Abstract
Lessons Learned
Pancreatic neuroendocrine tumors versus carcinoid tumors should be examined separately in clinical trials.
Progression-free survival is more clinically relevant as the primary endpoint (rather than response rate) in phase II trials for low-grade neuroendocrine tumors.
Background.
The most common subtypes of neuroendocrine tumors (NETs) are pancreatic islet cell tumors and carcinoids, which represent only 2% of all gastrointestinal malignancies. Histone deacetylase (HDAC) inhibitors have already been shown to suppress tumor growth and induce apoptosis in various malignancies. In NET cells, HDAC inhibitors have resulted in increased Notch1 expression and subsequent inhibition of growth. We present here a phase II study of the novel HDAC inhibitor panobinostat in patients with low-grade NET.
Methods.
Adult patients with histologically confirmed, metastatic, low-grade NETs and an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2 were treated with oral panobinostat 20 mg once daily three times per week. Treatment was continued until patients experienced unacceptable toxicities or disease progression. The study was stopped at planned interim analysis based on a Simon two-stage design.
Results.
Fifteen patients were accrued, and 13 were evaluable for response. No responses were seen, but the stable disease rate was 100%. The median progression-free survival (PFS) was 9.9 months, and the median overall survival was 47.3 months. Fatigue (27%), thrombocytopenia (20%), diarrhea (13%), and nausea (13%) were the most common related grade 3 toxicities. There was one grade 4 thrombocytopenia (7%). These results did not meet the prespecified criteria to open the study to full accrual.
Conclusion.
The HDAC inhibitor panobinostat has a high stable disease rate and reasonable PFS in low-grade NET, but has a low response rate.
Abstract
作者总结
经验
• 在临床试验中, 应该将胰腺神经内分泌瘤与类癌分开进行研究。
• 在低级别神经内分泌瘤的II期临床试验中, 无进展生存作为主要终点 (比缓解率) 更具有临床相关性。
摘要
背景. 神经内分泌瘤 (NET) 最常见的亚型是胰岛细胞肿瘤和类癌, 仅占所有胃肠道恶性肿瘤的2%。已有研究显示组蛋白去乙酰化酶 (HDAC) 抑制剂可以抑制肿瘤进展, 并诱导多种恶性肿瘤发生凋亡。在NET细胞中, HDAC抑制剂可增加Notch1表达以及随后的生长抑制。本文介绍了新型HDAC抑制剂帕比司他治疗低级别NET患者的II期研究。
方法. 入选患者要求: 成年患者, 组织学确诊的转移性低级别NET, 东部肿瘤协作组 (ECOG) 体能状态评分≤2。给予患者帕比司他20 mg每日一次口服, 每周给药三次。治疗直至患者发生不可接受的毒性事件或疾病进展。本研究依据Simon二阶段设计, 在按计划进行期中分析时终止了研究。
结果. 共招募了15例患者, 其中13例治疗反应可评价。未观察到有患者发生缓解, 但疾病稳定率为100%。中位无进展生存 (PFS) 为9.9个月, 中位总生存为47.3个月。最常见的3级毒性事件为疲劳 (27%)、血小板减少 (20%)、腹泻 (13%) 和恶心 (13%)。1例患者发生了4级血小板减少 (7%)。这些结果未能达到研究完成招募的预设标准。
结论. HDAC抑制剂帕比司他治疗低级别NET的疾病稳定率高, 并且具有合理的PFS, 但缓解率非常低。The Oncologist 2016;21:785–786g
Discussion
Therapeutic options for advanced low-grade NET are limited. First-line therapy involves a somatostatin analog, such as octreotide long-acting repeatable. Biologic therapies targeting mTOR pathway and vascular endothelial growth factor have been approved for the treatment of patients with well-differentiated pancreatic NET: sunitinib and everolimus, respectively. Both agents demonstrated the benefit of prolongation of PFS.
Panobinostat is a potent, orally active, pan-HDAC inhibitor that can affect multiple cancer-related pathways, including cell-cycle regulation, differentiation, and apoptosis. Several preclinical studies indicated that HDAC inhibitors, valproic acid and suberoyl bishydroxamic acid, can activate Notch1 signaling, suppress NET tumor markers, and inhibit NET cell growth. Based on the evidence that Notch1 activation can lead to NET differentiation and suppression of tumor growth, we opened a phase II clinical trial of panobinostat in patients with metastatic low-grade NETs.
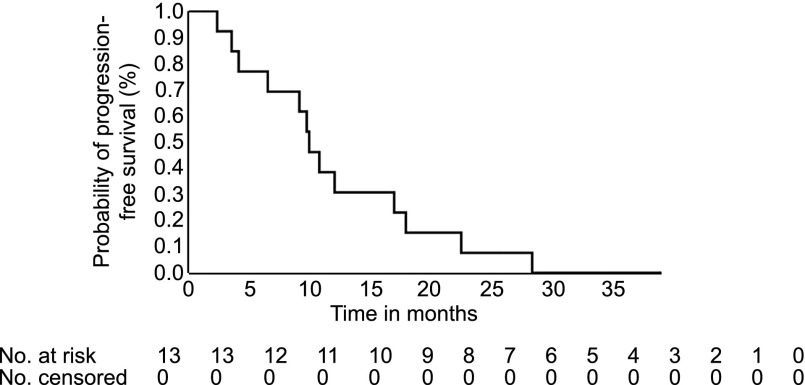
The total number of subjects initially planned in this study was 33. An interim analysis was planned when 13 evaluable patients had been accrued. All patients had stable disease as best response (Fig. 1). Because of lack of objective response, the study was closed to accrual. The median progression-free survival was 9.9 months (90% confidence interval [CI], 4.1–16.9), and the median overall survival was 47.27 months (90% CI, 17.87 to not reached), with the total follow-up time of 5 years. Panobinostat was tolerated relatively well in patients in our study. Fatigue, thrombocytopenia, anorexia, diarrhea, and nausea were the most common grade 3 treatment related toxicities (Adverse Events Table). Patients with pancreatic NETs (4 of 5 patients, 80%) underwent more than 10 cycles of panobinostat in this study. This would seem to be a clinically meaningful delay in time to progression of the cancer. It is increasingly clear that pancreatic NETs and carcinoid subtypes have different biology, respond differently to therapeutic agents, and should be evaluated as separate entities in clinical trials.
Figure 1.
Kaplan-Meier curve for median progression-free survival, which is 9.90 months with 90% confidence interval (4.10–16.9 months).
Our study was terminated early because of the use of objective response rate as the primary outcome measure, which is the shortcoming of this study. We would use progression-free survival (PFS) as the primary endpoint if the study should be repeated. Overall survival is not a practical endpoint for advanced NET studies.
Panobinostat showed favorable clinical activity in different hematologic malignancies, such as in relapsed Hodgkin lymphoma, myelofibrosis, refractory cutaneous T-cell lymphoma, and multiple myeloma, as either single-agent or combination treatment. However, HDAC inhibitors have not demonstrated effectiveness in clinical trials involving solid tumors. Their limitations in solid tumors could be related to drug instability because of short protein kinase half-life, tissue impermeability in tumor microenvironment, drug resistance due to activation of signal transducers and activators of transcription signaling pathway, antiapoptotic effect of nuclear transcription nuclear factor κB, and lack of the specific target in solid tumors. The question regarding the role of Notch1 in well-differentiated NET remains unanswered.
Trial Information
- Disease
Neuroendocrine – pancreatic
- Disease
Neuroendocrine – other
- Stage of disease / treatment
Metastatic / Advanced
- Prior Therapy
No designated number of regimens
- Type of study - 1
Phase II
- Type of study - 2
Single Arm
- Primary Endpoint
Overall Response Rate
- Secondary Endpoint
Progression-Free Survival
- Secondary Endpoint
Overall Survival
- Secondary Endpoint
Toxicity
- Secondary Endpoint
Tolerability
- Additional Details of Endpoints or Study Design
The primary endpoint was the tumor response rate (complete or partial response) using Response Evaluation Criteria in Solid Tumors (RECIST) criteria. A Simon optimal two-stage design was used to test the null hypothesis that the true response rate was 6% versus the alternative hypothesis that it was 20%. With a significance level of 10% and a power of 85%, at least one response was required among the first 13 evaluable patients to proceed to the second stage, where additional patients would be enrolled for a total of 30 evaluable patients.
- Investigator's Analysis
Level of activity did not meet planned clinical trial endpoint.
Drug Information
- Drug 1
- Generic/Working name
Panobinostat
- Trade name
Farydak
- Company name
Novartis
- Drug type
Small molecule
- Drug class
HDAC
- Dose
20 mg per flat dose
- Route
Oral (po)
- Schedule of Administration
Once daily, three times per week
Patient Characteristics
- Number of patients, male
10
- Number of patients, female
5
- Stage
Metastatic low-grade neuroendocrine tumors
- Age
Median (range): 57 (40–80)
- Number of prior systemic therapies
Median (range): Not collected
- Performance Status: ECOG
0 — 10
1 — 5
2 — 0
3 — 0
Unknown —
- Primary Site:
Lung and bronchus, 2
Pancreas, 5
Rectum, 1
Small Intestine, 5
Unknown, 2
- Cancer Types or Histologic Subtypes
Low,* 6
Well Differentiated,* 5
Grade unknown, not stated, or not applicable, 4
Primary Assessment Method
Control Arm: Total Patient Population
- Number of patients screened
15
- Number of patients enrolled
15
- Number of patients evaluable for toxicity
15
- Number of patients evaluated for efficacy
13
- Response assessment CR
n = 0
- Response assessment PR
n = 0
- Response assessment SD
n = 13
- Response assessment PD
n = 0
- (Median) duration assessments PFS
9.9 months, CI: 90
- (Median) duration assessments OS
47.3 months
- Kaplan-Meier time units
Months
Adverse Events
Assessment, Analysis, and Discussion
- Completion
Study completed
- Terminated reason
Did not fully accrue
- Pharmacokinetics / Pharmacodynamics
Not Collected
- Investigator's Assessment
Level of activity did not meet planned clinical trial endpoint
NETs are uncommon tumors arising from the neuroendocrine system. The gastrointestinal (GI) tract, pancreas, and lung are the most common primary tumor sites in patients with NETs [1], and gastroenteropancreatic (GEP) NETs represent approximately 2% of all gastrointestinal malignant neoplasms. The clinical evaluation for NETs should incorporate several key factors, such as anatomic site, histology, grade, differentiation, and hormone secretion. In particular, the anatomic site of origin is now recognized as a key determinant of treatment selection [2]. According to the 2010 World Health Organization grading system for GEP NETs, there are three grades (G1, G2, and G3) for differentiation on pathology report, based on Ki-67 and mitotic counts [3]. Well-differentiated NETs include G1 and G2; poorly differentiated NETs are G3.
For patients with well-differentiated and functional tumors, somatostatin analogs such as octreotide long-acting repeatable are the mainstay of treatment for control tumor growth as well as symptomatic control, with improved PFS and quality of life [4, 5]. Recent randomized studies for biologic therapies targeting mTOR pathway and vascular endothelial growth factor demonstrated prolongation of PFS compared with placebo for pancreatic NET; for example, everolimus (11 vs. 4.6 months) [6] or sunitinib (11.4 vs. 5.5 months) [7].
Early studies suggested that activation of the Notch pathway can lead to neuroendocrine differentiation in gastrointestinal carcinoid tumor [8] and inhibit NET cell growth [9]. Of note, it is well recognized that Notch can function as either an oncogene or a tumor suppressor, depending on the cell type [10–12]. Both valproic acid (VPA) and suberoyl bishydroxamic acid, two histone deacetylase (HDAC) inhibitors, can activate Notch1 signaling, suppress neuroendocrine tumor markers, and inhibit NET cell growth both in vitro and in vivo [13, 14]. Furthermore, our group previously conducted a pilot clinical trial of VPA for patients with advanced carcinoid cancer. Five of the six patients (62.5%) assessable for radiographic response were noted to have stable disease by RECIST, and one patient with an unconfirmed partial response was noted to have a 40-fold increase in Notch1 mRNA levels [15].
Panobinostat (LBH589) is a potent, orally active, pan-HDAC inhibitor that can affect multiple cancer-related pathways via nonhistone protein targets, including cell-cycle regulation, differentiation, and apoptosis [16, 17]. Based on the role of Notch1 activation in suppression of NET tumor markers and tumor growth, we opened a phase II clinical trial of HDAC inhibitor panobinostat in patients with metastatic low-grade NETs.
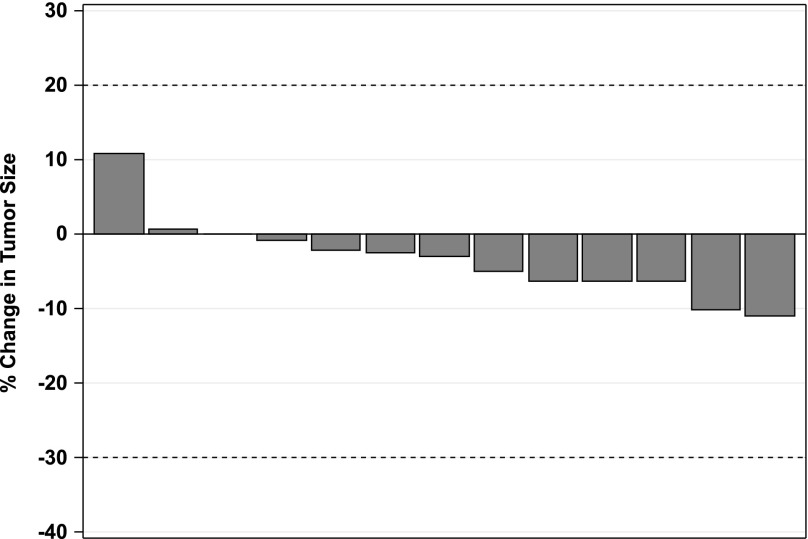
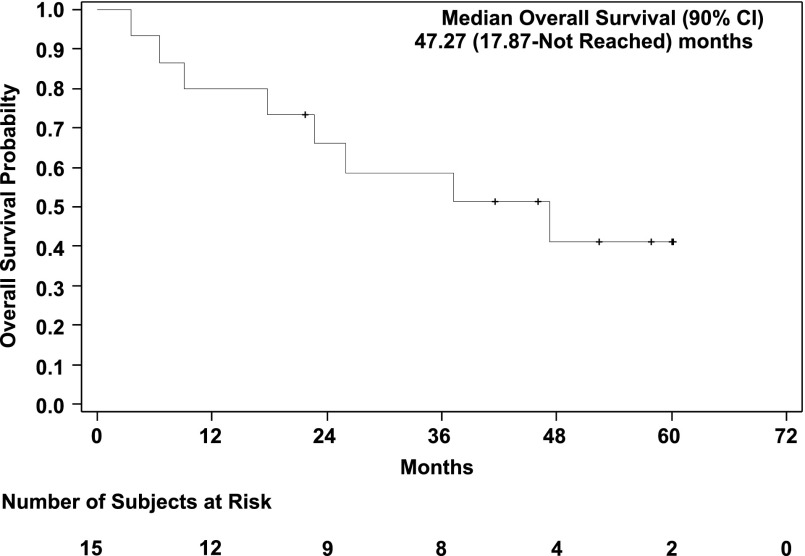
The total number of subjects initially planned for this study was 33. An interim analysis was planned when 13 evaluable patients had been accrued. At least one response was required among the first 13 evaluable patients to proceed to the second stage, where additional patients would be enrolled. Among 15 patients who were accrued, 66.6% were male, age range 40–80 years old, 54% carcinoid, and 33% pancreatic NET (Table 1). Because of the lack of objective response in the interim analysis, the study was closed. Panobinostat was tolerated relatively well in patients in our study. The most common toxicities of all grades were thrombocytopenia, fatigue, diarrhea, nausea, hyperglycemia, hypertriglyceridemia, and thyroid dysfunction. Fatigue (27%), thrombocytopenia (20%), anorexia (20%), diarrhea (13%), and nausea (13%) were the most common treatment-related grade 3 toxicities. There was one case (7%) of grade 4 toxicity of thrombocytopenia (Adverse Events Table). Eight patients needed dose modifications because of adverse events, such as thrombocytopenia, neutropenia, and fatigue, during their treatment courses (data not shown). All patients had stable disease as best response (Fig. 1). Three of 13 patients underwent only 2 cycles of treatment, whereas 7 patients underwent more than 10 cycles of treatment (Table 2). Median progression-free survival was 9.9 months (90% CI, 4.1–16.9) (Fig. 2), and median overall survival was 47.27 months (90% CI, 17.87 to not reached), with the total follow-up time of 5 years (Fig. 3).
Table 1.
Patient characteristics

Table 2.
Patient tumor characteristics and treatment outcome
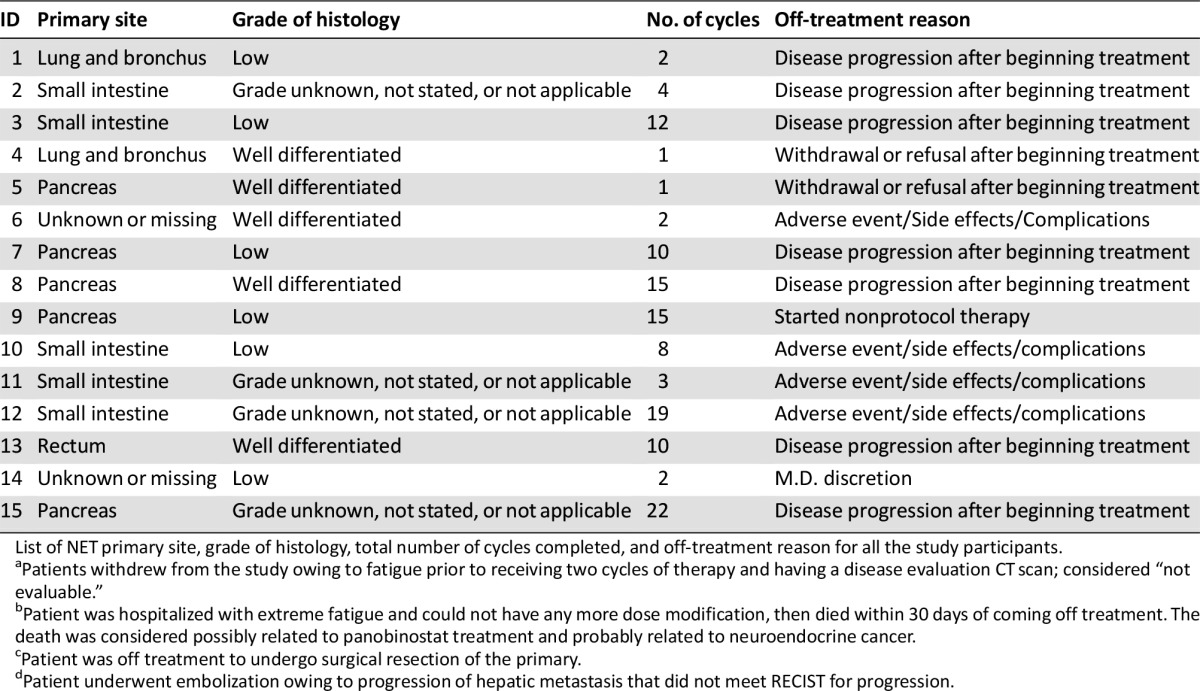
Figure 2.
Percentage change in tumor size based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Waterfall plot of radiographic changes from baseline to best response of 13 evaluable patients, revealing that every patient (100%) has stable disease. Stable disease is defined as neither sufficient shrinkage to qualify for partial response (less than 30% decrease in the sum of the longest diameters of target lesions) nor sufficient increase to qualify for progressive disease (at least 20% increase in the sum of the longest diameters of target lesions).
Figure 3.
Kaplan-Meier curve for overall survival, which is 47.27 months with 90% confidence interval, with a follow-up time of 5 years.
Abbreviation: CI, confidence interval.
Kaplan-Meier curve for median progression-free survival, which is 9.90 months with 90% confidence interval (4.10–16.9 months).
Patients with pancreatic NETs (four of five patients; one patient withdrew early) underwent more than 10 cycles of panobinostat in this study, although our sample size is too small to draw any conclusion. It is increasingly clear that pancreatic NETs (PNETs) and carcinoid subtypes have different biology, respond differently to therapeutic agents, and should be evaluated as separate entities in clinical trials [18]. Examination of the PNETs and GI carcinoid demonstrated only a few areas of overlap in the accumulation of genetic aberrations, using comparative genomic hybridization, microsatellite analysis, and sequencing techniques [19]. As to treatment, PNETs are more sensitive to cytotoxic chemotherapy than carcinoid tumors, such as streptozocin, capecitabine, and temozolomide, as shown in early clinical trials as well as recent retrospective clinical studies [20–22].
Our study was terminated early because of the use of objective response rate as the primary outcome measure, which is the shortcoming of this study. We would use progression-free survival as the primary endpoint if the study should be repeated. The challenge in phase II studies in NETs is the small patient population available and the often long survival postprogression [23]. Overall survival is not a practical endpoint for advanced NET studies, because of the nature of indolent disease and the availability of multiple sequential therapies [2].
Panobinostat showed favorable clinical activity in different hematologic malignancies, such as in relapsed Hodgkin lymphoma [24], myelofibrosis [25], refractory cutaneous T-cell lymphoma [26], and multiple myeloma [27], as either single-agent or combination treatment. However, the results of recent clinical trials of panobinostat in solid tumors are disappointing, including castration-resistant prostate cancer [28], metastatic renal cell cancer [29], and pancreatic cancer [30]. Generally, HDAC inhibitors have not demonstrated effectiveness in clinical trials involving solid tumors. Their limitations in solid tumors could be related to drug instability due to short protein kinase half-life [31, 32], tissue impermeability in tumor microenvironment, drug resistance due to activation of the signal transducers and activators of transcription signaling pathway [33, 34], antiapoptotic effect of nuclear transcription nuclear factor κB [35], and lack of the specific target in solid tumors [36]. There was no study participant in our trial who underwent pretreatment and posttreatment biopsy for Notch1 activity, which is the limitation of this study.
In conclusion, panobinostat has a high stable disease rate and reasonable PFS in low-grade NET. Oral panobinostat at a dose of 20 mg three times weekly was relatively well tolerated in patients in this study. Four of five patients with PNETs had durable stable disease on panobinostat, which is encouraging. Further studies of panobinostat in combination with other agents are indicated in patients with NETs. The questions regarding off-target effects of panobinostat and the role of Notch1 in well-differentiated NET remain unanswered. In future clinical trials, it is important to develop pharmacodynamics biomarkers to predict treatment response in patients.
Supplementary Material
Acknowledgments
This work was supported by University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520 and the Novartis Pharmaceutics Corporation.
Footnotes
ClinicalTrials.gov Identifier: NCT00985946
Sponsor: Novartis
Principal Investigator: Noelle K. LoConte
IRB Approved: Yes
Click here to access other published clinical trials.
Disclosures
Kyle D. Holen: AbbVie (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Kunz PL. Carcinoid and neuroendocrine tumors: Building on success. J Clin Oncol. 2015;33:1855–1863. doi: 10.1200/JCO.2014.60.2532. [DOI] [PubMed] [Google Scholar]
- 3.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 4.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 5.Toumpanakis C, Caplin ME. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol. 2013;40:56–68. doi: 10.1053/j.seminoncol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 8.Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90:4350–4356. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Yan S, Wong F, et al. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138:1137–1142; discussion 1142. doi: 10.1016/j.surg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61:3200–3205. [PubMed] [Google Scholar]
- 11.Radtke F, Raj K. The role of Notch in tumorigenesis: Oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto Y, Maitra A, Ghosh B, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 13.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. The Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 14.Greenblatt DY, Cayo M, Ning L, et al. Suberoyl bishydroxamic acid inhibits cellular proliferation by inducing cell cycle arrest in carcinoid cancer cells. J Gastrointest Surg. 2007;11:1515–1520; discussion 1520. doi: 10.1007/s11605-007-0249-1. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed TA, Holen KD, Jaskula-Sztul R, et al. A pilot phase II study of valproic acid for treatment of low-grade neuroendocrine carcinoma. The Oncologist. 2011;16:835–843. doi: 10.1634/theoncologist.2011-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): Successes and challenges. Cancer Lett. 2009;280:233–241. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JM, Hackanson B, Lübbert M, et al. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenetics. 2010;1:117–136. doi: 10.1007/s13148-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: Consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zikusoka MN, Kidd M, Eick G, et al. The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer. 2005;104:2292–2309. doi: 10.1002/cncr.21451. [DOI] [PubMed] [Google Scholar]
- 20.Murray-Lyon IM, Eddleston AL, Williams R, et al. Treatment of multiple-hormone-producing malignant islet-cell tumour with streptozotocin. Lancet. 1968;2:895–898. doi: 10.1016/s0140-6736(68)91058-1. [DOI] [PubMed] [Google Scholar]
- 21.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71:663–670. doi: 10.1007/s00280-012-2055-z. [DOI] [PubMed] [Google Scholar]
- 23.Yao JC, Lagunes DR, Kulke MH. Targeted therapies in neuroendocrine tumors (NET): Clinical trial challenges and lessons learned. The Oncologist. 2013;18:525–532. doi: 10.1634/theoncologist.2012-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younes A, Sureda A, Ben-Yehuda D, et al. Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: Results of a phase II study. J Clin Oncol. 2012;30:2197–2203. doi: 10.1200/JCO.2011.38.1350. [DOI] [PubMed] [Google Scholar]
- 25.DeAngelo DJ, Mesa RA, Fiskus W, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013;162:326–335. doi: 10.1111/bjh.12384. [DOI] [PubMed] [Google Scholar]
- 26.Duvic M, Dummer R, Becker JC, et al. Panobinostat activity in both bexarotene-exposed and -naïve patients with refractory cutaneous T-cell lymphoma: Results of a phase II trial. Eur J Cancer. 2013;49:386–394. doi: 10.1016/j.ejca.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: Panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122:2331–2337. doi: 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 28.Rathkopf D, Wong BY, Ross RW, et al. A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2010;66:181–189. doi: 10.1007/s00280-010-1289-x. [DOI] [PubMed] [Google Scholar]
- 29.Hainsworth JD, Infante JR, Spigel DR, et al. A phase II trial of panobinostat, a histone deacetylase inhibitor, in the treatment of patients with refractory metastatic renal cell carcinoma. Cancer Invest. 2011;29:451–455. doi: 10.3109/07357907.2011.590568. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Cao Q, Dudek AZ. Phase II study of panobinostat and bortezomib in patients with pancreatic cancer progressing on gemcitabine-based therapy. Anticancer Res. 2012;32:1027–1031. [PubMed] [Google Scholar]
- 31.Morita S, Oizumi S, Minami H, et al. Phase I dose-escalating study of panobinostat (LBH589) administered intravenously to Japanese patients with advanced solid tumors. Invest New Drugs. 2012;30:1950–1957. doi: 10.1007/s10637-011-9751-0. [DOI] [PubMed] [Google Scholar]
- 32.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fantin VR, Loboda A, Paweletz CP, et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer Res. 2008;68:3785–3794. doi: 10.1158/0008-5472.CAN-07-6091. [DOI] [PubMed] [Google Scholar]
- 35.Fantin VR, Richon VM. Mechanisms of resistance to histone deacetylase inhibitors and their therapeutic implications. Clin Cancer Res. 2007;13:7237–7242. doi: 10.1158/1078-0432.CCR-07-2114. [DOI] [PubMed] [Google Scholar]
- 36.Slingerland M, Guchelaar HJ, Gelderblom H. Histone deacetylase inhibitors: An overview of the clinical studies in solid tumors. Anticancer Drugs. 2014;25:140–149. doi: 10.1097/CAD.0000000000000040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.