Abstract
Introduction
We describe a family with two first-degree cousins who presented with similar phenotypes characterized by neonatal intracranial hemorrhage and subsequent onset of thrombosis.
Patients/Methods
We enrolled the two affected patients, five unaffected family members and fifty-five normal controls. Clinical, laboratory, and radiological characteristics of patients were obtained. Exome sequencing was performed for the older affected child. PROC c.811 C>T was genotyped by PCR in patients, family members, and controls. Protein C amidolytic activity and antigen were measured using the STACHROM® protein C kit and ELISAs. To define functional abnormalities caused by the patients' mutation, recombinant wildtype protein C and its mutants R229W, R229Q and R229A were studied.
Results
For the two cousins, Protein C amidolytic activity was 61% and 59% and antigen was 57% and 73% (nl 70–140%), respectively. Exome sequencing revealed a homozygous variant in exon 9 of the protein C (PROC) gene c.811 C>T (R229W). The R229W mutation is located in the calcium binding loop of protein C's protease domain that mediates thrombomodulin interactions. Recombinant R229W-protein C mutant was strikingly defective in rate of activation by thrombin: thrombomodulin, suggesting an in vivo deficit in these children for generation of activated protein C.
Conclusions
These cases emphasize that protein C and activated protein C are important in maintaining the integrity of the brain vascular endothelium in humans. Moreover, routine protein C assays utilizing snake venom protease fail to detect protein C mutants that are resistant to thrombin:thrombomodulin activation.
Keywords: Intracranial Hemorrhage, Mutation, Neonate, Protein C, Thrombosis
Introduction
Protein C (PC) deficiency is an autosomal trait in which individuals with homozygous protein C gene (PROC) mutations usually have very low PC levels and present soon after birth with massive purpura fulminans whereas heterozygous PC deficient adults have increased risk for venous thrombosis[1–4]. Activated protein C (APC) is anticoagulant by inactivating factors Va and VIIIa. APC also exerts remarkable cytoprotective activities that dampen inflammatory response and apoptosis, regulate gene expression, and reduce vascular permeability by stabilizing endothelial barrier[5, 6].
Here we report two first cousins with a homozygous PROC R229W mutation associated with a unique phenotype of massive perinatal intracranial bleeding and delayed onset of clinical thrombosis until the age of 4 months. Both PC antigen and functional amidolytic activity, based on standard commercially available tests, ranged from 57–73%, so diagnosis was established only after gene sequencing which identified the PROC R229W mutation. Remarkably, this mutation reduced by >99% its rate of activation by thrombin:thrombomodulin (TM).
Methods
Patients
Two patients, first-degree cousins who were affected by perinatal intracranial hemorrhage and subsequent severe thrombosis, were enrolled in this study as were five unaffected family members and 55 Saudi controls. Details of clinical, laboratory, and radiological characteristics of patients were obtained. PC amidolytic activity was measured using STACHROM® protein C kit and antigen was determined using commercial ELISA techniques. The study was approved by the institutional review board of the College of Medicine, King Saud University.
Exome Sequencing and PROC c.811 C>T Genotyping
Exome capture sequencing was performed in patient IV:1 using the Agilent SureSelect Target Enrichment System All Exon kit (Agilent Technologies, Santa Clara, CA) according to the company’s protocol. High-throughput sequencing of captured library was done using Illumina Hiseq2000 platform. Raw image files were processed by Illumina basecalling Software 1.7. The reads were mapped against the UCSC hg19 (http://genome.ucsc.edu/) reference using OAPaligner/SOAP2 (http://soap.genomics.org.cn/) and BWA (http://bio-bwa.sourceforge.net/) for SNP and Indel analysis, respectively.
Sanger sequencing was used to validate PROC c.811 C>T mutation identified by exome sequencing. PROC c.811 C>T (R271W equivalent to R229W in mature PC numbering that Is used hereafter) was genotyped by PCR in patients, family members, and controls. The region was amplified using forward primer 5'-CTACCTCTTTGGGATTGACACCT-3' and reverse primer 5'-GGGAATCTTGATGAAGTTGAGG-3' with product size of 552 bp. PCR reaction (25 µl) contained 1 µl of genomic DNA, 0.5 µl (10 µM) of each primer, 10.5 µl of H2O, and 12.5 µl of PCR mastermix (KAPA Biosystems, Woburn, MA). Initial denaturation at 94°C for 5 min followed by 35 cycles (94°C for 60 sec, 54°C for 60 sec, and 72°C for 30 sec) then final 10 min at 72°C. PCR products were purified and subsequently sequenced using both primers. Sequencing was performed using a 96 capillary gene analyzer (3730 Applied Biosystems, CA). Data was analyzed using sequencing analysis software (v5.3.1) from Applied Biosystems.
Proteins and Reagents
Thrombin and prothrombin were purchased from Enzyme Research Laboratories (South Bend, IN), factor Va and factor Xa from Hematologic Technologies (Burlington VT), rabbit-lung TM from American Diagnostica, Inc. (Greenwich, CT), chromogenic substrate H-D-lysyl (g-Cbo)-prolyl-argininyl-p-nitroanilide (Pefachrome PCa) from Centerchem Inc. (Norwalk, CT), and chromogenic substrate CBS 34–47 from American Bioproducts (Parsippany, NJ). Phospholipid vesicles (80 % phosphatidylcholine, 20 % phosphatidylserine) were prepared as described[9]. Recombinant PC antigen concentration was determined using the Asserachrom Protein C ELISA assay from American Bioproducts (Parsippany, NJ).
Recombinant R229W Protein C Mutant
To define protein functional abnormalities, recombinant wildtype PC and PC with the mutations, R229W, R229Q and R229A, were studied for rate of activation by thrombin:TM and for anticoagulant activities. PC mutants were prepared, purified and studied as previously described[7, 8]. For functional assays protein C was activated by thrombin in the absence of TM and in the presence of 5 mM EDTA as previously described [7,8] to generate activated protein C (APC). In these non-physiologic conditions the protein C variants were activated normally. The half-life of APC activity in plasma was measured by mixing each APC variant in human citrated plasma and then assaying aliquots of the mixture for cleavage of an APC chromogenic substrate as described[9].
Functional Assays
APC was also quantified by active site titration with p-nitrophenol-guanidino benzoate adapted from Chase and Shaw[7, 10]. The relative rate of activation of PC by thrombin:TM was determined as described with human thrombin and rabbit lung TM in the presence of CaCl2 [11]. Briefly, the reaction was initiated by adding protein C (0.5 µM) to a mix of 6 nM thrombin and 10 nM thrombomodulin at 37 °C. Aliquots were removed at time points and assayed for APC activity with the chromogenic pefachrome PCa in the presence of hirudin to block thrombin activity. For APC functional assays protein C was activated by 110 nM thrombin in EDTA without thrombomodulin[11]. APC anticoagulant activity was determined in a standard activated partial thromboplastin time (APTT) assay and in a dilute prothrombin time (PT) assay as previously described[7, 8]. The dilute PT assay isolates factor V inactivation and is performed as follows: plasma (50 µL) and 50 µL of defined concentration of APC were pre-incubated for 3 minutes at 37 °C, then coagulation was initiated by addition of 50 µL of 1:60 diluted Innovin (Dade Behring Inc., Newark, DE) in HEPES buffered saline with 25 mM CaCl2[8].
Results and Discussion
Perinatal Intracranial Hemorrhage and Delayed Onset of Thrombosis
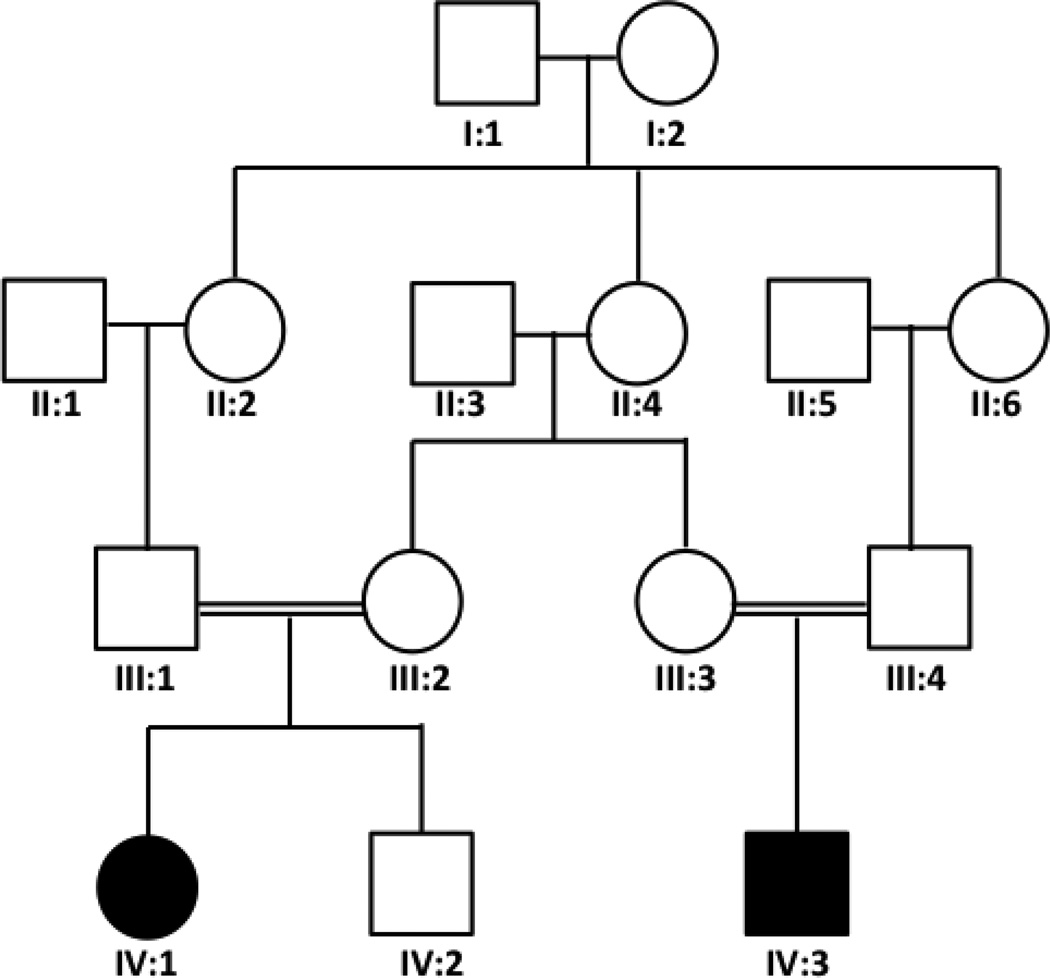
Two first-degree cousins (IV:1 and IV:3) presented with a similar hemorrhagic phenotype seen upon brain MRI imaging (Figure 1), and the family pedigree (Figure 2) was suggestive of autosomal recessive inheritance. The first child (IV:1) was a 10 year old girl who presented at 13 days of age with seizures and brain CT scan showing intracranial hemorrhage. At 4 months of age, she presented with multiple thrombotic lesions over both hands and feet that required extensive debridement. She was started on unfractionated heparin and fresh frozen plasma (FFP) and had no new necrotic lesions afterward except for transient skin discoloration. She was later switched to enoxaparin. FFP was slowly tapered off for the last 4 years. At 9 years of age, PC amidolytic activity was 61% (nl 70–140%) and protein C antigen was 57% (nl 70–140%). Protein S, antithrombin III, protein Z, FVIII, and fibrinogen levels were normal. FV Leiden and prothrombin nt20210 mutations were absent. The second child (IV:3) was a 2 year old boy who was delivered by Cesarean section because of fetal distress. The mother gave history of decreased fetal movement for a few weeks prior to his birth and was noted to be hypotonic. Brain MRI in the first week of life for Subject IV:3 showed massive subacute hemorrhage in the brain parenchyma and upper spinal canal (Figure 1). Bleeding stopped with appropriate supportive care. He did not have purpura fulminans or any evidence of thrombosis during the neonatal period. At 4 months of age, a ventriculoperitoneal shunt was placed because of hydrocephalus, and post operatively he developed a thrombotic lesion in his left foot that resolved after starting enoxaparin and plasma replacement. He remains on enoxaparin only with no new significant lesions. At 10 months of age PC amidolytic activity was 59% and protein C antigen was 73%. A definitive diagnosis of PC deficiency was not made until we identified a genetic mutation in PROC (see below). Both patients suffer from severe permanent neurological deficit and blindness. Parents of both patients report no history of thrombosis in themselves or other family members.
Figure 1.
MRI performed at 6 days of age for Subject IV:3 is shown in panels A+B+C. There is diffused reduction of signal intensities on [A] Axial T2 weighted images (WI) and [B] Axial gradient echo images of both cerebral hemispheres, more predominantly white matter, consistent with hemorrhage in the background of severe brain parenchymal atrophic changes and cystic encephalomalcia. Dark signals in the dependent portions of retrocerebellar cistern, 4th ventricle and lateral ventricles also represents blood. [C] Sagittal non-contrast T1 WI show bright signal of subacute hemorrhage in both cerebral and cerebellar hemispheres, retrocerebellar cistern, and upper spinal canal. [D] For MRI performed at 10 months of age, axial FLAIR (left) and T2 WI (middle) show grossly dilated ventricles with very thin brain parenchyma. Axial susceptibility WI (right) show extensive iron deposition over cerebellar surface and around the ependymal lining of ventricle.
Figure 2.
The family pedigree of the two affected children.
Exome Sequencing Revealed Homozygous PROC c.811 C>T Mutation
A total of 40,601 homozygous variants (SNPs and Indels) were identified, and of those 247 were not present in dbSNP137, HapMap, and 1000 genome. Fifty-six variants were in the coding or splicing regions and only one was in a candidate gene i.e. homozygous variant in exon 9 of PROC gene c.811 C>T. The C>T polymorphism leads to the amino acid change, arginine (R) to tryptophan (W), at residue 229 (mature protein numbering) of the serine protease domain. Sanger sequencing validated this mutation (Figure 3). Both patients were homozygous (T/T) while tested parents and three siblings were heterozygous (C/T), and 55 Saudi controls were normal (C/C). No family history of thrombosis among family members who are heterozygous for this mutation was reported. Complete exome sequence analysis also showed no mutations for ATIII, protein S, factors V or VIII, prothrombin, plasminogen, protein Z, or any ADAMTS genes. The PROC gene (c.811 C>T) mutation was reported previously as a compound heterozygous with another PROC mutation with PC antigen level 75% (normal range) and PC activity ranging between 32–70% of normal; there has been no prior report of individuals homozygous for this PROC variant[12]. This PROC gene mutation was also reported in one patient who additionally was heterozygous for a mutation of K196E in protein S (PROS1). This patient presented with venous thrombosis and had 77% of normal APC amidolytic activity[13]. In the ExAC database the 229Trp allele has a frequency of 8.423 X 10-6 with no reports of homozygotes (http://exac.broadinstitute.org/variant/2-128185947-C-T). The PROC R229Q mutation was previously reported[14].
Figure 3.
A PROC c.811 C>T genotyping in the two affected patients (IV:1 and IV:3), IV:3 father (C/T), and control (C/C). B Schematic protein C structure showing the site of mutation in the serine protease domain.
Recombinant R229W-protein C is Resistant to Thrombin:TM Activation
The R229W mutation is located in the calcium binding loop of PC's protease domain that mediates TM interactions[11]. Recombinant PC with the mutations R229W, R229Q and R229A were studied. Each was strikingly defective in rate of activation by thrombin:TM, showing an activation rate that was only <1%, 2% and 4%, respectively, compared to wildtype protein C (Figure 4A). The R229W, R229Q and R229A PC mutants were all activated normally by thrombin alone, either in the presence of CaCl2 or EDTA. This activation was confirmed with activity towards chromogenic substrates and by active site titration of all variants, both of which were normal (not shown). Once activated, each APC mutant was only moderately affected in its anticoagulant activity as measured in dilute PT assays, which involves factor Va inactivation (Figure 4B) or even less affected in anticoagulant activity as measured in APTT assays which involve inactivation of factors VIIIa and Va (Figure 4C). The amidolytic activity half-life of each mutant APC in plasma (not shown) was similar to that of wildtype APC (21 min at 37 °C[8,9]), showing the mutation did not affect reactivity with plasma serpins. These properties of recombinant R229W-PC suggest a severe in vivo deficit in these children for the activation of PC, implying a severe deficiency of circulating APC levels in spite of half-normal levels of PC zymogen. Unfortunately family members were not available for making measurements of circulating APC levels.
Figure 4.
The recombinant mutants, R229W-, R229A- and R229Q-protein C, showed major defects in the rate of activation by thrombin:TM compared to wildtype (WT) recombinant protein C (graph shown is average of 3 replicates, error bars are left out for clarity). The Y axis is in units of percentage of wildtype APC activity, with 100% defined according to a hyperbolic curve fit of the data. A. Anticoagulant activities of the R229W-, R229A- and R229Q-APC mutants were compared to WT-APC using dilute prothrombin time (PT) assays (n = 5) B and APTT assays (n = 5). C. Error bars are standard deviation.
Patients with severe PC deficiency present soon after birth with purpura fulminans and disseminated intravascular coagulation with or without intracranial bleeding or stroke[3, 15–18]. In our two cases here, the PC defect was associated with severe long term detrimental effects on the central nervous system (CNS) secondary to the massive perinatal intracranial hemorrhage. The presence of extensive brain atrophy, as shown in Figure 1D, on follow up MRI might reflect a role for APC not only as a stabilizer of CNS vascular endothelium but potentially also as direct neuroprotectant in the rowing embryo. This latter speculation is consistent with growing evidence for APC’s multiple cytoprotective activities and neurogenerative activities of APC as evidenced by its ability to promote neurogenesis in the murine brain and in cultured human embryo-derived neuroprogenitor cell populations[19–21]. It seems unlikely that etiology of intracranial hemorrhage in our patient was related to presence of extensive CNS vascular thrombosis given the delayed onset of clinical thrombosis for several months. As most routine laboratory coagulation assays for plasma PC utilize a snake venom protease to activate PC, they fail to detect TM-activation-resistant PC mutants. It seems that standard lab tests for PC functional activities are inadequate when physicians are faced with striking neonatal or postnatal hemorrhagic neuropathologies which might reflect some currently unappreciated neuroprotective activities of APC[19, 20]. Thus, we recommend that PROC genotyping and/or more advanced PC bioassays be considered for efforts made to understand the pathophysiology of such clinical presentations.
In summary, homozygosity for the PC R229W mutation in two related cousins was associated with significant leaky brain blood vessels in the perinatal period and significant neurological insult even prior to the first evidence of clinical thrombosis. This highlights the importance of PC and APC in maintaining the integrity of the brain vascular endothelium in humans.
Highlights.
Protein C is a natural anticoagulant and its deficiency increases risk of thrombosis
Homozygous Protein C gene (PROC) c.811 C>T mutation (R229W) was identified in one family
Protein C R229W mutation was linked to perinatal intracranial bleeding and delayed onset of thrombosis
Recombinant protein C mutant R229W is resistant to thrombin:thrombomodulin activation
Acknowledgments
This work was supported by the National Institutes of Health grants HL031950, HL052246, and HL021455 (J.H.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in abstract form at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 9, 2013.
Authorship
A.A., K.K., M.K., and F.B.A. participated in the conception of the clinical and genetic study. A.A. was responsible for interpretation of exome sequencing data. A.A., A.J.G. and J.H.G. were responsible for conceptualizing and performing the studies of recombinant protein C mutants. A.A., A.J.G. and J.H.G. were responsible for writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia. 2008;14:1214–1221. doi: 10.1111/j.1365-2516.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 2.Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease. J. Clin. Invest. 1981;68:1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branson HE, Katz J, Marble R, Griffin JH. Inherited protein C deficiency and coumarin-responsive chronic relapsing purpura fulminans in a newborn infant. Lancet. 1983;2:1165–1168. doi: 10.1016/s0140-6736(83)91216-3. [DOI] [PubMed] [Google Scholar]
- 4.Seligsohn U, Berger A, Abend M, Rubin L, Attias D, Zivelin A, Rapaport SI. Homozygous protein C deficiency manifested by massive venous thrombosis in the newborn. N. Engl. J. Med. 1984;310:559–562. doi: 10.1056/NEJM198403013100904. [DOI] [PubMed] [Google Scholar]
- 5.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 6.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015 doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale AJ, Heeb MJ, Griffin JH. The autolysis loop of activated protein C interacts with factor Va and differentiates between the Arg506 and Arg306 cleavage sites. Blood. 2000;96:585–593. [PubMed] [Google Scholar]
- 8.Gale AJ, Tsavaler A, Griffin JH. Molecular characterization of an extended binding site for coagulation factor Va in the positive exosite of activated protein C. J. Biol. Chem. 2002;277:28836–28840. doi: 10.1074/jbc.M204363200. [DOI] [PubMed] [Google Scholar]
- 9.Heeb MJ, Bischoff R, Courtney M, Griffin JH. Inhibition of activated protein C by recombinant alpha 1-antitrypsin variants with substitution of arginine or leucine for methionine358. J. Biol. Chem. 1990;265:2365–2369. [PubMed] [Google Scholar]
- 10.Chase T, Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem. Biophys. Res. Commun. 1967;29:508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- 11.Gale AJ, Griffin JH. Characterization of a thrombomodulin binding site on protein C and its comparison to an activated protein C binding site for factor Va. Proteins. 2004;54:433–441. doi: 10.1002/prot.10627. [DOI] [PubMed] [Google Scholar]
- 12.Reitsma PH, Bernardi F, Doig RG, Gandrille S, Greengard JS, Ireland H, Krawczak M, Lind B, Long GL, Poort SR. Protein C deficiency: a database of mutations, 1995 update. On behalf of the Subcommittee on Plasma Coagulation Inhibitors of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1995;73:876–889. [PubMed] [Google Scholar]
- 13.Miyata T, Sato Y, Ishikawa J, Okada H, Takeshita S, Sakata T, Kokame K, Kimura R, Honda S, Kawasaki T, Suehisa E, Tsuji H, Madoiwa S, Sakata Y, Kojima T, Murata M, Ikeda Y. Prevalence of genetic mutations in protein S, Protein C and antithrombin genes in Japanese patients with deep vein thrombosis. Thromb. Res. 2009;124:14–18. doi: 10.1016/j.thromres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Gandrille S, Alhenc-Gelas M, Juban-Vague I, Fiessinger JN, Goossens M, Aiach M. Two qualitative protein C (PC) deficiencies due to Arg 229 ->GLN (PC Marseille) and Ser 252 ->ASN (PC Paris) mutations. Thromb. Haemost. 1991;65:1196. [Google Scholar]
- 15.Marlar RA, Montgomery RR, Broekmans AW. Diagnosis and treatment of homogyzous protein C deficiency. Report of the Working Party on Homozygous Protein C Deficiency of the Subcommittee on Protein C and Protein S, International Committee on Thrombosis and Haemostasis. J. Pediatr. 1989;114:528–534. doi: 10.1016/s0022-3476(89)80688-2. [DOI] [PubMed] [Google Scholar]
- 16.Unal S, Gumruk F, Yigit S, Tuncer M, Tavil B, Cil O, Takci S, Urata M, Hotta T, Kang D, Cetin M. A novel mutation in protein C gene (PROC) causing severe phenotype in neonatal period. Pediatr. Blood Cancer. 2014;61:763–764. doi: 10.1002/pbc.24782. [DOI] [PubMed] [Google Scholar]
- 17.Tarras S, Gadia C, Meister L, Roldan E, Gregorios JB. Homozygous protein C deficiency in a newborn. Clinicopathologic correlation. Arch. Neurol. 1988;45:214–216. doi: 10.1001/archneur.1988.00520260102029. [DOI] [PubMed] [Google Scholar]
- 18.Ohga S, Kang D, Kinjo T, Ochiai M, Doi T, Ishimura M, Kayamori Y, Urata M, Yamamoto J, Suenobu SI, Kanegane H, Ikenoue T, Shirahata a, Hara T. Paediatric presentation and outcome of congenital protein C deficiency in Japan. Haemophilia. 2013;19:378–384. doi: 10.1111/hae.12097. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, Zhao Z, Yang Q, Wang M, Bell RD, Wang S, Chow N, Davis TP, Griffin JH, Goldman SA, Zlokovic BV. An activated protein C analog stimulates neuronal production by human neural progenitor cells via a PAR1-PAR3-S1PR1-Akt pathway. J. Neurosci. 2013;33:6181–6190. doi: 10.1523/JNEUROSCI.4491-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosnier LO, Zlokovic BV, Griffin JH. Cytoprotective-selective activated protein C therapy for ischaemic stroke. Thromb. Haemost. 2014;112:883–892. doi: 10.1160/TH14-05-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends in Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]