Abstract
Background
Percutaneous coronary intervention (PCI) aims to increase coronary blood flow by relieving epicardial obstruction. However, no study has objectively confirmed this and assessed changes in flow over different phases of the cardiac cycle. We quantified the change in resting and hyperemic flow velocity after PCI in stenoses defined physiologically by fractional flow reserve and other parameters.
Methods and Results
Seventy-five stenoses (67 patients) underwent paired flow velocity assessment before and after PCI. Flow velocity was measured over the whole cardiac cycle and the wave-free period. Mean fractional flow reserve was 0.68±0.02. Pre-PCI, hyperemic flow velocity is diminished in stenoses classed as physiologically significant compared with those classed nonsignificant (P<0.001). In significant stenoses, flow velocity over the resting wave-free period and hyperemic flow velocity did not differ statistically. After PCI, resting flow velocity over the wave-free period increased little (5.6±1.6 cm/s) and significantly less than hyperemic flow velocity (21.2±3 cm/s; P<0.01). The greatest increase in hyperemic flow velocity was observed when treating stenoses below physiological cut points; treating stenoses with fractional flow reserve ≤0.80 gained Δ28.5±3.8 cm/s, whereas those fractional flow reserve >0.80 had a significantly smaller gain (Δ4.6±2.3 cm/s; P<0.001). The change in pressure-only physiological indices demonstrated a curvilinear relationship to the change in hyperemic flow velocity but was flat for resting flow velocity.
Conclusions
Pre-PCI physiology is strongly associated with post-PCI increase in hyperemic coronary flow velocity. Hyperemic flow velocity increases 6-fold more when stenoses classed as physiologically significant undergo PCI than when nonsignificant stenoses are treated. Resting flow velocity measured over the wave-free period changes at least 4-fold less than hyperemic flow velocity after PCI.
Keywords: angioplasty, blood flow velocity, percutaneous coronary intervention
The purpose of percutaneous coronary intervention (PCI) is to relieve epicardial stenoses and thereby increase coronary flow ostensibly to relieve symptoms of angina. However, some studies suggest that PCI offers little clinical benefit over medical therapy,1 whereas others show that when PCI is guided by markers of physiological severity such as fractional flow reserve (FFR) outcomes can be improved.2–4 Because physiological parameters offer additional information about ischemia over angiographic assessment,5 the disparity in findings and improved outcome by application of physiology is likely because of improved differentiation of lesions into those with the highest likelihood of ischemia, and deferring those with lowest likelihood of ischemia.6 However, the uptake of physiology before PCI remains low.7 Even in centers with high-volume use, clinicians may choose to stent vessels in which physiological parameters, such as FFR, are above their thresholds (for example, FFR>0.80).7 Furthermore, it remains unconfirmed whether coronary physiological parameters identify stenoses, which will demonstrate an improvement in coronary flow and whether the increase in flow after PCI is predicted by physiological parameters.
In addition, new resting measures of stenosis severity have been proposed, such as the basal stenosis resistance (BSR) index and the instantaneous wave-free ratio (iFR). Although intracoronary pressure alone is known to increase after intervention,8 it is unclear how resting flow velocity, either over the whole cycle or the wave-free period, relates to each or behaves after intervention. Furthermore, because most application of physiology in clinical practice has been focused on pressure-only methodology, the precise relationship between pressure and flow velocity after PCI must be determined.
To investigate these issues, we assessed the change in both hyperemic and resting flow velocity after coronary intervention to a wide spectrum of stenoses in patients referred for PCI as a part of the Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity (JUSTIFY) study.9 Stenoses were treated according to anatomic and clinical information, and the change in flow velocity after PCI was assessed in relation to the physiological significance of the stenoses before PCI. Stenoses were defined physiologically by the reference standard pressure-only index (FFR). Further assessment was performed in relation to other physiological indices available in the catheter laboratory. Specifically, we sought to assess what increase in flow velocity should be expected for a change in a pressure index.
Methods
Study Population
The JUSTIFY family of studies incorporate pressure and flow velocity data collected prospectively for research purposes from patients scheduled for elective percutaneous coronary angioplasty at the Amsterdam Medical Centre, the Netherlands, and Imperial College London, United Kingdom.9 The purpose of the JUSTIFY studies is to better understand the relationship between different indices of coronary stenosis. In this analysis, only cases using a single Combowire to acquire simultaneous pressure and flow velocity data before and after angioplasty were assessed. In total, 75 stenoses underwent PCI.
Patients with significant valvular disease or previous coronary artery bypass grafts were not included in this study. The local ethical review boards approved the respective study protocols, and all subjects gave written informed consent.
Study Protocol: Coronary Catheterization
Coronary angiography and pressure-flow velocity assessments of coronary stenoses were performed using conventional approaches via the femoral artery. Intracoronary nitrates (300 µg) were administered in all cases before the introduction of coronary wires. Combined pressure and flow velocity wires (Combowire XT; Volcano Corporation, San Diego, CA) were normalized at the coronary ostia before every pressure recording. Measurements were made in the proximal vessel and distal to the stenosis. Adenosine was administered by central femoral vein in 43 stenoses (140 µg/kg per minute) and by intracoronary bolus in 32 stenoses (60 µg). The dose of intracoronary adenosine exceeds the dose of adenosine originally validated for use in man (20–40 µg).10 The same dose was used before and after intervention. Coronary intervention was performed at the operators discretion based on usual clinical care, including angiographic and noninvasive findings. For postangioplasty measurements, all stents were optimized with postdilation where angiographically indicated before further assessment with pressure wire. Repeated measurements after angioplasty were performed at the same coronary location as preangioplasty.
Hemodynamic Recordings
The ECG, pressures, and flow velocity signals were directly extracted from the digital archive of the device console (ComboMap; Volcano Corporation). At the end of each recording the pressure sensor was returned to the catheter tip to ensure there was no pressure drift. Where drift was identified the measurements were repeated. An adequate flow velocity envelope was obtained in all patients permitting the calculation of flow-based indices. Data were analyzed off-line, using a custom software package designed with Matlab (Mathworks, Inc, Natick, MA).
Calculation of Pressure-Only Indices
iFR was calculated as a ratio of the distal coronary pressure:proximal coronary pressure at rest, using automated algorithms acting over the diastolic wave-free period as previously described in Adenosine Vasodilator Independent Stenosis Evaluation Study (ADVISE)11 and validated in the Multicenter Core Laboratory Comparison of the Instantaneous Wave-Free Ratio and Resting Pd/Pa With Fractional Flow Reserve (RESOLVE).12 iFR is measured using intracoronary pressure-only, at baseline, without adenosine administration (Figure 1); its clinical cut point is 0.90 and has an ischemic cut point of 0.86.13 FFR measurements were performed using a standard technique,14 using the ratio of distal coronary pressure:proximal pressure during stable hyperemia; the clinical cut point is 0.80 and has an ischemic cut point of 0.75.15
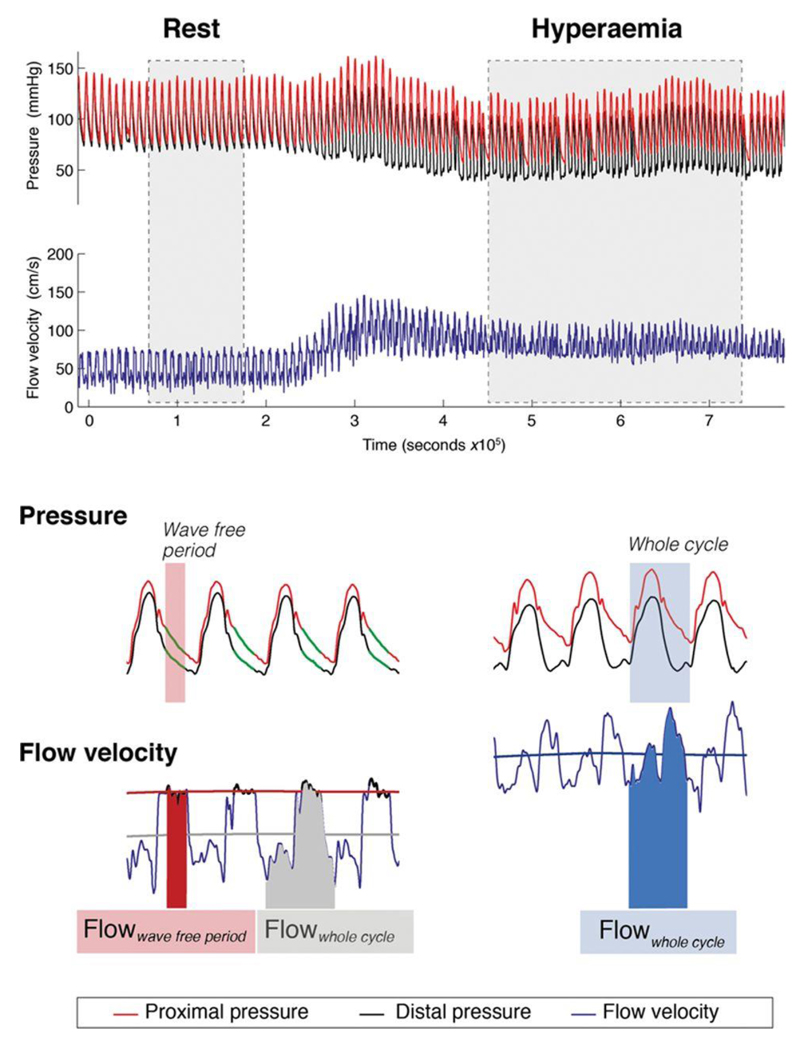
Figure 1.
Flow velocity across a stenosis before percutaneous coronary intervention (PCI). Simultaneous trans-stenotic pressure and flow velocity measurements permit assessment of hemodynamic change before and after PCI. Flow velocity can be measured during adenosine-mediated hyperemia over the entire cardiac cycle (Hyperemic Flowwhole cycle). It can also be measured under basal conditions, over the whole cardiac cycle (Rest Flowwhole cycle) or over the specific part in diastole known as the wave-free period (Rest Flowwave-free period).
Calculation of Flow Velocity–Based Indices
Lesions were categorized by coronary flow reserve (CFR), calculated by the ratio of whole cycle resting flow velocity:hyperemic flow velocity16; values below 2 and 1.7 have been considered abnormal previously, and both thresholds were tested. The hyperemic stenosis resistance was calculated using the hyperemic transtenotic gradient indexed by the hyperemic flow velocity; values >0.80 mm Hg/cm s are abnormal.17 The BSR was similarly calculated but uses resting transtenotic gradient and resting flow velocity; values >0.66 mm Hg/cm s are abnormal.18
Equations for the intracoronary indices are as follows:
where Pa is the mean aortic pressure and Pd is the mean intracoronary pressure distal to stenosis
Calculation of Flow Velocity Over Different Windows
Flow velocity was assessed at rest over entire cardiac cycle (Rest Flowwhole cycle; Figure 1) and over the specific diastolic wave-free period during which iFR is calculated (Rest Flowwave-free period). Flow velocity was also assessed during adenosine-mediated hyperemia over the whole cardiac cycle (Hyperemic Flowwhole cycle) and the wave-free period (Hyperemic Flowwave free period). Flow velocity is reported for the whole group or FFR strata as mean±SE. In addition, it is reported as a ratio of flow velocity before and after PCI: for example, a prepost hyperemic flow velocity ratio of 1 would suggest no increase in flow velocity after PCI, whereas a ratio of 2 would suggest flow velocity had doubled.
Microvascular resistance was calculated before and after PCI according to the following equations (where flow indicates flow velocity [cm/s] and Pd indicates distal coronary pressure [mm Hg]).
Microvascular resistance was used to estimate the presence of PCI-related microembolization which can affect post-PCI measurements. Because microembolization would prevent the microcirculation from responding to adenosine, the degree of embolization can be quantified by measuring the capacity of adenosine to reduce microvascular resistance (vasodilator reserve) post PCI. This was compared with the reduction in resistance offered by adenosine in stenoses with an FFR>0.80 pre-PCI because physiologically nonflow limiting stenoses are the most responsive to adenosine. In addition, CFR was measured post PCI to provide an additional manner to assess for the impact of embolization.
Data Analysis
Data are expressed as mean±SEM, unless otherwise stated. Patient demographics are presented as counts and percentages where appropriate. Correlations were assessed by Pearson correlation coefficient. Linear regression analysis was used to determine the coefficient of determination (R2) between quantitative variables. Regression analysis was used with polynomial best-fit curves to determine the relationship between quantitative variables. Relationships were determined to be linear or curvilinear based on appearance. Independent data were compared using Student t test and Mann–Whitney U test. Where data was paired, for example, before and after PCI, paired t tests were used. Comparisons of means between multiple groups were performed using ANOVA with Bonferroni, Sidak, and Scheffe corrections for multiple testing; this was followed by pairwise analysis using the Tukey HSD test. Repeated measures correction was applied where appropriate. For all analyses, a value of P<0.05 was considered significant. Statistical analysis was performed using Matlab (Mathworks, Inc) and STATA version 11 (StataCorp, College Station, TX).
Results
Patient Characteristics
Measurements were made before and after coronary PCI in 67 patients (75 stenoses; 76% men; 62±9 years old). Demographics are shown in Table 1. Physiological parameters before and after PCI are shown in Table 2. The mean FFR pre-PCI was 0.68±0.02, which was comparable with Fractional Flow Reserve Guided Percutaneous Coronary Intervention Plus Optimal Medical (OMT) Treatment Verses OMT (FAME-2).4 The mean stenosis diameter was 56±1.3% by quantitative coronary angiography. Mean arterial pressure had only a limited relationship with flow velocity; this was true of flow velocity measurements made at rest and hyperemia and also true before and after PCI (R2 for all comparisons were <0.04).
Table 1. Patient Demographic Data for Patients From the JUSTIFY Study in Which Paired Assessments Before and After PCI Were Made.
| PCI Patients n |
% | |
|---|---|---|
| Patients | 67 | |
| Age, y | 62± | 9 |
| Men | 50 | 74.6 |
| Hypertension | 36 | 53.7 |
| Hyperlipidemia | 56 | 83.6 |
| Current or ex-smoker | 31 | 46.3 |
| Diabetes mellitus | 19 | 28.4 |
| Chronic renal impairment | 4 | 6.0 |
| Previous myocardial infarction | 8 | 11.9 |
| Family history of CAD | 29 | 43.3 |
| Impaired LV function EF<30% | 1 | 1.5 |
| Stable angina | 64 | 95.5 |
| Unstable angina | 3 | 4.5 |
| Single-vessel disease | 42 | 62.7 |
| Multivessel disease | 25 | 37.3 |
| Stenoses | 75 | |
| Coronary vessel | ||
| Left anterior descending | 44 | 58.7 |
| Circumflex | 13 | 17.3 |
| Right coronary | 18 | 24.0 |
| Lesion characteristics | ||
| Lesion severity (QCA %) | 61.4 ± | 13.9 |
| Adenosine administration | ||
| Central intravenous | 43 | 57.3 |
| Intracoronary bolus | 32 | 42.7 |
Values are n, mean±SD or n (%). CAD indicates coronary artery disease; EF, ejection fraction; JUSTIFY, Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity; LV, left ventricle; PCI, percutaneous coronary intervention; and QCA, quantitative coronary angiography.
Table 2. Comparison of Flow Velocities Measured Either Over the Whole Cycle or the Diastolic Wave-Free Period, Either at Rest or During Hyperemia.
| Pre-PCI Flow Velocities, cm/s | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyperemic Flowwfp | Hyperemic Flowwhole cycle | Rest Flowwfp | Rest Flowwhole cycle | Comparison | |||||||||
| n | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Hyperemic Flowwfp vs Hyperemic Flowwhole cycle | Hyperemic Flowwhole cycle vs Rest Flowwfp | Rest Flowwhole cycle vs Rest Flowwfp | ||
| Physiologically significant stenoses | |||||||||||||
| FFR | ≤0.75 | 44 | 27.4 | 2.4 | 23.6 | 2.1 | 21.8 | 1.9 | 16.8 | 1.4 | <0.001 | 0.54 | <0.001 |
| ≤0.8 | 52 | 29.4 | 2.3 | 25.0 | 2.0 | 22.2 | 1.7 | 17.2 | 1.3 | <0.001 | 0.29 | <0.001 | |
| iFR | ≤0.86 | 42 | 27.7 | 2.3 | 23.6 | 2.1 | 23.0 | 1.9 | 17.8 | 1.4 | <0.001 | 0.82 | 0.002 |
| ≤0.9 | 53 | 31.2 | 3.0 | 25.8 | 2.3 | 23.9 | 2.0 | 18.3 | 1.4 | <0.001 | 0.55 | <0.001 | |
| iFRa | <0.66 | 47 | 27.9 | 2.3 | 23.8 | 2.0 | 22.0 | 1.8 | 17.0 | 1.4 | <0.001 | 0.53 | <0.001 |
| HSR | >0.80 | 45 | 25.0 | 1.7 | 21.2 | 1.5 | 19.0 | 1.3 | 14.9 | 1.1 | <0.001 | 0.30 | <0.001 |
| BSR | >0.66 | 38 | 23.3 | 1.6 | 19.6 | 1.5 | 19.0 | 1.5 | 14.8 | 1.2 | <0.001 | 0.80 | <0.001 |
| CFR | <2.0 | 49 | 29.0 | 2.2 | 24.0 | 1.9 | 24.1 | 1.8 | 18.7 | 1.4 | <0.001 | 0.97 | <0.001 |
| <1.7 | 41 | 29.2 | 2.6 | 24.3 | 2.2 | 25.8 | 2.1 | 19.9 | 1.5 | <0.001 | 0.97 | <0.001 | |
| Physiologically nonsignificant stenoses | |||||||||||||
| FFR | >0.75 | 31 | 47.9* | 4.6 | 37.5* | 4.2 | 24.2 | 2.3 | 18.8 | 1.6 | <0.001 | 0.001 | <0.001 |
| >0.8 | 23 | 50.5* | 5.9 | 39.1* | 5.4 | 24.3 | 2.8 | 18.8 | 1.7 | <0.001 | 0.002 | <0.001 | |
| iFR | >0.86 | 33 | 46.3* | 4.6 | 37.7* | 4.0 | 22.6 | 2.2 | 17.5 | 1.5 | <0.001 | 0.002 | <0.001 |
| >0.9 | 22 | 47.0* | 4.6 | 39.6* | 5.0 | 20.1 | 1.2 | 16.2 | 0.9 | <0.001 | 0.001 | <0.001 | |
| iFRa | >0.66 | 28 | 49.3* | 5.0 | 40.0* | 4.5 | 24.1 | 2.5 | 18.7 | 1.6 | <0.001 | 0.003 | <0.001 |
| HSR | <0.80 | 30 | 52.1* | 4.7 | 42.8* | 4.2 | 28.5 | 2.7 | 21.8 | 1.8 | <0.001 | 0.006 | <0.001 |
| BSR | <0.66 | 37 | 48.8* | 4.1 | 40.4* | 3.6 | 26.7 | 2.3 | 20.6 | 1.5 | <0.001 | 0.002 | <0.001 |
| CFR | ≥2.0 | 26 | 48.7* | 5.6 | 40.8* | 4.9 | 20.4 | 2.3 | 15.8 | 1.5 | <0.001 | <0.001 | <0.001 |
| ≥1.7 | 34 | 43.9* | 4.6 | 36.5* | 4.0 | 19.2 | 1.9 | 14.9 | 1.2 | <0.001 | <0.001 | <0.001 | |
Flow velocities are compared according to physiological stenosis significance as determined by many different parameters. Where different thresholds have been proposed, both thresholds have been assessed. CFR indicates coronary flow reserve; FFR, fractional flow reserve; HSR, hyperemic stenosis resistance; iFR, instantaneous wave-free ratio; iFRa, adenosine-mediated hyperemia; PCI, percutaneous coronary intervention; and wfp, wave-free period.
P<0.001 difference between flow for physiologically significant stenoses and nonsignificant stenoses for the given index.
Assessment of Coronary Flow Velocity Before PCI
Mean flow velocities at rest and during hyperemia were assessed (Figure 1) and assessed in relation to physiological parameters typically used to categorize stenoses as significant or not (Table 2). In all cases, regardless of the physiological parameter used to stratify the study population, by restricting the flow velocity measurement to the wave-free period (Resting Flowwave free period) elicited flow velocities that were significantly higher than those measured over the whole cardiac cycle at rest (Resting Flowwhole cycle). Hyperemic Flowwhole cycle was not different from Rest Flowwave free period in stenoses classed as physiologically significant by any of the parameters and cut points assessed (Table 2), but was greater when stenoses were classed as nonsignificant. Measuring flow velocity only during the wave-free period under conditions of hyperemia elicited significantly greater flow velocities than over the whole-cycle (P<0.001).
Change in Hyperemic Flow Velocity After Intervention
After PCI, the mean Hyperemic Flowwhole cycle across all stenoses rose significantly to 51.0±2.87 cm/s. Stratification by FFR demonstrated that when stenoses had pre-PCI FFR values >0.80, Hyperemic Flowwhole cycle velocity increased by 4.6±2.3 cm/s; this was significantly less than when FFR values ≤0.80 (28.5±3.8 cm/s; P<0.001; Figure 2). Expressed as a ratio of flow velocity pre:post PCI, a similar finding was noted (FFR ≤0.80, pre:post flow velocity ratio of 2.83±0.0.29 versus 1.17±0.08; P<0.001). The behavior of hyperemic flow velocity over the wave-free period was similar to the whole cycle. Stratification by the other physiological parameters elicited a similar relationship (Figure 3).
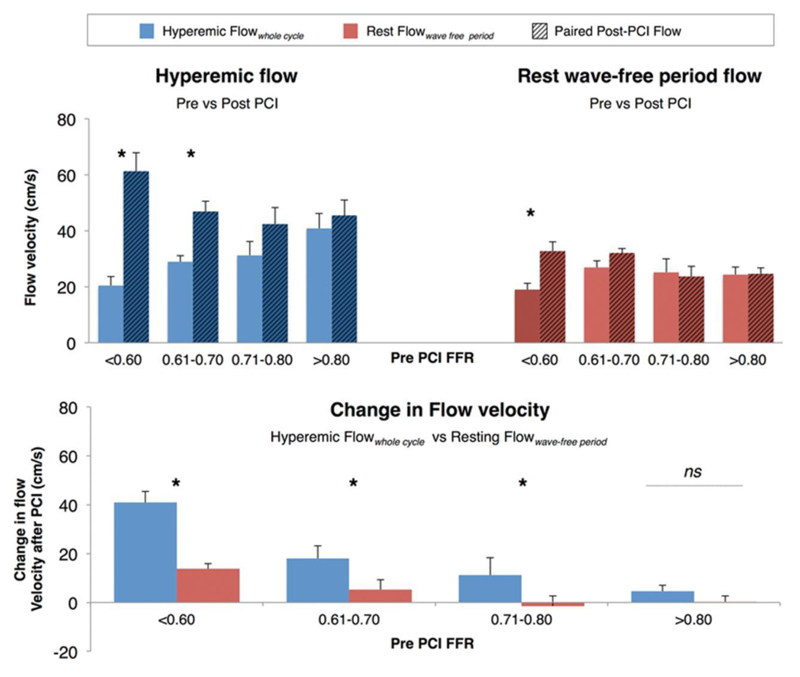
Figure 2.
A, Paired mean flow velocity shown before and after percutaneous coronary intervention (PCI) measured during hyperemia (blue) and the wave-free period (red). Fractional flow reserve (FFR) ≤0.60 had 28 stenoses; FFR 0.61 to 0.70: 10 stenoses; FFR 0.71 to 0.80: 14 stenoses; FFR >0.80: 23 stenoses. Hyperemic flow increases most for highly physiologically significant lesions (FFR≤0.70). Flow over the iFR-window is remarkably stable throughout all levels of lesion severity and changes little after PCI. Rest Flowwave-free period has little variability between FFR categories of stenosis severity. B, The change in flow velocity after PCI. Hyperemic Flowwhole cycle increases significantly more than the increases in Rest Flowwave-free period for every FFR category of stenosis severity. *P≤0.01.
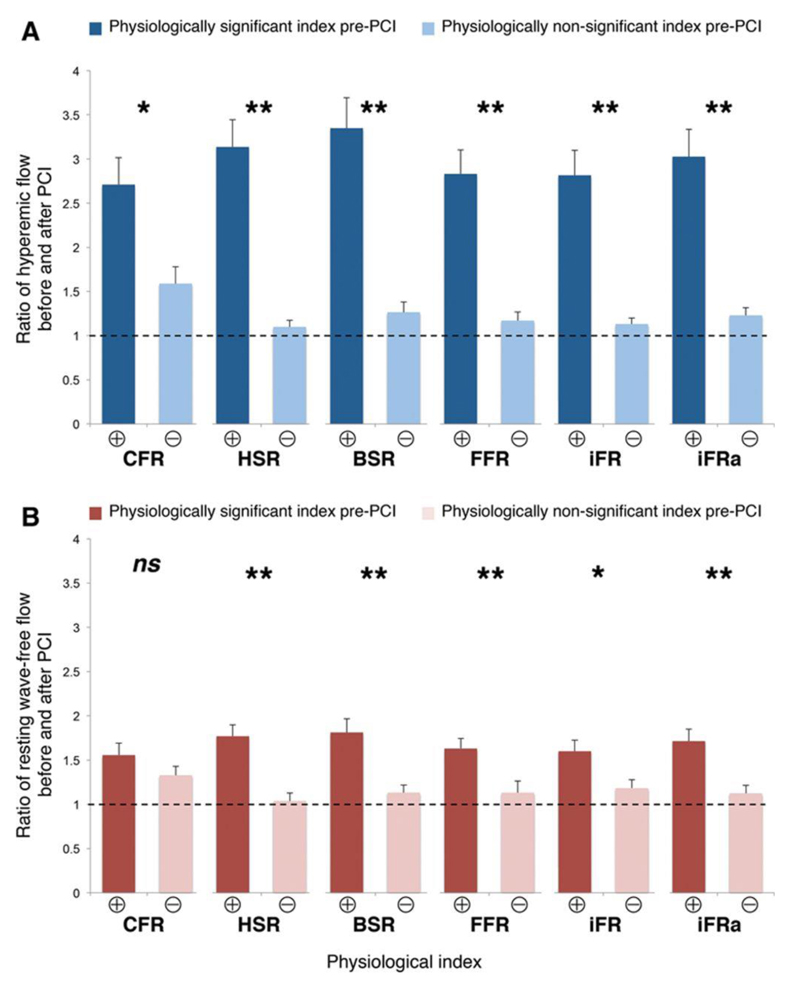
Figure 3.
The change in hyperemic flow after percutaneous coronary intervention (PCI) according to different pre-PCI indices. Coronary flow reserve (CFR), fractional flow reserve (FFR), instantaneous wave-free ratio measured at rest (iFR), or during adenosine-mediated hyperemia (iFRa), basal stenosis resistance (BSR), and hyperemic stenosis resistance (HSR) were used to classify stenoses as physiologically significant (⊕) or physiologically nonsignificant (Ө) according to their respective cut points, as described in the Methods section of this article. The change in hyperemic flow velocity after PCI (top) was significantly higher when stenoses were physiologically significant (⊕) than when nonsignificant (Ө), regardless of the index used. *P=0.01, **P<0.001. Bottom, The change in resting wave-free period flow using the same annotation.
Stenoses with an FFR 0.71 to 0.80 had a small but statistically nonsignificant increase in hyperemic flow velocity (Δ11.2±7.1 cm/s; P=0.14). Much larger increases in flow velocity were observed for stenoses with FFR 0.61 to 0.70 (Δ18.0±3.2 cm/s; P=0.007) and FFR≤0.60, Δ40.8±4.6 cm/s; P<0.001; Figure 2).
Change in Resting Flow Velocity After Intervention
The change in resting flow velocity measured over the wave-free period was assessed (Figure 2A and 2B). In stenoses with FFR>0.80 before PCI, no significant increase in flow velocity was observed (0.3±2.4 cm/s; P=0.90). Similar small changes were noted for stenoses with FFRs of 0.6 to 0.70 and 0.70 to 0.80 (Figure 2A). Only when stenoses had FFR values of ≤0.60 was a significant increase in Rest Flowwave free period observed (13.7±2.2 cm/s); this was 3-fold smaller than the change in hyperemic flow velocity for this range. In all cases, when the pre-PCI FFR was <0.80, the change in hyperemic flow velocity after PCI was significantly greater than observed at rest (Figure 2B; P<0.01 for all).
Using Pressure Indices to Predicting the Change in Flow Velocity Before PCI
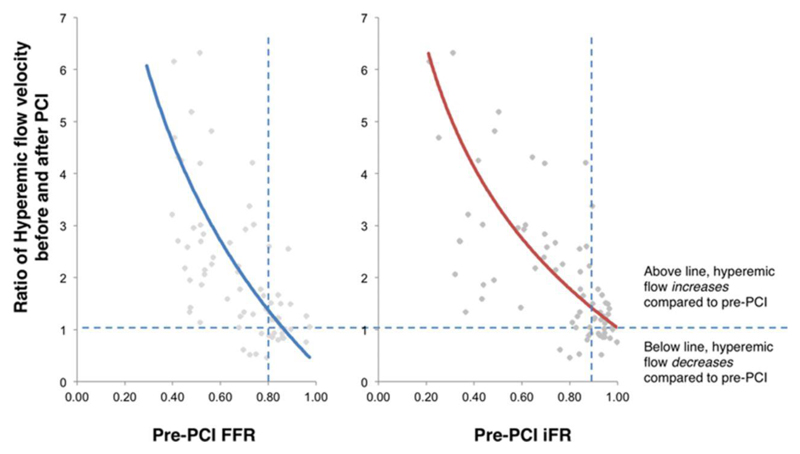
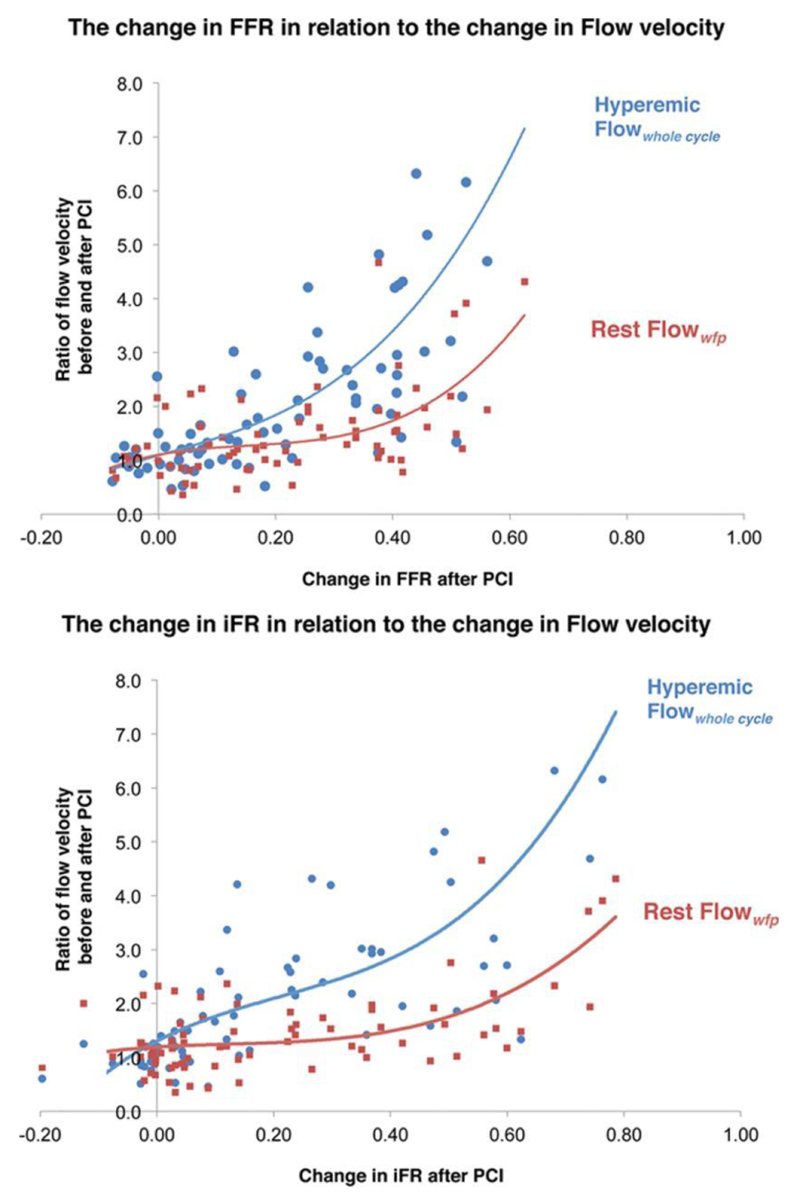
All the physiological parameters demonstrated significant increases after PCI (Table 3). The change in hyperemic flow velocity after PCI had a curvilinear relationship with both pre-PCI FFR and iFR values (Figure 4). Plotting the change in either pressure index with the change in flow velocity (Figure 5) shows that over the typical range of improvement in the index seen in clinical practice (≈0.20), then hyperemic flow velocity is likely to double while resting flow velocity had little rise. For larger changes in either index, then the increase hyperemic flow rises exponentially while resting flow shows significant rises only when increments in pressure are large.
Table 3. Change in Physiological Indices Before and After Coronary Intervention.
| ΔFFR | ΔiFR | ΔHSR | ΔBSR | ΔCFR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-PCI | Post-PCI | Overall δ | Pre-PCI FFR≤0.80 | Pre-PCI FFR>0.80 | Pre-PCI IFR<0.90 | Pre-PCI IFR≥0.90 | Pre-PCI HSR≥0.80 | Pre-PCI HSR<0.80 | Pre-PCI BSR≥0.66 | Pre-PCI BSR<0.66 | Pre-PCI CFR<2.0 | Pre-PCI CFR≥2.0 | |
| Basal indices | |||||||||||||
| iFR | 0.73± | 0.94± | 0.21± | 0.29± | 0.01± | 0.29± | 0.02± | 0.34±0. | 0.01± | 0.38± | 0.03± | 0.27± | 0.08± |
| 0.03 | 0.01 | 0.03 | 0.03 | 0.01* | 0.03 | 0.01* | 04 | 0.01 | 0.04 | 0.01* | 0.04 | 0.03* | |
| BSR | 1.42± | 0.22± | −1.20 | −1.71 | −0.05 | −1.67 | −0.07 | −2.00± | 0.00± | −2.29 | −0.07 | −1.51 | −0.62 |
| 0.20 | 0.03 | ±0.21 | ±0.27 | ±0.04* | ±0.27 | ±0.04* | 0.29 | 0.04 | ±0.32 | ±0.04* | ±0.29 | ±0.20* | |
| * | |||||||||||||
| Hyperemic indices | |||||||||||||
| FFR | 0.68± | 0.89± | 0.22± | 0.29± | 0.04± | 0.27± | 0.08± | 0.33±0. | 0.04± | 0.34± | 0.09± | 0.25± | 0.15± |
| 0.02 | 0.01 | 0.03 | 0.02 | 0.01* | 0.03 | 0.02* | 02 | 0.01 | 0.03 | 0.02* | 0.03 | 0.03* | |
| CFR | 1.75± | 2.46± | 0.71± | 1.02± | 0.00± | 1.03± | −0.07 | 1.06±0. | 0.17± | 1.15± | 0.26± | 0.89± | 0.36± |
| 0.09 | 0.10 | 0.20 | 0.12 | 0.19* | 0.10 | ±0.22* | 12 | 0.18 | 0.12 | 0.17* | 0.11 | 0.24* | |
| HSR | 1.64± | 0.26± | −1.38 | −1.94 | −0.13 | −1.86 | −0.24 | −2.25± | −0.08 | −2.48 | −0.26 | −1.77 | −0.64 |
| 0.19 | 0.03 | ±0.19 | ±0.25 | ±0.04* | ±0.26 | ±0.06* | 0.26 | ±0.05 | ±0.30 | ±0.07* | ±0.28 | ±0.16* | |
Data show mean and SE. CFR indicates coronary flow reserve; FFR, fractional flow reserve; HSR, hyperemic stenosis resistance; iFR, instantaneous wave-free ratio; and PCI, percutaneous coronary intervention.
P<0.001 for difference in the size of delta when comparing significant and nonsignificant stenoses.
Figure 4.
The relationship between pre-percutaneous coronary intervention (PCI) pressure-only physiological indices and the increase in hyperemic flow velocity after PCI. Hyperemic flow increases significantly following PCI. A ratio of pre-PCI and post-PCI hyperemic flow velocity was plotted using a third-order polynomial against the pre-PCI fractional flow reserve (FFR) value. Ratios above 1 suggest an increase in flow velocity, whereas those below 1 suggest a fall. iFR indicates instantaneous wave-free ratio.
Figure 5.
Estimating the change in hyperemic flow velocity based on the δ or change in pressure-only index after percutaneous coronary intervention (PCI). Pressure-only indices increase after PCI and demonstrate a curvilinear relationship with the change in resting and hyperemic flow velocity. For a change in instantaneous wave-free ratio (iFR), the change in wave-free flow velocity was predicted by the curve y=7.522x3−2.863x2+0.6754x+1.1943, whereas the change in hyperemic flow velocity was predicted by the curve y=18.96x3−11.94x2+5.6048x+1.304. For fractional flow reserve (FFR), the change in resting wave-free flow velocity was predicted by a curve y=20.7x3−9.1852x2+2.0422x+1.1019, whereas the change in hyperemic flow was y=17.175x3−0.1665x2−3.0192x+1.0898.
Change in Microvascular Resistance After Intervention
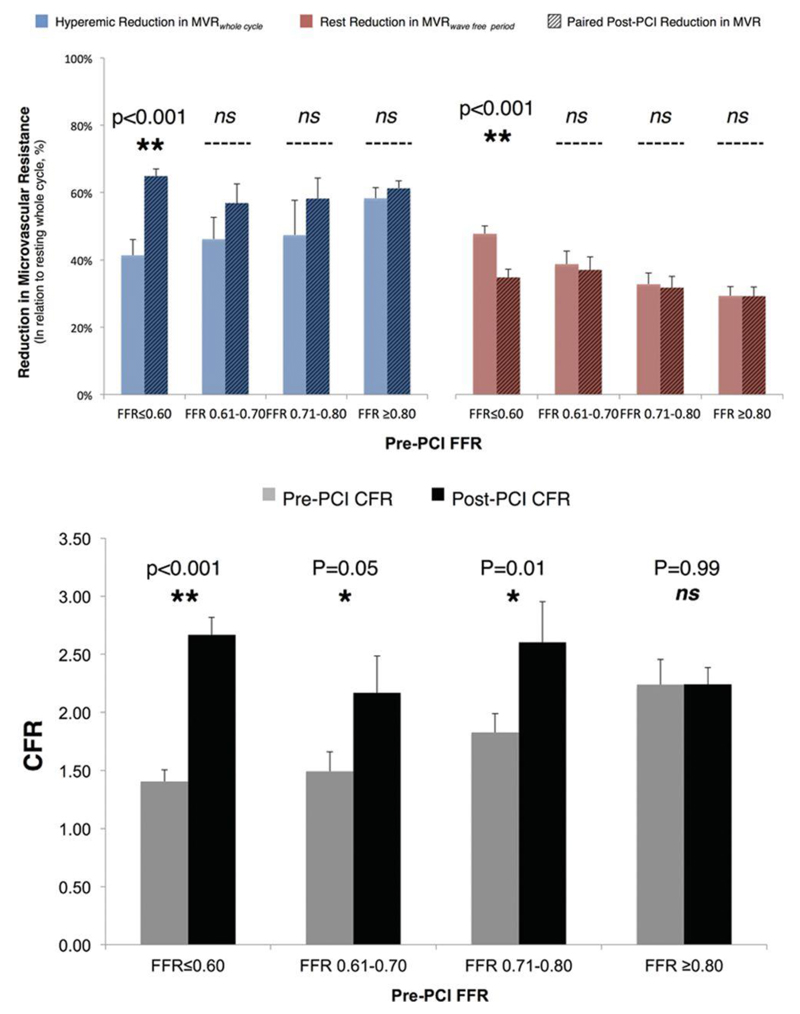
To assess the impact of microembolization on the post-PCI measurements, the gain in resistance reduction offered by hyperemia over the whole cycle and the wave-free period at rest was compared with the resting whole cycle (Figure 6, top). A significant reduction in the vasodilator reserve would suggest embolization. Hyperemic vasodilator reserve rose after PCI (48±3%–61±2%; P<0.001), and there was no difference between post-PCI vasodilator reserve and that observed in stenoses with pre-PCI FFR values >0.80 (61±2% versus 58±3%; P=0.44), suggesting that there was no important embolization. This was confirmed by measuring CFR, which significantly improved across all strata of FFR-positive stenoses (Figure 6, bottom) and had no significant relationship with either pre- or post-PCI FFR values (all P=ns). Under resting conditions, the wave-free period offered a consistent reduction in resistance across all stenoses (33±01%), and there was no difference per strata when pre-PCI FFR was >0.60. In the most significant stenoses (FFR≤0.60), pre-PCI, the wave-free period offered a greater reduction in resistance than those with FFR>0.60 (P<0.001). It is likely severe stenoses are maximally vasodilated at rest to maintain resting flow. In this strata alone, post-PCI resting resistance is lower than before PCI (P<0.001); however, the value is numerically consistent with that of other strata. Because vasodilator reserve is maintained in these stenoses (as shown by the adenosine response post-PCI in this strata), this does not suggest embolization, rather a relative physiological vasoconstriction in response to the increased resting flow observed in these stenoses after the removal of a flow-limiting stenosis.
Figure 6.
Change in microvascular resistance and flow reserve after percutaneous coronary intervention (PCI). Top, The capacity for hyperemia and the wave-free period to reduce resistance compared with the resting whole cycle was compared before and after PCI as a marker of microembolization. Post PCI, the hyperemic effect was not significantly blunted, suggesting no impact of embolization. Bottom, This was confirmed by assessing the capacity of coronary flow reserve (CFR) to increase after PCI. FFR indicates fractional flow reserve.
Discussion
In this study, we have demonstrated that (1) physiological severity of the lesion predicts the likelihood of coronary flow velocity increasing after coronary intervention, (2) hyperemic flow velocity changes most after PCI, whereas resting flow velocity changes little. We also find that the increase in hyperemic flow velocity is similar regardless of the physiological parameter used to dichotomize stenoses. In addition, we have determined a model for estimating the change in flow velocity after a change in pressure index.
Pressure-only indices are known to improve after intervention to a stenosis8,19 but because they use pressure as a surrogate estimate of flow, the increase in distal pressure could reflect other changes caused by PCI, such as microembolization or epicardial vessel spasm. Flow velocity is also known to change, but previous reports were limited by categorizing stenoses based on visual estimation of severity.20,21 In this study, we report the change in flow velocity according to pre-PCI physiological parameters, which overcomes the considerable limitations of visual or anatomic definitions of lesion severity. Furthermore, because the microvasculature continued to respond to adenosine after PCI in a manner similar to physiologically unobstructed vessels before PCI, then any impact of microembolization on the flow velocity post-PCI was small.
Hyperemic Flow Velocity Before and After Intervention
Hyperemic flow considers all the potential blood flow that can occur when the tightly controlled myocardial-coronary autoregulatory processes are uncoupled by the use of a vasodilator. Hyperemic flow velocity declines in presence of stenoses occupying 50% of the vessel lumen.22,23 Conceptually, if a stenosis can be reduced to <50%, one would expect an improvement in hyperemic flow velocity, to similar levels seen in vessels when there is no stenosis. Because the angiographic appearance of stenoses can reflect poorly its importance, we chose to classify stenoses by FFR, which is a familiar and easily understood alternative. Furthermore, the concept of FFR should lead to predictable increases in coronary flow velocity after PCI.
Before PCI, in physiologically significant stenoses, hyperemic flow velocity measured over the whole cardiac cycle was greater than resting flow velocity measured over the whole cycle. However, it was not significantly different from resting flow velocity measured over the wave-free period. This demonstrates that constraining flow analyses to a period in diastole provides a higher flow velocities than measurable over the whole cardiac cycle and also that in significant stenoses there is little difference between what is calculable at rest and hyperemia. This is in keeping with the Classification Accuracy of Pressure-Only Ratios Against Indices Using Flow Study (CLARIFY) study that showed ischemic stenoses demonstrated comparable microvascular resistance both over the resting wave-free period and during whole-cycle hyperemia.13
A higher flow velocity in the pre-PCI setting is pertinent for the diagnostic sensitivity of pressure-only indices, which rely on the highest transtenotic gradient possible to sufficiently distinguish between mild, moderate, and severe stenoses.22 FFR uses exogenous hyperemia to increase flow velocity to a level, where it is more easy to distinguish stenosis severities than possible by Pd/Pa at rest. For iFR, a resting pressure index measured only over the wave-free period, the higher velocities would mean greater stenosis discrimination at rest than a whole cycle resting index.
Because the resting wave-free flow velocity and hyperemic whole cycle flow velocity were statistically similar for stenoses classed as significant whether by FFR, iFR, BSR, hyperemic stenosis resistance, or CFR, then it likely that this finding is valid by whatever means is used to stratify stenoses.
The findings of hyperemic stenosis resistance and BSR are pertinent because these parameters index transtenotic gradients by flow velocity and thereby limit possible false-positives that may occur with pressure-only indices.9,24,25 Although rarely considered and not previously detected without simultaneous flow velocity measurement, false-positive pressure-only indices will occur when flow velocity can increase significantly during hyperemia (which by definition, cannot be flow limiting) to generate an apparently important pressure gradient.26 Most importantly, these lesions carry the same prognosis >10 years as those which are negative using FFR guidance.25
After PCI, hyperemic flow velocities were broadly similar to the values seen in the presence of stenoses labeled as physiologically nonsignificant (either by FFR>0.80 or any of the other indices; Figure 2A). That is, stenoses with FFR>0.80 had a mean hyperemic flow velocity of 41±5 cm/s; after PCI, the entire cohort had a flow velocity of 53±3 cm/s, whereas those with FFR 0.61 to 0.70 had a mean of 47±5 cm/s and those with FFR 0.71 to 0.80 had a mean 42±6 cm/s. All of these values are statistically similar, suggesting that this value of flow velocity should be expected, on average, after intervention. The greatest increment in hyperemic flow velocity was seen in stenoses when FFR≤0.70 pre-PCI (Figure 2B), which is similar to the FFR value that is most closely related to ischemia on noninvasive testing (FFR=0.75).15 This value is also remarkably similar to the FFR value (0.67) determined to have prognostic value on a large meta-regression.27
Smaller, less significant increments in hyperemic flow velocity are noted for higher FFR values, with little increase in stenoses typically considered nonischemic (FFR>0.80). These findings are in keeping with the basic tenets of FFR and likely account for the findings of DEFER and FAME—concentrating PCI to stenoses most likely to increase flow should lead to more favorable outcomes. At present, it remains unclear what degree of flow velocity increase is required to achieve symptomatic benefit or reduce clinical events.
Other indices of stenoses significance also predicted similar increases in hyperemic flow velocity after PCI: when stratified by CFR, hyperemic stenosis resistance, BSR, and iFR, the change in flow velocity was always significantly higher when stenoses were classed as significant by the given parameter than when classed as nonsignificant (Figure 3).
Higher values of post-PCI flow velocity may be achievable in animal models, which use external constrictors to mimic a lesion; typically in those models there can be a lack of generalized atheroma, and a lack of microcirculatory disease. In this study, in humans with coronary artery disease, the post-PCI flow velocity will be modulated by many factors including atheroma and the completeness of PCI which will differ from the release of an external constrictor. The values of post-PCI hyperemic flow velocity are consistent with those measured in unobstructed vessels in humans.10,28
Resting Flow Velocity Changes Little After Coronary Intervention
Previous studies assessing Doppler flow velocity suggested that post-PCI CFR may be underestimated because of a rise in resting flow velocity.29 Others showed modest change in resting flow velocity immediately after balloon angioplasty.30 In this modern cohort, undergoing PCI as per current practice, resting flow velocity only changed significantly after stent placement and optimization in highly significant lesions. The maintenance of resting flow velocity at a steady figure despite the removal of a stenosis likely demonstrates in vivo coronary autoregulation. Only profoundly significant stenoses (with FFR<0.60 or iFR<0.50) demonstrated significant change in resting flow velocity. Nonetheless, mean post-PCI CFR values exceed normal levels and increased significantly for all stenoses with pre-PCI FFR<0.80 (Figure 6, bottom). Note that CFR has been criticized because a value of 3 may be half of normal if a given vessel has a CFR of 6.31 However, although normal unobstructed vessels in young patients or animals may have exceptionally high CFRs, in this cohort of patients with coronary disease requiring PCI, such high CFRs are not observed. It is possible diffuse epicardial resistance limited exceedingly high post-PCI CFRs.
Predicting the Change in Flow Velocity Using Pressure-Only Indices
Both the pre-PCI FFR and the iFR values significantly predict the change in hyperemic flow velocity significantly, in a curvilinear fashion. Both indices had similar predictive power, despite the difference in calculation (iFR measured at rest and FFR measured during hyperemia). Similarly, the δ of the pressure index demonstrated a complex curvilinear relationship with the change in flow velocity. However, for both iFR and FFR, change in the index over the typical range seen clinically (0.22 for FFR and 0.20 for iFR8) lead to little change in resting flow velocity and a more linear increase in hyperemic flow velocity after PCI. This means for typical stenoses showing average changes in either pressure index, interventionalists can be reassured that increments in the pressure ratio directly reflect increments in flow velocity increase.
Practical Clinical Implications
Our findings strongly support the use of physiological techniques to detect lesions which are both likely ischemia producing, but also likely to benefit from PCI, and provide mechanistic support to the established clinical trials (FAME, FAME-2).3,4 By reducing the number of stents being implanted, the likelihood of procedural complications is reduced. This is particularly emphasized whenever compared with an approach that attempts to stent all stenoses whereby the theoretical risks of stenting apply to each one placed while the potential benefit of flow increase may be confined to a few. By focusing on treating stenoses most likely to lead to a measurable flow increase, PCI may be more likely to improve symptoms and potentially reduce cardiac events.
The relative little change in resting flow velocity may have important clinical applications for the interrogation of tandem lesions or in diffuse disease, particularly for planning intervention, where the greater change in hyperemic flow velocity can limit practical prediction of the impact of stenting a given stenosis when multiple stenoses are present.32
Limitations
This study is comparatively smaller than pressure-only studies that assess change in pressure before and after PCI. However, it is one of the largest reported using simultaneous pressure–flow velocity wire before and after PCI in the modern era.
In this data set, assessments were paired, and the doses of adenosine used post-PCI measurements were consistent with pre-PCI doses allowing direct comparisons of FFR values and hyperemic flow velocities. Those stenoses assessed by intracoronary adenosine were done so using a conservative dose that although exceeding original validation work, has been superseded in clinical practice (doses 100–150 µg are now favored). Higher doses of adenosine may elicit higher flow velocities, but this is dependent on the true severity of the underlying stenosis: truly flow-limiting stenoses are likely to have little gain in flow velocity regardless of the dose (eg, stenoses with FFR≤0.60), whereas those not truly flow limiting will have a greater increase in flow velocity (eg, those stenoses with FFR>0.80). Higher doses may also make FFR values become marginally lower,33 with greatest impact on stenoses with borderline FFR values most (those just >0.80 may be reclassified as just <0.80). This may mean that the marginal increase in hyperemic flow velocity observed for stenoses with pre-PCI FFR values of 0.70 to 0.80 may be even smaller than shown here. For the change in resting flow velocity, no difference in results is expected because measurements are made without adenosine and change in resting flow was only observed for stenoses with pre-PCI FFR≤0.60.
Physiologically nonsignificant stenoses demonstrated some improvement in flow velocity; on average, this was a small amount compared with when stenoses were significant. Because it is still unclear what degree of flow velocity increase will improve symptoms or produce prognostic benefit, we cannot be sure whether such increases are worthwhile.
Coronary flow velocity measurements in truly significant stenoses can be difficult to perform as velocities are diminished. The 2 centers involved in stenosis assessment have >10 years of experience each in performing these measurements meaning the an adequate wave-form for phasic analysis was possible.
Flow velocity measurements were taken once operators had completed PCI. It is unclear whether flow velocity would continue to improve at a later date.
Wedge pressure recordings to estimate the impact of collateral flow were not routinely performed in this study. Visible collaterals were avoided during this study, but nonvisible vessels may have been present in those with significant FFR values. Because collaterals are expected to close on removal of the stenosis, it is not expected that they would significantly alter the findings. Wedge occlusion can also be used to correct hyperemic resistance measurements, which may otherwise be overestimated in severe stenoses (FFR<0.60). In this study, because vasodilator response was used only to exclude a significant impact of embolization, the overall interpretation is not altered.
Volumetric absolute coronary flow was not measured because the potential error, imposed by the technical limitations of measuring vessel size, is large. Although coronary size will change around the lesion, flow velocity measurements were made at the same location distal to the region of interest, in an area that did not change size. Thereby the changes in flow velocity are likely to equate to changes in absolute flow.
This study was performed in humans with coronary artery disease referred to the catheter laboratory for assessment and treatment. As such, the results are applicable to other patients. The results may differ from animal studies, however, particularly in young animals with distensible microcirculation and external constrictors used for simulating a stenosis simulation. In these models, removal of a stenosis may manifest even higher flow velocities than seen in humans although the clinical relevance is unclear.
Conclusions
Physiological assessment tools can strongly predict the likely increase in coronary flow velocity after PCI. This change is most marked in physiologically more severe lesions where the difference between resting and hyperemic flow velocity measurements is small.
What is Known.
Fractional flow reserve is a pressure-based index, which in animal models of stenoses has a close relationship with flow velocity. Patients undergoing stenting as directed by coronary physiological parameters, such as fractional flow reserve, have improved outcomes compared with less discriminate stenting based on angiography.
What the Study Adds.
Hyperemic flow velocity improves significantly after removal of a coronary stenosis in humans. Stenoses classified as physiologically important demonstrate the greatest gain in flow velocity, regardless of the index chosen for stratification. The incremental gain in hyperemic flow velocity is related to the pre–percutaneous coronary intervention physiological severity with the greatest gain seen in the strata in which fractional flow reserve was shown to predict hard outcomes in a large metaregression. Resting coronary flow velocity, over the wave-free period, shows only small changes after stenting. Coronary intervention in stable disease does not significantly alter microvascular responsiveness to adenosine or the wave-free period.
Acknowledgments
We thank the catheter laboratory staff at the Academic Medical Centre, Amsterdam, and Hammersmith Hospital, London, for their support. This study would not be possible without their ongoing commitment to research.
Sources of Funding
Dr Nijjer (G1100443) and Dr Sen (G1000357) are Medical Research Council fellows. Dr Petraco (FS/11/46/28861), Dr JE Davies (FS/05/006), and Dr Francis (FS 10/038) are British Heart Foundation fellows. Additional support was provided by the National Institute of Health Research Imperial Biomedical Research Centre.
Footnotes
Disclosures
Imperial College London, Dr Davies, and Mayet hold intellectual property pertaining to identification of the wave-free period in an automated manner. Drs Davies, Escaned, and Piek have undertaken consultancy work for Volcano Corporation.
References
- 1.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, et al. COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 2.Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928–2934. doi: 10.1161/01.cir.103.24.2928. [DOI] [PubMed] [Google Scholar]
- 3.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, et al. FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 4.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Mobius-Winckler S, Rioufol G, Witt N, et al. FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 5.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van’t Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 6.Fox KAA. COURAGE or FAME…? Who should have percutaneous coronary intervention in stable coronary artery disease? Heart. 2013;99:442–444. doi: 10.1136/heartjnl-2012-303029. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Elrashidi MY, Flammer AJ, Lennon RJ, Bell MR, Holmes DR, Bresnahan JF, Rihal CS, Lerman LO, Lerman A. Long-term outcomes of fractional flow reserve-guided vs. angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013;34:1375–1383. doi: 10.1093/eurheartj/eht005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijjer SS, Sen S, Petraco R, Sachdeva R, Cuculi F, Escaned J, Broyd C, Foin N, Hadjiloizou N, Foale RA, Malik I, et al. Improvement in coronary haemodynamics after percutaneous coronary intervention: assessment using instantaneous wave-free ratio. Heart. 2013;99:1740–1748. doi: 10.1136/heartjnl-2013-304387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petraco R, van de Hoef TP, Nijjer S, Sen S, van Lavieren MA, Foale RA, Meuwissen M, Broyd C, Echavarria-Pinto M, Foin N, Malik IS, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve) Circ Cardiovasc Interv. 2014;7:492–502. doi: 10.1161/CIRCINTERVENTIONS.113.000926. [DOI] [PubMed] [Google Scholar]
- 10.De Bruyne B, Pijls NH, Barbato E, Bartunek J, Bech JW, Wijns W, Heyndrickx GR. Intracoronary and intravenous adenosine 5’-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation. 2003;107:1877–1883. doi: 10.1161/01.CIR.0000061950.24940.88. [DOI] [PubMed] [Google Scholar]
- 11.Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, Tarkin J, Petraco R, Broyd C, Jabbour R, Sethi A, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59:1392–1402. doi: 10.1016/j.jacc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Jeremias A, Maehara A, Généreux P, Asrress KN, Berry C, De Bruyne B, Davies JE, Escaned J, Fearon WF, Gould KL, Johnson NP, et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2014;63:1253–1261. doi: 10.1016/j.jacc.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 13.Sen S, Asrress KN, Nijjer S, Petraco R, Malik IS, Foale RA, Mikhail GW, Foin N, Broyd C, Hadjiloizou N, Sethi A, et al. Diagnostic classification of the instantaneous wave-free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (Classification Accuracy of Pressure-Only Ratios Against Indices Using Flow Study) J Am Coll Cardiol. 2013;61:1409–1420. doi: 10.1016/j.jacc.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Pijls NH, Kern MJ, Yock PG, De Bruyne B. Practice and potential pitfalls of coronary pressure measurement. Catheter Cardiovasc Interv. 2000;49:1–16. doi: 10.1002/(sici)1522-726x(200001)49:1<1::aid-ccd1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 16.Piek JJ, Boersma E, di Mario C, Schroeder E, Vrints C, Probst P, de Bruyne B, Hanet C, Fleck E, Haude M, Verna E, et al. Angiographical and Doppler flow-derived parameters for assessment of coronary lesion severity and its relation to the result of exercise electrocardiography. DEBATE study group. Doppler Endpoints Balloon Angioplasty Trial Europe. Eur Heart J. 2000;21:466–474. doi: 10.1053/euhj.1999.1871. [DOI] [PubMed] [Google Scholar]
- 17.Meuwissen M, Siebes M, Chamuleau SA, van Eck-Smit BL, Koch KT, de Winter RJ, Tijssen JG, Spaan JA, Piek JJ. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation. 2002;106:441–446. doi: 10.1161/01.cir.0000023041.26199.29. [DOI] [PubMed] [Google Scholar]
- 18.Van de Hoef TP, Nolte F, Damman P, Delewi R, Bax M, Chamuleau SAJ, Voskuil M, Siebes M, Tijssen JGP, Spaan JAE, Piek JJ, et al. Diagnostic accuracy of combined intracoronary pressure and flow velocity information during baseline conditions: adenosine-free assessment of functional coronary lesion severity. Circ Cardiovasc Interv. 2012;5:508–514. doi: 10.1161/CIRCINTERVENTIONS.111.965707. [DOI] [PubMed] [Google Scholar]
- 19.Bech GJ, De Bruyne B, Akasaka T, Liïstro F, Bonnier HJ, Koolen JJ, Pijls NH. Coronary pressure and FFR predict long-term outcome after PTCA. Int J Cardiovasc Intervent. 2001;4:67–76. doi: 10.1080/146288401753258303. [DOI] [PubMed] [Google Scholar]
- 20.Verhoeff BJ, Siebes M, Meuwissen M, Atasever B, Voskuil M, de Winter RJ, Koch KT, Tijssen JG, Spaan JA, Piek JJ. Influence of percutaneous coronary intervention on coronary microvascular resistance index. Circulation. 2005;111:76–82. doi: 10.1161/01.CIR.0000151610.98409.2F. [DOI] [PubMed] [Google Scholar]
- 21.Piek JJ, Boersma E, Voskuil M, di Mario C, Schroeder E, Vrints C, Probst P, de Bruyne B, Hanet C, Fleck E, Haude M, et al. DEBATE Study Group. The immediate and long-term effect of optimal balloon angioplasty on the absolute coronary blood flow velocity reserve. A subanalysis of the DEBATE study. Doppler Endpoints Balloon Angioplasty Trial Europe. Eur Heart J. 2001;22:1725–1732. doi: 10.1053/euhj.2000.2587. [DOI] [PubMed] [Google Scholar]
- 22.Gould KL. Pressure-flow characteristics of coronary stenoses in unsedated dogs at rest and during coronary vasodilation. Circ Res. 1978;43:242–253. doi: 10.1161/01.res.43.2.242. [DOI] [PubMed] [Google Scholar]
- 23.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782–1788. doi: 10.1056/NEJM199406233302503. [DOI] [PubMed] [Google Scholar]
- 24.Van de Hoef TP, Nolte F, Rolandi MC, Piek JJ, van den Wijngaard JPHM, Spaan JAE, Siebes M. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol. 2012;52:786–793. doi: 10.1016/j.yjmcc.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 25.van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SAJ, Voskuil M, Henriques JPS, Koch KT, de Winter RJ, Spaan JAE, et al. Physiological Basis and Long-Term Clinical Outcome of Discordance Between Fractional Flow Reserve and Coronary Flow Velocity Reserve in Coronary Stenoses of Intermediate Severity. Circ Cardiovasc Interv. 2014;7:301–311. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 26.Van de Hoef TP, Meuwissen M, Escaned J, Davies JE, Siebes M, Spaan JAE, Piek JJ. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat Rev Cardiol. 2013;10:439–452. doi: 10.1038/nrcardio.2013.86. [DOI] [PubMed] [Google Scholar]
- 27.Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino L, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–1654. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]
- 28.Chamuleau SA, Siebes M, Meuwissen M, Koch KT, Spaan JA, Piek JJ. Association between coronary lesion severity and distal microvascular resistance in patients with coronary artery disease. Am J Physiol Heart Circ Physiol. 2003;285:H2194–H2200. doi: 10.1152/ajpheart.01021.2002. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RF, Johnson MR, Marcus ML, Aylward PE, Skorton DJ, Collins S, White CW. The effect of coronary angioplasty on coronary flow reserve. Circulation. 1988;77:873–885. doi: 10.1161/01.cir.77.4.873. [DOI] [PubMed] [Google Scholar]
- 30.Serruys PW, di Mario C, Piek J, Schroeder E, Vrints C, Probst P, de Bruyne B, Hanet C, Fleck E, Haude M, Verna E. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe) Circulation. 1997;96:3369–3377. doi: 10.1161/01.cir.96.10.3369. [DOI] [PubMed] [Google Scholar]
- 31.Fearon WF. Invasive coronary physiology for assessing intermediate lesions. Circ Cardiovasc Interv. 2015;8:e001942. doi: 10.1161/CIRCINTERVENTIONS.114.001942. [DOI] [PubMed] [Google Scholar]
- 32.Nijjer SS, Sen S, Petraco R, Escaned J, Echavarria-Pinto M, Broyd C, Al-Lamee R, Foin N, Foale RA, Malik IS, Mikhail GW, et al. Pre-angioplasty instantaneous wave-free ratio pullback provides virtual intervention and predicts hemodynamic outcome for serial lesions and diffuse coronary artery disease. JACC Cardiovasc Interv. 2014;7:1386–1396. doi: 10.1016/j.jcin.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 33.De Luca G, Venegoni L, Iorio S, Giuliani L, Marino P. Effects of increasing doses of intracoronary adenosine on the assessment of fractional flow reserve. JACC Cardiovasc Interv. 2011;4:1079–1084. doi: 10.1016/j.jcin.2011.08.004. [DOI] [PubMed] [Google Scholar]