Abstract
Glucocorticoids (GCs) are the standard therapy for treating multiple sclerosis (MS) patients suffering from an acute relapse. One of the main mechanisms of gC action is held to be the induction of T cell apoptosis leading to reduced lymphocyte infiltration into the CNS, yet our analysis of experimental autoimmune encephalomyelitis (EAE) in three different strains of genetically manipulated mice has revealed that the induction of T cell apoptosis is not essential for the therapeutic efficacy of GCs. Instead, we identified the redirection of T cell migration in response to chemokines as a new therapeutic principle of GC action. GCs inhibited the migration of T cells towards CCL19 while they enhanced their responsiveness towards CXCL12. Importantly, blocking CXCR4 signaling in vivo by applying Plerixafor® strongly impaired the capacity of GCs to interfere with EAE, as revealed by an aggravated disease course, more pronounced CNS infiltration and a more dispersed distribution of the infiltrating T cells throughout the parenchyma. Our observation that T cells lacking the GC receptor were refractory to CXCL12 further underscores the importance of this pathway for the treatment of EAE by GCs. Importantly, methylprednisolone pulse therapy strongly increased the capacity of peripheral blood T cells from MS patients of different subtypes to migrate towards CXCL12. This indicates that modulation of T cell migration is an important mechanistic principle responsible for the efficacy of high-dose GC therapy not only of EAE but also of MS.
Keywords: Multiple sclerosis, EAE, MS patients, Glucocorticoids, Apoptosis, CXCR4
Introduction
Administration of synthetic glucocorticoids (GCs) is an integral part of multiple sclerosis (MS) therapy. Experimental autoimmune encephalomyelitis (EAE) is a valuable tool with which to investigate the pathomechanism of MS and has substantially helped to develop new therapeutic regimens [42]. In addition, EAE has been instrumental in dissecting how GCs exert their beneficial effects in the treatment of neuroinflammatory diseases [40]. It has been long known that GCs increase T cell apoptosis in the CNS and peripheral lymphoid organs and indeed this function is currently thought to play a pivotal role for their therapeutic efficacy [31, 35]. However, GCs also reduce the expression of cytokines and adhesion molecules, modulate macrophage and microglia functions and tighten the blood–brain barrier (BBB). While there are a variety of cell types that are potential targets of GC therapy of EAE, recent findings suggest that T lymphocytes are the most critical ones [50, 51].
Directed T cell migration is guided by chemokines, many of which have been implicated in the pathogenesis of EAE and MS [17]. Inflammatory chemokines such as CCL2 and CCL5 are generally up-regulated during EAE while constitutively expressed ones such as CXCL10, CXCL12, CCL19, CCL20 and CCL21 are expressed by endothelial cells of the BBB as well as other areas of the CNS under steady state conditions [1, 10, 27, 33, 43]. Although the importance of chemokine pathways in EAE and MS is well established, little is known concerning their regulation by GCs. It has been found that CXCL10 and CCL20, two chemokines involved in the migration of Th1 and Th17 cells to the CNS, were reduced in human patients after GC application [21, 25]. Furthermore, the diminished numbers of peripheral blood T cells after physical stress correlated with high cortisol levels and an increased migratory capacity towards CXCL12 [30]. This suggested an involvement of CXCR4 in T cell redistribution. Indeed, GCs were found to increase surface expression of the chemokine receptor CXCR4 on different cell types [8, 28] and to augment signaling by CXCR4 in cell culture [15]. We, therefore, hypothesized that there could be a link between the modulation of chemokine signaling, in particular the CXCL12/CXCR4 pathway, by GCs and their efficacy in the treatment of EAE and MS.
GCs mediate the majority of their functions via the cytosolic GC receptor (GR). Upon hormone binding, the GR translocates into the nucleus, where it modulates gene expression by two major modes of action [4]. It can either dimerize and bind to regulatory DNA elements found in many genes, or it can interact as a monomer with transcription factors such as NF-κB thereby leading to gene repression. GRdim mice carry a point mutation in the DNA-binding domain of the GR which interferes with dimerization but not with protein–protein interaction [34]. Consequently, repression of pro-inflammatory cytokines such as IFNγ or TNFα by GCs is unaffected in GRdim mice while induction of T cell apoptosis for instance is abrogated [34, 36].
Here, we provide surprising evidence that induction of T cell apoptosis is dispensable for the therapeutic efficacy of the synthetic GC dexamethasone (Dex) in EAE therapy and that GCs acting via the monomeric GR, which is unable to transactivate gene expression and to induce apoptosis, are sufficient to ameliorate the disease. In addition, we found that GCs profoundly change the responsiveness of T cells to chemokines, and that in particular it is the increased migration of CXCR4-expressing T cells that is crucial for the beneficial effect of GC therapy. Our analysis of MS patients of different ages and disease courses confirmed that after methylprednisolone (MP) therapy peripheral blood T cells migrated more efficiently towards CXCL12 than did T cells isolated before treatment. The combined studies in animal models and MS patients, therefore, indicate that GCs redirect T cells away from the CNS and thereby reduce the deleterious inflammatory foci found in the CNS in both EAE and MS. This adds an additional important mechanism to the current model of how GCs interfere with neuroinflammatory diseases.
Materials and methods
Animal experimentation
Wildtype C57Bl/6 and Balb/c mice were purchased from Harlan (Borchen, Germany). The following mutant mouse strains were bred in our animal facility in Göttingen: GRflox/flox control mice (designated GRflox) on a C57Bl/6 background, GRflox/flox mice crossed with lckCre mice (designated GRlck) on a C57Bl/6 background and GRdim/dim mice (designated GRdim) on either a C57Bl/6 or a Balb/c background [37, 47, 51]. Breeding efficacy of GRdim mice on a C57Bl/6 background was very poor, resulting in 1–2 % of homozygous offspring. This phenomenon was not observed on the Balb/c background, where 20–25 % of homozygous offspring were obtained. GRflox/dim mice crossed with lckCre mice (designated GRlckdim) were generated by intercrossing GRlck and GRdim mutants on a C57Bl/6 background, resulting in mice in which only T cells express the GRdim receptor, whereas all other cells are heterozygous for the GRdim allele and thus resemble wildtype cells [3]. GRflox/dim mice without Cre recombinase did not show any phenotype and served as controls. For EAE induction mice were used at an age of 10–12 weeks. Vav-Bcl-2 transgenic mice (designated Bcl-2tg) were previously described and bred in our animal facility in Innsbruck [29].
Bone marrow chimeric vav-Bcl-2 transgenic or GRhscdim mice were generated according to established protocols [48]. In brief, the bone marrow was isolated by flushing the femurs of 8–12-week-old mice. In parallel, CD45.1 congenic C57Bl/6 mice (Jackson laboratory, Bar Harbor, USA) were γ-irradiated at 10 or 11.5 Gray. Subsequently, 2 × 106 bone marrow cells were resuspended in 200 μl PBS and injected i.v. or retro-orbitally. The reconstituted mice were kept in individually ventilated cages for 3 weeks with neomycin-supplemented water (Sigma, St. Louis, MO). After 6 weeks, the mice were analyzed for successful reconstitution by flow cytometry and subjected to the EAE experiments.
All animal experiments were performed in accordance with accepted standards of animal welfare and approved by the responsible German authorities in Lower Saxony (LAVES) or the Austrian Ministry of Education, Science and Research.
EAE induction, treatment and analysis
C57Bl/6 mice were immunized with 50 μg myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35–55) in CFA and treated twice with 400 ng pertussis toxin in total as described [51]. C57Bl/6 chimeric mice were immunized in a similar manner with the exception that pertussis toxin was applied only once at a dose of 200 ng. Balb/c mice were immunized with 50 μg proteolipid protein peptide 180–199 (PLP180–199) in CFA and treated twice with 600 ng pertussis toxin in total as described [22]. Animals were weighed daily and scored for clinical signs of the disease on a scale from 0 to 10 depending on severity; scores were as follows: 0, normal; 1, reduced tone of tail; 2, limp tail, impaired righting; 3, absent righting; 4, gait ataxia; 5, mild paraparesis of hindlimbs; 6, moderate paraparesis; 7, severe paraparesis or paraplegia; 8, tetraparesis; 9, moribund; 10, death. Treatment with Dexamethasone-dihydrogen-phosphate (Dexa-ratiopharm®, Ratiopharm) was performed by i.p. or i.v. injection at a dose of either 100 or 4 mg/kg, starting once the mice had reached an average clinical score of 2–3 [51]. Blockade of CCR7 was achieved by i.v. injection of 50 μg of an anti-CCR7 antibody (eBioscience, Frankfurt, Germany) on two consecutive days. In some cases mice were killed on the day after the last Dex treatment and the peripheral lymphoid organs or the spinal cord prepared.
Implantation of osmotic minipumps and administration of Plerixafor®
Alzet Osmotic Pumps (Model 1002; Durect Corporation, Cupertino, CA) were implanted s.c. near the spine of C57/Bl6 mice under sterile conditions at day 8 after induction of EAE. The pumps were filled with Plerixafor® (IBL International, Hamburg, Germany) to allow a constant release of 4 mg/kg/day. Mice of the control groups were sham operated since preliminary tests had revealed that implantation of osmotic pumps with PBS did not interfere with EAE.
Isolation of infiltrating cells from the spinal cord and purification of T cells by magnetic cell sorting
Lymphocytes were isolated from the spinal cord by density centrifugation following perfusion of the mice with NaCl as described [46]. In brief, the dissected tissue was homogenized and resuspended in a three-layer Percoll gradient. After centrifugation the lymphocytes were harvested at the interphases between the layers, washed with PBS and analyzed by flow cytometry. Single cell suspensions from lymph nodes and spleens were prepared by passing the cells through a 40 μm Nylon mesh. T cells were purified using the Pan T Cell Isolation Kit II and the autoMACS system (both from Miltenyi Biotech, Bergisch Gladbach, Germany); their purity was assessed via FACS analysis for βTCR, B220, CD4 and CD8α and routinely greater than 95 %.
Flow cytometry
All antibodies and reagents were obtained from BioLegend (London, UK): anti-mCD3 (145-2C11), anti-mCD4 (RM4-5), anti-mCD8 (53-6.7), anti-mCD11b (M1/70), anti-mCD25 (PC61), anti-mCD44 (IM7), anti-mMHC class II (H2b, AF6-120.1), anti-mMac-3 (M3/84), anti-mGITR (DTA-1), anti-hCD3 (HIT3a), anti-hCD4 (OKT4), anti-hCD8 (HIT8a), anti-hCD19 (HIB19), AnnexinV and 7-AAD. Stainings were performed as previously described [51]. Analysis was carried out using a FACSCanto II device (BD Biosciences, Heidelberg, Germany) in combination with FlowJo software (Treestar, Ashland, OR, USA).
Immunohistochemistry
Histological analysis of PFA-fixed paraffin-embedded tissue sections for T cell infiltration was performed as previously described [51]. In brief, 3 μm cross-sections were stained with a rat anti-humanCD3 antibody (1:200; Serotec, Düsseldorf, Germany) followed by incubation with a biotinylated rabbit anti-rat antibody (1:200; Vector Laboratories, Burlingame, CA). Antigen unmasking was achieved by pre-treating the sections in 1 mM EDTA, pH 8.0 for 30 min in a microwave oven at 850 W. The peroxidase-based ABC detection system (Dako, Hamburg, Germany) and DAB were used for visualization. Quantification was achieved by taking pictures with an Olympus BX51 microscope at a 200-fold magnification and counting individual cells with ImageJ (http://rsb.info.nih.gov/ij/).
Fluorescent immunohistochemical staining was performed using a rabbit anti-humanCXCL12 antibody (1:200; Peprotech, Hamburg, Germany) and a rat anti-humanCD3 antibody (1:200) which were developed with a FITC-labeled goat anti-rabbit (1:750) and a Cy3-labeled goat anti-rat (1:500) secondary antibody (Dianova, Hamburg, Germany), respectively. DAPI served as a counterstain. Analysis was performed using a Zeiss fluorescent microscope Axio Observer Z1 and AxioVision software.
In vitro apoptosis assay
Splenocytes were isolated from untreated mice and put in culture with different concentrations of Dex for 24 h. Kinetic analysis was performed by FACS staining with AnnexinV-Cy5 and 7-AAD as described [44].
In vitro T cell migration assay
Single cell suspensions were prepared from lymph nodes and spleens and used for negative MACS isolation with the Pan T Cell Isolation Kit II (Miltenyi Biotec). Purified T cells were either kept for 3 h in 0.5 % fatty acid-free BSA under serum-starved conditions or additionally treated with 10−7 M Dex. Subsequently, 1 × 106 T cells were added to the upper chamber of a transwell insert with a pore size of 5 μm (Corning Life Sciences, Acton, MA) and allowed to migrate. The mouse chemokines CXCL12 (PeproTech, Hamburg, Germany) or CCL19 (Biomol, Hamburg, Germany) were added to the lower chamber at different concentrations. T cells in the lower chamber were counted after 3 h by flow cytometry using Calibrite Beads (BD Bioscience). Effects on CD4+CD44+ memory T cells were assessed by FACS analysis of total T cells. In some assays, 50 ng/ml recombinant murine IFNγ and 5 ng/ml recombinant murine IL-17A (both from ImmunoTools, Friesoythe, Germany) were added to the migration assays.
Proliferation assay and cytokine ELISA
Splenocytes were harvested on the day after the last Dex application from EAE mice and restimulated in the presence of 20 μg/ml of MOG35–55 peptide as described [50]. Supernatants were collected after 72 h and cytokine levels were determined by commercially available ELISA kits for IFNγ, IL-10 (BD Bioscience), GM-CSF and IL-17 (R&D Systems, Wiesbaden, Germany) according to the manufacturers’ instructions. T cell proliferation was assessed after a total of 64 h of incubation time using a 3[H]-thymidine incorporation assay as previously described [50].
Analysis of peritoneal macrophages
Mice were injected i.p. with 1 ml 4 % thioglycolate solution 4 days prior to the isolation of peritoneal exudate cells by repeated flushing with PBS as described [39]. Following centrifugation, the cells were seeded in 48-well plates in RPMI medium with 10 % FCS and standard antibiotics and incubated for 3 h. Non-adherent cells were removed by repeated washings with PBS/0.1 % BSA and the adhered macrophages used for experimentation. Purity of the preparations was usually around 90 % as determined by flow cytometry based on CD11b staining.
Western blot analysis
T cells were centrifuged, lysed in a denaturing sample buffer for 1.5 h on ice, boiled at 95 °C for 5 min and separated on a 7.5 % SDS-PAGE gel. After transfer to a nitrocellulose membrane (Hybond-ECL, Amersham, Freiburg, Germany) the proteins were stained with the indicated primary antibodies followed by incubation with an HRP-conjugated secondary antibody. For visualization, H2O2 was used as an oxidizing agent in combination with an ECL plus substrate (Thermo Scientific, Bonn, Germany); chemiluminescence was detected using the ChemiLux Imager (Intas, Göttingen, Germany). Densitometric quantification of the band intensities was achieved using gelPro analyzing software (Media Cybernetics, Bethesda, MD, USA); the specific background was individually subtracted in each case. The following antibodies were used: anti-phospho-Focal Adhesion Kinase-Tyr397 (P-FAK, Cell Signaling, Danvers, MA, USA), anti-ERK (Santa Cruz, Heidelberg, Germany) and goat anti-rabbit-Igg-HRP (Pierce, Bonn, Germany).
RNA isolation and quantitative RT-PCR
Total RNA from CNS infiltrating lymphocytes was isolated using the Quick-RNA MiniPrep kit (Zymo Research, Irvine, CA, USA) and reverse transcribed into cDNA by the help of the iScript kit (Bio-Rad, Munich, Germany). Quantitative real-time PCR was performed on an ABI 7500 instrument (Applied Biosystems, Darmstadt, Germany) using SYBR green mastermix from the same company according to the manufacturer’s instruction. The results were normalized to the mRNA expression of HPRT and evaluated using the ΔΔCt method. The primer sequences are available upon request.
Human T cell preparation and migration
All experiments using human cells were approved by the local ethics committee. Buffy coats were obtained from healthy male and female human donors (Department of Hematology and Oncology, University of Göttingen Medical School). Initially, the cells were subjected to a Ficoll density gradient (Biochrom, Berlin, Germany) to obtain PBMCs, and monocytes were subsequently depleted by means of a 46 % Percoll gradient (GE Healthcare, Munich, Germany). Further purification of the T cell subset was achieved by magnetic cell sorting through negative depletion with the EasySep Human T cell Enrichment Kit (Stemcell Technologies, Grenoble, France). FACS analysis with antibodies against CD3, CD4, CD8 and CD19 indicated that the purity was >95 %.
T cells from healthy blood donors were kept for 3 h in 0.5 % fatty acid-free BSA under serum-starved conditions or additionally treated with 10−7 M Dex. Subsequently, 1 × 106 T cells were subjected to a transwell assay using a pore size of 5 μm (Corning Life Sciences) and allowed to migrate for 3 h against a gradient of 50 ng/ml human CXCL12 (ImmunoTools). Quantification of migrated cells was achieved by flow cytometry using Calibrite Beads (BD Bioscience).
Blood samples from MS patients were obtained with informed consent immediately before high-dose pulse therapy with MP (1 g/day) as well as 24 h later. T cells from MS patients were isolated by Ficoll density gradient in combination with the EasySep Human T cell Enrichment Kit (Stemcell Technology). Purified T cells were directly subjected to a transwell assay against CXCL12 as described above.
Statistical analysis
Analysis of all animal experiments where more than two EAE groups had to be compared was done using the Kruskal–Wallis test followed by Dunn’s multiple comparison test (Prism Version 5.0, GraphPad Software, La Jolla, USA) [13]. To determine differences referring to the disease course, the whole curves rather than individual time points were compared between experimental groups. Strictly speaking, statistical analysis was performed from the day after the first Dex treatment until the end of the observation period. All other analyses were performed by unpaired t test except for human samples that were analyzed using the paired t test. Data are depicted as mean ± SEM; p values above 0.05 were considered as nonsignificant (ns); *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Induction of T cell apoptosis and GR dimerization are dispensable for high-dose GC therapy of EAE
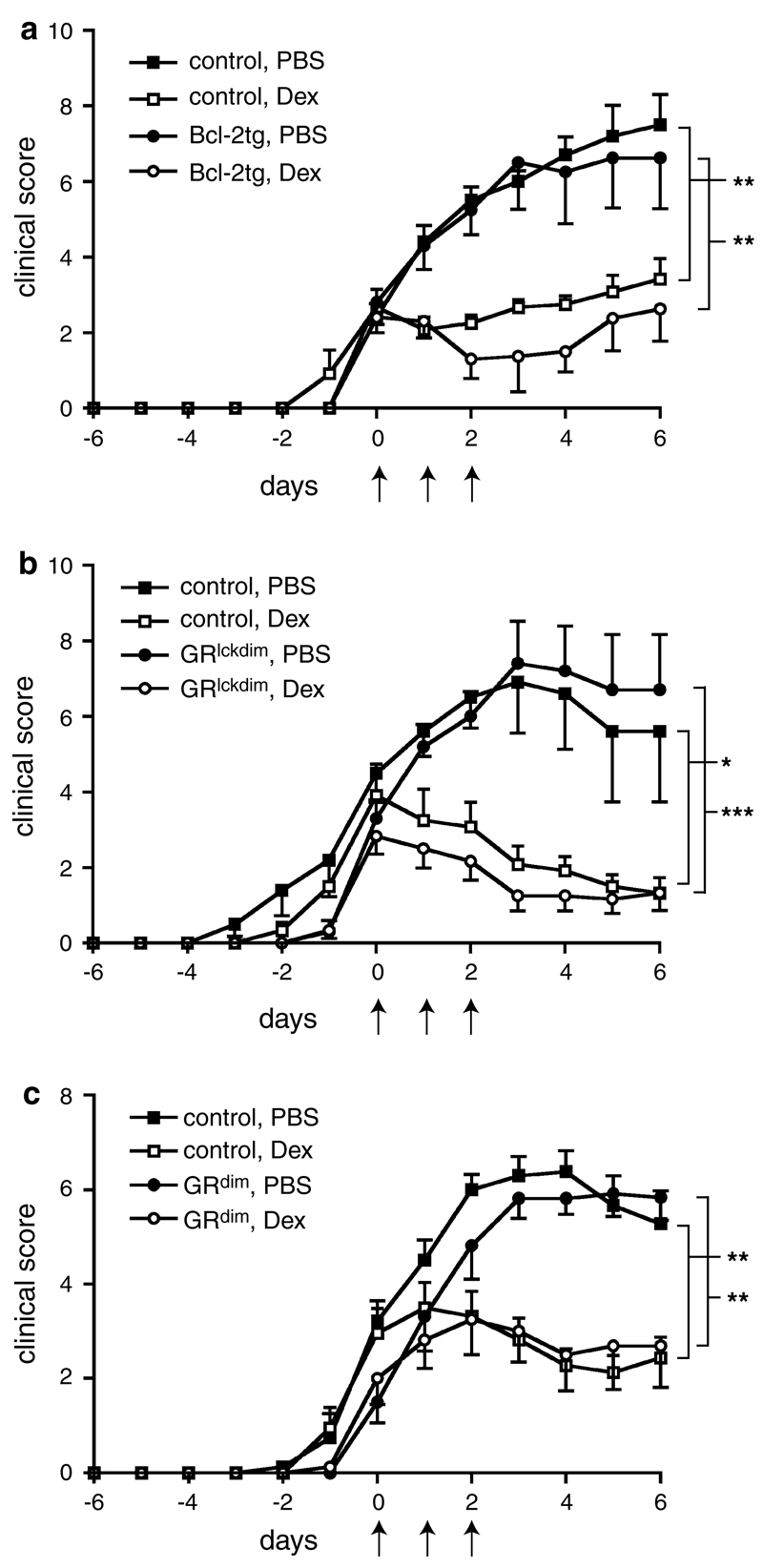
To test the role of apoptosis induction in T cells for the therapeutic efficacy of GCs we evoked EAE in mice that overexpress Bcl-2 in T cells. Similarly to wildtype controls, the Bcl-2 transgenic mice were fully susceptible to EAE induction by immunization with MOG35–55 (Fig. 1a). Surprisingly, Dex ameliorated the disease in Bcl-2 transgenic mice to a similar extent as in wildtype control animals (Fig. 1a), although T cells from the transgenic mice were refractory to GC-induced apoptosis (supplemental Fig. 1a, b). To confirm these results we employed GRlckdim mice that express a dimerization-defective GR in T cells. Notably, the monomeric GR allows only transrepression but not transactivation of genes, an effect that is required for GC-induced cell death [34]. Indeed, CD4+ T cells from GRlckdim mice were refractory to apoptosis induction by Dex (supplemental Fig. 2a), while expectedly, induction of B cell apoptosis and down-regulation of MHC class II levels on peritoneal macrophages by Dex were unaffected (supplemental Fig. 2b, c). The disease course of EAE was similar in GRlckdim and control mice and Dex treatment efficiently ameliorated it regardless of the genotype (Fig. 1b).
Fig. 1.
GC-induced T cell apoptosis and GR dimerization are dispensable for the treatment of EAE with Dex. EAE was induced by immunization with MOG35–55 peptide. After reaching a clinical score of about 3, mice of each genotype were randomly divided into two groups, one of which was treated on three consecutive days with 100 mg/kg Dex and the other one with PBS as a control (indicated by arrows). The start of the treatment was defined as day 0. The following types of mice were used: a chimeric mice reconstituted with bone marrow from Bcl-2 transgenic or control mice; data are pooled from two independent experiments, N = 5−6. b GRlckdim mice expressing the GRdim receptor exclusively in T cells or the respective GRflox/dim littermate controls; data are pooled from two independent experiments, N = 5−6. c GRdim mice and phenotypically normal GR+/dim control mice; data are pooled from three independent experiments, N = 11−12. All values are depicted as mean ± SEM. Statistical analysis was performed by comparing the disease courses starting on day 1 after the beginning of the treatment until the end of the observation period using the Kruskal–Wallis test followed by Dunn’s multiple comparison test
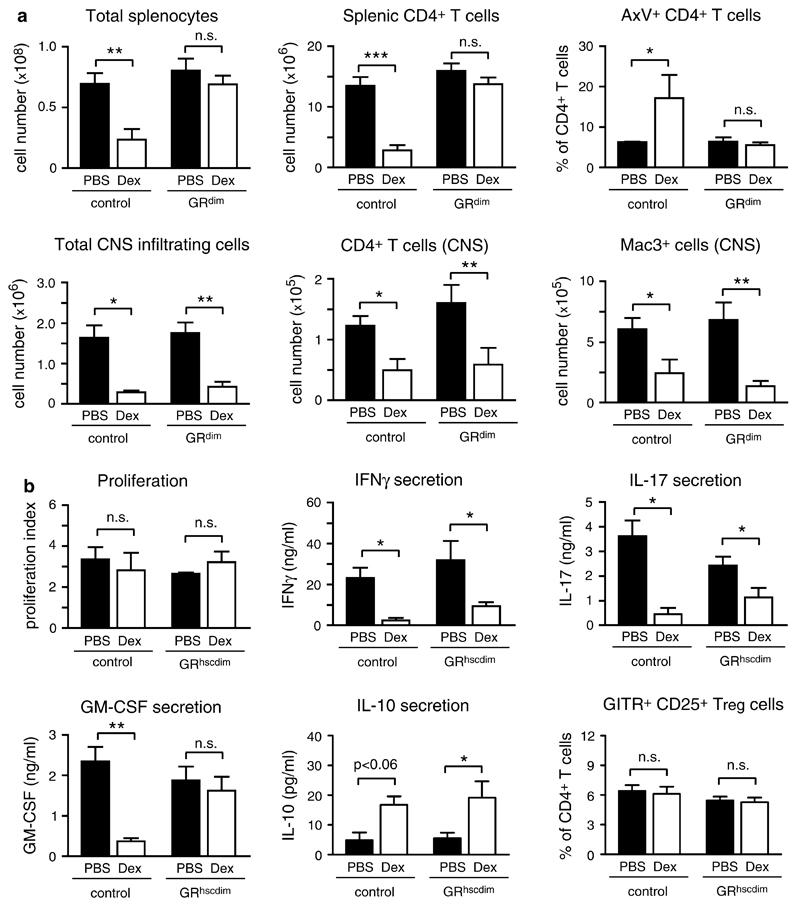
To exclude that apoptosis induction in cells other than T cells might account for the therapeutic GC effects, we analyzed mice that ubiquitously express the dimerization-defective GRdim receptor. Immunization with MOG35–55 resulted in a similar disease course and a comparable efficacy of Dex treatment in GRdim and control mice (Fig. 1c). Similar findings were made in GRdim mice on a Balb/c background immunized with PLP180–199 peptide (supplemental Fig. 3). To test how the clinical findings were reflected at the cellular level, we analyzed splenocytes and CNS infiltrating leukocytes in GRdim and control mice immunized with MOG35–55 on the day after the last Dex application. Flow cytometric quantification revealed that GC treatment of wildtype mice strongly diminished total splenocyte and splenic CD4+ T cell numbers by inducing apoptosis while this did not happen in GRdim mice expressing only a monomeric GR (Fig. 2a). In contrast, the total number of CNS infiltrating leukocytes, in particular the number of Mac3+ myeloid cells and CD4+ T cells in the spinal cord, were strongly diminished after Dex treatment in mice of both genotypes (Fig. 2a). Hence, inhibition of leukocyte infiltration into the CNS by GCs is independent of their capacity to reduce T cell numbers in secondary lymphoid organs, which suggests that GCs act via different mechanisms.
Fig. 2.
Differential requirements of the dimerized GR for the modulation of EAE by Dex. a EAE was induced in GRdim as well as GR+/dim control mice by immunization with MOG35–55 peptide followed by treatment with 100 mg/kg Dex on three consecutive days or PBS as a control. Analysis was performed on the day after the last treatment. The spleens were removed and analyzed by FACS. The total number of splenocytes, the number of splenic CD4+ T cells (N = 6−8, pool of two independent experiments) and the percentage of apoptotic AnnexinV+ CD4+ T cells in the spleen (N = 3) are depicted in the upper panel. In addition, infiltrating leukocytes were isolated from the spinal cord by density gradient centrifugation and analyzed by FACS. The total number of CNS infiltrating cells, the number of CD4+ T cells and Mac3+ myeloid cells in the spinal cord are depicted in the lower panel (N = 4−6, pool of two independent experiments). b EAE was induced in chimeric mice reconstituted with bone marrow either from GRdim (GRhscdim) or GR+/dim (control) mice followed by treatment with 100 mg/kg Dex or PBS on three consecutive days. Splenocytes were isolated on the day after the last treatment and restimulated in vitro with MOG35–55 peptide for 72 h. The proliferation rate is shown as the index of proliferation in the presence of the antigen divided by proliferation in its absence (N = 3−5). Levels of IFNγ, IL-17, GM-CSF and IL-10 in cell culture supernatants were determined by ELISA (N = 3−8, pool of two independent experiments). The percentages of GITR+CD25+CD4+ Treg cells in the spleen were analyzed by flow cytometry immediately after dissection (N = 5−9, pool of two independent experiments). The values in all panels are depicted as mean ± SEM. Statistical analysis was performed using the unpaired t test
Distinct requirements of GR dimerization for the control of cytokine release
The retained therapeutic efficacy of GCs observed in GRdim mice could have been explained by their impact on the proliferation and cytokine secretion of pathogenic T cells or by the expansion of regulatory T (Treg) cells. To test these functions, we used GRdim bone marrow chimeric mice on a C57Bl/6 background (designated GRhscdim), an approach taken to overcome the poor breeding efficiency of GRdim mice on the C57Bl/6 background (see “Materials and methods”).
EAE induced in GRhscdim chimeras could be efficiently ameliorated by Dex treatment although splenic CD4+ T cell numbers were unaffected (supplemental Fig. 4a, b). These findings confirm that GRhscdim mice behave similarly in EAE experiments as GRdim mice (Figs. 1c, 2a). The proliferation rate of wildytpe and GRhscdim splenocytes after restimulation with MOG35–55 peptide ex vivo was comparable and unaffected by GC therapy (Fig. 2b). Dex treatment of wildtype as well as GRhscdim mice led to a significantly reduced secretion of the pro-inflammatory cytokines IFNγ and IL-17 by splenocytes of both genotypes after antigen-restimulation ex vivo. In contrast, the GM-CSF release was only reduced in cultures from Dex-treated wildtype but not from GRhscdim mice (Fig. 2b). Of note, the mRNA expression of IL-17 and GM-CSF in CNS infiltrating cells followed a similar pattern as their secretion by splenocytes (supplemental Fig. 4c). Furthermore, GC therapy led to an increased release of the anti-inflammatory cytokine IL-10 by splenocytes after restimulation with MOG35–55 peptide ex vivo, an effect which was independent of the genotype of the mice (Fig. 2b). In agreement with previous findings [51], Dex treatment did not alter the percentage of Treg cells in the spleen of EAE mice (Fig. 2b). Collectively, these experiments indicate that GCs acting via the monomeric GR are able to modulate IFNγ, IL-17 and IL-10 secretion albeit they neither inhibit T cell proliferation nor induce Treg cells nor globally suppress all relevant pro-inflammatory cytokines. These findings, therefore, stimulated our search for other potential mechanisms of GC action in EAE.
GCs repress T cell migration towards CCL19 in vitro but blockade of its receptor CCR7 does not impact the therapeutic effect of Dex on EAE in vivo
To explore other potential therapeutic mechanisms of GCs, we analyzed their effects on the responsiveness of T cells to various chemokines. Initially, we investigated CCL19 which is one of the main ligands of CCR7 and expressed by endothelial cells of the BBB [1]. CCL19 enhanced T cell migration in a transwell assay while preincubation with Dex reduced this effect. The diminished responsiveness to CCL19 was observed irrespectively of whether total T cells or CD44+CD4+ memory T cells were studied (Fig. 3a). Of note, CCR7 surface levels on T lymphocytes were unaltered by Dex (supplemental Fig. 5a), suggesting that GCs interfere with intracellular CCR7 signaling rather than its expression on the cell surface.
Fig. 3.
Dex represses CCL19-directed T cell migration in vitro while blocking CCR7 in vivo has no significant effect on EAE. a Total T cells were isolated from spleens and lymph nodes of C57Bl/6 mice, pretreated for 3 h with or without Dex and tested in a transwell assay for their capacity to migrate towards 100 ng/ml mouse CCL19 during a 3-h period without further presence of Dex. Cell numbers were determined by FACS using reference beads (left panel, N = 8). results for CD44+ CD4+ T cells were calculated by FACS analyses of transwell assays of total T cells (right panel, N = 3). All values are depicted as mean ± SEM. Statistical analysis was performed using the unpaired t test. b C57Bl/6 mice were immunized with MOG35–55 peptide and either treated with 100 mg/kg Dex i.v. on three consecutive days (dashed arrows) or 50 μg anti-CCR7 antibody i.v. on two consecutive days (arrows) after reaching an approximate clinical score of 2. Application of PBS served as a control; the onset of the treatment was defined as day 0 (N = 4−5). c EAE was induced in C57Bl/6 mice followed by treatment either with 4 mg/kg Dex i.p. (dashed arrows), a combination of 4 mg/kg Dex i.p. and 50 μg anti-CCR7 antibody i.v. (arrows) or PBS as a control (N = 5−6). All values in b and c are depicted as mean ± SEM. Statistical analysis was performed by comparing the disease courses between days 1 and 6 after the beginning of the treatment using the Kruskal–Wallis test followed by Dunn’s multiple comparison test
To investigate whether modulation of the CCL19/CCR7 chemotactic pathway plays any role in vivo, we induced EAE in C57Bl/6 mice and injected them with an anti-CCR7 antibody, either alone or in combination with Dex (Fig. 3b, c). While GC therapy strongly ameliorated the disease at a high dose and moderately at a low dose as expected, inhibition of CCR7 alone had no impact on EAE. Administration of anti-CCR7 in combination with a low dose of Dex improved the disease slightly more than Dex alone but without reaching statistical significance. The lack of therapeutic potency was not because CCR7 had been insufficiently blocked, as T cells from mice treated with the anti-CCR7 antibody in vivo were significantly impaired in migrating towards CCL19 in vitro (supplemental Fig. 5b). Taken together, our findings suggest that repression of CCL19-directed migration by GCs does not significantly contribute to their therapeutic efficacy, which encouraged us to investigate other chemokine receptor signaling pathways which potentially could be more important.
The monomeric GR is both necessary and sufficient for CXCL12-directed T cell migration and its enhancement by GCs
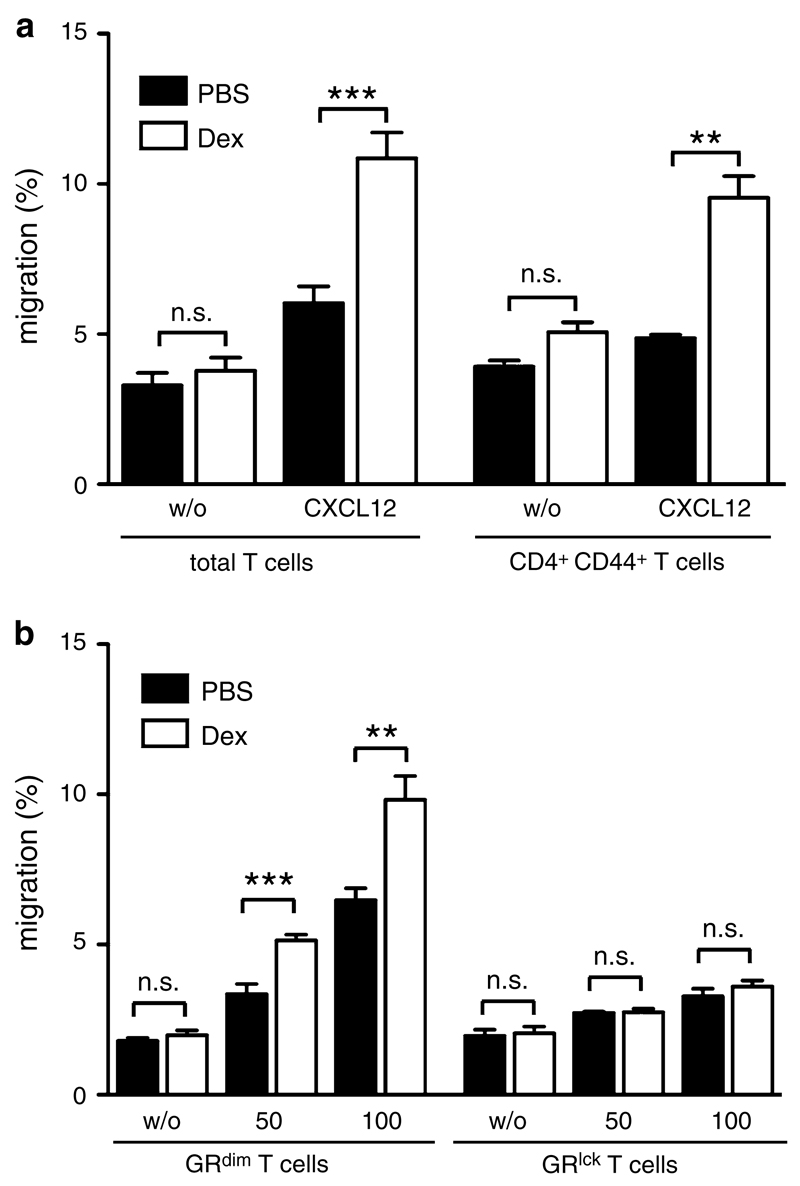
We then tested the effect of GCs on the responsiveness of T cells to CXCL12, which is also constitutively expressed in the CNS and whose receptor CXCR4 is present on both naïve and memory T cells [23]. T cells from C57Bl/6 mice efficiently migrated towards CXCL12 in a transwell assay. However, in contrast to CCL19, Dex enhanced rather than diminished the migratory capacity of total and CD44+CD4+ memory T cells (Fig. 4a). The potentiating effect of Dex could be confirmed in T cells from Balb/c mice (supplemental Fig. 6a) and was not influenced by the addition of IFNγ and IL-17A (supplemental Fig. 7). We conclude that the changes in proinflammatory cytokine secretion caused by Dex therapy of EAE (see Fig. 2b) were unrelated to the observed migratory phenotype.
Fig. 4.
CXCL12-directed T cell migration is enhanced after Dex treatment. a Total T cells were isolated from spleens and lymph nodes of C57Bl/6 mice, pretreated for 3 h with or without Dex and tested in a transwell assay for their capacity to migrate towards 50 ng/ml mouse CXCL12 during a 3-h period without further presence of Dex. Cell numbers were determined by FACS using reference beads (left panel, N = 15). Results for CD44+ CD4+ T cells were calculated by FACS analyses of transwell assays using total T cells (right panel, N = 3). All values are depicted as mean ± SEM. Statistical analysis was performed using the unpaired t test. b Isolated T cells from spleens and lymph nodes of GRdim or GRlck mice, both on a C57Bl/6 background, were tested in a transwell assay for their capacity to migrate towards 50 or 100 ng/ml mouse CXCL12. The cells were pretreated for 3 h with or without Dex and subsequently allowed to migrate for another 3 h without further presence of Dex. Cell numbers were determined by FACS using reference beads (N = 6−8 for GRdim T cells, N = 10−12 for GRlck T cells). All values are depicted as mean ± SEM. Statistical analysis was performed using the unpaired t test
To evaluate the requirements of GR signaling for CXCR4 function, we isolated T cells from GRdim mice and tested their migration towards CXCL12. Interestingly, the enhancing effect of Dex treatment was retained in these cells, indicating that the loss of apoptosis induction by GCs in GRdim mice is uncoupled from their retained ability to potentiate CXCL12-directed T cell migration (Fig. 4b). The same held true for T cells from GRdim mice of the Balb/c background (supplemental Fig. 6b). Surprisingly, T cells isolated from GRlck mice, i.e., cells that completely lack the GR, did not migrate along a CXCL12 gradient at all and Dex had no effect on their migration (Fig. 4b). Thus, the monomeric GR is sufficient but also necessary to endow T cells with the capacity to migrate towards CXCL12.
The modified T cell migration in response to Dex could not be explained by altered CXCR4 surface levels (supplemental Fig. 8a), suggesting that GCs might impact CXCL12-directed migration by enhancing CXCR4 signaling. Indeed, we found evidence that GCs influence focal adhesion kinase (FAK), which is involved in the rearrangement of the cytoskeleton, facilitates T cell migration and is part of the CXCR4 signaling pathway [20, 38]. Treatment of total T cells with CXCL12, Dex or a combination of both led to a marked increase in FAK phosphorylation (Fig. 5a, b) which mirrors the increased T cell migration after CXCL12 treatment and its enhancement by Dex (see Fig. 4a). The basal phosphorylation level of FAK was higher in T cells from GRlck mice but it was hardly altered by addition of CXCL12 or Dex (Fig. 5a, b). This observation is in line with the inability of GRlck T cells to migrate towards CXCL12, suggesting that the GR is essential for the functioning of the CXCL12/CXCR4 signaling pathway.
Fig. 5.
Treatment of T cells with CXCL12 and Dex leads to the activation of FAK. a Isolated T cells from spleens and lymph nodes of C57Bl/6 wildtype (wt) or GRlck mice were treated with 10−7 M Dex, 100 ng/ml mouse CXCL12 (C12) or a combination of both (D + C) for 15 min or left untreated (con). Protein lysates were analyzed by western blot using antibodies against p-FAK and ERK as a loading control. One representative example out of three is depicted. b Western blots were quantified by densitometry followed by normalization of p-FAK to ERK. Protein levels of p-FAK in wt control cells were set equal to 1 and all other values were calculated accordingly. N = 3. The values are depicted as mean ± SEM. Statistical analysis was performed using the unpaired t test
CXCR4 is critical for GC therapy of EAE
To evaluate whether the modulation of T cell migration towards CXCL12 plays a role for GC therapy of EAE in vivo, we applied Plerixafor® to C57Bl/6 mice after induction of EAE. Plerixafor® is a potent and specific CXCR4 antagonist [14] that is also active in the mouse system (supplemental Fig. 8b). Permanent in vivo application was achieved by implanting osmotic minipumps at day 8 after immunization which resulted in the release of a constant dose of the inhibitor over a 14-day period. Plerixafor® alone had no significant impact on clinical symptoms, whereas Dex treatment of control mice, as predicted, ameliorated the disease course (Fig. 6a). Blocking CXCR4 by Plerixafor® significantly diminished the therapeutic effect of GC therapy, although it did not completely abolish it (Fig. 6a).
Fig. 6.
The CXCR4 signaling pathway is a target of GCs in the treatment of EAE. a At day 8 after immunization with MOG35–55 peptide, C57Bl/6 mice were implanted with an osmotic minipump under their back skin which delivered Plerixafor® over a 14-day period (indicated by a bar) or sham operated as a control (con). After the onset of first disease symptoms, mice receiving Plerixafor® and sham-operated controls were divided into two groups, one receiving 100 mg/kg Dex on three consecutive days and the other one PBS as a control (indicated by arrows). N = 6−9 (pool of two separate experiments). All values are depicted as mean ± SEM. Statistical analysis was performed by comparing the disease courses starting on day 1 after the beginning of the treatment until the end of the observation period using the Kruskal–Wallis test followed by Dunn’s multiple comparison test. Spinal cords were dissected at the end of the experiment (a) and analyzed by immunohistochemistry using an anti-CD3 antibody. Quantification of infiltrating cells per square millimeter (mm2) was performed separately for the white (b) and the gray (c) matter; N = 4−7. The values are depicted as mean ± SEM. Statistical analysis was performed using the unpaired t test
CXCL12 is highly expressed in the meninges of the CNS, for which reason T cells expressing its receptor CXCR4 were found to be retained in the perivascular space during EAE [23, 41]. In addition, CXCL12 is expressed by secondary lymphoid and non-lymphoid organs and redirects CXCR4-expressing T cells away from inflammatory lesions [28]. Histological studies revealed that Dex strongly reduced the total number of infiltrating T cells in control mice but only slightly in mice additionally receiving Plerixafor® (Figs. 6b, 7). Furthermore, Plerixafor® application (with or without the addition of Dex) resulted in a more dispersed distribution of the infiltrating T cells throughout the parenchyma including the gray matter, which is different from the focal lesions that are predominantly found in close proximity to the meninges and the blood vessels in control mice (Figs. 6c, 7). These findings were corroborated by fluorescent immunohistochemical analysis. Regardless of the treatment with Dex, T cells in Plerixafor®-treated mice were located remotely from the CXCL12-positive endothelial cells and diffusely distributed throughout the spinal cord parenchyma (Fig. 8). Taken together, blocking of CXCR4 during Dex therapy results in an increased T cell homing to the CNS and a more pronounced parenchymal invasion of the infiltrated T cells, and both effects presumably contribute to the reduced therapeutic efficacy of GCs after Plerixafor® administration.
Fig. 7.
Immunohistochemical analysis of T cell infiltration after treatment of EAE with or without Dex and/or Plerixafor®. C57Bl/6 mice were immunized with MOG35–55 peptide and at day 8 they were either implanted with an osmotic minipump which delivered Plerixafor® over a 14-day period or sham operated as a control. After the onset of first disease symptoms mice receiving Plerixafor® and control mice were further randomly divided into two groups: one of them received 100 mg/kg Dex on three consecutive days and the other one received PBS (corresponding to the experiment shown in Fig. 6a). Spinal cords were obtained at the end of the experiment and analyzed by immunohistochemistry using an anti-CD3 antibody. One representative staining out of four of each group is depicted as an overview of the whole spinal cord (left side) and an enlarged picture taken from the upper middle part (right side). The length of the bar corresponds to 500 or 200 μm, respectively
Fig. 8.
T cell infiltrates in the spinal cord are more dispersed after treatment with Plerixafor®. C57Bl/6 mice were immunized with MOG35–55 peptide and at day 8 they were either implanted with an osmotic minipump which delivered Plerixafor® over a 14-day period or sham operated as a control. After the onset of first disease symptoms mice receiving Plerixafor® and control mice were further randomly divided into two groups: one of them received 100 mg/kg Dex on three consecutive days and the other one received PBS (corresponding to the experiment shown in Fig. 6a). Spinal cords were obtained at the end of the experiment and analyzed by fluorescent immunohistochemical analysis with an anti-CXCL12 antibody that was developed with a FITC-conjugated secondary antibody, an anti-CD3 antibody that was developed with a Cy3-conjugated secondary antibody and DAPI as a counterstain. Representative images of spinal cord sections of one control mouse (upper left), one mouse treated only with Dex (lower left), one mouse receiving exclusively Plerixafor® (upper right) and one mouse co-treated with Dex and Plerixafor® (lower right) are depicted (N = 4). The large arrows point towards structures positively staining for CXCL12, presumably endothelial cells of blood vessels, whereas the small arrows indicate infiltrating T cells. The length of the bar corresponds to 100 μm
GC treatment enhances CXCL12-directed migration of human T cells in vitro
To confirm that the newly identified mode of GC action was indeed of clinical relevance we initially analyzed peripheral blood T cells from healthy human donors. Purified T lymphocytes consisting of a mixture of CD4+ and CD8+ cells (ratio 2:1) strongly migrated towards CXCL12 in a transwell assay and Dex further enhanced their migratory capacity significantly (Fig. 9a). Thus, the potentiating effect of GC treatment on CXCL12-directed T cell migration also applies to humans, which suggests that T cell redirection through the CXCR4/CXCL12 axis might occur during GC therapy of MS patients.
Fig. 9.
Migration of human T cells towards CXCL12 is enhanced by GCs in vitro and in vivo. a Purified T cells from peripheral blood of healthy human donors were pretreated for 3 h with or without 10−7 M Dex and tested in a transwell assay for their capacity to migrate towards 50 ng/ml human CXCL12 during a 3-h period without further presence of Dex. A transwell assay without CXCL12 served as a control (w/o). Cell numbers were determined by FACS using reference beads. T cells from individual human donors were tested in quadruplicates (N = 4/6); each symbol represents a single donor. Statistical analysis was performed using the paired t test. a, b T cells were purified from the peripheral blood of patients with diagnosed MS (for clinical details see Table 1), both immediately before (pre MP) and 24 h after high-dose MP pulse therapy (post MP). Afterwards the T cells were subjected to a transwell assay to test their capacity to migrate towards 50 ng/ml human CXCL12 during a 3 h period. A transwell assay where CXCL12 was omitted (w/o) served as a control. Cell numbers were determined by FACS using reference beads. b The percentage of migrated cells in each condition is collectively depicted for all patients irrespective of their clinical subtype. Each symbol represents one MS patient. Statistical analysis was performed using the paired t test (N = 10). c In addition, the change in the T cells’ migratory capacity in response to CXCL12 following MP therapy is depicted individually for each patient. In this case, the patients were classified according to their MS disease course, namely RRMS in adults (N = 4) and children (N = 3) as well as PPMS in adults (N = 3)
MP pulse therapy enhances T cell migration towards CXCL12 in MS patients
To investigate whether the well-known beneficial effect of MP pulse therapy correlated with an enhanced T cell migration towards CXCL12 in MS patients, we performed a longitudinal study using purified T cells obtained from peripheral blood immediately before and 24 h after MP pulse therapy. We included adult patients with a relapsing-remitting (RRMS) and a primary progressive (PPMS) disease course as well as children suffering from RRMS. The clinical characteristics of the patients are summarized in Table 1. Importantly, migration of purified peripheral blood T cells of MS patients towards CXCL12 was significantly enhanced after MP pulse therapy as compared to T cells isolated before the treatment (Fig. 9b). In fact, a qualitatively similar effect was seen in every single patient irrespective of the disease course or the age of the patient (Fig. 9c). In summary, standard MP pulse therapy strongly enhances T cell migration towards CXCL12 and thus might provide a plausible explanation for the therapeutic effects of GCs in MS patients.
Table 1.
Clinical characteristics of MS patients analyzed for the migratory capacity of their peripheral blood T cells after methylprednisolone pulse therapy
| Age | Gender | Disease duration (years) | Immunomodulatory medication | Time since last GC pulse | |
|---|---|---|---|---|---|
| RRMS adult | |||||
| #1 | 29 | Male | 1 | Copaxone® | 10 months |
| #2 | 31 | Female | None | NA | NA |
| #3 | 33 | Female | 1 | Rebif® | 10 months |
| #4 | 36 | Male | 6 | Betaferon® | 6 months |
| PPMS adult | |||||
| #1 | 43 | Female | 10 | None | 3 years |
| #2 | 59 | Male | 10 | None | 3 months |
| #3 | 67 | Male | 2 | None | 10 weeks |
| RRMS children | |||||
| #1 | 11 | Female | 5 | None | 10 weeks |
| #2 | 15 | Female | 2 | Rebif® | 6 months |
| #3 | 17 | Female | 2 | Rebif® | 5 months |
Discussion
This study is focused on the therapeutic effects of GCs in CNS autoimmunity. In our previous work, we showed that it is primarily the GCs’ action on peripheral T cells which is essential for ameliorating EAE [51]. In particular, we had found a massive induction of T cell apoptosis, a down-regulation of adhesion molecules [51] and lower amounts of IFNγ and IL-17 [50] after treatment of EAE with either GCs or the selective GR ligand CpdA. The overall consequence was a reduced influx of autoreactive T cells into the CNS [51]. In this study, we now concentrated on one of these potential mechanisms, namely apoptosis induction, to determine whether and to what extent it was required for the successful therapy of EAE by GCs. Apoptosis of immune cells, both in the periphery and in the CNS, is widely held responsible for the beneficial effects of GCs [31, 35], and in support of this hypothesis we found dramatically reduced T cell numbers in secondary lymphoid organs after high-dose Dex treatment [51]. However, our data presented here argue against GCs acting solely by inducing cell death. We show that full therapeutic efficacy can be achieved even when T cells are completely refractory to apoptosis induction by Dex. This was initially shown in a model where overexpression of Bcl-2 in hematopoietic cells did not impede the therapeutic efficacy of GCs in EAE. Further evidence was obtained in GRdim mice which carry a point mutation that prevents GR dimerization and consequently gene transactivation and apoptosis induction [34]. When we induced EAE in mice expressing the GRdim receptor specifically in T cells, the therapeutic effect of GC application was similar to that seen in wildtype mice. To rule out any effect GCs could have on other cell types in this model, we used mice ubiquitously expressing the GRdim receptor but obtained similar results. Importantly, CNS infiltration in GRdim mice was strongly reduced albeit peripheral T cell numbers remaining unaltered. This meant that GCs acting via the monomeric GR were either able to alter cytokine production by pathogenic T cells, which would dampen the pro-inflammatory milieu required for their entry into the CNS, or they directly impacted T cell migration. In fact, repression of IFNγ and IL-17 was retained in GRdim mice, indicating that it was mediated by the monomeric GR. However, neither IFNγ nor IL-17 alone is essential for the pathogenic potential of effector T cells [12, 16] but rather GM-CSF seems to be a crucial factor in EAE [6, 11, 24]. Yet, this cytokine was not repressed in GRdim T cells by Dex. GCs could also act by up-regulating anti-inflammatory cytokines such as IL-10 [49] or by expanding Treg cells [2]. Indeed, we observed increased IL-10 levels in response to Dex in our model irrespective of the genotype. However, while GC treatment of EAE had been previously reported to increase the percentage and potency of Treg cells [5], this phenomenon could neither be reproduced in a former [51] nor in the current study.
In view of the ambivalent findings concerning the regulation of pro-inflammatory cytokines and Treg cells by Dex, we searched for other mechanisms that might be important for GC therapy. Due to the fact that chemokine/chemokine receptor interactions were suggested to be involved in the modulation of EAE [1, 10, 23, 41], we investigated whether GCs could directly influence chemokine-induced T cell migration. As a matter of fact, T cell responsiveness to CCL19 was significantly reduced by Dex. However, if this played a role in vivo, blockade of its main receptor CCR7 should also interfere with neuroinflammation. Administration of an anti-CCR7 antibody alone as well as in combination with Dex did not significantly ameliorate EAE. It is, therefore, unlikely that the inhibitory effect of Dex on CCL19-directed migration contributes to the therapeutic efficacy of GCs in a relevant manner.
Intriguingly, the effect of Dex on T cell migration towards CXCL12 was opposite of that towards CCL19. Total and CD4+CD44+ memory T cells migrated significantly better along a CXCL12 gradient when pretreated with Dex, and this effect was independent of the levels of pro-inflammatory cytokines such as IFNγ and IL-17, which are modulated by GC therapy of EAE. While the latter population comprises antigen-specific T cells responsible for the autoimmune attack, naïve T cells are recruited to the CNS as bystander cells and help sustain the inflammatory response [45]. Thus, both cell types contribute to the pathogenesis of EAE and are subject to GC action during therapy. Furthermore, the monomeric GR expressed in GRdim mice alone was fully capable of enhancing CXCL12-directed T cell migration, which is compatible with the retained ability of these mice to respond to GC therapy of EAE. The fact that the potentiating effect of GC treatment on CXCL12-directed migration was also found in human T cells from healthy donors as well as MS patients undergoing high-dose MP pulse therapy argues for its clinical relevance. Increased T cell migration towards CXCL12 in response to GCs could have at least two consequences. First, CXCL12 is constitutively expressed in peripheral lymphoid and other solid organs such as liver, lung and bone marrow [9, 28]. Enhanced migration could, therefore, lead to a redirection of autoreactive and bystander T cells away from inflammatory CNS lesions towards peripheral tissues. This would be in line with the observation that humans systemically treated with GCs show a reduction in the number of circulating T cells within hours, and that T cells are found instead sequestered within lymphoid organs and the bone marrow [32]. Second, T cells having an improved capacity to respond to CXCL12 might be trapped in the perivascular space of the CNS where CXCL12 is highly expressed, and thereby be prevented from entering the CNS parenchyma [23, 41]. Inhibition of CXCR4 strongly impaired the therapeutic effect of Dex on EAE, and indeed we found some evidence for both aforementioned mechanisms. CNS infiltration by T cells in general was markedly increased after the combined treatment with Dex and Plerixafor®, arguing that redirecting the T cells away from the inflamed CNS was crucial for the efficacy of GC therapy. Furthermore, in the presence of Plerixafor® infiltrating T cells were distributed over the whole CNS parenchyma including the gray matter. This indicates that the blockade of CXCR4 interferes with the accumulation of T cells in close vicinity to CXCL12-secreting endothelial cells, which might contribute to the therapeutic Dex effect. However, we do not have any direct evidence that T cells indeed become trapped within the perivascular space of CNS blood vessels after Dex therapy. CXCR7, which is expressed at the BBB, has been recognized as an alternative receptor for CXCL12 that scavenges CXCL12 and thereby facilitates T cell entry into the CNS parenchyma [7]. Blockade of CXCR7 ameliorates EAE and impedes leukocyte entry into the CNS due to the increase in CXCL12. Thus, increased responsiveness to CXCL12 after Dex treatment (our data) and higher CXCL12 levels after inhibition of CXCR7 [7] may have similar effects. Collectively, our data indicate that the CXCL12/CXCR4 chemokine/chemokine receptor pair plays an important role for the progression of EAE and MS.
Further support for our model that CXCR4 is of major importance for GC therapy of EAE comes from the observation that GR ablation abrogates both CXCL12-directed T cell migration and enhanced phosphorylation of FAK. This implies that the GR per se is essential for CXCR4 signaling. It was previously shown that CXCR4 physically associates with the TCR after CXCL12 stimulation and that it uses ZAP-70’s ITAM motif for signal transduction [19]. Dex enhances CXCR4 signaling by increasing ZAP-70 phosphorylation through interference of the GR with the TCR complex [15]. It was also found to enhance FAK phosphorylation in adenocarcinoma cells [18], which is in line with our findings for primary T cells. The aforementioned data from the literature suggest that CXCR4 and the TCR form a complex involving the GR after CXCL12 exposure that leads to the activation of FAK, the rearrangement of the cytoskeleton and enhanced T cell migration [26]. In the absence of the GR, none of these events occur and T cells fail to migrate along a CXCL12 gradient, which might explain why mice lacking the GR in T cells develop a severe EAE and are refractory to GC therapy [51].
Our clinical study of MS patients of different subtypes including adults with RRMS and PPMS as well as children with RRMS provides evidence that the alteration in T cell migration as a newly identified mechanism of GC action might be of considerable clinical relevance. Standard high-dose MP pulse therapy of MS patients from all three groups resulted in enhanced migration of peripheral T cells towards CXCL12 within 24 h. This effect was robust as it occurred in every patient and was independent of the age and the particular disease course. In our view, this observation indicates that the altered T cell migration mediated via the CXCL12/CXCR4 axis could be a plausible explanation for the beneficial effects of GC therapy. This brings about several important consequences. First, the search for improved GC derivatives to be used as MS therapeutics should concentrate on those that act via the DNA-binding-independent function of the GR as they can be expected to efficiently redirect T cells away from the CNS while having less side effects. Second, research on new drugs should consider that actively redirecting T cells away from the inflamed CNS seems to ameliorate disease symptoms in MS patients regardless of their disease course and age. Third, new biomarkers are needed that help to predict the efficacy of MP administration in RRMS and to decide upon escalating therapy. We believe that there might be a correlation between the responsiveness of the patients’ T cells towards CXCL12 and the clinical outcome of MP therapy. Therefore, prospective studies should be conducted to determine whether this feature could indeed serve as a biomarker.
In summary, our results have uncovered T cell migration as a process in the pathogenesis of EAE that is significantly influenced by therapeutic and, most likely, endogenous GCs as well. Based on our findings for human T cells, we expect that this regulatory principle also applies to the treatment of MS patients. While the observed effect presumably acts in concert with the suppression of T cell effector functions and the induction of apoptosis, the latter is per se dispensable for therapeutic efficacy of GCs.
Supplementary Material
Acknowledgments
We would like to thank Martina Weig, Birgit Curdt, Regine Kruse, Nancy Meyer, Amina Bassibas and Julian Koch for technical assistance, Cathy Ludwig for language corrections, Jerry Adams for vav-Bcl-2 tg mice and Meike Schaffrinski and Florian Klemm for help with buffy coats. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Lu634/8-1, Tu220/3-1, FOR 1336, SFB-TRR 43/B11 & B13), the Bundesministerium für Bildung und Forschung (UNDERSTAND MS) and the Austrian Science Fund (FWF), grant Y212-B13.
Footnotes
Conflict of interest The authors declare that they have no conflict of interests.
Contributor Information
Nils Schweingruber, Institute for Cellular and Molecular Immunology, University of Göttingen Medical School, Humboldtallee 34, 37073 Göttingen, Germany; Department of Neuroimmunology, Institute for Multiple Sclerosis research, The Hertie Foundation and MPI for Experimental Medicine, University of Göttingen Medical School, Waldweg 33, 37073 Göttingen, Germany.
Henrike J. Fischer, Institute for Cellular and Molecular Immunology, University of Göttingen Medical School, Humboldtallee 34, 37073 Göttingen, Germany
Lisa Fischer, Department of Neuroimmunology, Institute for Multiple Sclerosis research, The Hertie Foundation and MPI for Experimental Medicine, University of Göttingen Medical School, Waldweg 33, 37073 Göttingen, Germany.
Jens van den Brandt, Institute for Cellular and Molecular Immunology, University of Göttingen Medical School, Humboldtallee 34, 37073 Göttingen, Germany.
Anna Karabinskaya, Institute for Cellular and Molecular Immunology, University of Göttingen Medical School, Humboldtallee 34, 37073 Göttingen, Germany.
Alexander Flügel, Department of Neuroimmunology, Institute for Multiple Sclerosis research, The Hertie Foundation and MPI for Experimental Medicine, University of Göttingen Medical School, Waldweg 33, 37073 Göttingen, Germany.
Fred Lühder, Department of Neuroimmunology, Institute for Multiple Sclerosis research, The Hertie Foundation and MPI for Experimental Medicine, University of Göttingen Medical School, Waldweg 33, 37073 Göttingen, Germany.
Holger M. Reichardt, Institute for Cellular and Molecular Immunology, University of Göttingen Medical School, Humboldtallee 34, 37073 Göttingen, Germany
References
- 1.Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood–brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32(8):2133–2144. doi: 10.1002/1521-4141(200208)32:8<2133:AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 2.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195(5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baschant U, Frappart L, Rauchhaus U, Bruns L, Reichardt HM, Kamradt T, Brauer R, Tuckermann JP. Glucocorticoid therapy of antigen-induced arthritis depends on the dimerized glucocorticoid receptor in T cells. Proc Natl Acad Sci USA. 2011;108(48):19317–19322. doi: 10.1073/pnas.1105857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120(2-3):69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+) CD4(+) CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol. 2006;36(8):2139–2149. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- 6.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, Russell JH, et al. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med. 2011;208(2):327–339. doi: 10.1084/jem.20102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curnow SJ, Wloka K, Faint JM, Amft N, Cheung CM, Savant V, Lord J, Akbar AN, Buckley CD, Murray PI, Salmon M. Topical glucocorticoid therapy directly induces up-regulation of functional CXCR4 on primed T lymphocytes in the aqueous humor of patients with uveitis. J Immunol. 2004;172(11):7154–7161. doi: 10.4049/jimmunol.172.11.7154. [DOI] [PubMed] [Google Scholar]
- 9.D’Apuzzo M, Rolink A, Loetscher M, Hoxie JA, Clark-Lewis I, Melchers F, Baggiolini M, Moser B. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur J Immunol. 1997;27(7):1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- 10.Dos Santos AC, Roffe E, Arantes RM, Juliano L, Pesquero JL, Pesquero JB, Bader M, Teixeira MM, Carvalho-Tavares J. Kinin B2 receptor regulates chemokines CCL2 and CCL5 expression and modulates leukocyte recruitment and pathology in experimental autoimmune encephalomyelitis (EAE) in mice. J Neuroinflamm. 2008;5:49. doi: 10.1186/1742-2094-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156(1):5–7. [PubMed] [Google Scholar]
- 13.Fleming KK, Bovaird JA, Mosier MC, Emerson MR, LeVine SM, Marquis JG. Statistical analysis of data from studies on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;170(1–2):71–84. doi: 10.1016/j.jneuroim.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Fricker SP. Physiology and pharmacology of plerixafor. Transfus Med Hemother. 2013;40(4):237–245. doi: 10.1159/000354132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh MC, Baatar D, Collins G, Carter A, Indig F, Biragyn A, Taub DD. Dexamethasone augments CXCR4-mediated signaling in resting human T cells via the activation of the Src kinase Lck. Blood. 2009;113(3):575–584. doi: 10.1182/blood-2008-04-151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119(1):61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holman DW, Klein RS, Ransohoff RM. The blood–brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):220–230. doi: 10.1016/j.bbadis.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koukouritaki SB, Gravanis A, Stournaras C. Tyrosine phosphorylation of focal adhesion kinase and paxillin regulates the signaling mechanism of the rapid nongenomic action of dexamethasone on actin cytoskeleton. Mol Med. 1999;5(11):731–742. [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, Hedin KE. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25(2):213–224. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Le Y, Zhu BM, Harley B, Park SY, Kobayashi T, Manis JP, Luo HR, Yoshimura A, Hennighausen L, Silberstein LE. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity. 2007;27(5):811–823. doi: 10.1016/j.immuni.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Choi SC, Lee MH, Oh HM, Choi EY, Choi EJ, Yun KJ, Seo GS, Kim SW, Lee JG, Han WC, et al. Increased expression of MIP-3alpha/CCL20 in peripheral blood mononuclear cells from patients with ulcerative colitis and its down-regulation by sulfasalazine and glucocorticoid treatment. Inflamm Bowel Dis. 2005;11(12):1070–1079. doi: 10.1097/01.MIB.0000187576.26043.ac. [DOI] [PubMed] [Google Scholar]
- 22.Lyons JA, Ramsbottom MJ, Trotter JL, Cross AH. Identification of the encephalitogenic epitopes of CNS proteolipid protein in BALB/c mice. J Autoimmun. 2002;19(4):195–201. doi: 10.1006/jaut2002.0619. [DOI] [PubMed] [Google Scholar]
- 23.McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177(11):8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- 24.McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194(7):873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalowska-Wender G, Losy J, Szczucinski A, Biernacka-Lukanty J, Wender M. Effect of methylprednisolone treatment on expression of sPECAM-1 and CXCL10 chemokine in serum of MS patients. Pharmacol Rep. 2006;58(6):920–923. [PubMed] [Google Scholar]
- 26.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 27.Muzio L, Cavasinni F, Marinaro C, Bergamaschi A, Bergami A, Porcheri C, Cerri F, Dina G, Quattrini A, Comi G, Furlan R, et al. Cxcl10 enhances blood cells migration in the sub-ventricular zone of mice affected by experimental autoimmune encephalomyelitis. Mol Cell Neurosci. 2010;43(3):268–280. doi: 10.1016/j.mcn.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Nagase H, Miyamasu M, Yamaguchi M, Kawasaki H, Ohta K, Yamamoto K, Morita Y, Hirai K. Glucocorticoids preferentially upregulate functional CXCR4 expression in eosinophils. J Allergy Clin Immunol. 2000;106(6):1132–1139. doi: 10.1067/mai.2000.110923. [DOI] [PubMed] [Google Scholar]
- 29.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96(26):14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okutsu M, Ishii K, Niu KJ, Nagatomi R. Cortisolinduced CXCR4 augmentation mobilizes T lymphocytes after acute physical stress. Am J Physiol Regul Integr Comp Physiol. 2005;288(3):R591–R599. doi: 10.1152/ajpregu.00438.2004. [DOI] [PubMed] [Google Scholar]
- 31.Pender MP, Rist MJ. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia. 2001;36(2):137–144. doi: 10.1002/glia.1103. [DOI] [PubMed] [Google Scholar]
- 32.Pountain GD, Keogan MT, Hazleman BL, Brown DL. Effects of single dose compared with three days’ prednisolone treatment of healthy volunteers: contrasting effects on circulating lymphocyte subsets. J Clin Pathol. 1993;46(12):1089–1092. doi: 10.1136/jcp.46.12.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C–C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 34.Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93(4):531–541. doi: 10.1016/S0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 35.Reichardt HM, Lühder F. The ambivalent role of apoptosis in experimental autoimmune encephalomyelitis and multiple sclerosis. Curr Pharm Des. 2012;18(29):4453–4464. doi: 10.2174/138161212802502224. [DOI] [PubMed] [Google Scholar]
- 36.Reichardt HM, Tuckermann JP, Göttlicher M, Vujic M, Weih F, Angel P, Herrlich P, Schütz G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. Embo J. 2001;20(24):7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichardt SD, Föller M, Rexhepaj R, Pathare G, Minnich K, Tuckermann JP, Lang F, Reichardt HM. Glucocorticoids enhance intestinal glucose uptake via the dimerized glucocorticoid receptor in enterocytes. Endocrinology. 2012;153(4):1783–1794. doi: 10.1210/en.2011-1747. [DOI] [PubMed] [Google Scholar]
- 38.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123(Pt 7):1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 39.Schweingruber N, Haine A, Tiede K, Karabinskaya A, van den Brandt J, Wüst S, Metselaar JM, Gold R, Tuckermann JP, Reichardt HM, Lühder F. Liposomal encapsulation of glucocorticoids alters their mode of action in the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2011;187(8):4310–4318. doi: 10.4049/jimmunol.1101604. [DOI] [PubMed] [Google Scholar]
- 40.Schweingruber N, Reichardt SD, Lühder F, Reichardt HM. Mechanisms of glucocorticoids in the control of neuroinflammation. J Neuroendocrinol. 2012;24(1):174–182. doi: 10.1111/j.1365-2826.2011.02161.x. [DOI] [PubMed] [Google Scholar]
- 41.Siffrin V, Brandt AU, Radbruch H, Herz J, Boldakowa N, Leuen-berger T, Werr J, Hahner A, Schulze-Topphoff U, Nitsch R. Differential immune cell dynamics in the CNS cause CD4+ T cell compartmentalization. Brain. 2009;132(Pt 5):1247–1258. doi: 10.1093/brain/awn354. [DOI] [PubMed] [Google Scholar]
- 42.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60(1):12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 43.Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22(14):5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tischner D, Theiss J, Karabinskaya A, van den Brandt J, Reichardt SD, Karow U, Herold MJ, Lühder F, Utermöhlen O, Reichardt HM. Acid sphingomyelinase is required for protection of effector memory T cells against glucocorticoid-induced cell death. J Immunol. 2011;187(9):4509–4516. doi: 10.4049/jimmunol.1100911. [DOI] [PubMed] [Google Scholar]
- 45.Tischner D, van den Brandt J, Weishaupt A, Lühder F, Herold MJ, Reichardt HM. Stable silencing of the glucocorticoid receptor in myelin-specific T effector cells by retroviral delivery of shRNA: insight into neuroinflammatory disease. Eur J Immunol. 2009;39(9):2361–2370. doi: 10.1002/eji.200939490. [DOI] [PubMed] [Google Scholar]
- 46.Tischner D, Weishaupt A, van den Brandt J, Müller N, Beyersdorf N, Ip CW, Toyka KV, Hünig T, Gold R, Kerkau T, Reichardt HM. Polyclonal expansion of regulatory T cells interferes with effector cell migration in a model of multiple sclerosis. Brain. 2006;129(Pt 10):2635–2647. doi: 10.1093/brain/awl213. [DOI] [PubMed] [Google Scholar]
- 47.Tuckermann JP, Kleiman A, Moriggl R, Spanbroek R, Neumann A, Illing A, Clausen BE, Stride B, Förster I, Habenicht AJ, Reichardt HM, et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest. 2007;117(5):1381–1390. doi: 10.1172/JCI28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhmann A, van den Brandt J, Dittmann K, Hess I, Dressel R, Binder C, Lühder F, Christiansen H, Fassnacht M, Bhandoola A, Wienands J, et al. T cell development critically depends on prethymic stromal patched expression. J Immunol. 2011;186(6):3383–3391. doi: 10.4049/jimmunol.1001939. [DOI] [PubMed] [Google Scholar]
- 49.Varga G, Ehrchen J, Tsianakas A, Tenbrock K, Rattenholl A, Seeliger S, Mack M, Roth J, Sunderkoetter C. Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J Leukoc Biol. 2008;84(3):644–650. doi: 10.1189/jlb.1107768. [DOI] [PubMed] [Google Scholar]
- 50.Wüst S, Tischner D, John M, Tuckermann JP, Menzfeld C, Hanisch UK, van den Brandt J, Lühder F, Reichardt HM. Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis. PLoS One. 2009;4(12):e8202. doi: 10.1371/journal.pone.0008202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wüst S, van den Brandt J, Tischner D, Kleiman A, Tuckermann JP, Gold R, Lühder F, Reichardt HM. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol. 2008;180(12):8434–8443. doi: 10.4049/jimmunol.180.12.8434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.