Abstract
A critical challenge to the success of biodegradable vascular grafts is the establishment of a healthy endothelium. To establish this monolayer of endothelial cells (ECs), a variety of techniques have been developed, including cell seeding. Vascular grafts may be seeded with relevant cell types and allowed to mature before implantation. Due to the low proliferative ability of adult ECs and issues with donor site morbidity, there has been increasing interest in using endothelial progenitor cells (EPCs) for vascular healing procedures. In this work, we combined the proliferative and differentiation capabilities of a commercial cell line of early EPCs with an established bioreactor system to support the maturation of cell-seeded vascular grafts. All components of the vascular graft and bioreactor setup are commercially available and allow for complete customization of the scaffold and culturing system. This bioreactor setup enables the control of flow through the graft, imparting fluid shear stress on EPCs and affecting cellular proliferation and differentiation. Grafts cultured with EPCs in the bioreactor system demonstrated greatly increased cell populations and neotissue formation compared with grafts seeded and cultured in a static system. Increased expression of markers for mature endothelial tissues were also observed in bioreactor-cultured EPC-seeded grafts. These findings suggest the distinct advantages of a customizable bioreactor setup for the proliferation and maturation of EPCs. Such a strategy may be beneficial for utilizing EPCs in vascular tissue engineering applications.
Introduction
The most significant challenge in vascular tissue engineering is the development of small-diameter grafts with antithrombotic properties and high patency. Numerous attempts have been made to improve the patency and success of these grafts with inner diameters of <6 mm.1,2 The reduced patency is generally caused by thrombosis and intimal hyperplasia, and the prevailing notion is that the early establishment of a healthy endothelium can reduce the risk of these issues.3
One approach to expediting the growth of a functional endothelium is the seeding of cells on a vascular graft before implantation. To support cells seeded in vitro, a variety of cell types, materials, fabrication techniques, and bioreactors have been used to provide a mechanical and biological environment for the development of tissue-engineered vascular grafts (TEVGs).4–8 In this study, we focus on seeding, proliferating, and differentiating early endothelial progenitor cells (EPCs) on a biodegradable vascular graft within a tubular perfusion system (TPS) bioreactor.
Several strategies exist for the extraction and isolation of native endothelial cells (ECs) from autologous vessels.9 However, the clinical application of these techniques is made challenging by the limited number of available ECs and the limited proliferative potential of mature ECs, along with the donor site morbidity associated with EC harvest. In contrast, studies have shown that EPCs have improved proliferative potential.10,11 Methods of isolation and large-scale expansion of these cells have also been developed.12–14 Early- and late-outgrowth EPCs have been implicated in the repair and function of the endothelium, and the harvest of these cells is less invasive than that of ECs. Thus, these cells may prove to be promising candidates for the seeding of vascular grafts before implantation.15
Biomechanical stimuli serve as integral components to the development of a mature endothelium. Mechanical forces applied by blood flow can affect vascular remodeling, homeostasis, and disease.16 As one example, shear stress provides vital input toward the proliferation and maturation of vessel-related cells such as ECs and smooth muscles cells.6,17 More recently, investigations into EPCs show differentiation of progenitor cells into EC-like cells as a result of arterial shear stress conditions.18–22 Fluid flow through TEVGs can be simulated in systems such as TPS bioreactors, which can offer distinct advantages over static culture conditions, including providing cell waste removal, nutrient delivery, and mechanical stimuli.23–26
In this study, we demonstrate a methodology for the fabrication, seeding, and subsequent culture of TEVGs utilizing off-the-shelf products. Examples of these grafts are shown in Figure 1, and the process of seeding and culturing is demonstrated in Figure 2. Both the vascular graft and the bioreactor are constructed of commercially available components, which allows for the easy manufacture, repeatability, and modification of this total TEVG preparation system. For the scaffold portion of this work, we used a solvent-cast, poly(glycolic acid) (PGA)-based felt graft with a poly(DL-caprolactone-co-lactic acid) (PCLLA) solution characterized in a previous study.27 The graft is mechanically compatible with vascular tissues, porous, biodegradable, and demonstrated good cell adhesion and infiltration when implanted in a mouse model. This scaffold platform was chosen for its ease of production and modification.28 The next component of the TEVG preparation system is the TPS bioreactor. We have previously demonstrated the successful application of the TPS bioreactor in the development and support of various tissue engineering constructs.23,29–31 In contrast to work showing the differentiation of EPCs in arterial shear stress environments, we chose to demonstrate cell seeding and differentiation in venous shear stress conditions given the lack of available TEVGs for venous conditions and their application in conditions such as congenital heart disease. Thus, it may be advantageous to understand the fate of EPCs within the venous system. Finally, for the biological component, we used EPCs for cell seeding.
FIG. 1.
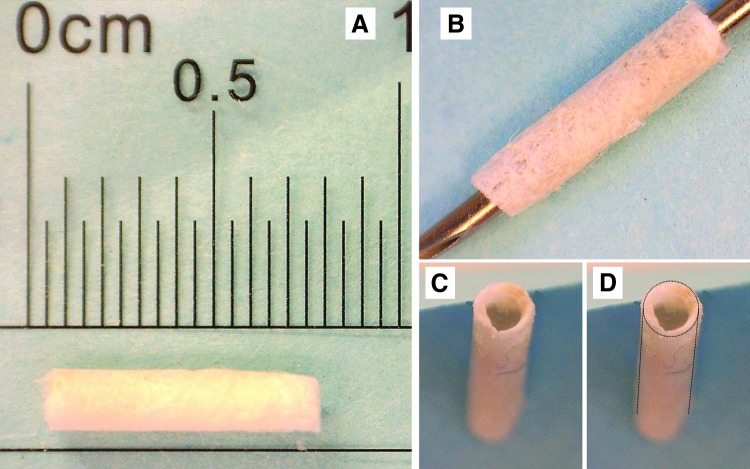
Gross appearance of vascular grafts before cell seeding. (A) A graft after freeze-drying to show scale and (B) graft still on a 21 g needle during fabrication. (C) and (D) show the inner lumen and overall shape of the finished graft.
FIG. 2.
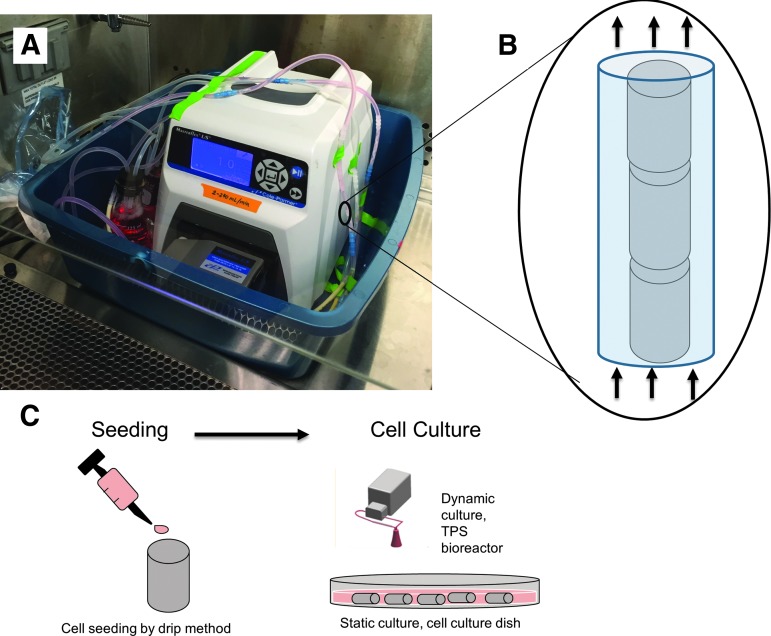
Schematic of seeding and culture process. (A) The TPS bioreactor set up shortly after vascular grafts and medium were introduced. (B) TEVGs seeded with EPCs placed in perfusion flow chambers. (C) Proposed process utilizing seeding and culturing of TEVGs. EPC, endothelial progenitor cell; TEVG, tissue-engineered vascular graft; TPS, tubular perfusion system.
The objective of this study was to determine whether this off-the-shelf TEVG preparation system would demonstrate successful neotissue formation and endothelial-like differentiation of EPCs within the biodegradable scaffolds in comparison with grafts seeded and cultured in a static environment. In addition, we explored the application of low flow rate conditions to simulate the effects of the venous environment on EPC differentiation. Any component of the bioreactor may easily be switched out with off-the-shelf components to accommodate vascular grafts of varying sizes and shapes. Likewise, the bioreactor system could be used with virtually any vascular graft and cell type.
Materials and Methods
Vascular graft fabrication
Grafts were fabricated according to previous studies.27,32 Rectangular sections of 6.00 × 4.00 mm were cut from a PGA polymer BIOFELT (Biomedical Structures). These sections were then inserted into a polypropylene tube with an inner lumen diameter of 1.4 mm. To maintain the patency of the inner lumen during the solvent-casting procedure, a 21 g stainless steel needle was inserted into the opposite end of the tube. A 40:60 copolymer PCLLA solution 15% w/v in 1,4-dioxane was then deposited into the tubes to cover the PGA scaffold. Grafts were then frozen at −20° C for 30 min and, subsequently, freeze-dried for 24 h. Afterward, grafts were stored at −20°C until they were used.
Bioreactor design
The bioreactor system consists of a design adapted from a previously described methodology.31 Briefly, an L/S multichannel pump system (Cole Palmer) was used to drive flow (2 mL/min) through a tubing circuit. The flow rate was chosen based on previous data regarding EPC differentiation into EC-like cells.18,19,21,33 Such a flow rate mimics physiologically relevant venous wall shear stresses of 0.6 dynes/cm2.
Pharmed BPT tubing (Cole Palmer) was used for the portion of the circuit passing through the pump. All other tubing comprised platinum-cured silicone and was joined by silver ion-lined microbial-resistant tubing connectors (Cole Palmer). The growth chamber where grafts were placed also consisted of platinum-cured silicone tubing with an inner diameter of 3.2 mm and a wall thickness of 0.8 mm. After the tubing and components were autoclaved, they were assembled inside a laminar flow hood. Each growth circuit was packed with 15 consecutive, cell-seeded grafts. After loading and assembly, the bioreactors were placed in a cell culture incubator at 37°C and 5% CO2. The cell medium was loaded into separate 125 mL Erlenmeyer flasks for each tubing circuit and topped with rubber stoppers. The medium within the flasks was replaced with fresh medium every 3 days.
Endothelial progenitor cell culture
Early EPCs were purchased from Celprogen. Cells were cultured in polystyrene flasks before seeding, and the medium was changed every 3 days. Human EPC Complete Growth Media with Serum and Antibiotics (Celprogen) were used.
EPC seeding
Cells were trypsinized, pelleted, and resuspended in fresh medium at 5.0 × 106 cells/mL. Grafts were placed on untreated tissue culture dishes. For each graft, 100 μL of cell suspension was pipetted through it. Excess solution was pipetted four additional times to ensure graft coverage. Grafts were then incubated for 30 min at 37°C and 5% CO2 to ensure cell attachment. Excess medium was then washed off with phosphate-buffered saline (PBS). Seeded grafts were either placed in a 34.8 mm-diameter tissue culture plate for static conditions (n = 5) or loaded into the growth chamber of a tubing circuit for continuous flow bioreactor conditions (n = 5).
DNA quantification
Cell pellets were isolated from grafts via trypsinization and thorough rinsing with PBS. Brightfield microscopy was used to qualitatively ensure cell detachment. Pellets were then resuspended in PBS, and the DNA was isolated using a DNeasy Tissue Kit (Qiagen). Standard manufacturer protocols were followed. Subsequent quantification of double-stranded DNA was accomplished with a Quant-iT Picogreen dsDNA Assay Kit (Invitrogen). After 5 min of incubation in the dark with the PicoGreen dsDNA reagent (n = 3 for each group in each time point), fluorescence was measured using an M5 SpectraMax plate reader (Molecular Devices) and using an excitation of 480 nm and an emission of 520 nm.
Quantitative reverse transcriptase polymerase chain reaction
Cell pellets were isolated from vascular grafts, and RNA was subsequently extracted via an RNeasy Mini Plus Kit (Qiagen). Reverse transcription of the isolated RNA and subsequent reactions were performed using a QuantiTect SYBR Green RT-PCR Kit (Qiagen). Quantitect primer assays targeted expression of CD34 (Quantitect primer assay ID: HS_cd34_1_SG), CD31 (HS_PECAM1_1_SG), von Willebrand's Factor (vWF, HS_VWF_1_SG), vascular endothelial growth factor (VEGF, HS_VEGFA_1_SG), and nitric oxide synthase 3 (NOS3, HS_NOS3_1_SG). Results were then analyzed using the comparative threshold cycle method and normalized using GAPDH as an endogenous reference. Relative values (ΔΔCT) to those of the control are reported.
Histological analysis
Grafts were removed from the bioreactor or static culture dishes at 3, 7, and 14 days after cell seeding. Samples were subsequently fixed in 4% para-formaldehyde and embedded in paraffin. The embedded grafts were then sectioned at 5 μm thickness. Samples underwent immunohistochemical staining to detect EC markers in the following antibody sets: CD31 antigens were detected via a rabbit anti-CD31 antibody with a goat anti-rabbit Texas Red-conjugated secondary antibody, CD34 was detected with a mouse anti-CD34 anti-body and a goat anti-mouse Cy5-conjugated secondary antibody, and, finally, with a goat anti-vWF antibody with a donkey anti-goat FITC-conjugated secondary (Abcam). The cell nucleus was stained with VectaShield plus DAPI (Vector Laboratories).
Fluorescent images were used to quantify the number of cells presenting each cellular marker. Five images were analyzed per experimental group and were taken following an unbiased image collection pattern. First, each image was transformed into black and white by using a consistent threshold in ImageJ. Each cell was then designated as a region of interest, and integrated density for each fluorescent channel was recorded on a per-cell basis. Cells expressing fluorescence over a set threshold of integrated density for each marker were counted as “positive.” Positive cells were then expressed as a percent of total cells counted per image.
Statistical analysis
Data were analyzed using single-factor analysis of variance with Student's t-Test or ANOVA assuming normal data distribution with a confidence of 95% (p < 0.05). Standard deviation error bars are reported on each figure along with relevant statistical relationships.
Results
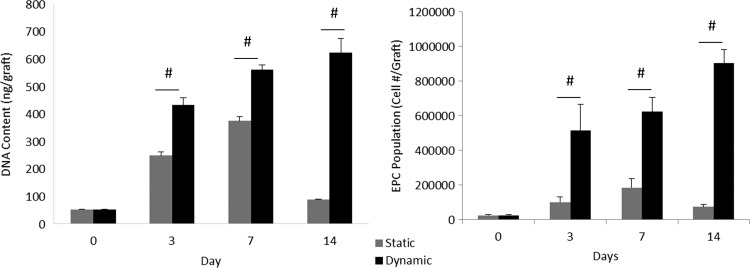
Initial cell seeding of a solution containing 5 × 105 cells resulted in an average of 2.425 ± 0.645 × 104 cells/graft, resulting in a seeding efficiency of 4.850% ± 1.290%. EPC populations cultured in static conditions achieved a total fold change of 7.629 ± 2.195 after 7 days and 3.339 ± 0.616 after 14 days. EPC populations cultured in dynamic conditions increased 25.670 ± 3.859 fold after 7 days and 37.165 ± 3.041 fold after 14 days. Total EPCs that attached to dynamically cultured TEVGs achieved a population of 9.013 × 105 ± 0.810 × 105 cells after 14 days in contrast to a total population of 0.735 × 105 ± 0.149 × 105 cells on statically cultured cells.
These observations were further supported by DNA quantification. Picogreen assays yielded a total DNA concentration of 622.65 ± 49.96 ng/graft for dynamically cultured TEVGs at 14 days and of 87.11 ± 2.45 ng/graft for statically cultured TEVGs. Overall, dynamically cultured TEVGs demonstrated a significant increase in long-term proliferation of EPCs, shown through cell and DNA quantification of cells attached to grafts, as observed in Figure 3.
FIG. 3.
DNA quantification and EPC population evaluation on static and dynamic grafts. Dynamic culturing of TEVGs provided clear improvements in overall cell population growth. Such trends were demonstrated both in the quantification of total DNA content in dynamically cultured cells and in simple cell counts of EPCs attached at various time points over the 2 week study. #Represents statistical significance compared with all other groups within the time point (p < 0.05).
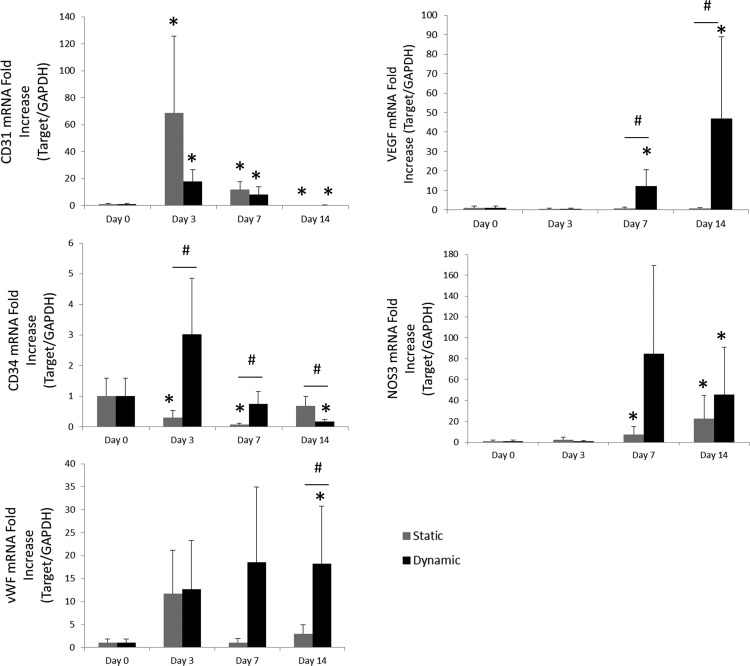
Relative mRNA expression was analyzed by comparing the fold increase of markers related to endothelial function and phenotype. Fold increase was evaluated by quantifying mRNA expression of dynamically and statically cultured TEVGs at various time points compared with EPCs after initial seeding. Results are summarized in Figure 4. NOS3 expression was shown to increase over time compared with initial grafts, although there was no statistically significant difference between dynamic and static EPC populations. On the other hand, both VEGF and vWF expression demonstrated increases by day 14 when comparing dynamically cultured TEVGs with statically cultured TEVGs. Both static and dynamic populations exhibited increased CD31 expression after days 3 and 7 compared with EPCs after initial seeding. However, at day 14, CD31 expression is reduced. Dynamically cultured TEVGs demonstrated decreased CD34 expression on day 14 compared with statically cultured samples, whereas statically cultured TEVGs demonstrated decreased CD34 expression on days 3 and 7. CD34 expression in dynamically cultured TEVGs decreased from days 3 to 14. In contrast, CD34 expression in statically cultured TEVGs increased from days 3 to 14.
FIG. 4.
Relative expression of markers related to EPCs and endothelialization. mRNA fold increases are presented over 14 days of culturing in either static or dynamic cultures. TEVGs generally demonstrated expression of endothelial markers consistent with mature ECs over time (increased CD31, NOS3, and vWF, decreased CD34); whereas dynamically cultured TEVGs showed increased expression of vWF and VEGF, indicative of endothelial maturation and function. *Represents statistical significance compared with initial (day 0), and #represents statistical significance compared with all other groups within the time point (p < 0.05). EC, endothelial cell; NOS3, nitric oxide synthase 3; VEGF, vascular endothelial growth factor; vWF, von Willebrand Factor.
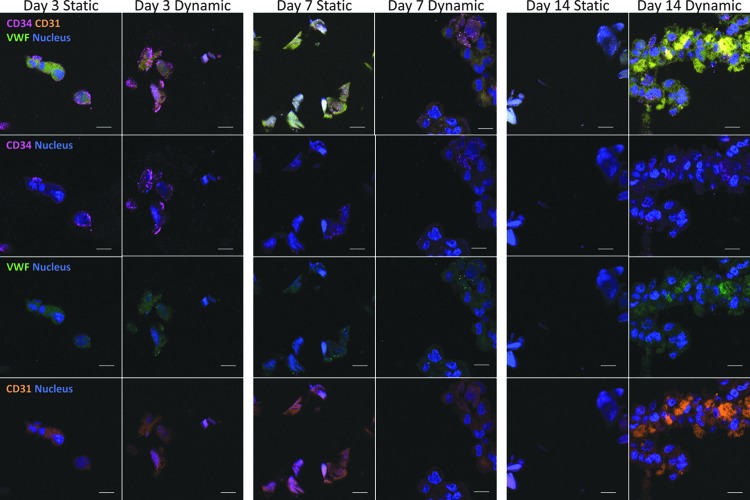
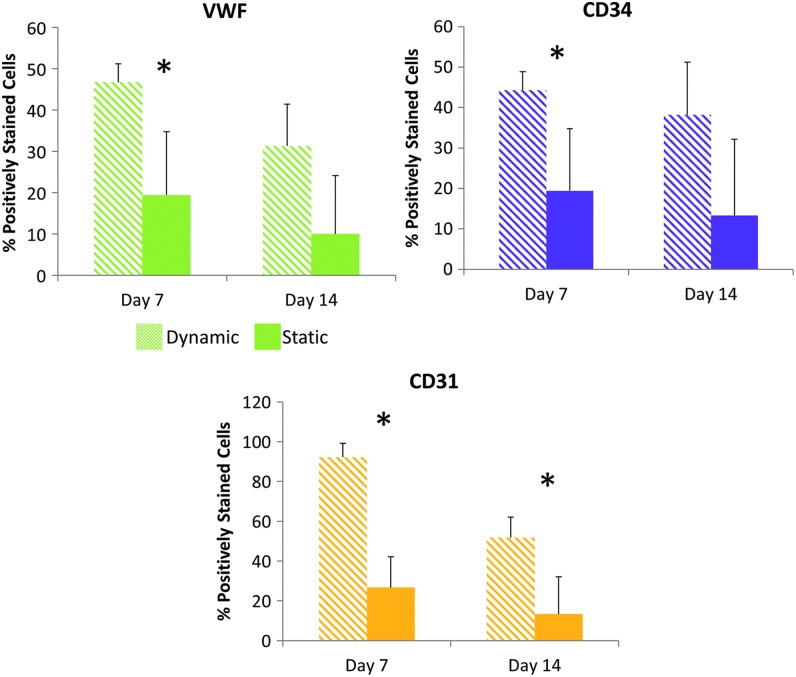
Immunohistochemistry provided insight into the effects of culturing on EPC-based tissue formation within the TEVGs. Figure 5 demonstrates a more thorough distribution of EPCs throughout TEVG cross-sections in dynamically cultured samples. In contrast, statically cultured TEVGs demonstrated less distributed EPC populations and sparser tissue formation. These immunohistochemistry results showed expression of endothelial markers, CD31 and vWF, along with EPC marker CD34. Figure 6 provides a representation of graft cross-sections to visualize the distribution of DAPI-stained EPCs within grafts. In Figure 7, the percent of stained cells expressing vWF, CD34, and CD31 was quantified. Dynamically cultured grafts demonstrated a significant increase in cells stained positively for vWF at day 7, CD34 at day 7, and CD31 at both days 7 and 14 compared with statically cultured TEVGs.
FIG. 5.
Immunohistochemical staining of cross-sectional cuts of the grafts cultured in either static or dynamic cultures. CD31 is a marker for mature ECs and is shown in orange; CD34 is a marker for EPCs and is shown in purple; and vWF is a marker for ECs and is shown in green. Images suggest that a higher expression of EC markers is found in dynamic cultures and is intensified over time. The scale bar shows 10 μm.
FIG. 6.
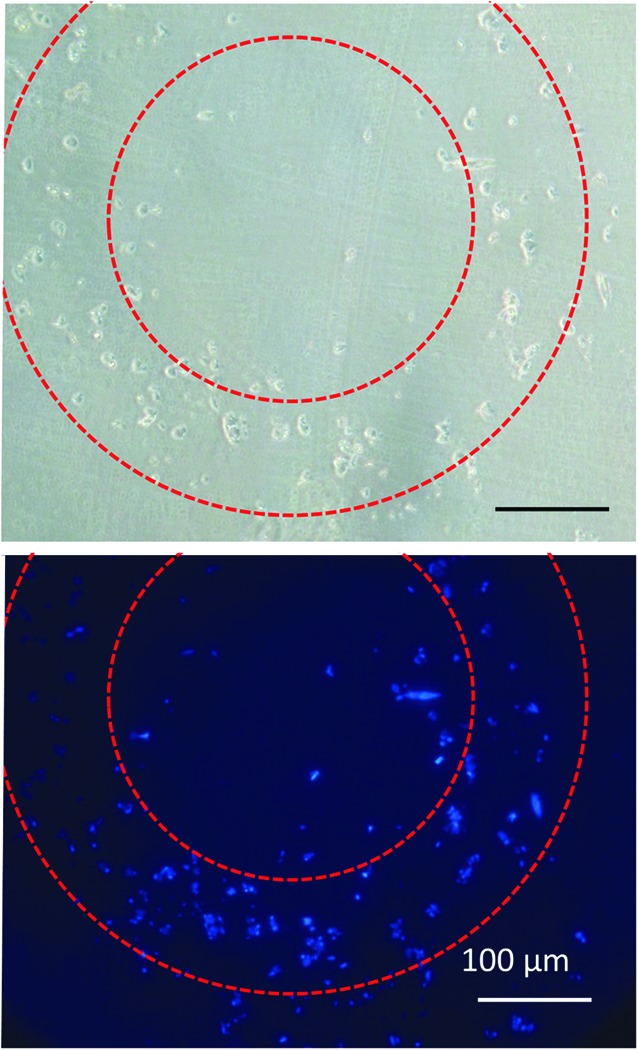
Cross-sectional overview of grafts. An overview image using phase contrast (top) and DAPI (bottom) to show the nucleus of cells cultured around the graft after 7 days in dynamic culture. Dotted lines suggest the region of the grafts, and the scale bar shows 100 μm.
FIG. 7.
Quantitative immunohistochemical analysis. Graphs show the percent of cells that are designated as positive for each marker detected with the immunohistochemical stain. An asterisk indicates significantly different experimental groups for each day as demonstrated with a Student's T-Test (p < 0.05).
Discussion
EPCs provide a promising cell type for the seeding of TEVGs. However, like other cell types, expansion time and differentiation may be limited in vitro. We sought to determine whether we could improve the proliferation and function of EPCs seeded on an established TEVG platform by culturing these grafts in a TPS bioreactor system. In addition, we used the TPS bioreactor to apply low-level shear stress to the TEVGs to simulate venous conditions.
A low flow rate was chosen for several reasons. First, we hoped to establish an appropriate bioreactor setup to culture grafts that are suitable for the venous system. Much research focuses on the arterial environment for TEVG applications, but there are more limited vascular graft materials available for the venous system. Thus, we hoped to determine the effects of shear stresses experienced by the endothelium in conditions similar to the venous system. Second, it has been shown that a low flow rate supports better cell adhesion on graft surfaces during in vitro culture.34 Prolonged adhesion is crucial to cell proliferation and eventual endothelialization of a TEVG.
Overall, our results indicated that dynamically cultured TEVGs in a low-shear stress environment provide a robust platform for cell population growth and function compared with a static environment. The marked increases in cell number and DNA content in dynamically cultured grafts demonstrate the TPS bioreactor's superiority over static culture conditions in improving cell proliferation and population growth.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) results also demonstrated the efficacy of dynamically cultured EPC-seeded TEVGs in terms of endothelial function, which is critical to vascular homeostasis and health. This includes the expression of molecules such as endothelial NOS3. This protective enzyme contributes to the inhibition of platelet aggregation and adhesion, which mediates inflammation and thrombosis.35 In addition, VEGF is an essential growth factor for ECs. VEGF, which may also be mediated by NOS3, can induce EC migration and proliferation, aiding the formation of a healthy endothelium.36,37 NOS3 expression did not significantly differ between static and dynamic culture conditions, though both cultured populations demonstrated an increase in NOS3 expression compared with initially seeded EPC populations. Dynamically cultured TEVGs seeded with EPCs demonstrated improved VEGF and vWF expression over statically cultured TEVGs. Thus, dynamically cultured EPCs may not only provide better neotissue formation over TEVGs through proliferation but also provide a more functional endothelium than statically cultured grafts immediately on implantation.
The presence of these functional EPCs may also mediate the normal growth of vascular smooth muscle cells within the graft, effectively preventing or reducing issues of intimal hyperplasia.38 Importantly, dynamically cultured TEVGs also expressed increased functional endothelial markers, vWF and VEGF, compared with statically cultured EPCs, as demonstrated by PCR results, and immunohistochemistry seemed to support these findings.
One particularly interesting result is the apparent decrease in CD31 mRNA expression as found by qRT-PCR and shown in Figure 4. However, immunohistochemistry staining demonstrates high expression of CD31 proteins on cultured cells. It should first be noted that there is not always a direct correlation between mRNA expression and actual presence of the protein.39 In fact, inverse correlations can and do occur. In the case of CD31, it has been observed that mRNA expression of CD31 declines as EPCs and ECs become more confluent and establish cell–cell junctions.40 Our results seem to support such an observation. CD31 expression increases during maturation and differentiation of EPCs, then decreases as EPCs proliferate, and, finally, establishes confluence within the grafts.
Figure 5 demonstrates overall favorable expression of both vWF and CD31 on dynamically cultured grafts, especially compared with static controls. Further evidence of endothelial-like differentiation of EPCs in dynamically cultured grafts was observed in the reduction of CD34 mRNA and protein expression after 14 days. CD34, expressed in hematopoietic progenitor and stem cells along with microvascular ECs and EPCs, is generally downregulated during maturation of cell populations from progenitor stem cells.41,42 CD34 mRNA and protein expression may be reduced during differentiation of both hematopoietic lineages and endothelial lineages. Not all subsets of hematopoietic populations express CD31, whereas endothelial lineages do.43
In this study, bone marrow-derived early EPCs isolated from peripheral blood expressing CD34, VEGFR-2, and CD133 were used. These cells are capable of maturing along hematopoietic and endothelial lines.44 Thus, EPCs within static and dynamic cultures may have possessed heterogeneous populations, containing both endothelial and hematopoietic cells, which may be partially responsible for the lower overall CD31 protein marker presence in Figure 7 and changes in CD34 expression.45
Several challenges remain to be solved. This particular method of initial cell seeding yielded low rates of initial cell attachment. However, low attachment is not unexpected. Similar attachment rates have been observed on these materials in other studies.27,32 The physical method of seeding may be altered to enhance cell retention on grafts. Alternatively, techniques to improve cell adhesion to biomaterials have been employed to improve EPC attachment to vascular graft materials. These include modifications such as heparin-coated scaffolds, VEGF, antibodies, and various peptides.28,34,38,46 Such modifications may improve initial cell seeding, which, in turn, may expedite in vitro endothelialization and tissue formation on bioreactor-cultured TEVGs. In addition, there is still some controversy on the role of EPCs and the role of specific subsets of these cells.47 For example, late-outgrowth ECs may express markers that are more consistent with mature ECs compared with endothelial colony-forming cells.8,10 Other subsets of EPCs may perform differently in the low-shear stress environment that the EPC-seeded TEVGs were exposed to. Still, the TEVG and TPS bioreactor setups are amenable to multiple cell types and may foster the endothelialization of grafts utilizing various subtypes of EPCs.
Conclusion
In this study, we successfully demonstrated the enhanced proliferation, infiltration, and differentiation of EPCs into endothelial-like cells seeded on TEVGs and cultured in a dynamic TPS bioreactor system utilizing low-shear stresses akin to the venous system. This platform provides an elegant and effective method of enhancing endothelialization through the use of a readily available cell type to seed small-diameter TEVGs, which may drastically reduce complications such as intimal hyperplasia and thrombosis in experimental and clinical applications. Further optimization and development of this platform may offer an off-the-shelf clinical solution to improve implanted TEVG patency.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under the Award Number R01 AR061460 and through a seed grant from the Children's National Sheikh Zayed Institute for Pediatric Surgical Innovation and the A. James Clark School of Engineering at the University of Maryland. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
J.P.F. is a founder and co-owner of the company 3DBioWorks, which focuses on the use of bioreactors for cell proliferation and differentiation.
References
- 1.Melchiorri A.J., Hibino N., and Fisher J.P. Strategies and techniques to enhance the in situ endothelialization of small-diameter biodegradable polymeric vascular grafts. Tissue Eng Part B 19, 292, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmedlen R.H., Elbjeirami W.M., Gobin A.S., and West J.L. Tissue engineered small-diameter vascular grafts. Clin Plast Surg 30, 507, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Wang X., Lin P., Yao Q., and Chen C. Development of small-diameter vascular grafts. World J Surg 31, 682, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Tondreau M.Y., Laterreur V., Gauvin R., Vallières K., Bourget J.-M., Lacroix D., et al. . Mechanical properties of endothelialized fibroblast-derived vascular scaffolds stimulated in a bioreactor. Acta Biomater 18, 176, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Ahn H., Ju Y.M., Takahashi H., Williams D.F., Yoo J.J., Lee S.J., et al. . Engineered small diameter vascular grafts by combining cell sheet engineering and electrospinning technology. Acta Biomater 16, 14, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Mun C.H., Jung Y., Kim S.H., Kim H.C., and Kim S.H. Effects of pulsatile bioreactor culture on vascular smooth muscle cells seeded on electrospun poly (lactide-co-ɛ-caprolactone) scaffold. Artif Organs 37, E168, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Bérard X., Rémy-Zolghadri M., Bourget C., Turner N., Bareille R., Daculsi R., et al. . Capability of human umbilical cord blood progenitor-derived endothelial cells to form an efficient lining on a polyester vascular graft in vitro. Acta Biomater 5, 1147, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Punshon G., Sales K.M., Vara D.S., Hamilton G., Seifalian A. M. Assessment of the potential of progenitor stem cells extracted from human peripheral blood for seeding a novel vascular graft material. Cell Prolif 41, 321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain R.K., Au P., Tam J., Duda D.G., and Fukumura D. Engineering vascularized tissue. Nat Biotechnol 23, 821, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Ingram D.A., Mead L.E., Tanaka H., Meade V., Fenoglio A., Mortell K., et al. . Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104, 2752, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Prasain N., Meador J.L., and Yoder M.C. Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J Vis Exp pii: , 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann N.A., Reinisch A., and Strunk D. Isolation and large scale expansion of adult human endothelial colony forming progenitor cells. J Vis Exp 1524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinisch A., Hofmann N.A., Obenauf A.C., Kashofer K., Rohde E., Schallmoser K., et al. . Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood 113, 6716, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann N.A., Reinisch A., and Strunk D. Endothelial colony-forming progenitor cell isolation and expansion. Methods Mol Biol 879, 381, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Glynn J.J., and Hinds M.T. Endothelial outgrowth cells: Function and performance in vascular grafts. Tissue Eng Part B Rev 20, 294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphrey J.D. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50, 53, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Ando J., and Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J 73, 1983, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Egorova A.D., DeRuiter M.C., De Boer H.C., Van De Pas S., Gittenberger-De Groot A.C., Van Zonneveld A.J., et al. . Endothelial colony-forming cells show a mature transcriptional response to shear stress. Vitr Cell Dev Biol Anim 48, 21, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Obi S., Masuda H., Shizuno T., Sato A., Yamamoto K., Ando J., et al. . Fluid shear stress induces differentiation of circulating phenotype endothelial progenitor cells. J Appl Physiol 303, C595, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y., Yamamoto K., Ando J., Matsumoto K., and Matsuda T. Arterial shear stress augments the differentiation of endothelial progenitor cells adhered to VEGF-bound surfaces. Biochem Biophys Res Commun 423, 91, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Ankeny R.F., Ankeny C.J., Nerem R.M., and Jo H. Maturing EPCs into endothelial cells: may the force be with the EPCs. Focus on “Fluid shear stress induces differentiation of circulating phenotype endothelial progenitor cells.” AJP Cell Physiol 303, C589, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obi S., Yamamoto K., Shimizu N., Kumagaya S., Masumura T., Sokabe T., et al. . Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol 106, 203, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Yeatts A.B., Both S.K., Yang W., Alghamdi H.S., Yang F., Fisher J.P., et al. . In vivo bone regeneration using tubular Perfusion system bioreactor cultured nanofibrous scaffolds. Tissue Eng Part A 20, 139, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotenberg M.Y., Ruvinov E., Armoza A., and Cohen S. A multi-shear perfusion bioreactor for investigating shear stress effects in endothelial cell constructs. Lab Chip 12, 2696, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Huang A.H., and Niklason L.E. Engineering biological-based vascular grafts using a pulsatile bioreactor. J Vis Exp 2646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Teoh S., Chong M., Yeow C., Kamm R., Choolani M., et al. . Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engine. Tissue Eng Part A 19, 893, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Melchiorri A.J., Hibino N., Brandes Z.R., Jonas R.A., and Fisher J.P. Development and assessment of a biodegradable solvent cast polyester fabric small-diameter vascular graft. J Biomed Mater Res A 102, 1972, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchiorri A.J., Hibino N., Yi T., Lee Y.U., Sugiura T., Tara S., et al. . Contrasting Biofunctionalization strategies for the enhanced endothelialization of biodegradable vascular grafts. Biomacromolecules 16, 437, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pisanti P., Yeatts A.B., Cardea S., Fisher J.P., and Reverchon E. Tubular perfusion system culture of human mesenchymal stem cells on poly-L-lactic acid scaffolds produced using a supercritical carbon dioxide-assisted process. J Biomed Mater Res A 100, 2563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeatts A.B., Geibel E.M., Fears F.F., and Fisher J.P. Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnol Bioeng 109, 2381, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeatts A.B., and Fisher J.P. Tubular perfusion system for the long-term dynamic culture of human mesenchymal stem cells. Tissue Eng Part C Methods 17, 337, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Roh J.D., Nelson G.N., Brennan M.P., Mirensky T.L., Yi T., Hazlett T.F., et al. . Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 29, 1454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto K., Takahashi T., Asahara T., Ohura N., Sokabe T., Kamiya A., et al. . Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol 95, 2081, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Wang X., and Cooper S. Adhesion of endothelial cells and endothelial progenitor cells on peptide-linked polymers in shear flow. Tissue Eng Part A 19, 1113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Förstermann U., and Münzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 113, 1708, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Cai J., Jiang W.G., Ahmed A., and Boulton M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc Res 71, 20, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Hutchings H., Ortega N., and Plouët J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J Off Publ Fed Am Soc Exp Biol 17, 1520, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Zhou M., Liu Z., Liu C., Jiang X., Wei Z., Qiao W., et al. . Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J Biomed Mater Res B Appl Biomater 100, 111, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Greenbaum D., Colangelo C., Gernstein M., and Williams K. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4, 117, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rae P.C., Kelly R.D., Egginton S., and St John J.C. Angiogenic potential of endothelial progenitor cells and embryonic stem cells. Vasc Cell 3, 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., et al. . Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Urbich C., and Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95, 343, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Woodfin A., Voisin M.B., and Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 27, 2514, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Ciraci E., Della Bella S., Salvucci O., Rofani C., Segarra M., Bason C., et al. . Adult human circulating CD34-Lin-CD45-CD133- cells can differentiate into hematopoietic and endothelial cells. Blood 119, 2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Case J., Mead L.E., Bessler W.K., Prater D., White H.A., Saadatzadeh M.R., et al. . Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol 35, 1109, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Chen X., Wang J., An Q., Li D., Liu P., Zhu W., et al. . Electrospun poly(l-lactic acid-co-ɛ-caprolactone) fibers loaded with heparin and vascular endothelial growth factor to improve blood compatibility and endothelial progenitor cell proliferation. Colloids Surf B Biointerfaces 128, 106, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Basile D.P., and Yoder M.C. Circulating and tissue resident endothelial progenitor cells. J Cell Physiol 229, 10, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]