Identifying and correctly managing endocrine immune-related adverse events are essential to provide optimal care and maximize the potential of immune checkpoint inhibitors. This review discusses the endocrine toxicities associated with this new cancer therapy, emphasizing clinical aspects and management.
Keywords: Thyroiditis, Autoimmune hypophysitis, Monoclonal antibodies, Cytotoxic T-lymphocyte antigen 4, Programmed cell death 1 receptor
Abstract
In recent years, immune checkpoint inhibitors have emerged as effective therapies for advanced neoplasias. As new checkpoint target blockers become available and additional tumor locations tested, their use is expected to increase within a short time. Immune-related adverse events (irAEs) affecting the endocrine system are among the most frequent and complex toxicities. Some may be life-threatening if not recognized; hence, appropriate guidance for oncologists is needed. Despite their high incidence, endocrine irAEs have not been fully described for all immunotherapy agents available. This article is a narrative review of endocrinopathies associated with cytotoxic T lymphocyte-associated antigen-4, blockade of programmed death receptor 1 and its ligand inhibitors, and their combination. Thyroid dysfunction is the most frequent irAE reported, and hypophysitis is characteristic of ipilimumab. Incidence, timing patterns, and clinical presentation are discussed, and practical recommendations for clinical management are suggested. Heterogeneous terminology and lack of appropriate resolution criteria in clinical trials make adequate evaluation of endocrine AEs difficult. It is necessary to standardize definitions to contrast incidences and characterize toxicity patterns. To provide optimal care, a multidisciplinary team that includes endocrinology specialists is recommended.
Implications for Practice:
Immune checkpoint inhibitors are already part of oncologists’ therapeutic arsenal as effective therapies for otherwise untreatable neoplasias, such as metastatic melanoma or lung cancer. Their use is expected to increase exponentially in the near future as additional agents become available and their approval is extended to different tumor types. Adverse events affecting the endocrine system are among the most frequent and complex toxicities oncologists may face, and some may be life-threatening if not recognized. This study reviews endocrinopathies associated to immune checkpoint inhibitors available to date. Incidence, timing patterns, and clinical presentation are discussed, and practical recommendations for management are proposed.
Introduction
The response of the immune system to foreign antigens and autoantigens requires precise and balanced reactions to eliminate pathogenic microorganisms and cancerous cells, while at the same time maintaining tolerance. In recent years, anticancer therapies based on blocking of immune checkpoints have emerged as promising options for otherwise untreatable conditions. This new drug generation enhances the immune system to combat cancer cells, resulting in significant long-lasting responses.
However, the toxicities associated with these new therapies differ from those seen with classic cytotoxics because of their acting mechanism [1]. By using these new drugs to aid the immune system to control neoplastic cells, immunologic tolerance can be altered and a higher risk for reactions mediated by self-directed antigens can be incurred. These reactions have been termed immune-related adverse events (irAEs). Along with the skin and gastrointestinal system, the endocrine system is one of the most frequently affected. Endocrine irAEs may present with serious or life-changing symptoms, as in the case of hypophysitis [2]. Unlike with other irAEs, adequate hormone replacement rapidly improves any symptoms, making immune suppression generally unnecessary. This allows patients to continue therapy, from which they may obtain substantial clinical benefit. Therefore, identifying and correctly managing endocrine irAEs are essential to provide optimal care and maximize the potential of these emerging drugs. Herein, we present a narrative review of the endocrine toxicities associated with this new cancer therapy, emphasizing clinical aspects and their management.
Search Methods
We searched PubMed and MEDLINE for clinical trials published before June 30, 2015. Electronic early-release publications were also included. Only articles in English were included. The search terms were “thyroid dysfunction,” “hypothyroidism,” “thyroid toxicity,” “thyroiditis,” “endocrine adverse events,” “Graves,” “hypophysitis,” “adrenal insufficiency,” and “diabetes mellitus, type 1” in association with “immune checkpoint inhibitors” and the names of the immune checkpoints inhibitors available to date. Clinical trials combining agents other than immune checkpoint inhibitors were excluded. Abstracts were reviewed and relevant articles were assessed in full. We searched the proceedings of the 2013–2015 conferences of the American Society of Clinical Oncology for relevant abstracts.
Cytotoxic T Lymphocyte-Associated Antigen-4 Inhibitors
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is a key receptor expressed on the surface of T lymphocytes that transmits an inhibitory signal through its ligand B7-1/2, downregulating T-cell activation [3]. By blocking of CTLA-4, the inhibitory signal is removed and T-cell activation is enhanced. Ipilimumab was the first CTLA-4 inhibitor to demonstrate an overall improvement in survival [4, 5] in metastatic melanoma.
Frequency and severity of irAE with ipilimumab are dose-dependent [6]; endocrine adverse events (AEs) have been reported in 0%–29% of patients (supplemental online Table 1) [7, 8]. Hypophysitis is the most frequent grade 3/4 and dose-limiting endocrine AE, having emerged as a distinctive irAE of ipilimumab; hypothyroidism and hyperthyroidism are next in frequency (Table 1). Fewer data are available for tremelimumab, which is associated with fewer reported endocrinopathies overall (0%–8.3%) [9, 10].
Table 1.
Ranges of reported endocrine adverse events
Hypophysitis is the most frequent grade 3/4 and dose-limiting endocrine AE, having emerged as a distinctive irAE of ipilimumab; hypothyroidism and hyperthyroidism are next in frequency.
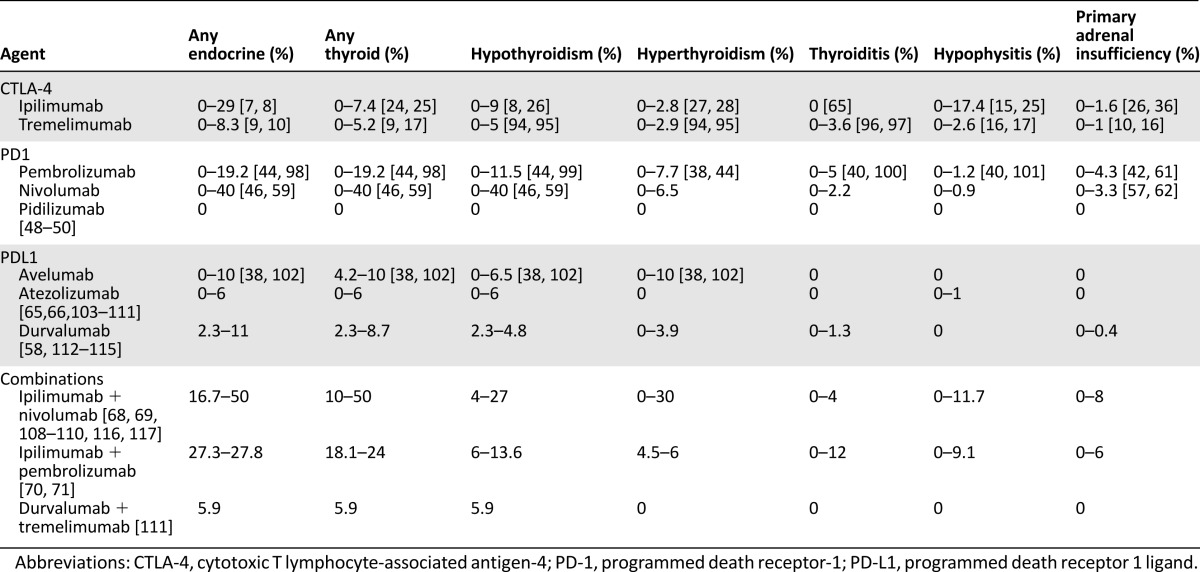
Timing Pattern
IrAEs related to ipilimumab occur in a well-defined characteristic timing pattern. The first to appear are usually those affecting the skin; they develop during the second to third week after starting treatment and can occur up to the 10th week. Digestive system AEs usually occur between weeks 5 and 10, and hepatic AEs from weeks 6 to 14. Endocrine AEs are typically expected to appear after the sixth or seventh week, with a median time to onset of 7–20 weeks (Fig. 1) [11]. Endocrinopathies are not resolved because the function of the gland is often permanently damaged, although hormone production can be successfully substituted. Time to resolution has not been reported for ipilimumab because of the lack of a valid definition in studies.
Figure 1.
Timing pattern of endocrine adverse events.
Hypophysitis
The incidence with ipilimumab varies between 0% and 17.4% [12, 13], with a clear dose-dependent relationship [14, 15]. With tremelimumab, the maximum incidence reported has been 2.6% [17]. Screening for central endocrine toxicity is not always required in clinical trials, and therefore some oligosymptomatic or transitory cases may have been missed. Unlike sporadic hypophysitis, which is more common in women, CTLA-4 blockade-related hypophysitis is more frequently reported in men. A retrospective systematic analysis identified an overall incidence of 8% and described a similar incidence per gender with a male/female ratio of 11:8 [18]; therefore, male predisposition remains to be confirmed. The median time to onset is 11 weeks [12], but onset has been reported as early as 4 weeks after starting treatment [19]. Although the pathogenic mechanism is unknown, the clinical picture and imaging findings resemble those seen with primary lymphocytic hypophysitis. The concomitant use of a drug that triggers autoimmunity suggests that an autoimmune mechanism is involved [20]. Pituitary autoantibodies in sporadic lymphocytic hypophysitis remain to be fully identified. The study of immunotherapy-induced cases can help to improve knowledge of this entity.
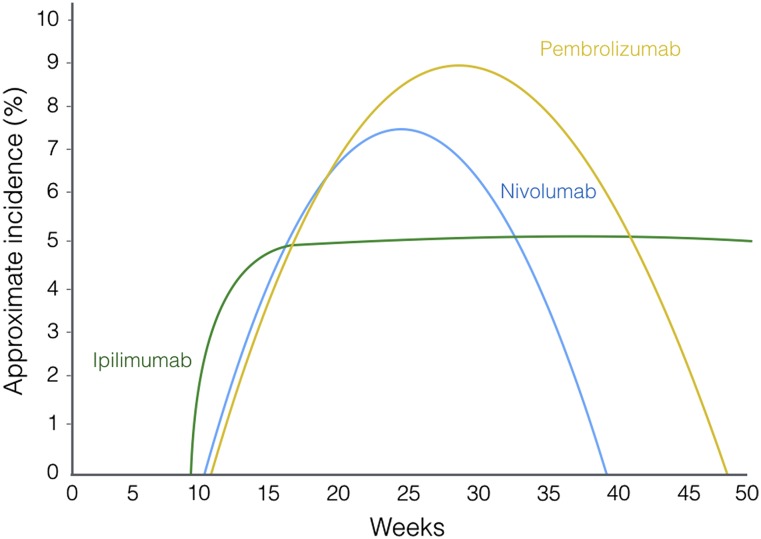
Melanocyte differentiation, melanogenesis, and the pituitary gland share a common pathway. The melanocyte-stimulating hormones (MSHs), which start the process, are neuropeptides produced in the intermediate lobe of the hypophysis [21]. The three forms of MSH are α-MSH, β-MSH, and γ-MSH; α-MSH is the most important melanocortin in pigmentation. They are all derivatives of a large common precursor: proopiomelanocortin (POMC). Interestingly, adrenocorticotropic hormone (ACTH) is also a derivative of POMC and, in fact, MSH-α is a direct derivative of ACTH. ACTH and α-MSH both bind to the melanocortin 2 receptor, triggering melanocyte differentiation (Fig. 2).
Figure 2.
Melanocyte differentiation pathway dependent on MSHα and ACTH. MSHα and ACTH can both bind to MC2R. MC2R is coupled to G-protein α-s, which stimulates adenylate cyclase type I, increasing production of cAMP, hence activating PKA. PKA phosphorylates CREB1, which in turn activates the expression of MITF. MITF regulates transcription of genes coding mitochondrial ribosomal proteins through interactions with TYRP1 and MLANA.
Abbreviations: ACTH, adrenocorticotropic hormone; AMP, adenosine monophosphate; CREB1, cAMP response element-binding protein 1; MC2R, melanocortin 2 receptor; MITF, microphthalmia-associated transcription factor; MLANA, melan-A; MSHα, melanocyte-stimulating hormone α; PKA, cAMP-dependent protein kinase; TYRP1, tyrosinase-related protein 1.
Recent evidence suggests that various members of this pathway may be acting as antigens to T cells activated by CTLA-4 blockade. In a patient who responded to ipilimumab for advanced melanoma, regressing tumor tissue and a skin rash were infiltrated with CD8+ T cells specific for melan-A, also known as MART-1, a melanocyte-differentiation specific antigen regulated by microphthalmia-associated transcription factor (MITF) [22]. Tyrosinase-related protein 1 (TYRP1) is also regulated by MITF and has also been reported in activated T cells in melanoma. Melan-A and TRYP1 are both final steps in the melanocyte differentiation pathway. The relationship between this pathway and tumors that respond to CTLA-4 blockers is not exclusive to melanomas: POMC is also expressed in lung cancer tissue [23]. Most cases of hypophysitis present with ACTH deficiency; hence, there could be a common peptide acting as an antigen for ipilimumab-induced T cells that could help to identify autoantibodies to the pituitary gland that are unknown to date. To date, no pathologic study of CTLA4-induced hypophysitis is available because it is unnecessary for diagnosis and thus would raise ethical issues.
Thyroid Disorders
Ipilimumab induces thyroid disorders in 0%–7.4% of the patients treated [24, 25]. Hypothyroidism (0%–9%) [8, 26] is the most frequent, followed by hyperthyroidism (0%–2.8%) [27, 28], whereas thyroiditis has not been reported. On the other hand, thyroid disorders are the most frequent endocrine irAE produced by tremelimumab (0%–5.2%), with a pattern similar to that seen with ipilimumab [9, 17].
Rare Endocrinopathies
A rare AE is Graves’ ophthalmopathy. Different CTLA-4 polymorphisms are associated with Graves’ ophthalmopathy [29, 30]. This condition is characterized by T-lymphocyte infiltration of the retrobulbar tissue [31]. However, thyroid-stimulating hormone (TSH) receptor antibodies (anti-TSIAbs) play an important role through the activation of fibroblasts and adipocytes [32]. This could explain the low incidence described with CTLA-4 inhibitors. Interestingly, reported cases have developed in euthyroid patients, with dramatic clinical presentations and positive anti-thyroid peroxidase (anti-TPOAbs), thyroglobulin, and anti-TSIAbs [33–35]. No ophthalmopathy cases have been reported with other immune checkpoint inhibitors. In addition, CTLA-4 blockade has been associated with autoimmune adrenalitis in rare cases (0%–1.6%) [16, 36].
Programmed Death 1 Receptor and Ligand Inhibitors
These second-generation monoclonal antibodies target programmed death 1 receptor (PD1) or its ligand (PD-L1). PD1 is another negative regulatory receptor expressed by T and B lymphocytes and natural killer (NK) cells, whose role is limiting their response, hence protecting healthy tissues [37]. Two of these drugs, pembrolizumab and nivolumab, are approved in metastatic melanoma and lung cancer [38–40]. They are being tested in a variety of other solid tumors, including renal cell cancer, ovarian cancer, Hodgkin’s lymphoma, esophageal carcinoma, colorectal cancer, hepatocarcinoma, and head and neck cancers [41–47]. Pidilizumab is an anti-PD1 that induces limited response in solid tumors; only hyperglycemia has been reported as an AE, with no information about autoimmunity [48–50]. Toxicities of this group do not seem to be dose-related [51, 52].
The incidence of endocrinopathies with PD-1/PD-L1 inhibitors may be different from that reported with anti-CTLA-4 agents (supplemental online Table 2), probably because of their distinct mechanism of action. The induction of CTLA-4 in T cells occurs in the early stages of their response to antigens. On the other hand, the PD1/PD-L1 pathway regulates inflammatory reaction both in peripheral tissues and neoplastic microenvironment, being activated downstream of the immune response and in a more peripheral scenario [53]. In summary, blocking CTLA-4 pathway acts on the triggering stage of the autoimmune process, whereas PD1/PD-L1 blockade does it in the modulating phase.
Timing Pattern
Endocrinopathies due to pembrolizumab and nivolumab present similar median times to onset: 10 and 11 weeks, respectively [54, 55]. The main difference seems to be the time to resolution of the event, which is shorter for nivolumab (median time, 38 vs. 48 weeks). However, time to resolution is not defined in these studies, and therefore times may not be comparable.
Hypophysitis
Hypophysitis is infrequent, with a maximum incidence of 1.2% for pembrolizumab and 0.9% for nivolumab [56, 57].
Thyroid Disorders
Thyroid disorders appear to be particularly common in anti-PD1 trials, with a rate of 0%–19.2% for pembrolizumab [44, 58]. Forty percent of thyroidopathies were reported in a phase I study with nivolumab that involved 20 patients [59]. Besides this early trial, the rate of thyroid disorders for nivolumab was reported to be 0%–18.5% [46, 55], similar to the rate with pembrolizumab. For both agents, hypothyroidism is the most prevalent toxicity, followed by hyperthyroidism and thyroiditis; severity is rarely higher than grade 2. Thyroid disorders are more frequent in women, which is consistent with the higher incidence observed in the general population. In a nivolumab series, 26% of the patients who presented thyroid dysfunction presented thyroid autoantibodies at baseline and 36% developed them during treatment; these data support an immune-mediated cause [55]. Hypothyroidism due to chronic autoimmune thyroiditis (also known as Hashimoto’s thyroiditis) and painless thyroiditis have an autoimmune basis; they involve both cellular and humoral immunity, which is not fully understood. Histologic evaluation of autoimmune thyroiditis shows lymphocytic infiltration by both B cells and cytotoxic T cells. Because PD1 is expressed by T and B lymphocytes and NK cells, these cells proliferate when they are blocked. Therefore, anti-PD1 monoclonal antibodies (mAbs) could induce more thyroidopathies than CTLA4 mAbs, which induce only T-lymphocyte proliferation. To our knowledge, neither PD-L1 expression in healthy thyroid tissue nor pathologic thyroid lymphocytic infiltration in cases induced by immune checkpoint inhibitors has been reported.
Histologic evaluation of autoimmune thyroiditis shows lymphocytic infiltration by both B cells and cytotoxic T cells. Because PD1 is expressed by T and B lymphocytes and NK cells, these cells proliferate when they are blocked. Therefore, anti-PD1 mAbs could induce more thyroidopathies than CTLA4 mAbs, which induce only T-lymphocyte proliferation.
Rare Endocrinopathies
Autoimmune adrenalitis is more frequent with the use of PD1/PD-L1 antibodies than with CTLA-4 inhibitors, although the incidences are low: 0%–4.3% with pembrolizumab [42, 61] and 0%–3.3% with nivolumab [57, 62]. Type 1 diabetes mellitus (DM1) has been rarely reported, with only four cases known to date [39, 63, 64]. Nevertheless, because most clinical trials offer information about irAEs developed in a minimum percentage of patients, the incidence could be higher.
PD-L1
Fewer endocrine AEs have been reported with PD-L1 inhibitors, with maximum reported incidence of 10% with avelumab [65], 6% with atezolizumab [66], and 11% with durvalumab [67]. Endocrinopathies due to PD-L1 antibodies are almost exclusively thyroid-related (supplemental online Table 3).
CTLA-4 and PD1/PD-L1 Combined Blockade
Experimental and clinical observations suggest that blocking both the CTLA-4 and the PD1/PD-L1 pathways has a synergistic effect. Ipilimumab and nivolumab are the first combined agents studied for treating patients with metastatic melanoma. Confirmed response was achieved in 59% of the patients, including 11.5% complete response, versus 2% in those receiving ipilimumab monotherapy [68] (Table 2). These results imply a revolution in the treatment of metastatic melanoma. However, as expected for the high response rates, drug-related AEs in studies of combination treatment present a parallel exponential increase in toxicity prevalence and severity. Endocrinopathies occur in 14%–50% of patients treated with these drug combinations, with thyroid AEs the most frequent (7%–28%), followed by hypophysitis (0%–12.8%), with grade 3/4 events occurring in 1%–20% of the cases (supplemental online Table 4).
Table 2.
Endocrine adverse events in phase III studies
Other combinations of CTLA-4 and PD1/PD-L1 pathways are being looked at, as are other diseases, such as renal cell cancer, glioblastoma, and lung cancer [69–71]. The combination of pembrolizumab and ipilimumab has been associated with endocrine AEs in 28% of the patients treated [70, 71]; the thyroid gland is the most affected organ (18.1%–24%), mostly in the form of hypothyroidism (6%–13.6%). As other target blockers become available, such as Lag-3 and Tim-3, new combinations will become an interesting field of study.
Clinical Presentation and Practical Management
Management of endocrine-related AEs is independent of the immune inhibitor type that produces the event. Routine screening with thyroid function tests is recommended at baseline, before each dose, and every 6–12 weeks for the first 6 months after completion of treatment because of the high incidence of thyroidopathies. Evaluating pituitary hormone levels at baseline is useful, although periodic tests are probably not necessary unless symptoms arise.
Hypophysitis
Symptoms derive from hormonal deficiencies and by the mass effect due to the swelling of the gland. The most common presentation includes headache, asthenia, fatigue, nausea, weakness, lethargy, erectile dysfunction, and loss of libido [12, 72]. Visual disturbance is rare because swelling is not usually large enough to affect the optic chiasma [2]. The main differential diagnosis is the appearance of brain metastases; therefore, a brain scan is mandatory. A selective pituitary magnetic resonance imaging scan with gadolinium contrast may show an enlarged pituitary gland, enhancement of the stalk, and heterogeneous enhancement or may be strictly normal [19, 20] (Fig. 3). A rare but crucial possibility to be considered is pituitary metastasis: Such metastases appear in the posterior lobe in 69%–79% of cases, causing diabetes insipidus as a result of the loss of antidiuretic hormone production [73]. Diabetes insipidus in CTLA4-induced hypophysitis is extremely rare [12]; therefore, the presence of a sellar mass in the posterior lobe or the onset of diabetes insipidus should suggest pituitary metastasis and a pituitary biopsy could be considered.
Figure 3.
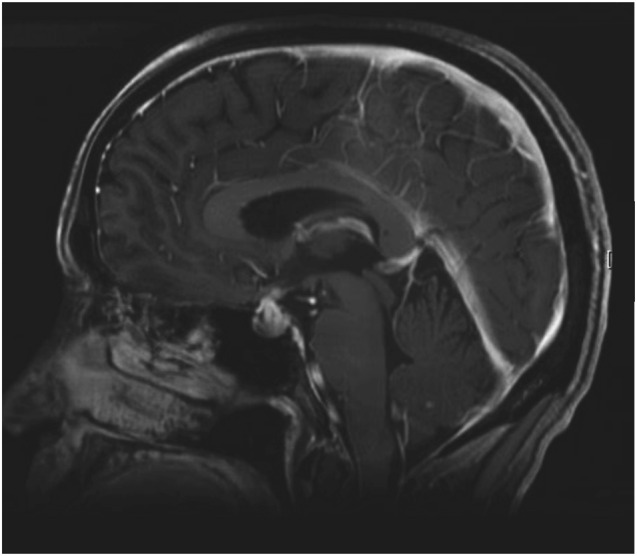
Brain magnetic resonance image of a patient with CTLA4 mAb-induced hypophysitis. Sagital T1-weighted postcontrast image showing an enlarged and intensely enhancing pituitary gland as well as thickening of the stalk affecting the optic quiasm.
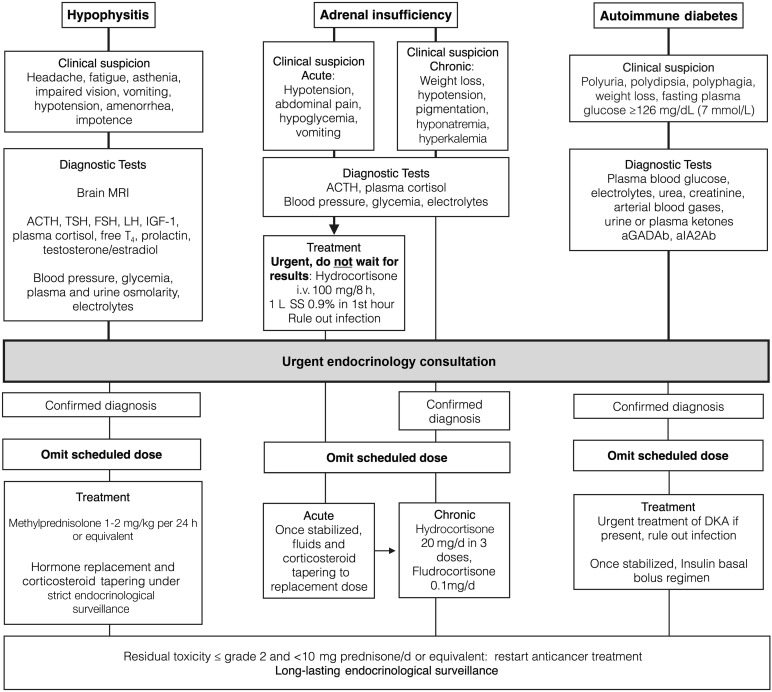
When hypophysitis is suspected, programmed dose should be withheld (Fig. 4). It is necessary to measure both the pituitary hormones and the target tissue hormones for an adequate diagnosis: ACTH, cortisol, TSH, free triiodothyronine, free thyroxine (T4), follicle-stimulating hormone, luteinizing hormone, prolactin, testosterone in men, and estradiol in women. If treatment with steroids is started before blood samples are obtained, an accurate diagnosis of all axes will not be possible, complicating evaluation as to whether long-standing replacement therapy is needed. In order, affected axes are typically the corticotroph, thyrotroph, and gonadotroph axes; not all of them have to be affected simultaneously [19].
Figure 4.
Suggested management of serious endocrine adverse events.
Abbreviations: ACTH, adrenocorticotropic hormone; aGADAb, anti-glutamic acid decarboxilase antibodies; aIA2Ab, anti-tyrosine phosphatase IA2 antibodies; DKA, diabetic ketoacidosis; FSH, follicle-stimulating hormone; IGF-1, insulin-like growth factor-1; LH, luteinizing hormone; MRI, magnetic resonance imaging; SS, saline solution 0.9%; T4, thyroxine; TSH, thyroid-stimulating hormone.
Acute treatment is based on high-dose glucocorticoids. A suggested regimen is methylprednisolone, 1–2 mg/kg per day i.v. for 3–5 days, followed by prednisone, 1–2 mg/kg per day gradually tapered over 4 weeks. An alternative regimen is dexamethasone, 4 mg every 6 hours for 1 week, gradually tapered to 0.5 mg/d, with substitution to replacement doses of hydrocortisone. Slow tapering is imperative because early reduction of glucocorticoids may induce relapse or trigger an adrenal crisis [74]. Treatment with checkpoint inhibitors may be resumed once the corticosteroid dose has been reduced to less than 10 mg prednisone or equivalent per day [75]. As steroids are tapered, hormone replacement therapy should be started when deficiency is present: Cortisol, thyroxine, and testosterone/estradiol should be replaced. Growth hormone replacement is contraindicated in patients with active neoplasias. Hormone deficiencies frequently recover over time, especially those of the thyrotroph and gonadotroph axes. The corticotroph axis is permanently affected in a large proportion of patients [76]. As weaning off steroid replacement is rare [77], careful reassessment of this axis is necessary before discontinuation of steroid substitution. At diagnosis, which patients will develop permanent hypopituitarism cannot be predicted.
Development of hypophysitis does not imply discontinuation of cancer treatment: Replacing pituitary hormones is a safe and common practice among endocrinologists. In addition, limited data suggest that the antitumor activity of CTLA-4 and PD1/PD-L1 mAbs does not seem to be affected by the temporary use of high-dose glucocorticoids [6, 78].
Thyroid Dysfunction
In cancer patients, symptoms of thyroid dysfunction may be difficult to recognize because they can be attributed to the underlying disease or medications. Moreover, distinguishing central hypothyroidism, sick euthyroid syndrome, and thyrotropin suppression due to previous exogenous steroids may be complicated.
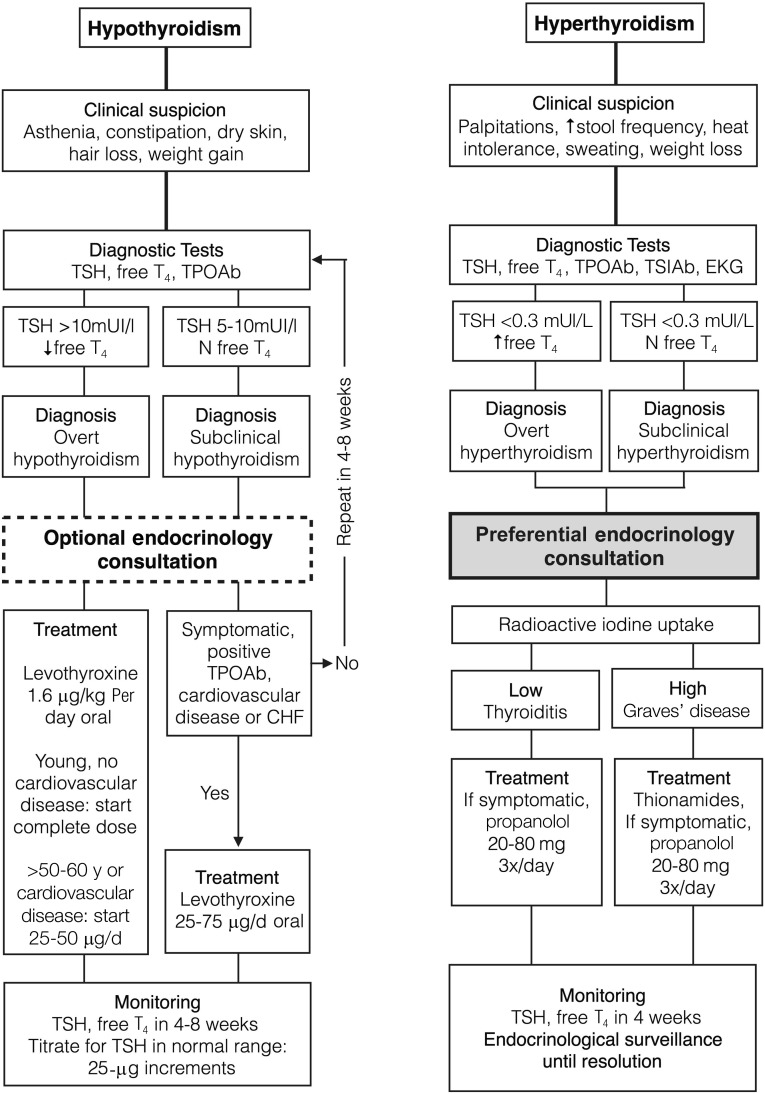
In overt hypothyroidism, treatment is based on substitution with levothyroxine at the usual dose (1.6 μg/kg per day) [34]. Symptoms may take several weeks to resolve, and TSH usually takes even longer. If hypothyroidism is subclinical, treatment is usually not necessary unless the patient becomes symptomatic, anti-TPOAbs are positive, or the patient has a history of cardiovascular disease or heart failure. In this setting, lower doses of levothyroxine are needed to achieve normal TSH (Fig. 5). TSH control is recommended 4–8 weeks after starting or titrating treatment until the normal range is reached [79].
Figure 5.
Suggested management of thyroid dysfunction.
Abbreviations: CHF, congestive heart failure; T4, thyroxine; TPOAb, anti-peroxidase antibodies; TSH, thyroid-stimulating hormone; TSIAb, anti-TSH receptor antibodies.
Hyperthyroidism usually presents with palpitations, increased stool frequency, heat intolerance, sweating, and weight loss. Laboratory investigations reveal a suppressed TSH with high or normal free T4. Primary autoimmune hyperthyroidism or Graves’ disease is caused by anti-TSIAbs, which activate TSH receptors, increasing thyroid hormone synthesis. A second cause of hyperthyroidism reported with immunotherapy is silent or painless thyroiditis, also known as lymphocytic thyroiditis. It classically develops with an initial transient hyperthyroid phase, followed by hypothyroidism, and usually returns to euthyroidism. However, it is not rare for patients with thyroiditis to finally develop permanent hypothyroidism. In painless thyroiditis, hyperthyroidism is produced by destruction of follicular thyroid cells with consequent liberation of thyroid hormone. In clinical trials with immune checkpoint inhibitors, incidence of thyroiditis is as high as 10% with a single agent [80] and 13% with combinations [81]. To distinguish both forms of hyperfunction, radioactive iodine uptake scintigraphy may be performed: An increased uptake >25% indicates an overstimulated gland, usually Graves’ disease; a low uptake favors thyroiditis. Control computed tomography in these patients must be done separately from scintigraphy because i.v. iodine contrast agents saturate the thyroid gland and could invalidate this test. Furthermore, anti-TSIAbs are elevated in Graves’ hyperthyroidism but not in painless thyroiditis, in which anti-TPOAbs may be present.
The treatment varies depending on the cause. In Graves’ disease, antithyroid drugs effectively block thyroid hormone synthesis. Radioactive iodine could be exceptionally used in selected patients with long survival who are symptomatic and intolerant of oral treatment. These treatments have no role in thyroiditis because synthesis does not increase. In symptomatic patients or those at risk for atrial fibrillation, β-blockers may be used.
Trained oncologists can diagnose and treat hypothyroidism. Hyperthyroidism, however, presents diagnostic and management difficulties; thus, endocrinologist consultation is recommended. For most thyroid dysfunctions, interruption of anticancer treatment is not necessary. In rare cases of extreme life-threatening dysfunctions (myxedematous coma and thyrotoxic crisis), treatment should be withheld until the episode is under control.
Graves’ Ophthalmopathy
The typical signs of Graves’ ophthalmopathy are proptosis and periorbital edema. Symptomatic patients will report eye irritation, intolerance to bright lights, eye or retro-orbital pressure or pain, diplopia, and blurred vision. Treatment varies depending on the severity of the condition. Mild symptoms require only local measures (artificial tears, use of eye shades, and raising the head of the bed). However, cases reported with ipilimumab have developed dramatic and severe presentations; these cases have required high-dose glucocorticoid treatment, which led to resolution of symptoms [34].
Primary Adrenal Insufficiency
Adrenal insufficiency can be classified as primary (PAI) if the adrenal glands are impaired or as secondary if it is due to a failure of the hypothalamic-pituitary axis [82]. If adrenal failure due to autoimmune adrenalitis is suspected, blood samples for serum cortisol, ACTH, aldosterone, and renin must be obtained. An early morning serum cortisol of less than 3 μg/dL (80 nmol/L) strongly suggests adrenal insufficiency [83]. ACTH levels distinguish between PAI, in which ACTH is high, and secondary AI due to pituitary impairment, in which ACTH is low or inappropriately normal for a low cortisol. Symptoms of PAI result from the lack of glucocorticoids and mineralocorticoids and are often nonspecific, such as nausea, weakness, fatigue, anorexia, abdominal pain, and weight loss. This clinical presentation could be attributed to the underlying neoplasia or the treatment itself, delaying diagnosis and adequate hormone replacement, which would increase the risk for adrenal crisis. Autoantibodies to the adrenal cortex and 21-hydroxylase are present in more than 90% of patients with autoimmune adrenalitis [84]. There are no data available about the presence of autoantibodies in cases associated with immunotherapy.
Treatment is based on glucocorticoid replacement in both primary and secondary adrenal insufficiency. The most widely used regimen for glucocorticoid substitution is with oral hydrocortisone, aiming to mimic the physiological circadian rhythm. The broadly recommended dose is 10–12 mg/m2 per day [85]. The adrenal cortex produces not only glucocorticoids but also mineralocorticoids; therefore, in PAI mineralocorticoids must be substituted as well.
Adrenal Crisis
The most life-threatening endocrinopathy is adrenal crisis, which may be difficult to diagnose. In healthy persons, the adrenal cortex responds to stressful events with an increase in endogenous cortisol secretion, which is essential for an adequate reactive response. In patients with adrenal insufficiency (primary or secondary), this increase does not occur or is not potent enough. Adrenal crisis usually presents as hypovolemic shock and may be accompanied by fever and generalized abdominal pain and tenderness. In addition, nonspecific symptoms may be present, such as nausea, vomiting, fatigue, lethargy, confusion, or coma [86]. Clinical suspicion is of vital importance given that shock, abdominal pain, and fever could lead to an incorrect diagnosis of acute surgical abdomen and the indication of a potentially fatal surgical intervention.
If suspected, blood samples for serum cortisol, ACTH, electrolytes, and renal function tests should be obtained and treatment initiated immediately thereafter, without waiting for results. Management includes i.v. hydrocortisone, 100 mg every 8 hours, and aggressive fluid replacement: 1–3 L of physiologic saline solution i.v. during the first 12–24 hours with continuous cardiac monitoring and strict control of urine output and volume status. It is also essential to evaluate for infection or sepsis. Endocrinology consultation is highly recommended for acute management, differential diagnosis, and evaluation for long-term replacement needs [87].
In cases of adrenal insufficiency, adequate education is essential to avoid adrenal crisis [88]. Patients must learn essential concepts: how to increase the steroid dose during illness or a medical procedure, the need to obtain medical assistance if the patient is not able to take oral medication, and the importance of wearing a medical alert necklace or bracelet. Patients and their families must be provided with hydrocortisone emergency injections and taught how and when to administer them.
Diabetes Mellitus
Autoimmune diabetes mellitus, known as type 1 (DM1), is characterized by an absolute insulin deficiency caused by autoimmune destruction of pancreatic β cells, implying dependence on insulin therapy. Positivity for autoantibodies to glutamic acid decarboxylase or to the tyrosine phosphatase IA-2 is characteristic of DM1. DM1 patients usually present with ketotic hyperglycemia, which, if left untreated, may develop in diabetic ketoacidosis [89]. Insulinopenia and ketosis produce polyuria, polydipsia, polyphagia, abdominal pain, and weight loss. However, patients receiving immunotherapy are evaluated by health professionals often and may present in a less evolved clinical picture, such as simple mild hyperglycemia. Because differential diagnosis in oligosymptomatic patients may be complicated, we suggest consulting an endocrinologist whenever hyperglycemia presents in a patient who is not known to have diabetes, especially if insulinopenic symptoms are present (Fig. 4).
Management of DM1 includes insulin in a basal-bolus scheme, appropriate education on insulin use, and an approach for hyperglycemia and hypoglycemia. This requires a team of experienced endocrinologists and diabetes nurses for adequate support. Therapeutic objectives in DM1 must be chosen in light of the short life expectancy of these patients. For the same reason, because screening for chronic complications typically starts 5 years from diagnosis [90] in these patients, screening is probably not necessary unless they reach this survival point.
Discussion
The study of endocrine AEs is made difficult by inconsistent terminology in clinical trials. For instance, some trials use terms such as “hypopituitarism,” “hypophysitis,” “pituitary disorder,” and “decrease in serum corticotropin level” as separate entities [4, 10]. Reporting terms separately results in confusion. In the absence of exogenous steroids as assumed in this setting, a decreased ACTH is the laboratory definition of secondary adrenal insufficiency and, hence, of pituitary corticotroph dysfunction; in this context, this dysfunction is probably due to hypophysitis. Moreover, when referring to thyroid disorders, some studies define “decreased blood TSH” [39] as a distinct irAE. These cases probably represent transient thyroiditis or sick euthyroid syndromes, considered a physiological response to illness with no pathological relevance. In addition, the incidence of thyroiditis could be substantially higher because cases reported as hyper- or hypothyroidism could correspond to thyroiditis in the temporary dysfunctional phases. Furthermore, some clinical trials with no endocrine AEs reported offer information only about toxicities occurring above a particular incidence as high as 15% or even 17.5% [24, 91–93]. These aspects contribute to over- and under-reporting of irAEs, limiting determination of precise incidence.
Moreover, most studies addressing endocrinopathies do not define what resolution criteria have been used. The majority of endocrine AEs imply permanent impairment of the function of a gland; therefore, restoration of the function is not applicable as a resolution criterion. An alternative definition could be the resolution of signs and symptoms or normalization of hormone levels through hormone replacement.
Conclusion
IrAEs affecting the endocrine system are frequent and generally mild. Thyroid disorders are the most frequent AE, while hypophysitis is more characteristic of ipilimumab. Combining agents increases the frequency and severity of endocrinopathies. Oncologists and endocrinologists need to be cautious and maintain a high degree of awareness because some conditions could be life-threatening if not recognized. Heterogenicity in clinical trials makes appropriate evaluation of endocrine AEs difficult; therefore, establishing clear definitions is imperative to standardize incidences and characterize toxicity patterns. Despite the irreversibility of most endocrinopathies, correctly managing them allows continuation of immunotherapy with a fairly small impact on the patient’s quality of life. However, laboratory interpretation, the use of specific diagnostic tests, management of substitution medication, and the need for thorough patient education increase the complexity of this toxicity group. From the endocrinologist’s point of view, irAEs offer a novel and unique model for the study of autoimmune endocrine diseases. As the use of immunotherapy increases, the endocrinologist will likely become a valuable member of the multidisciplinary team caring for these patients.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgment
The authors take full responsibility for the content of this review and have not received financial compensation or grants for conducting it.
Footnotes
For Further Reading: Gregory K. Pennock, Laura Q.M. Chow. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. The Oncologist 2015;20:812–822.
Implications for Practice: Immunotherapy is an evolving treatment approach based on the role of the immune system in eradicating cancer. An example of an immunotherapeutic is ipilimumab, an antibody that blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4) to augment antitumor immune responses. Ipilimumab is approved for advanced melanoma and induced long-term survival in a proportion of patients. The programmed death-1 (PD-1) checkpoint inhibitors are promising immunotherapies with demonstrated sustained antitumor responses in several tumors. Because they harness the patient’s own immune system, immunotherapies have the potential to be a powerful weapon against cancer.
Author Contributions
Conception/Design: Elisa González-Rodríguez, Delvys Rodríguez-Abreu
Collection and/or assembly of data: Elisa González-Rodríguez
Data analysis and interpretation: Elisa González-Rodríguez, Delvys Rodríguez-Abreu
Manuscript writing: Elisa González-Rodríguez, Delvys Rodríguez-Abreu
Final approval of manuscript: Elisa González-Rodríguez, Delvys Rodríguez-Abreu
Disclosures
Delvys Rodríguez-Abreu: Bristol-Myers Squibb, Merck Sharp & Dohme, Roche (C/A, SAB). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Pennock GK, Waterfield W, Wolchok JD. Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: How different are these from conventional treatment responses? Am J Clin Oncol. 2012;35:606–611. doi: 10.1097/COC.0b013e318209cda9. [DOI] [PubMed] [Google Scholar]
- 2.Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 6.Boasberg P, Hamid O, O’Day S. Ipilimumab: unleashing the power of the immune system through CTLA-4 blockade. Semin Oncol. 2010;37:440–449. doi: 10.1053/j.seminoncol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Chiarion Sileni V, Pigozzo J, Ascierto PA, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J Exp Clin Cancer Res. 2014;33:30. doi: 10.1186/1756-9966-33-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 9.Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;16:1662–1672. doi: 10.1158/1078-0432.CCR-09-2870. [DOI] [PubMed] [Google Scholar]
- 10.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 12.Dillard T, Yedinak CG, Alumkal J, et al. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 13.Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: A phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maker AV, Yang JC, Sherry RM, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29:455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 17.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 18.Ryder M, Callahan M, Postow MA, et al. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juszczak A, Gupta A, Karavitaki N, et al. Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: A case report and review. Eur J Endocrinol. 2012;167:1–5. doi: 10.1530/EJE-12-0167. [DOI] [PubMed] [Google Scholar]
- 20.Min L, Vaidya A, Becker C. Association of ipilimumab therapy for advanced melanoma with secondary adrenal insufficiency: a case series. Endocr Pract. 2012;18:351–355. doi: 10.4158/EP11273.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 22.Klein O, Ebert LM, Nicholaou T, et al. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res. 2009;15:2507–2513. doi: 10.1158/1078-0432.CCR-08-2424. [DOI] [PubMed] [Google Scholar]
- 23.Hao L, Zhao X, Zhang B, et al. Positive expression of pro-opiomelanocortin (POMC) is a novel independent poor prognostic marker in surgically resected non-small cell lung cancer. Tumour Biol. 2015;36:1811–1817. doi: 10.1007/s13277-014-2784-1. [DOI] [PubMed] [Google Scholar]
- 24.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 25.Di Giacomo AM, Danielli R, Calabrò L, et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy) Cancer Immunol Immunother. 2011;60:467–477. doi: 10.1007/s00262-010-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 28.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagawa T, Hidaka Y, Guimaraes V, et al. CTLA-4 gene polymorphism associated with Graves’ disease in a Caucasian population. J Clin Endocrinol Metab. 1995;80:41–45. doi: 10.1210/jcem.80.1.7829637. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya B, Imrie H, Perros P, et al. Cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphism confers susceptibility to thyroid associated orbitopathy. Lancet. 1999;354:743–744. doi: 10.1016/S0140-6736(99)01465-8. [DOI] [PubMed] [Google Scholar]
- 31.Grubeck-Loebenstein B, Trieb K, Sztankay A, et al. Retrobulbar T cells from patients with Graves’ ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93:2738–2743. doi: 10.1172/JCI117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies TF. The thyrotropin receptors spread themselves around. J Clin Endocrinol Metab. 1994;79:1232–1233. doi: 10.1210/jcem.79.5.7962313. [DOI] [PubMed] [Google Scholar]
- 33.Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg. 2011;27:e87–e88. doi: 10.1097/IOP.0b013e3181ef72a1. [DOI] [PubMed] [Google Scholar]
- 34.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164:303–307. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McElnea E, Ní Mhéalóid A, Moran S, et al. Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab. Orbit. 2014;33:424–427. doi: 10.3109/01676830.2014.949792. [DOI] [PubMed] [Google Scholar]
- 36.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 40.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 41.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch repair deficiency J Clin Oncol 20153315 supplLBA100a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doi T, Piha-Paul SA, Jalal SI, et al. Pembrolizumab (MK-3475) for patients (pts) with advanced esophageal carcinoma: Preliminary results from KEYNOTE-028 J Clin Oncol 20153315 suppl4010a. [Google Scholar]
- Seiwert TY, Haddad RI, Gupta S et al. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): Preliminary results from KEYNOTE-012 expansion cohort. J Clin Oncol 2015;33(15 suppl):LBA6008a.
- Varga A, Piha-Paul SA, Ott PA et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. J Clin Oncol 2015;33(15 suppl):5510a.
- 45.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khoueiry AB, Melero I, Crocenzi TS et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol 2015;33(15 suppl):LBA101a.
- 47.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II Trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J Clin Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins MB, Kudchadkar RR, Sznol M et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. J Clin Oncol 2014;32(15 suppl):9001a.
- 50.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 51.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Joshua AM, Kefford R et al. Association of immune-related thyroid disorders with pembrolizumab (pembro, MK-3475) in patients (pts) with advanced melanoma treated in KEYNOTE-001. J Clin Oncol 2015;33(15 suppl):9050a.
- Weber JS, Antonia SJ, Topalian SL et al. Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): A pooled analysis. J Clin Oncol 2015;33(15 suppl):9018a.
- 56.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 57.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell PH, Plimack ER, Bellmunt J et al. Pembrolizumab (Pembro; MK-3475) for advanced urothelial cancer: Results of a phase IB study. J Clin Oncol 2015;33(7 suppl):296.
- Hamanishi J, Mandai M, Ikeda T et al. Durable tumor remission in patients with platinum-resistant ovarian cancer receiving nivolumab. J Clin Oncol 2015;33(15 suppl):5570a. [DOI] [PubMed]
- 60.Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348:2646–2655. doi: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- 61.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, Gibney GT, Yu B et al. Survival, biomarker, and toxicity analysis of nivolumab (NIVO) in patients that progressed on ipilimumab (IPI). J Clin Oncol 2015;33(15 suppl):9055a.
- 63.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Nishina T, Iwasa S et al. A phase I dose expansion trial of avelumab (MSB0010718C), an anti-PD-L1 antibody, in Japanese patients with advanced gastric cancer. J Clin Oncol 2015;33(15 suppl):4047a.
- Spira AI, Park K, Mazieres J et al. Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol 2015;33(15 suppl):8010a.
- Rizvi NA, Brahmer JR, Ou S-HI et al. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33(15 suppl):8032a.
- 68.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or Monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JH, Vlahovic G, Sahebjam S et al. Preliminary safety and activity of nivolumab and its combination with ipilimumab in recurrent glioblastoma (GBM): CHECKMATE-143. J Clin Oncol 2015;33(15 suppl):3010a.
- Atkins MB, Choueiri TK, Hodi FS et al. Pembrolizumab (MK-3475) plus low-dose ipilimumab (IPI) in patients (pts) with advanced melanoma (MEL) or renal cell carcinoma (RCC): Data from the KEYNOTE-029 phase 1 study. J Clin Oncol. 2015;33(15 suppl):3009a.
- Patnaik A, Socinski MA, Gubens MA et al. Phase 1 study of pembrolizumab (pembro; MK-3475) plus ipilimumab (IPI) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 cohort D. J Clin Oncol 2015;33(15 suppl):8011a.
- 72.Kaehler KC, Piel S, Livingstone E, et al. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: Identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37:485–498. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Kim YH, Lee BJ, Lee KJ, et al. A case of pituitary metastasis from breast cancer that presented as left visual disturbance. J Korean Neurosurg Soc. 2012;51:94–97. doi: 10.3340/jkns.2012.51.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lammert A, Schneider HJ, Bergmann T, et al. Hypophysitis caused by ipilimumab in cancer patients: Hormone replacement or immunosuppressive therapy. Exp Clin Endocrinol Diabetes. 2013;121:581–587. doi: 10.1055/s-0033-1355337. [DOI] [PubMed] [Google Scholar]
- 75.European Medicines Agency: Keytruda. EPAR. 2015. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003820/human_med_001886.jsp&mid=WC0b01ac058001d124. Accessed April 14, 2016.
- 76.Albarel F, Gaudy C, Castinetti F, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172:195–204. doi: 10.1530/EJE-14-0845. [DOI] [PubMed] [Google Scholar]
- 77.Sarnaik AA, Yu B, Yu D, et al. Extended dose ipilimumab with a peptide vaccine: Immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17:896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A, DePril V, Hamid O et al. Evaluation of the effect of systemic corticosteroids for the treatment of immune-related adverse events (irAEs) on the development or maintenance of ipilimumab clinical activity. J Clin Oncol 2009;27(15 suppl):9037a.
- 79.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- 80.Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: A retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119:3687–3695. doi: 10.1002/cncr.28282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, Postow MA, Chesney JA et al. Clinical response, progression-free survival (PFS), and safety in patients (pts) with advanced melanoma (MEL) receiving nivolumab (NIVO) combined with ipilimumab (IPI) vs IPI monotherapy in CheckMate 069 study. J Clin Oncol 2015;33(15 suppl):9004a.
- 82.Neary N, Nieman L. Adrenal insufficiency: Etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes. 2010;17:217–223. doi: 10.1097/MED.0b013e328338f608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hägg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf) 1987;26:221–226. doi: 10.1111/j.1365-2265.1987.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 84.Falorni A, Laureti S, De Bellis A, et al. Italian addison network study: Update of diagnostic criteria for the etiological classification of primary adrenal insufficiency. J Clin Endocrinol Metab. 2004;89:1598–1604. doi: 10.1210/jc.2003-030954. [DOI] [PubMed] [Google Scholar]
- 85.Purnell JQ, Brandon DD, Isabelle LM, et al. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89:281–287. doi: 10.1210/jc.2003-030440. [DOI] [PubMed] [Google Scholar]
- 86.Burke CW. Adrenocortical insufficiency. Clin Endocrinol Metab. 1985;14:947–976. doi: 10.1016/s0300-595x(85)80084-0. [DOI] [PubMed] [Google Scholar]
- 87.Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383:2152–2167. doi: 10.1016/S0140-6736(13)61684-0. [DOI] [PubMed] [Google Scholar]
- 88.Wass JAH, Arlt W. How to avoid precipitating an acute adrenal crisis. BMJ. 2012;345:e6333. doi: 10.1136/bmj.e6333. [DOI] [PubMed] [Google Scholar]
- 89.Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: Etiology, presentation, and management. Pediatr Clin North Am. 2005;52:1553–1578. doi: 10.1016/j.pcl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl):S58–S56. [DOI] [PubMed]
- Cho DC, Sosman JA, Sznol M et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2013;31(15 suppl):4505a.
- Herbst RS, Gordon MS, Fine GD et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol 2013;31(15 suppl):3000a.
- Hamid O, Sosman JA, Lawrence DP et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol 2013;31(15 suppl):9010a.
- 94.Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750–1755. doi: 10.1093/annonc/mdu205. [DOI] [PubMed] [Google Scholar]
- 95.Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 96.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 97.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- Plimack ER, Bellmunt J, Gupta S et al. Pembrolizumab (MK-3475) for advanced urothelial cancer: Updated results and biomarker analysis from KEYNOTE-012. J Clin Oncol 2015;33(15 suppl):4502a.
- Goldberg SB, Gettinger SN, Mahajan A et al. Activity and safety of pembrolizumab in patients with metastatic non-small cell lung cancer with untreated brain metastases. J Clin Oncol 2015;33(15 suppl):8035a.
- Ott PA, Fernandez MEE, Hiret S et al. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol 2015;33(15 suppl):7502a.
- Shitara K, Yamada Y, Yoh K et al. Phase I, open-label, multi-ascending dose trial of avelumab (MSB0010718C), an anti-PD-L1 monoclonal antibody, in Japanese patients with advanced solid tumors. J Clin Oncol 2015;33(15 suppl):3023a.
- Gulley JL, Spigel D, Kelly K et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: A phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J Clin Oncol 2015;33(15 suppl):8034a.
- Kim JW, Bellmunt J, Powles T et al. Clinical activity, safety, and biomarkers of MPDL3280A in metastatic urothelial bladder cancer: Additional analysis from phase IA study. J Clin Oncol 2015;33(7 suppl):297a.
- Brahmer JR, Rizvi NA, Lutzky J et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 2014;32(15 suppl):8021a.
- Lutzky J, Antonia SJ, Blake-Haskins A et al. A phase 1 study of MEDI4736, an anti-PD-L1 antibody, in patients with advanced solid tumors. J Clin Oncol 2014;32(15 suppl):3001a.
- Segal NH, Antonia SJ, Brahmer JR et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol 2014;32(15 suppl):3002a.
- Segal NH, Ou S-HI, Balmanoukian AS et al. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J Clin Oncol 2015;33(15 suppl):3011a.
- 108.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers HJ, Plimack ER, Sternberg C et al. CheckMate 214: A phase III, randomized, open-label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2015;33(15 suppl):TPS4578a.
- 110.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia SJ, Goldberg SB, Balmanoukian AS et al. Phase Ib study of MEDI4736, a programmed cell death ligand-1 (PD-L1) antibody, in combination with tremelimumab, a cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) antibody, in patients (pts) with advanced NSCLC. J Clin Oncol 2015;33(15 suppl):3014a.
- 112.Chiarion-Sileni V, Pigozzo J, Ascierto PA, et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: the expanded access programme in Italy. Br J Cancer. 2014;110:1721–1726. doi: 10.1038/bjc.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Altomonte M, Di Giacomo A, Queirolo P, et al. Clinical experience with ipilimumab 10 mg/kg in patients with melanoma treated at Italian centres as part of a European expanded access programme. J Exp Clin Cancer Res. 2013;32:82. doi: 10.1186/1756-9966-32-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weber J, Hamid O, Amin A, et al. Randomized phase I pharmacokinetic study of ipilimumab with or without one of two different chemotherapy regimens in patients with untreated advanced melanoma. Cancer Immun. 2013;13:7. [PMC free article] [PubMed] [Google Scholar]
- 115.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia SJ, Bendell JC, Taylor MH et al. Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Clin Oncol 2015;33(15 suppl):7503a.
- Antonia SJ, Gettinger SN, Chow LQM et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol 2014;32(15 suppl):8023a.
- 118.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- Bauer TM, McCleod M, Chandler JC et al. An ongoing phase IIIb/IV safety trial of nivolumab (NIVO) in patients (pts) with advanced or metastatic non-small-cell lung cancer (NSCLC) who progressed after receiving 1 or more prior systemic regimens. J Clin Oncol 2015;33(15 suppl):3013a.
- Paz-Ares L, Horn L, Borghaei H et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33(15 suppl):LBA109a.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.