Sir
Hyperhidrosis is a common ailment which greatly affects quality of life. There are several treatment options, but there is a lack of consensus and clear guidelines for treatment of these patients.[1,2] Medical (e.g., Botulinum toxin type A) and surgical treatments have been used to control or reduce excessive sweating with variable success.[3,4,5] Only surgical modalities have been capable of conferring a permanent solution.[4] Recently, few studies have been done for clinical evaluation of radiofrequency (RF) energy in the treatment of primary axillary hyperhidrosis(PAH).[6,7] But there is limited published evidence. We report in this paper, successful treatment of refractory PAH with fractionated microneedle radiofrequency (FMR) in a 29-year-old female patient, confirmed with routine histopathological staining.
A 29-year-old female patient had severe recalcitrant bilateral axillary hyperhidrosis since puberty (grade 4 in Hyperhidrosis Disease Severity Scale). Personal history was otherwise uneventful. The patient identified many triggers such as emotion, temperature, or physical effort for the hyperhidrosis that occurred in more than ten attacks per day. Topical treatment with aluminium chloride had produced an unsatisfactory response and she stopped the use of this agent 6 months prior to meeting us for treatment. Systemic therapy using an anticholinergic preparation had to be stopped because of adverse effects. Botulinum toxin type A was used in the affected area in frequent sessions with limited results. We then started the patient on four treatment sessions of FMR (INFINI; Lutronic, Goyang, Korea) at 2-week intervals [Figure 1]. Results of the Iodine starch test (Minor sweat test) are shown in Figure 2. The INFINI procedure is performed in foursteps [Figure 3]. After marking the area to be treated, a topical eutectic mixture of 2.5% lidocaine hydrochloric acid and 2.5% prilocaine (EMLA; Astra- Zeneca, Sodertalje, Sweden) was applied to the axilla under occlusion 30 minutes before therapy. The treatment settings were 2.5-to 3-mm Microneedle penetrating depth, 6 to 9 level intensity, and 140- to 180-ms RF time. Multiple placements with the hand piece are required to deliver Microneedle RF [Figure 3]. Immediately after treatment minimal undesirable effects such as mild skin irritation and erythema and pin point bleeding were noted [Figure 3] but none were so severe as to cause termination of treatment. Results of the Iodine starch test after treatment are shown in Figure 4. During the 6 month follow-up, the patient had no complaints of hyperhidrosis. The FMR is a minimally invasive method for delivering thermal energy to the target tissue without destroying the epidermis, by using rapid penetration with microneedle. This treatment has demonstrated excellent efficacy for skin rejuvenation, face lifting, large pores and acne scars.[8,9] The novel bipolar radiofrequency device can destroy eccrine glands which are considered as a thermolysis at the interface of the deep dermis and subcutis, minimizing damage to surrounding tissue. The results demonstrate that microwave technology is well suited for targeting sweat glands while allowing for protection of both the upper skin layers and the structures beneath the subcutaneous fat.[10] The mechanism of reduced hyperhidrosis by way of the radiofrequency device is that the sweat glands are destroyed by heating the interface where they are located, that is, the hypodermal interface. Therefore, the device works by heating and cooling at the same time.[7,10,11] The radiofrequency from the tips of the microneedles causes direct thermal injury which decreases the size and density of the apocrine glands.[12] In our patient, a clear reduction of viable sweat gland structures was seen when her before treatment (“baseline”) sample was compared with the follow-up time samples. There was no significant histopathologic evidence of adverse effects on other cutaneous structures in this study. Thus, FMR can be considered as a safe, effective and non-invasive technique for treatment of primary axillary hyperhidrosis but more studies are needed to confirm the persistent effect and histopathological alternation during treatment with FMR.
Figure 1.
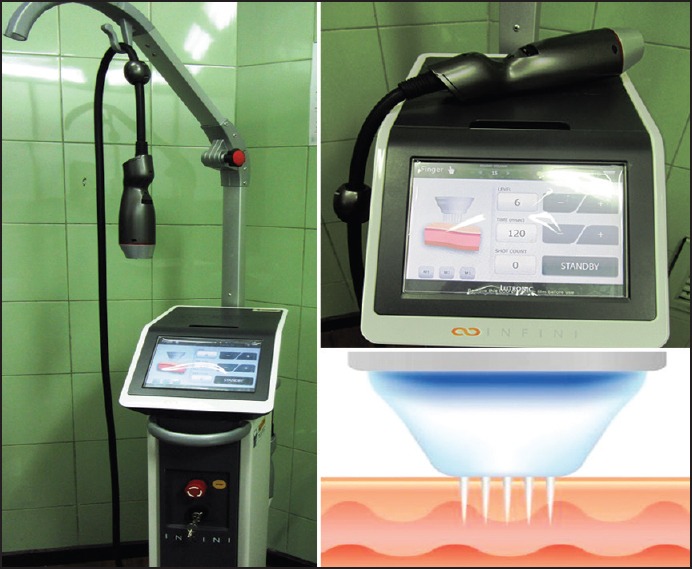
The INFINI console, headpiece. (INFINI; Lutronic, Goyang, Korea)
Figure 2.

Starch-iodine tests. Starch-iodine photographs of the left axilla of subject at baseline (blue-black sedimentation)
Figure 3.
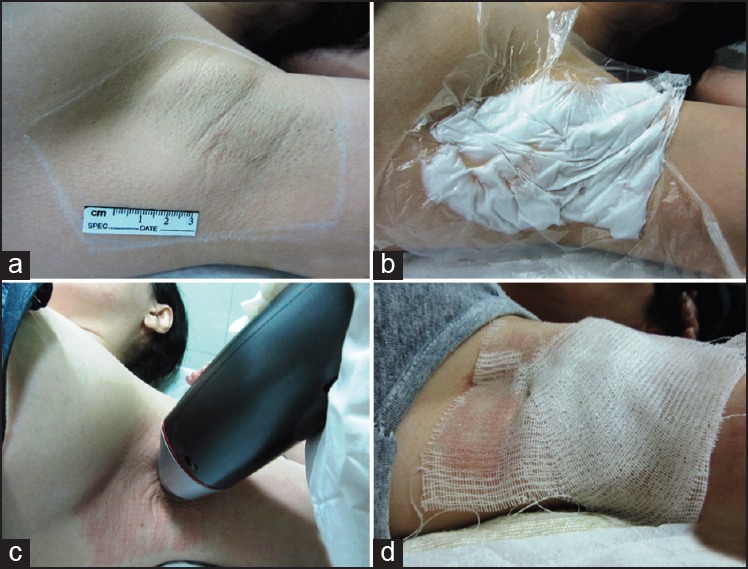
Procedure description. The INFINI procedure is performed in foursteps. (a) Step 1 –sizing the area to be treated and marking that area after starch-iodine test. (b) Step 2 –Topical anaesthesia under occlusion. (c) Step 3 –place hand piece on designated areas to deliver the therapy. (d) Step 4 –dressing with sterile vaseline gauzes
Figure 4.
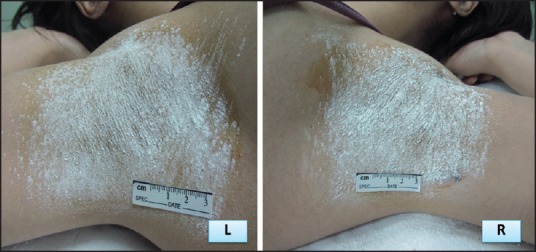
Starch-iodine test. Normal results of starch-iodine testafter foursessions of fractionated microneedle radiofrequency. (Wight sedimentation)
References
- 1.Amini M, Harmsze AM, Tupker RA. Patient's estimation of efficacy of various hyperhidrosis treatments in a dermatological clinic. Acta Derm Venereol. 2008;88:356–62. doi: 10.2340/00015555-0440. [DOI] [PubMed] [Google Scholar]
- 2.Yadalla HK, Ambika H, Chawla S. A case of idiopathic unilateral circumscribed hyperhidrosis. Indian J Dermatol. 2013;58:163. doi: 10.4103/0019-5154.108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doft MA, Hardy KL, Ascherman JA. Treatment of hyperhidrosis with botulinum toxin. Aesthet Surg J. 2012;32:238–44. doi: 10.1177/1090820X11434506. [DOI] [PubMed] [Google Scholar]
- 4.Solish N, Bertucci V, Dansereau A, Hong HC, Lynde C, Lupin M, et al. Canadian Hyperhidrosis Advisory Committee. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: Recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33:908–23. doi: 10.1111/j.1524-4725.2007.33192.x. [DOI] [PubMed] [Google Scholar]
- 5.Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55:8–14. doi: 10.4103/0019-5154.60343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong HC, Lupin M, O’shaughnessy KF. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol Surg. 2012;38:728–35. doi: 10.1111/j.1524-4725.2012.02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim M, Shin JY, Lee J, Kim JY, Oh SH. Efficacy of fractional microneedle radiofrequency device in the treatment of primary axillary hyperhidrosis: A pilot study. Dermatology. 2013;227:243–9. doi: 10.1159/000354602. [DOI] [PubMed] [Google Scholar]
- 8.Lolis MS, Goldberg DJ. Radiofrequency in cosmetic dermatology: A review. Dermatol Surg. 2012;38:1765–76. doi: 10.1111/j.1524-4725.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- 9.Cho SI, Chung BY, Choi MG, Baek JH, Cho HJ, Park CW, et al. Evaluation of the clinical efficacy of fractional radiofrequency microneedle treatment in acne scars and large facial pores. Dermatol Surg. 2012;38:1017–24. doi: 10.1111/j.1524-4725.2012.02402.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JE, O’shaughnessy KF, Kim S. Microwave thermolysis of sweat glands. Lasers Surg Med. 2012;44:20–5. doi: 10.1002/lsm.21142. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Chang KY, Suh DH, Song KY, Ryu HJ. The efficacy of a microwave device for treating axillary hyperhidrosis and osmidrosis in Asians: A preliminary study. J Cosmet Laser Ther. 2013;15:255–9. doi: 10.3109/14764172.2013.807114. [DOI] [PubMed] [Google Scholar]
- 12.Grice K, Sattar H, Baker H. The effect of ambient humidity on transepidermal water loss. J Invest Dermatol. 1972;58:343–6. doi: 10.1111/1523-1747.ep12540526. [DOI] [PubMed] [Google Scholar]


