysis.
Tamoxifen and Endoxifen Concentrations
Blood samples were collected at enrollment, at which point all patients had been on tamoxifen 20 mg/day for at least 4 months. An additional sample was collected after 4 months of genotype-guided treatment, with EM/UM patients continuing on 20 mg/day and PM or IM patients taking 40 mg/day. At each time point, plasma concentrations of tamoxifen (Z isomer only), (Z)-4-hydroxy-tamoxifen, N-desmethyl-tamoxifen (Z isomer only), and (Z)-4-hydroxy-N-desmethyl-tamoxifen (endoxifen; 10% E, 90% Z) were measured using a high-performance liquid chromatography/tandem mass spectrometry (API 3200) assay method as previously described [19]. This analysis includes only endoxifen concentrations at baseline and 4 months for the 380 patients on the expansion study, which used a slightly modified endoxifen assay, as previously described [13].
Quality-of-Life Data
Quality-of-life data were collected using the Functional Assessment of Cancer Therapy-Breast, including the Endocrine Subscale (FACT-B [ES]), Version 3, and the Breast Cancer Prevention Trial Menopausal Symptom Scale (BCPT-MSS) at the time of consent and after 4 months of genotype-guided treatment. These validated scales collect patient-reported toxicity relevant to breast cancer endocrine therapy [20, 21]. The FACT-B [ES] is subdivided into several subscales (physical, social/family, emotional, functional, relationship with doctor, and additional concerns) and asks patients to rate how true a statement has been for the past 7 days on a scale from 0 (not at all) to 4 (very much). The BCPT-MSS asks how much a patient was bothered on a scale of 0 (not at all) to 4 (extremely) in the past 4 weeks.
Statistical Analysis
Two separate analyses were conducted and reported in this article. The first is the comparison of endoxifen concentrations at baseline and after 4 months of genotype-guided treatment between CYP2D6 phenotype groups and at baseline between black and white patients. Endoxifen concentration was compared between groups using analysis of variance and two-group t tests. All analyses of endoxifen concentrations were conducted in the expansion cohort only, as the analysis in the original cohort has been previously published [19].
The second analysis is a comparison of toxicity at baseline and the change in quality of life from baseline to 4 months. FACT was scored as recommended, yielding scores for FACT-B total, FACT-B trial outcome index, FACT-Endocrine subscale total, and several subscales (physical, social/family, emotional, functional, breast cancer, endocrine symptom). Additionally, each individual toxicity item was analyzed separately. For all FACT summary scores, higher scores indicate better quality of life; however, for individual items on the endocrine symptom subscale, higher scores indicate worse symptoms. BCPT-MSS was also scored as recommended, by averaging across the individual items, each subscale (hot flashes, nausea, bladder control, vaginal problems, musculoskeletal, cognitive problems, weight problems, arm problems), and each individual toxicity item. For BCPT-MSS, all items range from 0 (not at all) to 4 (extremely); thus, higher scores on both summary and individual items indicate worse symptoms. All individual and combined toxicity scores were compared across CYP2D6 phenotypes (PM, IM, and EM/UM) at baseline using linear regression models, assuming an additive genetic effect. The change in mean toxicity from baseline to 4 months within each phenotype group was evaluated using paired t tests. A standard significance threshold of p < .05 was used for all analyses, including the many hypothesis-generating quality- of-life comparisons. Analyses were conducted using SAS statistical software, version 9.3 (SAS Institute, Cary, NC, http://www.sas.com).
Results
Patient Characteristics by Genotype
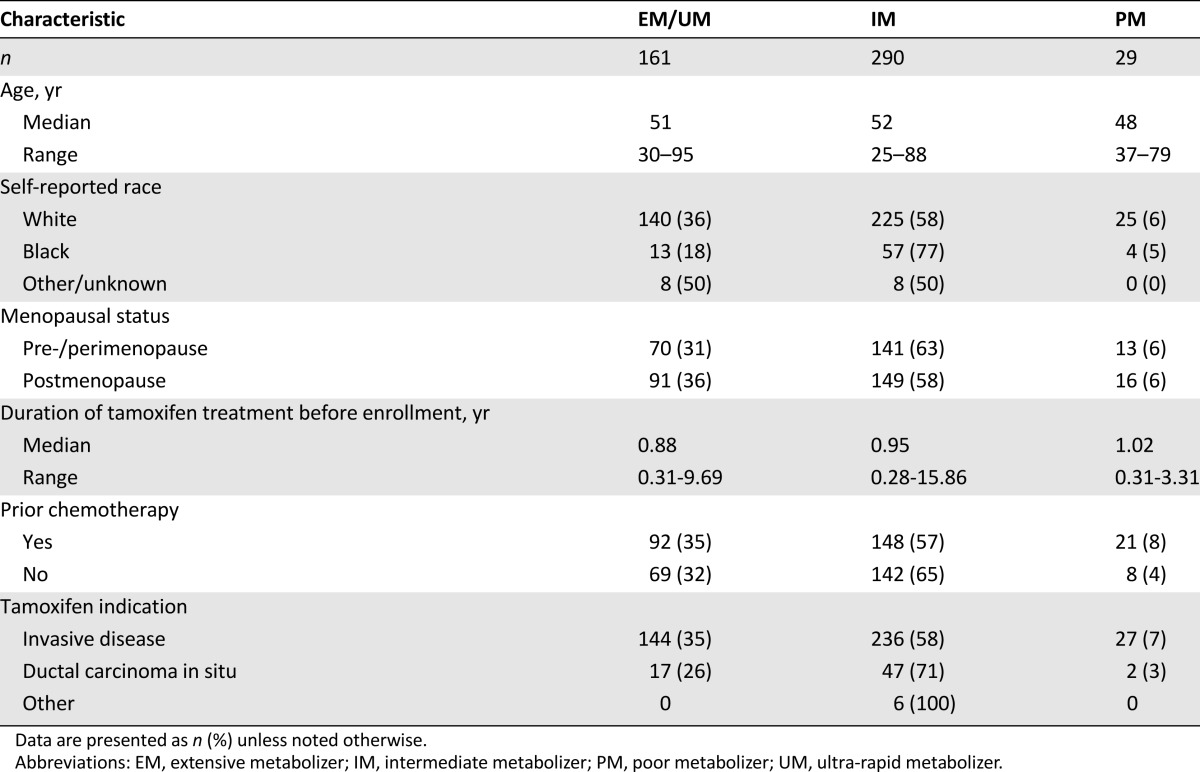
A total of 500 patients were enrolled on the prospective CYP2D6 genotype-guided tamoxifen clinical study. After excluding patients who were ineligible or had missing genotype data, 480 were evaluable in the toxicity analysis. Only patients enrolled on the expansion cohort are included in the comparison of endoxifen concentration at baseline and after 4 months of genotype-guided treatment, because the original 120 patients have previously been published [19]. After elimination of patients who were ineligible or had missing data, 353 patients were evaluable in this follow-up analysis, of whom 344 self-reported their race as white or black and were included in the race analysis (supplemental online Fig. 1). Demographic data for the 480 patients evaluable in the toxicity analysis, including age, race, menopausal status, duration of tamoxifen treatment before enrollment, prior chemotherapy use, and tamoxifen indication, can be found in Table 1.
Table 1.
Demographic data for all 480 subjects evaluable in toxicity analysis
Endoxifen at Baseline and 4 Months
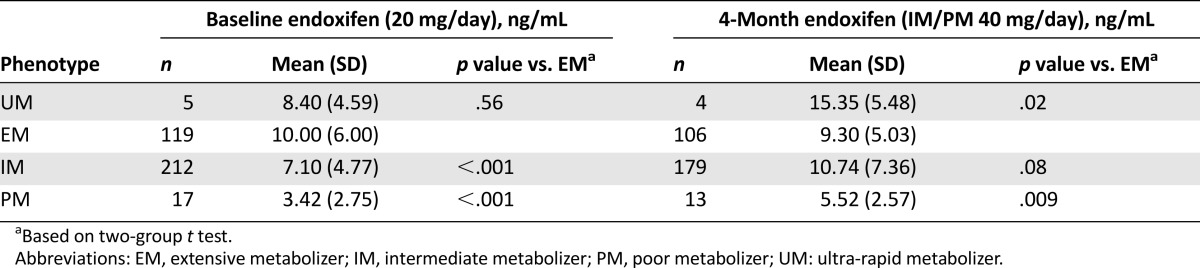
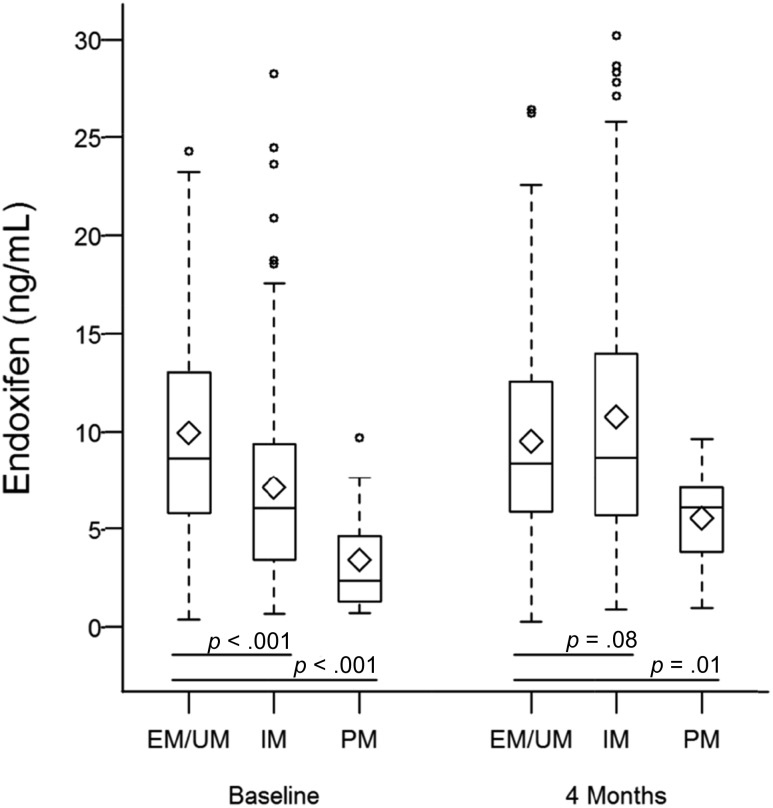
All patients on this study were taking 20 mg/day of tamoxifen at study entry. Steady-state endoxifen concentrations were significantly higher in patients with EM/UM genotypes (mean concentration 10.00 ng/mL) than in those with IM (7.10 ng/mL, p < .001) or PM (3.42 ng/mL, p < .001) genotypes (Table 2). After 4 months of CYP2D6 genotype-dosed therapy, in which PM and IM patients received double the dose, although a significant difference in endoxifen concentration remained (p = .01), it was markedly attenuated and was actually eliminated in IM patients. Significant differences still existed, however, in IM patients, in whom endoxifen concentrations had risen by 48% to 10.74 ng/mL, which was no longer different from, and was in fact nominally greater than, the endoxifen concentrations in EM/UM patients (9.30 ng/mL, p = .08). Endoxifen concentrations in genotype-dosed PM patients also rose by approximately 61% but remained significantly lower than in EM patients (5.52 ng/mL, p = .009) or genotype-dosed IM patients (Table 2, Fig. 1). The endoxifen concentration estimates or statistical comparisons were not meaningfully altered by excluding the 51 patients who had baseline endoxifen concentration data but did not have 4-month concentrations (data not shown).
Table 2.
Endoxifen concentrations at baseline (n = 353) and 4 months (n = 302) by CYP2D6 phenotype
Figure 1.
Endoxifen concentrations at baseline and 4 months by CYP2D6 phenotype group. At baseline, with all patients receiving 20 mg/day of tamoxifen, there were significant differences in endoxifen concentrations in EM patients compared with IM or PM patients (p < .001). After 4 months of dose escalation to 40 mg/day in the IM and PM patients, only the IM patients had endoxifen concentrations similar to EM patients (p = .08), whereas the PM patients continued to have lower endoxifen concentrations (p = .01), recapitulating the findings reported in the analysis of the first 120 patients enrolled on this trial [18].
Abbreviations: EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; UM, ultra-rapid metabolizer.
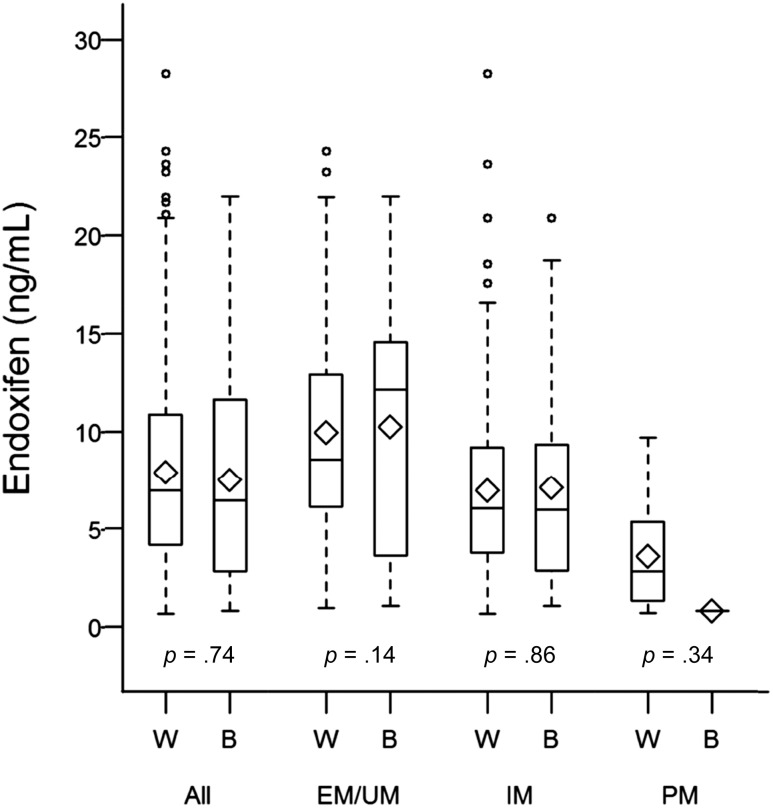
A total of 344 self-described white (n = 292) or black (n = 52) patients were evaluable in the baseline comparison of endoxifen concentration by race. At baseline, the mean endoxifen concentration was similar in white and black patients (7.90 ng/mL vs. 7.51 ng/mL, p = .63). As expected, there were no statistically significant differences in endoxifen concentration at baseline in the CYP2D6 UM, EM, IM, or PM phenotype groups when comparing white and black patients (all p > .05) (supplemental online Table 1; Fig. 2).
Figure 2.
Comparison of baseline endoxifen concentration in white and black patients combined and stratified by CYP2D6 phenotype. White and black patients had similar median endoxifen concentrations at baseline compared overall or within CYP2D6 phenotype groups (all p > .05).
Abbreviations: B, black; EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; UM, ultra-rapid metabolizer; W, white.
Quality of Life at Baseline
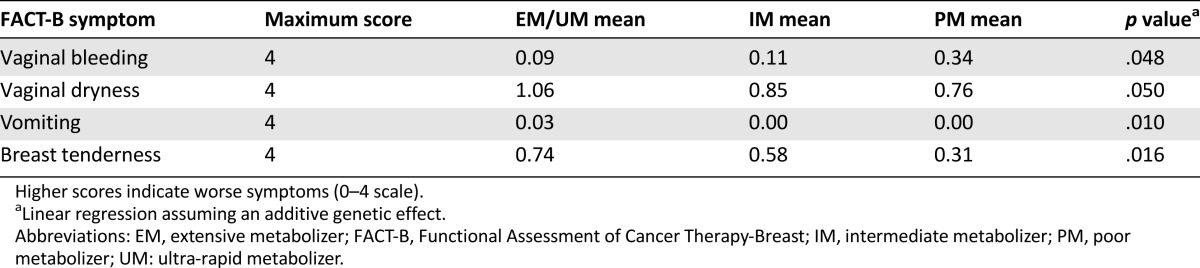
In the 480 patients evaluable, there was no identifiable trend in baseline toxicity across CYP2D6 phenotype groups for any of the combined FACT or BCPT-MSS scales or subscales. Patients reported decent overall quality of life (FACT-B scores ranging from 116 to 119, out of 144) and relatively minor decreases in quality of life as a result of their endocrine therapy (FACT [ES] scores ranging from 59 to 60, out of 72). As metabolizer phenotype increased, there were significant increases in several toxicities on the FACT scale, including breast tenderness (p = .016), vaginal dryness (p = .050), and vomiting (p = .010), whereas there was a significant decrease in vaginal bleeding (p = .048) (Table 3). No differences were identified by the BCPT-MSS, including lack of significance for vaginal dryness (p = .25) and vomiting (p = .60), whereas breast tenderness and vaginal bleeding are not assessed by this scale. Comparisons across CYP2D6 phenotype for all subscales and individual toxicities from both scales are reported in supplemental online Table 2.
Table 3.
Statistically significant differences in toxicity across phenotype groups at baseline
Change in Quality of Life From Baseline to 4 Months
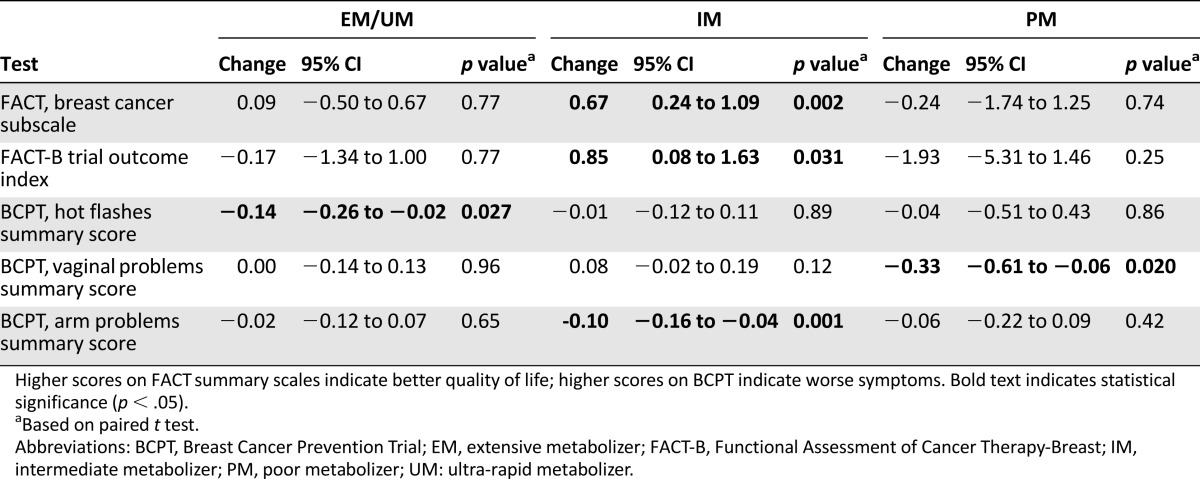
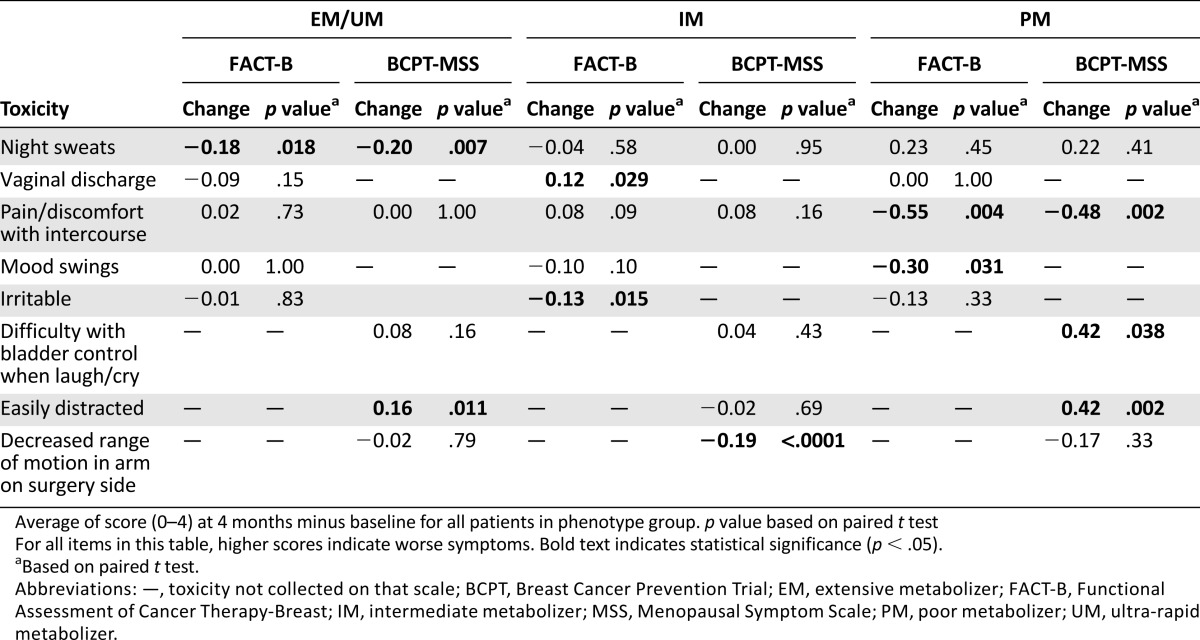
Increasing the dose from 20 to 40 mg/day did not cause a concurrent increase in toxicity or decrease in patient quality of life in PM and IM groups based on comparisons of the overall scales or subscales (Table 4). However, there were increases in individual toxicity items for individual phenotype groups (Table 5). The PM group reported an increase in distractedness on the BCPT-MSS (Δ = 0.42, 95% confidence interval [CI] 0.17–0.66, p = .002), although the EM group also reported an increase despite no dose change (Δ = 0.16, 95% CI 0.04–0.28, p = .011). The IM group reported an increase in vaginal discharge on FACT (Δ = 0.12, 95% CI 0.01–0.22, p = .029) that was not seen in either the PM or EM groups.
Table 4.
Statistically significant changes in overall toxicity and toxicity subscales from baseline to 4 months (n = 421)
Table 5.
Statistically significant changes in individual toxicities on the FACT-B and/or BCPT-MSS from baseline to 4 months (n = 421)
There were also improvements seen in individual toxicities or quality-of-life measures within phenotype groups (Table 5). The PM group experienced less pain with intercourse (FACT: p = .004, BCPT-MSS: p = .002) and fewer mood swings (FACT: p = .031) and vaginal problems (BCPT-MSS: p = .020). The IM group reported decreased irritability (FACT: p = .015), decreased limitation in range of motion in the arm on the surgery side (p < .0001), improvement on the BCPT arm problems scale (p = .001), and overall improvement on the FACT Trial Outcome Index (Δ = 0.85, 95% CI 0.08–1.63, p = .031) and Breast Cancer Subscale (Δ = 0.67, 95% CI 0.24–1.09, p = .002). Finally, there were decreases in reports of several toxicities in EM/UM patients who remained on 20 mg/day, including decreased BCPT-MSS hot flash summary score (Δ = −0.14, 95% CI −0.26 to −0.02, p = .027) and decreases in night sweats on both scales (FACT: p = .018, BCPT-MSS: p = .007). Changes for every subscale and individual toxicity within each phenotype group can be found in supplemental online Tables 3 and 4, respectively.
Discussion
This CYP2D6 genotype-guided tamoxifen dosing study allowed us to assess any potential associations between CYP2D6 phenotype and (a) active endoxifen concentrations and (b) toxicity when all patients were treated at the standard 20-mg/day dose and when patients with low-activity phenotype were escalated to 40 mg/day. In this expansion study, we validated our initial finding that increasing doses for IM patients normalizes their endoxifen concentration with that of EM/UM patients treated with standard doses, whereas endoxifen concentration remains significantly lower in PM patients after escalation. Other similar studies that increased doses in patients based on baseline endoxifen or CYP2D6 genotype have reported similar findings [22, 23]. Our relatively large study enabled detailed assessment of the consequences of dose escalation on treatment-related toxicity and overall quality of life. We did not detect any clinically meaningful increases in treatment-related toxicity or decreases in quality of life from tamoxifen dose escalation and could not replicate prior reports of associations between CYP2D6 phenotype and toxicity. Therefore, our prospective study confirms the clinical feasibility of genotype-directed tamoxifen treatment to safely eliminate well-established differences in endoxifen concentration in IM patients, which represents the majority of women receiving tamoxifen.
CYP2D6 phenotype is a strong predictor of endoxifen concentration; however, it is still unclear whether endoxifen concentration is associated with treatment efficacy [14]. Two studies have reported that patients below a threshold endoxifen concentration (<5.9 ng/mL) have increased risk of recurrence [8, 9]. This threshold is below the mean endoxifen concentration in IM patients receiving standard 20-mg/day dosing (7.10 ng/mL) but above that of PM patients after dose escalation to 40 mg/day (5.52 ng/mL) in our study. If recurrence risk increases below this endoxifen threshold, our data do not support the clinical utility of increasing doses in IM or PM patients to 40 mg/day. Alternatively, our study defined the endoxifen concentration of EM patients receiving standard dosing as the “target.” In that case, our data support dose escalation in IM patients to 40 mg/day, which our data suggests does not increase toxicity or decrease quality of life. Based on our results, PM patients would require even further dose escalation, the safety of which is unknown. We are not aware of any direct evidence that the use of higher tamoxifen doses for the general population improves treatment efficacy or enhances toxicity. Some European countries use 30 or 40 mg/day as the standard of care. The Early Breast Cancer Trialists’ Collaborative Group meta-analysis reported a higher recurrence risk reduction with higher daily doses (p = .02 for trend between relative risks for 20, 30, and 40 mg/day), but not for breast cancer mortality [24]. Lack of detectable benefit in the general population does not preclude the possibility that there is a subset of patients who would benefit from dose escalation, for instance those with low endoxifen concentration during treatment. However, at this time, we do not recommend any dose escalation approach, as the connection between endoxifen concentration and efficacy, or the effect on efficacy of genotype-directed dose escalation, has not been conclusively established [10]. Clinical trials of endoxifen currently under way (Alliance A011203; NCT02311933) could provide answers to some of these critical questions.
The link between endoxifen, tamoxifen, or any other metabolite concentration and toxicity is even less well established. Previous publications have reported that CYP2D6 phenotype may be a predictor of toxicity [25], particularly hot flashes [26, 27], but this has not been consistently found [28, 29]. In our dataset, there was no significant difference in overall toxicity or hot flashes across CYP2D6 phenotype groups all treated at standard doses. As CYP2D6 activity increased, breast tenderness, vaginal dryness, and vomiting increased on the FACT scale; however, these findings could not be replicated in the BCPT-MSS data and have not been previously reported to our knowledge. Our findings do not support a robust link between CYP2D6 phenotype and toxicity during treatment, suggesting that endoxifen concentration is unlikely to be predictive of treatment-related toxicity or vice versa. This again supports the clinical feasibility of dose escalation in patients achieving suboptimal endoxifen concentrations.
Similar to results previously reported in a study of 90 patients receiving genotype-guided tamoxifen dose escalation [22], we did not detect a meaningful increase in toxicity from dose escalation. These results are contrary to our prior report of a significant increase in “severe toxicity” in IM patients after dose escalation [30]. That preliminary analysis of this dataset dichotomized patients by whether they rated any toxicity 3 or 4 on the BCPT-MSS scale and compared the proportion at baseline and after 4 months of treatment. A small but statistically significant increase was reported by IM patients, providing the basis for the hypotheses tested formally in this analysis. Using the recommended scoring approaches for these validated quality- of-life assessments, we detected minor increases in distractedness and vaginal discharge in PM and IM patients, respectively. No composite toxicity scale increased in either group, nor did any single toxicity increase in both PM and IM groups. Many comparisons were conducted without proper statistical adjustment, and these two nominal increases are likely because of chance, not actual consequences of dose escalation. Furthermore, the number of toxicities that increased in PM and IM patients was exceeded by the number that decreased, including significant improvements in the FACT breast cancer subscale and trial outcome index in IM patients. These improvements in quality of life could be partially attributable to decreased adherence, particularly in patients with toxicity [31], or may be a consequence of remaining on treatment for an extended period of time. It is possible that over time patients learn to prevent, manage, or accept side effects associated with tamoxifen treatment; however, there was no association between time on tamoxifen treatment before enrollment and any of the composite toxicity measures at baseline (data not shown). Nevertheless, this explanation is partially supported by the preponderance of decreased toxicity (improved quality of life) reported by the EM/UM patients, who remained on standard dosing and serve as a pseudonegative control for this analysis. A more direct comparison could have been conducted if PM or IM patients were randomized to dose escalation (40 mg/day) or continued standard care (20 mg/day); however, given the negative findings, it is unlikely that this would have detected any meaningful changes in quality of life in the dose-escalated patients.
In a secondary analysis of baseline endoxifen concentration, we did not detect an overall difference in self-reported white and black patients or a difference between races within CYP2D6 phenotype groups. Our understanding of CYP2D6 genetic variation seems to be sufficient to accurately predict CYP2D6 phenotype from genotype information for white and black patients. This is not the case with all pharmacogenetic associations, as evidenced by the inferior performance of genetically guided warfarin dosing strategies in African-American patients [32], which has been attributed to racial differences in the effect of clinical and genetic factors [33]. Interestingly, greater endoxifen concentrations have been reported for Hispanic patients in comparison with non-Hispanic white patients [34]. This could be partially attributable to the lower frequency of CYP2D6 low-activity alleles found in the Hispanic cohort; however, within the CYP2D6 EM group, Hispanic patients had greater endoxifen concentrations. This suggests that there could be additional genetic variation within Hispanic patients that is not currently appreciated, or perhaps there could be other nongenetic differences that explain this finding. Unfortunately, we did not have a sufficient number of Hispanic patients to include this group in any analyses. Ongoing analyses of this robust dataset will identify additional clinical and genetic factors, such as concomitant treatment with weak CYP2D6 inhibitors, age, weight, or season of sample collection, that could contribute to the residual variability in endoxifen concentrations after accounting for CYP2D6 genotype [35].
Several additional limitations of this analysis warrant discussion. This study was not powered, designed, or intended to collect any data on treatment efficacy; therefore, the clinical utility of CYP2D6 genotype-guided tamoxifen treatment cannot be evaluated. Relatedly, the quality-of-life data were collected for only 6 months, whereas treatment-related toxicity, particularly severe toxicities such as thromboembolism, can take years to develop. Second, the present analysis did not specifically analyze the relationship between endoxifen concentration, or concentrations of any other metabolite, and treatment toxicity. This analysis may be conducted in the future but is beyond the scope of the current article. Finally, there is likely some inherent bias in our estimates of toxicity at baseline, given that only patients who are not experiencing unacceptable toxicity would continue on treatment long enough for enrollment to our study. Adherence with tamoxifen therapy is known to be suboptimal, and this may in fact be more pronounced in patients with high CYP2D6 activity [36], biasing our baseline comparison of toxicity across phenotypes. This limitation could be circumvented by enrolling patients at treatment initiation, as opposed to enrolling patients currently on treatment.
Conclusion
This prospective study verified that dose escalation in patients with low-activity CYP2D6 phenotypes increases endoxifen concentrations without any evidence of enhanced short-term treatment-related toxicity or deterioration in patient quality of life. Decisions regarding which patients should have doses increased, and by how much, require validation that endoxifen concentration is associated with treatment efficacy and identification of an optimal endoxifen concentration target, or prospective demonstration that genotype-guided tamoxifen dose escalation improves treatment outcomes.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We recognize the important contributions of Dr. David Flockhart, who passed away before the publication of this manuscript, to the understanding of the metabolism of tamoxifen and many other drugs that serves as the basis for much of the ongoing research into personalized therapy. Supported by Susan G. Komen Career-Catalyst Award KG100355 (W.J. Irvin), grant CA58223 from the National Cancer Institute Specialized Programs of Research Excellence, North Carolina University Cancer Research Fund, University of North Carolina at Chapel Hill Investments for the Future Grant 6231, Laboratory Corporation of America, Roche Diagnostics, American Society of Clinical Oncology Foundation and Breast Cancer Research Foundation (L.A. Carey), and National Institute of General Medical Sciences Pharmacogenomics Research Network Award (U-01GM061373) to the Consortium on Breast Cancer Pharmacogenomics (D.A. Flockhart). This study was funded in part by the Laboratory Corporation of America and Roche Diagnostics. Rachel Rabb is currently affiliated with Cancer Care of Western North Carolina, Asheville, NC. Steven Corso is currently affiliated with the Gibbs Cancer Center, Spartanburg, SC. Garry Schwartz is currently affiliated with Duke Cancer Institute, Raleigh, NC. Jeffrey M. Peppercorn is currently affiliated with Massachusetts General Hospital, Boston, MA.
This work was presented in part at the 2014 American Society of Clinical Oncology Annual Meeting, Chicago, IL.
Footnotes
For Further Reading: Oleg Gladkov, Vladimir Moiseyenko, Igor N. Bondarenko et al. A Phase III Study of Balugrastim Versus Pegfilgrastim in Breast Cancer Patients Receiving Chemotherapy With Doxorubicin and Docetaxel. The Oncologist 2016;21:7–15.
Implications for Practice: This paper provides efficacy and safety data for a new, once-per-cycle granulocyte colony-stimulating factor, balugrastim, for the prevention of chemotherapy-induced neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. In this phase III trial, balugrastim was shown to be not inferior to pegfilgrastim in the duration of severe neutropenia in cycle 1 of doxorubicin/docetaxel chemotherapy, and the safety profiles of the two agents were similar. Once-per-cycle balugrastim is a safe and effective alternative to pegfilgrastim for hematopoietic support in patients with breast cancer receiving myelosuppressive chemotherapy associated with a greater than 20% risk of developing febrile neutropenia.
Author Contributions
Conception/Design: Daniel L. Hertz, Joseph G. Ibrahim, Christine M. Walko, Karen E. Weck, Steven Anderson, Gustav Magrinat, Oludamilola Olajide, Susan Moore, Jeffrey M. Peppercorn, James P. Evans, Howard L. McLeod, Lisa A. Carey, William J. Irvin Jr.
Provision of study material or patients: Christine M. Walko, Gustav Magrinat, Susan Moore, Daniel R. Carrizosa, Steven Corso, Garry Schwartz, Mark Graham, Howard L. McLeod, Lisa A. Carey, William J. Irvin Jr.
Collection and/or assembly of data: Daniel L. Hertz, Christine M. Walko, Karen E. Weck, Steven Anderson, Oludamilola Olajide, Rachel Raab, Jeffrey M. Peppercorn, David R. Jones, Zeruesenay Desta, David A. Flockhart, Howard L. McLeod, Lisa A. Carey, William J. Irvin Jr.
Data analysis and interpretation: Daniel L. Hertz, Allison Deal, Joseph G. Ibrahim, Christine M. Walko, Jeffrey M. Peppercorn, David R. Jones, Zeruesenay Desta, David A. Flockhart, Howard L. McLeod, Lisa A. Carey, William J. Irvin Jr.
Manuscript writing: Daniel L. Hertz, Allison Deal, Christine M. Walko, Howard L. McLeod, Lisa A. Carey, William J. Irvin Jr.
Final approval of manuscript: Daniel L. Hertz, Allison Deal, Joseph G. Ibrahim, Karen E. Weck, Steven Anderson, Gustav Magrinat, Oludamilola Olajide, Susan Moore, Rachel Raab, Daniel R. Carrizosa, Steven Corso, Garry Schwartz, Mark Graham, Jeffrey M. Peppercorn, James P. Evans, Howard L. McLeod, Lisa A. Carey, William J. Irvin Jr.
Disclosures
Steven Anderson: Roche Molecular Systems, Pfizer (C/A), LabCorp (E, OI); Daniel R. Carrizosa: Boehringer-Ingelheim (C/A), Celgene, Merck (RF); Jeffrey M. Peppercorn: GlaxoSmithKline (E, OI [spouse]); Howard L. McLeod: Cancer Genetics Inc. (C/A, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Early Breast Cancer Trialists’ Collaborative Group Tamoxifen for early breast cancer: An overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 2.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31(suppl):5a. [Google Scholar]
- 4.Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Shen D, Shao J, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Reports. 2013;4:1116–1130. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 7.Lim YC, Desta Z, Flockhart DA, et al. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55:471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 8.Saladores P, Murdter T, Eccles D, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J. 2014;15:84–94. doi: 10.1038/tpj.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love RR, Desta Z, Flockhart D, et al. CYP2D6 genotypes, endoxifen levels, and disease recurrence in 224 Filipino and Vietnamese women receiving adjuvant tamoxifen for operable breast cancer. SpringerPlus. 2013;2:52. doi: 10.1186/2193-1801-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mürdter TE, Schroth W, Bacchus-Gerybadze L, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 12.Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther. 2012;91:321–326. doi: 10.1038/clpt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertz DL, Snavely AC, McLeod HL, et al. In vivo assessment of the metabolic activity of CYP2D6 diplotypes and alleles. Br J Clin Pharmacol. 2015;80:1122–1130. doi: 10.1111/bcp.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries Schultink AH, Zwart W, Linn SC, et al. Effects of pharmacogenetics on the pharmacokinetics and pharmacodynamics of tamoxifen. Clin Pharmacokinet. 2015;54:797–810. doi: 10.1007/s40262-015-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz MP, Suman VJ, Hoskin TL, et al. CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res. 2013;19:500–507. doi: 10.1158/1078-0432.CCR-12-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rae JM, Drury S, Hayes DF, et al. Lack of correlation between gene variants in tamoxifen metabolizing enzymes with primary endpoints in the ATAC trial. 33rd Annual San Antonio Breast Cancer Symposium; December 8–10, 2010; San Antonio, TX.; 2010. [Google Scholar]
- 18.Regan MM, Leyland-Jones B, Bouzyk M, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104:441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irvin WJ, Jr, Walko CM, Weck KE, et al. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: A multicenter study. J Clin Oncol. 2011;29:3232–3239. doi: 10.1200/JCO.2010.31.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 21.Fallowfield LJ, Leaity SK, Howell A, et al. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: Validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55:189–199. doi: 10.1023/a:1006263818115. [DOI] [PubMed] [Google Scholar]
- 22.Kiyotani K, Mushiroda T, Imamura CK, et al. Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res Treat. 2012;131:137–145. doi: 10.1007/s10549-011-1777-7. [DOI] [PubMed] [Google Scholar]
- 23.Barginear MF, Jaremko M, Peter I, et al. Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: Effect on active metabolite isomers and the antiestrogenic activity score. Clin Pharmacol Ther. 2011;90:605–611. doi: 10.1038/clpt.2011.153. [DOI] [PubMed] [Google Scholar]
- 24.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorizio W, Wu AH, Beattie MS, et al. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat. 2012;132:1107–1118. doi: 10.1007/s10549-011-1893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry NL, Rae JM, Li L, et al. Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat. 2009;117:571–575. doi: 10.1007/s10549-009-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 28.Dezentje V, Gelderblom H, Schaik RN, et al. CYP2D6 genotype in relation to hot flashes as tamoxifen side effect in a Dutch cohort of the tamoxifen exemestane adjuvant multinational (TEAM) trial. Breast Cancer Res Treat. 2014;143:171–179. doi: 10.1007/s10549-013-2777-6. [DOI] [PubMed] [Google Scholar]
- 29.Sestak I, Kealy R, Nikoloff M, et al. Relationships between CYP2D6 phenotype, breast cancer and hot flushes in women at high risk of breast cancer receiving prophylactic tamoxifen: Results from the IBIS-I trial. Br J Cancer. 2012;107:230–233. doi: 10.1038/bjc.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hertz DL, Snavely AC, Evans JP, et al. Does increasing the daily tamoxifen dose in patients with diminished CYP2D6 activity increase toxicity? J Clin Oncol. 2014;32(suppl):561a. [Google Scholar]
- 31.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimmel SE, French B, Kasner SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369:2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limdi NA, Brown TM, Yan Q, et al. Race influences warfarin dose changes associated with genetic factors. Blood. 2015;126:539–545. doi: 10.1182/blood-2015-02-627042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangel LB, Taraba JL, Frei CR, et al. Pharmacogenomic diversity of tamoxifen metabolites and estrogen receptor genes in Hispanics and non-Hispanic whites with breast cancer. Breast Cancer Res Treat. 2014;148:571–580. doi: 10.1007/s10549-014-3191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teft WA, Gong IY, Dingle B, et al. CYP3A4 and seasonal variation in vitamin D status in addition to CYP2D6 contribute to therapeutic endoxifen level during tamoxifen therapy. Breast Cancer Res Treat. 2013;139:95–105. doi: 10.1007/s10549-013-2511-4. [DOI] [PubMed] [Google Scholar]
- 36.Rae JM, Sikora MJ, Henry NL, et al. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J. 2009;9:258–264. doi: 10.1038/tpj.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.