Abstract
Effective solubilization of proteins by chaotropes in proteomic applications motivates their use in solubilization-based antigen removal/decellularization strategies. A high urea concentration has previously been reported to significantly reduce lipophilic antigen content of bovine pericardium (BP); however, structure and function of the resultant extracellular matrix (ECM) scaffold were compromised. It has been recently demonstrated that in vivo ECM scaffold fate is determined by two primary outcome measures as follows: (1) sufficient reduction in antigen content to avoid graft-specific adaptive immune responses and (2) maintenance of native ECM structural proteins to avoid graft-specific innate responses. In this work, we assessed residual antigenicity, ECM architecture, ECM content, thermal stability, and tensile properties of BP subjected to a gradient of urea concentrations to determine whether an intermediate concentration exists at which both antigenicity and structure–function primary outcome measures for successful in vivo scaffold outcome can simultaneously be achieved. Alteration in tissue structure–function properties at various urea concentrations with decreased effectiveness for antigen removal makes use of urea-mediated antigen removal unlikely to be suitable for functional scaffold generation.
Introduction
The use of chaotropes for the extraction of proteins in proteomics and other analytical chemistry applications is well established.1,2 Chaotropes facilitate greater interaction between water molecules and hydrophobic protein moieties, promoting increased solubilization of proteins into aqueous solution. Effectiveness in isolating proteins of interest from a cell suspension or homogenized tissues motivates application of chaotropes to intact tissue to promote solubilization-based antigen removal and/or decellularization toward extracellular matrix (ECM) scaffold generation. Indeed, ethanol,3–7 isopropanol,8 sodium perchlorate,9 sodium dodecyl sulfate,4–7 and urea10 have been utilized for ECM scaffold generation from bovine pericardium (BP),10 aortic valve,4 umbilical vein,6,7 trachea,9 gall bladder,3 omentum,8 and temporomandibular joint.5
Solubilization of antigenic components is critical in promoting their removal from intact tissues.10,11 At high concentrations, urea and thiourea effectively solubilize proteins, which are otherwise difficult to extract into aqueous solution (i.e., highly hydrophobic proteins).2,12,13 Toward the goal of antigen removal from intact tissue, the use of urea and thiourea has been previously demonstrated to significantly reduce residual lipophilic antigenicity of BP.10 However, urea and thiourea are also associated with protein denaturation.14 Indeed, urea and thiourea at high concentrations substantially changed the gross morphology, histological structure, biochemical composition, and tensile properties of BP.10 Therefore, the objective of this work was to determine whether adjustment of urea and thiourea concentration would permit a balance between effective antigen removal and structure–function property preservation in the resultant ECM scaffolds.
Materials and Methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
Tissue harvest
Fresh BP from adult cattle (Spear Products, Inc., Coopersburg, PA) was harvested as previously described,10,11 trimmed into circumferential strips, and stored in Dulbecco's modified Eagle's medium (DMEM) with 15% (v/v) dimethyl sulfoxide (DMSO) at −80°C.
Antiserum production
All animal procedures were conducted in accordance with the guidelines established by the University of California, Davis IACUC and the Guide for the Care and Use of Laboratory Animals.15 Antinative BP serum was generated from New Zealand white rabbits (n = 4) as previously described.10,11 Briefly, following subcutaneous injection of BP homogenate and Freund's adjuvant at a 1:1 ratio into New Zealand white rabbits (n = 4) on days 0, 14, and 28, blood was collected at day 84. Serum was isolated following centrifugation and stored at −80°C.
Stepwise solubilization-based antigen removal
Each set of five anatomically-adjacent intact BP pieces (∼1.0 × 1.5 cm and 0.2 g) was generated from a single circumferentially oriented strip of BP as follows: one piece for native control and one for each of four antigen removal treatments. Antigen removal was performed on intact BP in 2 mL working volume at 4°C and 125 rpm unless otherwise stated and as previously described.10,16
Briefly, hydrophilic solubilization was achieved through incubation in optimized solubilizing antigen removal buffer (opt SARB; 10 mM Tris-HCl, pH 8.0 containing 100 mM dithiothreitol, 100 mM KCl, 2 mM MgCl2, 0.5 mM Pefabloc, and 1% [v/v] antibiotic–antimycotic solution) for 48 h. The first step of antigen removal was followed by lipophile solubilization at room temperature using one of six chaotrope solutions in opt SARB for 48 h: (1) 10% (v/v) isopropanol, 5% (v/v) glycerol; (2) 10% (v/v) isopropanol, 5% (v/v) glycerol, and 12.5% (v/v) water-saturated isobutanol; (3) 0 M urea and 0 M thiourea; (4) 4 M urea and 1 M thiourea; (5) 6 M urea and 1.5 M thiourea; and (6) 8 M urea and 2 M thiourea. Following nucleic acid digestion for 24 h and washout for 48 h, samples were stored in DMEM with 15% (v/v) DMSO at −80°C.
For Western blot analysis of residual antigens, an anatomically adjacent piece of BP subjected to lipophile solubilization for 1 min served as a negative antigen removal control for biological tissue variability and effects of antigen removal additives as previously described.10,11
Protein extraction
Protein extraction from minced samples (n = 6 per group) was performed as previously described10,11 using standard extraction solution (10 mM Tris-HCl [pH 8.0] containing 1 mM dithiothreitol, 2 mM magnesium chloride hexahydrate, 10 mM potassium chloride, and 0.5 mM Pefabloc SC [Roche, Indianapolis, IN]) containing 0.1% (w/v) SDS (Bio-Rad, Hercules, CA) to isolate residual hydrophilic proteins and standard extraction solution containing 1% (w/v) SDS to isolate residual lipophilic proteins.
One-dimensional electrophoresis and Western blot
One-dimensional electrophoresis and Western blot were performed using equal volumes of residual lipophilic protein extract (n = 6 per group) and day 84 antiserum as previously described.10,11 Residual antigenicity for a given group was calculated as band density with 48 h lipophile solubilization normalized to 1 min lipophile solubilization. Normalized residual antigenicity ratio was defined as residual antigenicity for a given group normalized to opt SARB vehicle only, time-matched control.
Histology
Histological assessment of samples was performed from two 1 mm wide strips (n = 6 per group) as previously described.10,11 Formalin-fixed paraffin-embedded sections underwent hematoxylin and eosin staining for assessment of general tissue ECM architecture, Verhoeff van Gieson staining for assessment of gross collagen and elastin organization, and Picrosirius red staining (PSR) for assessment of collagen alignment.
Collagen alignment
PSR-stained sections were assessed for collagen alignment under polarized light. Collagen alignment was quantified as percent area of collagen birefringence from high powered fields (HPFs) taken randomly throughout samples (n = 3 HPF per sample, n = 6 per group).
Quantitative biochemistry
ECM composition of samples was determined as previously described.10,11 Three 5 mm diameter discs (n = 6 per group) were weighed, frozen at −20°C overnight, lyophilized for 48 h, and weighed again.
One disc was subjected to digestion in 5 N HCl (1 mL per 10 mg dry weight [DW]) at 120°C for 24 h. Bovine collagen I (Chondrex, Inc., Redmond, WA) was digested similarly. Digest was quantified for collagen content per DW using collagen I standards and the Hydroxyproline Assay Kit (Chondrex, Inc.). A second disc was subjected to hot oxalic acid extraction (1 mL per 50 mg DW) at 99°C for 1 h four times. Digest was quantified for elastin content per DW using the Fastin elastin assay (Biocolor Ltd., Carrickfergus, UK). The third disc was subjected to papain digest (1 mL per 10 mg DW) at 65°C for 19 h. Digest was quantified for sulfated glycosaminoglycan (GAG) content per DW using the Blyscan sulfated GAG assay (Biocolor Ltd.).
Differential scanning calorimetry
Thermal stability of ECM proteins in the samples was determined using an adaptation of a method previously described.16,17 One 5 mm diameter disc (n = 6 per group) was weighed, frozen at −20°C overnight, lyophilized for 48 h, and weighed again (0.8–5.7 mg in DW). Lyophilized samples were crimped in aluminum pans and heated (30–280°C at 20°C/min and 10 min hold at 120°C) using a differential scanning calorimeter (DSC 6000; PerkinElmer, Waltham, MA). Denaturation temperature was determined with Pyris software (version 11.1.1.0497; PerkinElmer).
Tensile testing
Tensile properties of the samples were determined as previously described.10,11 Dog-bones (neck region 4.0 × 1.5 mm) were created from intact samples using pressed razor blades loaded into a custom 3D-printed handle (n = 6 per group). Samples were oriented with the anatomically circumferential direction parallel to the long axis of the dog-bone. Dog-bones were mounted under zero strain and subjected to a constant strain rate of 0.2 mm/s. Initial gauge length was defined as 4 mm. Initial gauge width and thickness were determined from images using ImageJ 1.42q software (Wayne Rasband; National Institutes of Health, Bethesda, MD). For each sample, a stress–strain curve was generated from the load-elongation curve, and the Young's modulus and ultimate tensile stress (UTS) were determined.
Statistical analysis
Nonrepeated measures ANOVA and Tukey–Kramer HSD post hoc analyses were performed on sample means. All data are presented as mean ± standard deviation unless otherwise stated using statistical significance of p < 0.05.
Results
Residual antigenicity
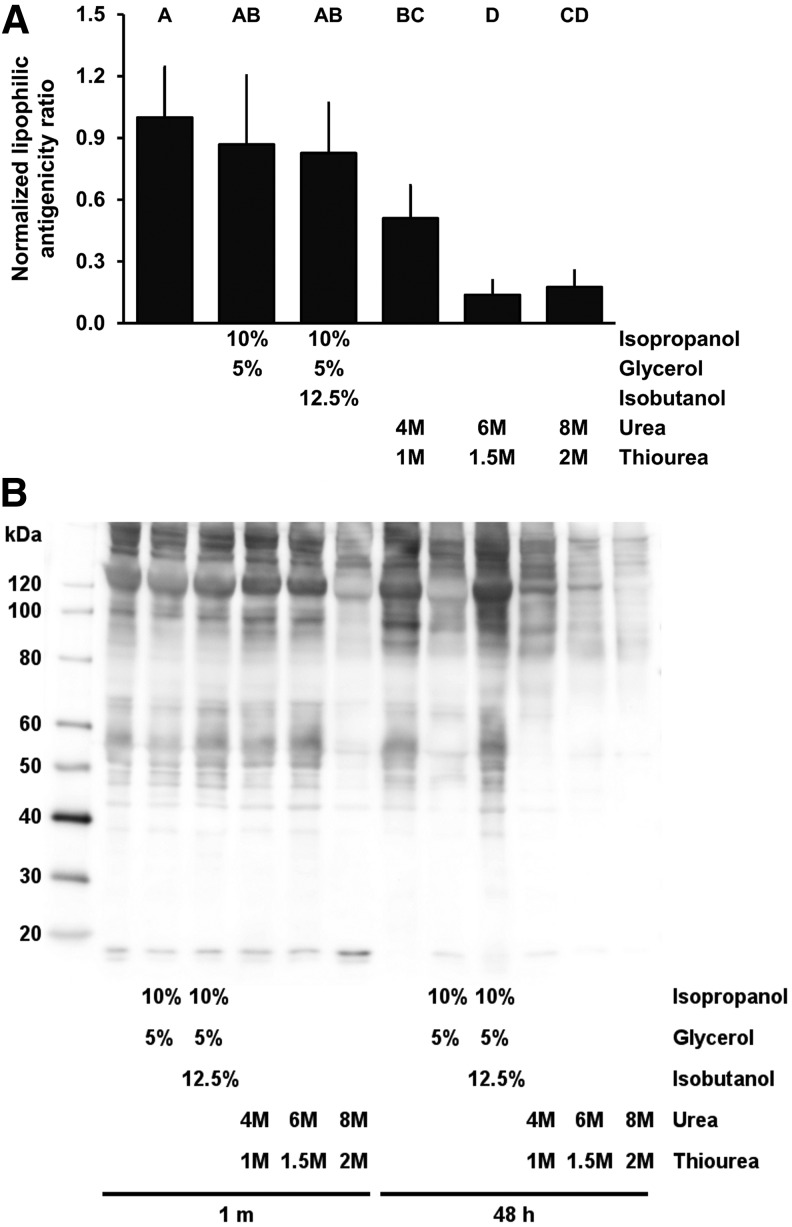
Use of neither isopropanol and glycerol in opt SARB (0.87 ± 0.33) nor isopropanol, glycerol, and isobutanol in opt SARB (0.83 ± 0.24) removed a significantly greater level of lipophilic antigens than achieved using opt SARB alone (1.00 ± 0.25, p = 0.8880 and p = 0.7124, respectively) (Fig. 1). Addition of urea and thiourea to opt SARB significantly reduced residual lipophilic antigenicity of BP: to 0.51 ± 0.16 with 4 M urea and 1 M thiourea (p = 0.0045), 0.14 ± 0.07 with 6 M urea and 1.5 M thiourea (p < 0.0001), and 0.18 ± 0.08 with 8 M urea and 2 M thiourea (p < 0.0001). As neither isopropanol and glycerol in opt SARB nor isopropanol, glycerol, and isobutanol in opt SARB significantly reduced residual antigenicity, they were excluded from subsequent structure–function assessments.
FIG. 1.
Residual lipophilic minor histocompatibility antigens in BP following antigen removal using chaotropes. Urea and thiourea decrease lipophilic antigenicity of BP (A). Representative Western blot of lipophilic BP-specific antigens following addition of chaotropes (B). Results plotted as mean ± standard deviation (n = 6 per group). Groups not connected by same letter are significantly different (p < 0.05). BP, bovine pericardium.
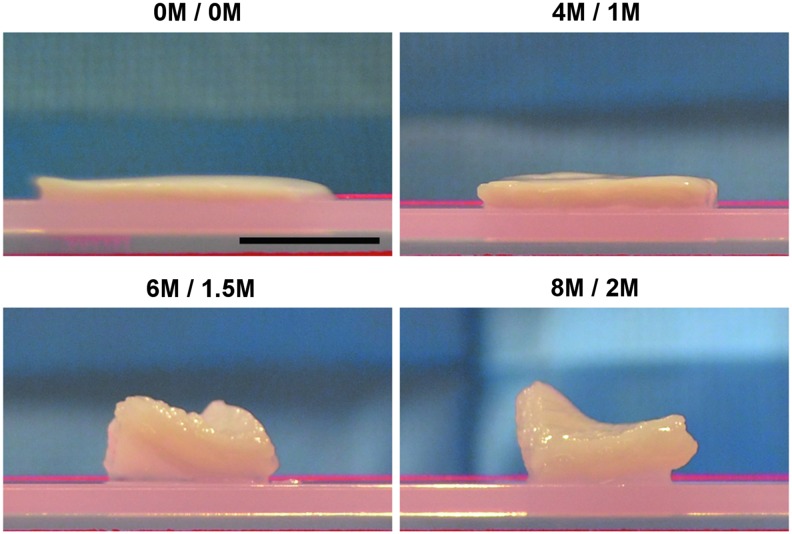
Gross morphology
Inclusion of urea and thiourea into opt SARB grossly changed the morphology of BP (Fig. 2). Application of 4 M urea and 1 M thiourea in opt SARB (4 M/1 M) resulted in thickening compared to opt SARB alone (0 M/0 M). At 6 M urea and 1.5 M thiourea (6 M/1.5 M) and 8 M urea and 2 M thiourea (8 M/2 M), BP underwent substantial thickening and twisting.
FIG. 2.
Gross morphology of BP following antigen removal using chaotropes. Urea and thiourea substantially thicken BP. Scale bar = 1 cm.
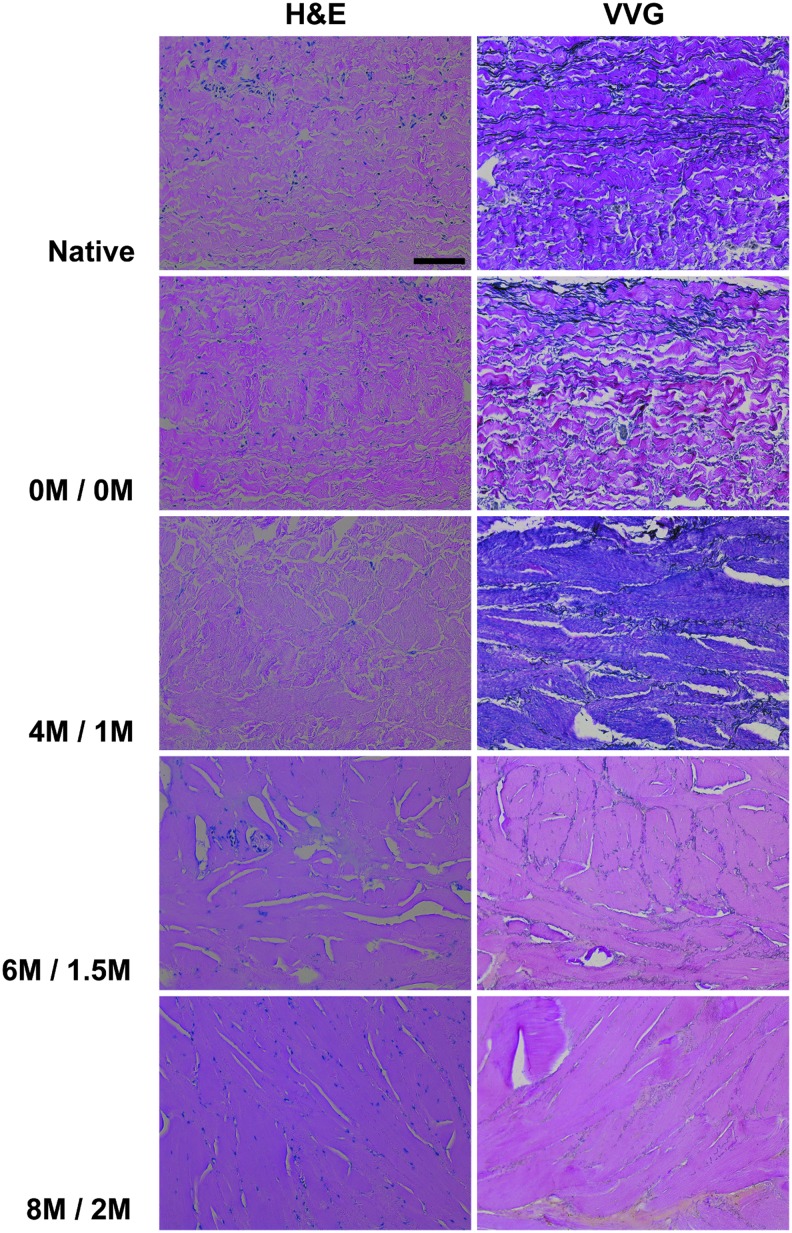
Histological ECM organization
Organization of BP collagen and elastin in the absence of urea and thiourea was not different from that in native BP: wavy collagen bundles were interspersed with elongated elastin molecules (Fig. 3). With addition of 4 M urea and 1 M thiourea, the wavy morphology of collagen characteristic in native BP was lost and elastin molecules became short and fragmented. Increasing chaotrope concentration to 6 M urea and 1.5 M thiourea further altered ECM organization. Small pores between collagen bundles, still apparent with 4 M urea and 1 M thiourea, were no longer detectable and elastin molecules became even more dispersed and fragmented. With 8 M urea and 2 M thiourea, only large pores between collagen bundles were evident, but occupied less area than those with 6 M urea and 1.5 M thiourea, and elastin molecules were even less apparent.
FIG. 3.
Histological architecture of BP following antigen removal using chaotropes. H&E staining (20 × ) and VVG staining (20 × ) reveal loss of BP collagen waviness and greater ECM compaction with increasing urea and thiourea concentrations. Elastin molecules fragment and decrease with increasing urea and thiourea concentrations. Scale bar = 100 μm. ECM, extracellular matrix. H&E, hematoxylin and eosin; VVG, Verhoeff van Gieson.
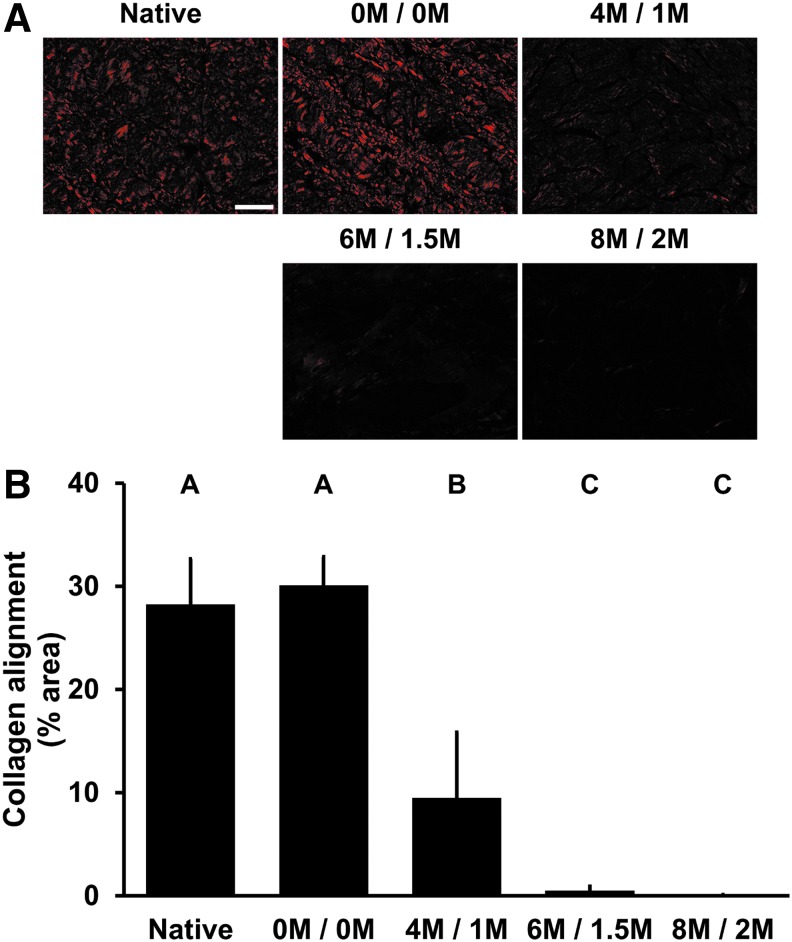
Collagen alignment
Alignment of collagen was significantly reduced with 4 M urea and 1 M thiourea (9.48% ± 6.37% area) compared to native BP (28.24% ± 4.41% area, p < 0.0001) (Fig. 4A, B). Both 6 M urea and 1.5 M thiourea (0.48% ± 0.44% area) and 8 M urea and 2 M thiourea (0.07% ± 0.04% area) had significantly less collagen alignment than native BP (p < 0.0001) and BP treated with 4 M urea and 1 M thiourea (p = 0.0014 and p = 0.0023, respectively).
FIG. 4.
Collagen alignment of BP following antigen removal using chaotropes. Picrosirius red staining under polarized light (20 × ) reveals loss of BP collagen alignment with increasing urea and thiourea concentrations (A). Percent area of collagen alignment is further decreased with high levels of urea and thiourea compared to low levels (B). Results plotted as mean ± standard deviation (n = 6 per group). Groups not connected by same letter are significantly different (p < 0.05). Scale bar = 100 μm.
Biochemical composition
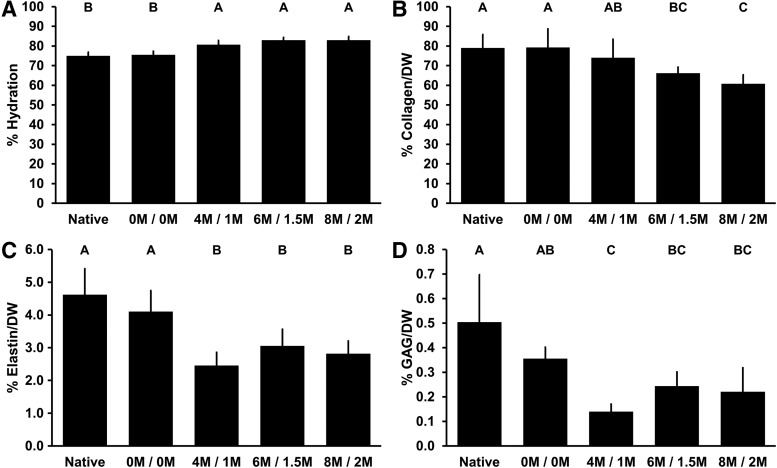
Water content increased significantly with exposure to urea and thiourea compared to native BP (74.97% ± 1.84%) to 80.63% ± 2.17% with 4 M urea and 1 M thiourea (p = 0.0001), 82.93% ± 1.45% with 6 M urea and 1.5 M thiourea (p < 0.0001), and 82.92% ± 1.84% with 8 M urea and 2 M thiourea (p < 0.0001) (Fig. 5A).
FIG. 5.
ECM composition of BP following antigen removal using chaotropes. Water content of BP increases with exposure to urea and thiourea (A). Collagen content of BP is not significantly different than native following treatment with 4 M urea and 1 M thiourea, but is decreased with 6 M urea and 1.5 M thiourea, and 8 M urea and 2 M thiourea (B). Both elastin (C) and GAG (D) content of BP are decreased in the presence of urea and thiourea. Results plotted as mean ± standard deviation (n = 6 per group). Groups not connected by same letter are significantly different (p < 0.05). GAG, glycosaminoglycan.
Collagen content of BP treated with 4 M urea and 1 M thiourea (74.00% ± 9.37%) was not significantly different from that of native BP (79.01% ± 6.86%, p = 0.7453) (Fig. 5B). However, both 6 M urea and 1.5 M thiourea (66.12% ± 3.12%) and 8 M urea and 2 M thiourea (60.72% ± 4.58%) significantly reduced collagen content of BP (p = 0.0325 and p = 0.0014, respectively). Interestingly, while collagen content of BP subjected to 6 M urea and 1.5 M thiourea was not significantly different from that of 4 M urea and 1 M thiourea (p = 0.3328), 8 M urea and 2 M thiourea significantly decreased collagen compared to that found in 4 M urea and 1 M thiourea (p = 0.0258).
Elastin content was significantly reduced compared to native BP (4.66% ± 0.76%) following any level of urea and thiourea treatment: to 2.49% ± 0.41% with 4 M urea and 1 M thiourea (p < 0.0001), 3.09% ± 0.50% with 6 M urea and 1.5 M thiourea (p = 0.0006), and 2.86% ± 0.43% with 8 M urea and 2 M thiourea (p < 0.0001) (Fig. 5C).
GAG content of BP subjected to any level of urea and thiourea was significantly reduced compared to native BP (0.50% ± 0.19%): to 0.14% ± 0.03% with 4 M urea and 1 M thiourea (p < 0.0001), 0.24% ± 0.06% with 6 M urea and 1.5 M thiourea (p = 0.0016), and 0.25% ± 0.10% with 8 M urea and 2 M thiourea (p = 0.0.0019) (Fig. 5D).
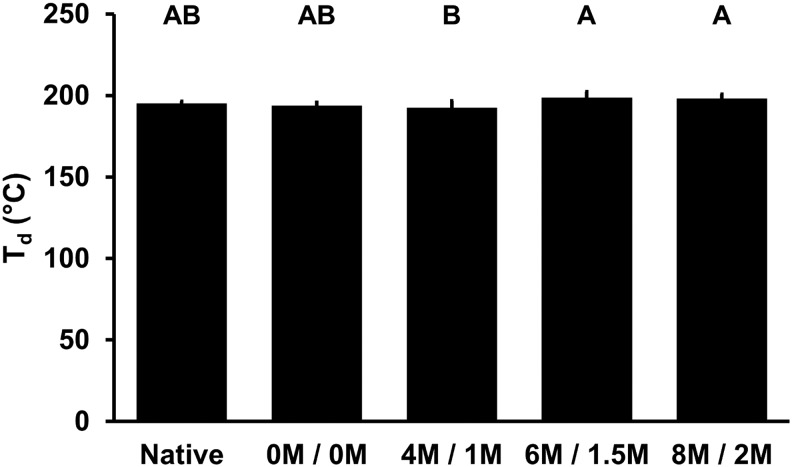
Thermal stability
The denaturation temperature of BP ECM proteins following treatment with urea and thiourea was not significantly different from native BP (195.08°C ± 1.47°C, p > 0.3440) (Fig. 6). The slight decrease in BP denaturation temperature with 4 M urea and 1 M thiourea (192.42°C ± 4.17°C) was significantly less than the slightly increased denaturation temperature of BP treated with 6 M urea and 1.5 M thiourea (198.43°C ± 3.86°C, p = 0.0178) and 8 M urea and 2 M thiourea (198.16°C ± 2.61°C, p = 0.0355).
FIG. 6.
Thermal stability of BP following antigen removal using chaotropes. Denaturation temperature (Td) of BP with exposure to urea and thiourea. Results plotted as mean ± standard deviation (n = 6 per group). Groups not connected by same letter are significantly different (p < 0.05).
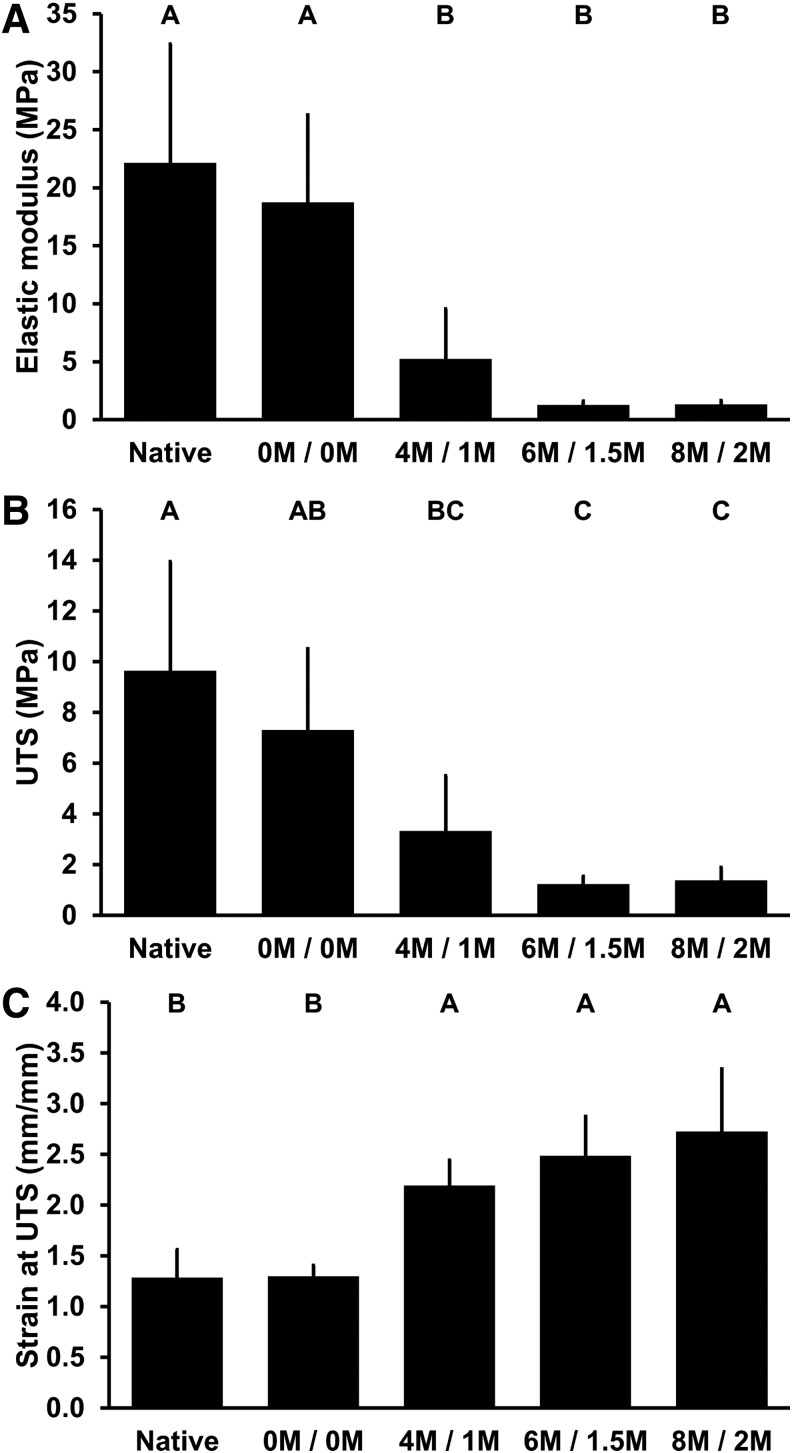
Tensile properties
The elastic modulus of BP following treatment with any level of urea and thiourea was significantly reduced compared to native BP (22.13 ± 10.26 MPa): to 5.20 ± 4.34 MPa with 4 M urea and 1 M thiourea (p = 0.0005), 1.25 ± 0.34 MPa with 6 M urea and 1.5 M thiourea (p < 0.0.001), and 1.32 ± 0.34 MPa with 8 M urea and 2 M thiourea (p < 0.0.001) (Fig. 7A).
FIG. 7.
Tensile properties of BP following antigen removal using chaotropes. Elastic modulus (A) and UTS (B) of BP decrease with exposure to urea and thiourea. Strain at UTS (C) of BP increases with exposure to urea and thiourea. Results plotted as mean ± standard deviation (n = 6 per group). Groups not connected by same letter are significantly different (p < 0.05). UTS, ultimate tensile stress.
The UTS of BP following treatment with any level of urea and thiourea was significantly reduced compared to native BP (9.64 ± 4.30 MPa): to 3.32 ± 2.19 MPa with 4 M urea and 1 M thiourea (p = 0.0025), 1.22 ± 0.31 MPa with 6 M urea and 1.5 M thiourea (p < 0.0.001), and 1.37 ± 0.51 MPa with 8 M urea and 2 M thiourea (p < 0.0.001) (Fig. 7B).
The strain at maximum stress of BP following treatment with any level of urea and thiourea was significantly increased compared to native BP (1.29 ± 0.28 mm/mm): to 2.19 ± 0.25 mm/mm with 4 M urea and 1 M thiourea (p = 0.0023), 2.49 ± 0.39 mm/mm with 6 M urea and 1.5 M thiourea (p < 0.0.001), and 2.72 ± 0.62 mm/mm with 8 M urea and 2 M thiourea (p < 0.0.001) (Fig. 7C).
Discussion
Two primary outcome measures must be simultaneously considered in functional xenogeneic scaffold generation for successful in vivo recipient responses as follows: (1) reduction in scaffold antigen content and (2) preservation of native ECM structure–function properties.16 Inadequate antigen removal has the potential to stimulate recipient graft-specific adaptive immune responses, while loss of native ECM structure–function properties has potential to stimulate deleterious recipient innate immune responses. The current work demonstrates that simultaneously achieving these two goals may not be possible with the use of urea and thiourea, as loss of native ECM structure–function properties was still observed despite decreasing ability to achieve antigen removal with decreasing chaotrope concentrations. By extension, such denaturing solubilizing agents may require that additional hurdles are overcome (e.g., protein refolding into native confirmation) before translational use in xenogeneic scaffold generation.
Decellularization and antigen removal strategies aim to overcome the immunological potential of xenogeneic ECM scaffolds by removing antigenic components from the tissue that elicit recipient adaptive immune response upon implantation. Indeed, recipient graft-specific immune responses toward xenogeneic tissues, in which antigen burden is insufficiently reduced, have been well documented. Implantation of immunogenic, epitope-containing peptide biomaterials in mice led to rising graft-specific IgG antibody titers and T-cell recruitment.18 Similarly, implantation of porcine-derived biomaterials in Old World primates led to rising graft-specific and anti-Galα1,3-Galβ1-4GlcNAc-R (α-Gal) IgG titers.19 In the clinical setting, IgG antibody titers for α-Gal IgG have been shown to increase in both juvenile and adult recipients of animal-derived, glutaraldehyde-fixed heart valve replacements.20,21 In proof-of-principle nonhuman primate studies, bioprostheses generated from Gal-knockout animals avoided elevation in α-Gal titers associated with bioprostheses generated from wild-type animals.22 The potentially catastrophic consequences of insufficient removal of antigenic components were most devastatingly demonstrated when implantation of decellularized porcine heart valves (Synergraft) in juvenile patients, later determined to contain cell remnants, led to lymphocytic response, calcification, and ultimately the deaths of 3 out of 4 children.23 In this study, we demonstrate the ability to achieve >80% reduction in lipophilic antigens using 6 and 8 M urea. However, 4 M urea was significantly less effective in achieving antigen removal compared to 6 M urea (49% vs. 86% reduction in residual lipophilic antigens, respectively). In an in vivo leporine subpannicular model, reduction of lipophilic minor histocompatibility antigens in BP by 60% using amidosulfobetaine-14 (ASB-14) was associated with a significant decrease in graft-specific adaptive immune responses compared to control scaffolds.10,16 Conversely, the mere 37% reduction in lipophilic antigens using 1% (w/v) SDS led to greater graft-specific T-cell response. It is possible that a 49% reduction in lipophilic antigens achieved using 4 M urea may be sufficient to ameliorate recipient adaptive immune responses, but evaluation using an in vivo model will be necessary to make this determination. Nonetheless, we anticipate that effectiveness in achieving the first outcome measure of in vivo scaffold success (i.e., reduction in scaffold antigen content) will continue to decrease with urea concentrations <4 M.
Maintenance of native ECM structure–function properties following decellularization and antigen removal processing preserves the ability for ECM scaffolds to withstand physiological mechanical forces. Treatment of BP with a spectrum of urea concentrations altered structure–function properties of the resultant biomaterial. Alteration of elasticity-related properties for urea-treated BP seemingly occurred in a concentration-independent manner. A similar reduction in elastin content and Young's modulus was observed for any urea-treated group. The increase in strain at maximum stress was also similar among all urea-treated groups. Conversely, the collagen-associated properties of BP were altered in a urea concentration-dependent manner: at high urea concentrations (i.e., 6 and 8 M), gross morphology, histological organization, collagen alignment, collagen content, and UTS of treated BP were significantly different than both native BP and BP that did not undergo treatment with urea (i.e., 0 M); however, at a lower urea concentration (i.e., 4 M), reduced collagen damage evidenced by gross morphology, histological organization, collagen alignment, collagen content, and UTS of treated BP resulted in a biomaterial with less severe ECM disruption. The differing association of urea with protein residues based on their hydrophobicity24 may account for the difference in effect on elastin- and collagen-based tissue properties. Alternatively, the absence of a concentration-dependent trend in elastin content, elastic properties, and GAG content with urea concentrations between 4 and 8 M may also be a result of elastin and GAG loss already having plateaued. Interestingly, elastin and GAG content were not observed to drop to 0%, perhaps owing to the loss of tissue porosity evident histologically preventing further elastin and GAG removal from the tissue. Overall damage to the structural organization of ECM proteins is anticipated to decrease thermal stability and denaturation temperature, while stabilization of ECM protein structure is expected to increase thermal stability and denaturation temperature. Although significance was not reached, addition of low levels of urea (i.e., 4 M) seemed to disrupt BP ECM structure enough to be visualized histologically and cause BP denaturation temperature to trend down. Interestingly, with greater concentrations of urea (i.e., 6 and 8 M), the increasing trend in denaturation temperature seems to suggest that the bound urea stabilizes the damaged ECM structure, as seen by tighter packing on histology. It has previously been demonstrated that maintenance of native ECM architecture using ASB-14 permitted in vivo recognition of the BP implant as self in origin and avoidance of foreign body fibrous encapsulation by the innate leporine immune response.16 Conversely, loss of native ECM architecture in glutaraldehyde-fixed BP or from using 1% (w/v) SDS was associated with significant innate immune-mediated foreign body response and fibrous encapsulation. Similarly, loss of biological and structural integrity of porcine-derived biomaterials resulted in foreign body response, as well as hindrance to normal tissue healing and integration processes, upon implantation in Old World primates.19 The innate immune response has also been implicated in stenosis of tissue engineered vascular grafts in murine studies performed to understand negative clinical outcomes.25 Regardless of the manifestation of the effect, it is apparent from altered tissue structure–function properties that urea indeed damages ECM proteins, which is undesirable for in vivo applications.
Two theories for the mechanism of urea-mediated protein solubilization have been described in the literature as follows: (1) indirectly through disruption of hydrogen bonds that mediate water–water interactions26 or (2) directly through interaction with the protein molecule.24 The indirect mechanism suggests that perturbation of the water network reduces the hydrophobic effect, thereby stabilizing hydrophobic moieties and promoting protein solubilization.26 If urea denatures and solubilizes proteins in an indirect manner, refolding of ECM proteins back to native confirmation may be necessary for successful in vivo outcomes with the resultant scaffold. Alternatively, the direct mechanism proposes that van der Waals forces between the protein and urea, being more favorable than water–protein interactions, draw urea molecules to the protein surface.24 Strong hydrogen bonds form between the carbonyl oxygen of urea and amide hydrogen molecules within the protein backbone. Hydrophilic protein-bound urea then recruits water molecules, much like a surfactant, to promote protein solubilization. The association of urea with the protein backbone breaks hydrogen bonds within the protein backbone, resulting in protein unfolding, denaturation, and solubilization.24 If urea denatures and solubilizes proteins in a direct manner, as suggested by the decreasing stability and denaturation temperature with 4 M urea and increasing stability and denaturation temperature with 6 and 8 M urea described above, removal of bound urea and refolding of ECM proteins back to native confirmation may be necessary for the resultant scaffold to achieve successful in vivo outcomes. Whether protein-bound urea can effectively be washed out of the ECM scaffold to recover native structure–function properties was not tested in this study and remains to be determined. However, according to the direct binding paradigm, the affinity between urea and proteins is favorable to that between water and protein.24 Thus, it is unlikely that urea could be sufficiently washed out from the protein backbone of ECM proteins to recover tissue structure–function properties. Even if urea could be sufficiently washed out from ECM proteins, it will need to be determined whether urea-mediated denaturation could be reversed through protein refolding back to native confirmation. Finally, even with achievement of protein refolding, any negative consequences associated with loss of ECM components (i.e., elastin and GAG) on in vivo outcomes will need to be identified.
Toward generation of a functional xenogeneic scaffold for clinical translation, the reduced ability for 4 M urea to achieve antigen removal, coupled with persistence of significant negative effects on scaffold structure–function properties, represents an inability of urea to achieve a balance between the competing goals for successful in vivo outcomes. By extension, it is unlikely that additional value will be gained from performing antigenicity and structure–function analyses for <4 M urea. As urea-mediated protein solubilization and denaturation likely cannot be uncoupled, there is unlikely to be a urea concentration that is compatible with functional ECM scaffold generation. Beyond just urea or even chaotropes (e.g., ethanol, isopropanol, sodium perchlorate, and sodium dodecyl sulfate), scientists and engineers seeking to enhance solubilization-based antigen removal for xenogeneic scaffold generation will need to consider a chemical's mechanism of action (i.e., whether protein solubilization is associated with denaturation) toward their end goal, particularly if native tissue properties are to be retained.
Authors’ Contributions
Conceived and designed experiments: M.L.W. and J.L.W. Performed experiments: M.L.W., J.L.W., R.M.H., K.C.S., and D.A.R. Analyzed the data: M.L.W., J.L.W., R.M.H., and K.C.S. Drafted article: M.L.W., J.L.W., and L.G.G. Funded the work: L.G.G.
Acknowledgments
This work was supported by the American Heart Association (SDG4980023, LGG) and NIH (R01HL115205, LGG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hill R.C., Calle E.A., Dzieciatkowska M., Niklason L.E., and Hansen K.C. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol Cell Proteomics 14, 961, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cordwell S.J. Sequential extraction of proteins by chemical reagents. Methods Mol Biol 424, 139, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Burugapalli K., Thapasimuttu A., Chan J.C.Y., Yao L., Brody S., Kelly J.L., and Pandit A. Scaffold with a natural mesh-like architecture: isolation, structural, and in vitro characterization. Biomacromolecules 8, 928, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Lim H.-G., Kim G.B., Jeong S., and Kim Y.J. Development of a next-generation tissue valve using a glutaraldehyde-fixed porcine aortic valve treated with decellularization, α-galactosidase, space filler, organic solvent and detoxification. Eur J Cardiothorac Surg 48, 104, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Lumpkins S.B., Pierre N., and McFetridge P.S. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater 4, 808, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Moore M., Sarntinoranont M., and McFetridge P. Mass transfer trends occurring in engineered ex vivo tissue scaffolds. J Biomed Mater Res Part A 100, 2194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uzarski J.S., Van De Walle A.B., and McFetridge P.S. Preimplantation processing of ex vivo-derived vascular biomaterials: effects on peripheral cell adhesion. J Biomed Mater Res Part A 101, 123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porzionato A., Sfriso M.M., Macchi V., Rambaldo A., Lago G., Lancerotto L., Vindigni V., and De Caro R. Decellularized omentum as novel biologic scaffold for reconstructive surgery and regenerative medicine. Eur J Histochem 57, e4, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiselevsky M.V., Anisimova N.Y., Lebedinskaya O.V., Polotskii B.E., and Davydov M.I. Optimization of a method for preparation and repopulation of the tracheal matrix for allogenic transplantation. Bull Exp Biol Med 151, 107, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Wong M.L., Wong J.L., Athanasiou K.A., and Griffiths L.G. Stepwise solubilization-based antigen removal for xenogeneic scaffold generation in tissue engineering. Acta Biomater 9, 6492, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Wong M.L., Leach J.K., Athanasiou K.A., and Griffiths L.G. The role of protein solubilization in antigen removal from xenogeneic tissue for heart valve tissue engineering. Biomaterials 32, 8129, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Rabilloud T., Adessi C., Giraudel A., and Lunardi J. Improvement of the solubilization of proteins in two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 18, 307, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabilloud T. Use of thiourea to increase the solubility of membrane proteins in two-dimensional electrophoresis. Electrophoresis 19, 758, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Gordon J.A., and Jencks W.P. The relationship of structure to the effectiveness of denaturing agents for proteins. Biochemistry 2, 47, 1963 [DOI] [PubMed] [Google Scholar]
- 15.National Research Council Institute of Laboratory Animal Resources Commission on Life Sciences. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, 1996 [Google Scholar]
- 16.Wong M.L., Wong J.L., Vapniarsky N., and Griffiths L.G. In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials 92, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samouillan V., Dandurand J., Lacabanne C., Thoma R.J., Adams A., and Moore M. Comparison of chemical treatments on the chain dynamics and thermal stability of bovine pericardium collagen. J Biomed Mater Res Part A 64A, 330, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Vigneswaran Y., Han H., De Loera R., Wen Y., Zhang X., Sun T., Mora-Solano C., and Collier J.H. Peptide biomaterials raising adaptive immune responses in wound healing contexts. J Biomed Mater Res Part A 2016; DOI: 10.1002/jbm.a.35767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandor M., Xu H., Connor J., Lombardi J., Harper J.R., Silverman R.P., and McQuillan D.J. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng 14, 2021, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Park C.S., Park S.-S., Choi S.Y., Yoon S.H., Kim W.-H., and Kim Y.J. Anti-Gal immune response following porcine bioprosthesis implantation in children. J Heart Valve Dis 19, 2010 [PubMed] [Google Scholar]
- 21.Park C.S., Oh S.-S., Kim Y.E., Choi S.Y., Lim H.-G., Ahn H., and Kim Y.J. Anti-alpha-Gal antibody response following xenogeneic heart valve implantation in adults. J Heart Valve Dis 22, 222, 2013 [PubMed] [Google Scholar]
- 22.McGregor C., Kogelberg H., Vlasin M., and Byrne G.W. Gal-knockout bioprostheses exhibit less immune stimulation compared to standard biological heart valves. J Heart Valve Dis 22, 383, 2013 [PubMed] [Google Scholar]
- 23.Simon P., Kasimir M.T., Seebacher G., Weigel G., Ullrich R., Salzer-Muhar U., Rieder E., and Wolner E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT(TM) in pediatric patients. Eur J Cardiothorac Surg 23, 1002, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Hua L., Zhou R., Thirumalai D., and Berne B.J. Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding. Proc Natl Acad Sci U S A 105, 16928, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hibino N., Mejias D., Pietris N., Dean E., Yi T., Best C., Shinoka T., and Breuer C. The innate immune system contributes to tissue-engineered vascular graft performance. FASEB J 29, 2431, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennion B.J., and Daggett V. The molecular basis for the chemical denaturation of proteins by urea. Proc Natl Acad Sci U S A 100, 5142, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]