Summary
Endothelial cells form an extensive network of blood vessels that has numerous essential functions in the vertebrate body. In addition to their well-established role as a versatile transport network, blood vessels can induce organ formation or direct growth and differentiation processes by providing signals in a paracrine (angiocrine) fashion. Tissue repair also requires the local restoration of vasculature. Endothelial cells are emerging as important signaling centers that coordinate regeneration and help to prevent deregulated, disease-promoting processes. Vascular cells are also part of stem cell niches and play key roles in hematopoiesis, bone formation and neurogenesis. Here, we will review these newly identified roles of endothelial cells in the regulation of organ morphogenesis, maintenance and regeneration.
Keywords: Endothelial cells, angiogenesis, lung, liver, bone marrow, organ morphogenesis, angiocrine signaling, vascular niche
Blood vessels – more than a transport network
Blood vessel formation occurs throughout reproductive life in females as well as during tissue repair or in certain disease conditions, but expansion of the vascular network is absolutely essential during development. Most of this vascular growth is mediated by angiogenesis, which involves processes such as endothelial cell (EC) proliferation and sprouting [1, 2]. Subsequent remodeling and blood vessel maturation generate a stable, hierarchically organized and efficient vascular network devoid of unnecessary connections [1, 3, 4]. The resulting vasculature, which consists of arteries, veins and interconnecting capillary beds, is optimized to fulfill its conventional and indispensable function in the living organism, namely the transport of gases, nutrients, metabolites, waste products, hormones, and cells. However, there is now increasing evidence that ECs are not only building blocks of the vascular transport network but also actively contribute to growth, differentiation, patterning, or repair processes in the surrounding tissue. The paracrine (also termed ‘angiocrine’) release of signaling molecules by vascular cells, which act on other cell types in the vicinity of blood vessels, has recently emerged as a fundamental mechanism in many different organ systems. This review will primarily focus on the nervous system, liver, lung, and bone to outline general as well as organ-specific principles for the regulation of tissue morphogenesis, homeostasis and regeneration by ECs.
Endothelial cells as mediators of organogenesis
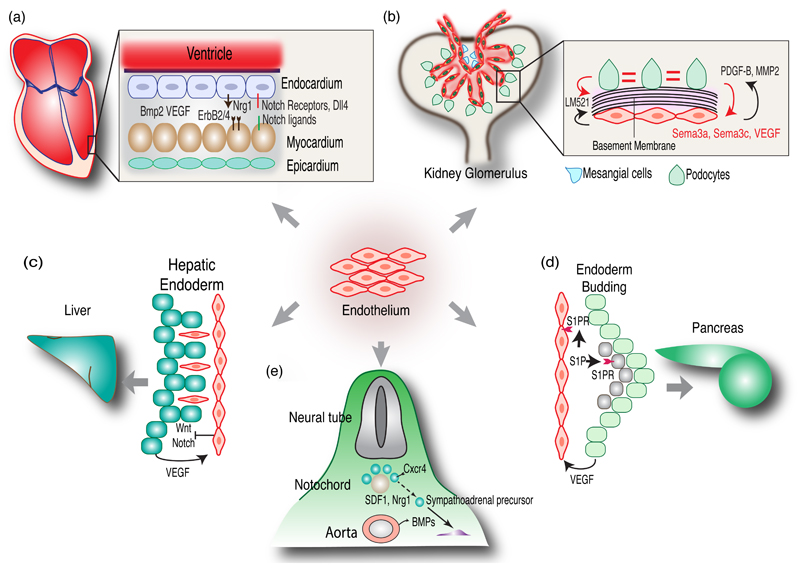
While angiogenesis is stimulated by tissue-derived signals, ECs, in return, release growth factors or even directly contribute to organ morphogenesis. For example, hemogenic endothelium in the embryonic dorsal aorta generates hematopoietic stem cells (HSCs) and thereby the adult hematopoietic system [5, 6]. The early vertebrate heart is a tube consisting of an inner endothelial layer that is separated from the outer myocardium by a gelatinous matrix termed cardiac jelly. ECs form the cardiac vasculature and endocardium, while the myocardium generates the muscular component of the heart. However, a subpopulation of cardiac progenitor cells expressing Flk1/KDR/VEGFR2, the receptor for vascular endothelial growth factor A (VEGF-A), delaminates from the endocardium and enters the cardiac jelly. In a process termed endothelial-mesenchymal transformation (EndMT), endocardial cells transdifferentiate and incorporate into the atrioventricular cushions [7–9]. Notch signaling in the endocardium controls the patterning of heart chambers and valves by limiting the myocardial expression of bone morphogenetic protein 2 (Bmp2), which is a crucial signal for endocardial EndMT but also induces the expression of target genes in the myocardium (Figure 1a) [10]. Endocardial cells release the growth factor neuregulin-1, which acts in a paracrine fashion on myocardial cells expressing the receptors ErbB2 and ErbB4 [11, 12] leading to the trabeculation of the heart ventricle [13]. Conversely, myocardial to endocardial VEGF-A signaling triggers coronary artery formation by VEGFR2-expressing endocardial cells (Figure 1a) [14].
Figure 1. Role of endothelial cells during organogenesis.
(a) Endothelial cells contribute to the coronary vasculature and endocardium during early heart development. Endocardial cells release Nrg1, which signals to myocardial cells expressing the receptors ErbB2 and ErbB4. Myocardial cells release VEGF acting on ECs. Notch ligands expressed in myocardium and endocardium signal through endocardial Notch receptors. Bmp2 released from the myocardium acts on the endocardium during heart valve formation.
(b) ECs play crucial role in the development of kidney glomeruli. Podocytes recruit ECs by expressing VEGF and, conversely, ECs secrete MMP2 and PDGF-B. Sema 3a and Sema 3c released by podocytes regulate kidney vascular morphogenesis. Both ECs and Podocytes synthesize glomerular basement membrane components such as Laminin-α5β2γ1 (LM521).
(c) During liver bud formation, hepatic endoderm migrates into the septum transversum mesenchyme along with ECs. Endoderm cells stimulate angiogenesis by releasing VEGF. ECs control Wnt and Notch signaling in the developing hepatic endoderm.
(d) ECs in close proximity to endoderm promote pancreatic bud formation. Sphingosine-1-phosphate (S1P) derived from vascular cells or the circulation promotes budding of pancreatic endoderm (green cells). S1P-receptors (S1PR) are expressed by endothelial cells (orange) and mesenchymal cells (grey).
(e) Dorsal aorta-derived BMPs induce mesenchymal SDF1 and neuregulin-1 expression, which attracts SA progenitors. BMP signaling also controls the segregation of progenitors forming the adrenal medulla and sympathetic ganglions, which involves neuregulin-ErbB signaling.
ECs also provide instructive signals during kidney development. In glomeruli, the units of blood ultrafiltration, capillaries are tightly enwrapped by highly branched podocytes and pericyte-like mesangial cells leaving only small filtration slits. Glomerular architecture is compromised in cloche mutant zebrafish [15], which lacks nearly all ECs and hematopoietic cells. Inactivation of the gene for VEGF-A or inhibition of VEGF in neonatal mice also led to renal defects [16]. While podocytes mediate EC recruitment into glomeruli via VEGF [17], ECs regulate podocyte and mesangial cell maturation in the glomerulus by local expression of matrix metalloproteinase-2 [18] and platelet-derived growth B (PDGF-B) (Figure 1b) [19]. Semaphorins, molecular guidance cues that control wiring in the nervous system and vascular patterning, are also involved in glomerular development. Sema3a, which is strongly expressed by podocytes in the adult kidney, inhibits EC migration and survival during early renal development. In contrast, Sema3c is a positive regulator of EC network formation and promotes branching of the ureteric bud epithelium [20]. The glomerular basement membrane, which is essential for renal function, is formed by extracellular matrix (ECM) proteins synthesized by ECs and podocytes. Laminin-521, a trimer of the α5, β2 and γ1 laminin subunits, is produced by both cell types and promotes glomerulogenesis (Figure 1b) [21].
Early liver development is another example for the importance of EC-derived signals. During liver bud formation and before the onset of blood circulation, ECs interact with hepatic endodermal cells that migrate into the mesenchyme of the septum transversum, a tissue that gives rise to the thoracic diaphragm and the ventral mesentery of the foregut. Flk1-/- mutant embryos showed normal formation of the hepatic endoderm and septum transversum, but lacked liver budding [22]. In contrast, cloche zebrafish mutants lacking ECs showed normal liver budding [23], which may be attributable to species-specific differences in organogenesis. Later in liver development, ECs are involved in the differentiation of the biliary duct epithelium by activating Notch signaling in hepatoblasts [24]. ECs have also been shown to act as niche cells that suppress Wnt and Notch signaling in endoderm and thereby promote hepatic specification in the early embryo (Figure 1c) [25]. Conversely, deletion of Hnf4, a nuclear receptor critical for liver development, from fetal hepatoblasts resulted in the loss of ECs and disrupted hepatocellular polarity [26].
During early pancreas development, dorsal and ventral buds develop from gut endoderm in close contact with the endothelium of the dorsal aorta and vitelline veins [27]. In Flk1-/- embryos, dorsal but not ventral pancreatic bud formation was severely impaired [28]. Thus, ECs play a differential role in dorsal versus ventral pancreatic bud development before their fusion into a single organ. Dorsal aorta-derived signals were also essential for the induction of Pdx1, a transcription factor that is indispensable for pancreas development, and insulin expression in embryonic endoderm ex vivo. Hypervascularization of the pancreas, induced through overexpression of VEGF, resulted in islet hyperplasia [27]. Furthermore, pancreatic islet progenitors express higher level of VEGF-A during early development, which enhances their vascularization compared to non-endocrine pancreas. Accordingly, inactivation of the gene encoding VEGF-A impaired pancreatic and islet vascularization, reduced β-cell proliferation and compromised glucose clearance. In contrast, similar experiments in adult mice led to reduced islet vascularization without affecting pancreas architecture or function [29]. During chick development, pancreatic endoderm has been shown to secrete the chemokine SDF1/Cxcl12 to attract CXCR4-expressing angioblasts, which, in turn, induce Pdx1 expression in endodermal cells [30]. Studies in zebrafish and chick support a mechanism involving retinoic acid-mediated, paracrine induction of Pdx1 in endodermal cells by angioblasts [31, 32]. N-cadherin-deficient (Cdh2-/-) mouse embryos lack a functional circulatory system and the dorsal pancreas, but pancreatic development was rescued after restoration of blood circulation by heart-specific re-expression of N-cadherin [33]. Indicating an important role of soluble factors present in plasma, pancreatic bud formation in Cdh2-/- embryos was also partially restored by administration of sphingosine-1-phosphate (S1P). Thus, blood vessel-derived S1P controls the proliferation or survival of dorsal pancreatic mesenchymal cells, which express the corresponding S1P-receptors (Figure 1d) [33]. Remarkably, ECs might also restrict endoderm expansion and thereby organ development. An excessive number of ECs and compromised growth of foregut-derived organs such as stomach, liver and pancreas were observed in mouse embryos lacking sphingosine-1-phosphate receptor 1 (S1P1). Conversely, pharmacological ablation of embryonic ECs led to expansion of the pancreatic epithelium [34].
The embryonic dorsal aorta also acts as a morphogenetic center for neural crest cell-derived neuroprogenitors forming the autonomic nervous system. The common progenitors (SA progenitors) of sympathetic ganglions and the adrenal medulla, which is part of the adrenal gland, first migrate ventrally towards the aorta before segregating into distinct migratory routes and lineages. This is controlled by bone morphogenetic proteins (BMPs) at two stages (Figure 1e). Initially, BMP4 and BMP7 produced by the dorsal aorta are required for the expression of SDF1 and neuregulin-1 in the paraaortic mesenchyme, which act as chemoattractants for SA progenitors [35]. Subsequently, BMP signaling activity persists in the progenitors forming the adrenal medulla, but not those giving rise to sympathetic ganglions, and controls the segregation of these cell populations, which involves signaling through neuregulin-1 and ErbB receptors [35].
Lung morphogenesis and regeneration
The pulmonary vasculature runs in parallel to airways and surrounds the intricate alveolar structure lined by respiratory epithelial cells. The close association of vascular and epithelial cells is essential for efficient gas exchange and respiration. Several studies have highlighted the importance of vasculature in lung morphogenesis [36, 37], in which vasculature and epithelium develop in an interdependent fashion to generate alveolar structures. Branching morphogenesis in the developing lung is highly stereotyped and involves a surprisingly small number of geometrically simple modes of epithelial branch formation [38]. Blood vessels play important but perfusion-independent roles in this process. While ablation of the lung vasculature both in vivo and ex vivo in lung explants did not perturb the rate of epithelial branching, branching stereotypy was altered due to preferential loss of a specific branching mode requiring rotation of growing epithelial buds. This defect led to altered lung morphology and ectopic branch formation at high frequency [39]. At the molecular level, spatial expression of branching regulators such as fibroblast growth factor 10, Sonic hedgehog and Sprouty2 was altered. While the molecular cues provided by the pulmonary vasculature are unknown, stereotypy of epithelial branching morphogenesis is not controlled by perfusion, blood flow or circulating factors [39].
Given the involvement of vessels in airway branching, it is not surprising that VEGF signaling is critical for lung development. Early disruption of the VEGF pathway causes strong structural abnormalities in lung [40, 41]. Analysis of VEGF mRNA expression showed the highest levels in animal and human lung samples and, in particular, in the alveolar epithelium [42, 43]. In addition to a protective function of VEGF for the pulmonary endothelium and the positive regulation of EC proliferation during lung growth and regeneration, the growth factor also has pneumotrophic activity that facilitates epithelial cell growth after lung injury in an autocrine fashion. VEGF treatment increased survival, promoted lung angiogenesis and was able to prevent alveolar damage in hyperoxia-induced lung injury [44]. In neonatal mice, reduced VEGF expression in alveolar cells or loss of matrix-binding VEGF isoforms led to fatal respiratory distress, impaired lung maturation and insufficient production of surfactant, a surface-active lipoprotein complex preventing alveolar collapse [45].
Pulmonary vasculature is also a crucial player during lung post-injury regeneration or in disease. For example, endothelial proliferation occurs in lung regeneration after H1N1 influenza infection and the function of distal airway stem cells, alveolar regeneration and restoration of alveolar capillaries are linked [46]. Coupling of alveolar morphogenesis to pulmonary vasculature was also observed during compensatory lung growth following unilateral lung lobe removal [47]. Shortly after pneumonectomy, bursts of proliferation and expansion of the progenitors of bronchiolar and alveolar epithelia occurred, which was associated with pulmonary EC proliferation [47]. VEGF and FGF signaling induced endothelial expression of matrix metalloproteinase 14 (MMP14), which led to the release of active EGF-like fragments from heparinbinding EGF-like growth factor (HB-EGF) and the laminin5 γ2 subunit (Figure 2). This led to the activation of EGF receptor in alveolar epithelial cells and bronchioalveolar stem cells (BASCs), expansion of BASCs and proliferation of alveolar epithelium. Systemic administration of EGF enabled compensatory alveologenesis and restored lung function in absence of endothelial VEGF signaling and MMP14 expression [47].
Figure 2. Endothelial cells in lung regeneration.
Compensatory lung growth following unilateral pneumonectomy occurs through VEGF and FGF signaling involving the receptors VEGFR2 and FGFR1 on pulmonary ECs. These signaling cascades induce expression of MMP14, which then generates EGF-like ligands by processing of heparin binding EGF-like growth factor (HB-EGF) and the laminin5 γ2 subunit. This leads to the activation of EGF receptor (EGFR) on alveolar epithelial cells and bronchioalveolar stem cells (BASCs), which drives expansion of BASCs and generation of epithelial cells. ECs also regulate BASC differentiation choices during regeneration. Post-injury release of BMP4 triggers NFAT-dependent TSP1 expression in ECs, which promotes alveolar differentiation and repair. Bottom: Release of pro-angiogenic signals after injury or infection in lung promotes endothelial cell expansion, which in turn causes enhanced release of angiocrine factors to promote regeneration.
The examples above show that repair of the pulmonary epithelium depends on stem and progenitor cell populations that are presumably located in specific niche microenvironments. ECs also regulate BASC differentiation during post-injury regeneration [48]. Analysis of colonies grown from single BASC clones in 3D culture revealed that ECs supported BASC differentiation into multiple epithelial lineages in vitro and after subcutaneous injection. This was linked to the expression of the ECM protein thrombospondin-1 (TSP1) by pulmonary endothelial cells and, accordingly, Tsp1-/- ECs were unable to support alveolar differentiation [48]. Analysis of the upstream signaling crosstalk showed that injury-induced BMP4 production triggered calcineurin/NFATc1-dependent TSP1 expression in ECs, which then promoted alveolar differentiation and repair (Figure 2) [48]. These findings highlight the importance of endothelial-epithelial crosstalk in lung stem cell differentiation during pulmonary regeneration.
Hepatic endothelium in liver regeneration and fibrosis
Liver sinusoidal endothelial cells (LSECs) are highly specialized to contribute to a variety of physiological processes. Liver regeneration requires the proliferation of LSECs and hepatocytes, which involves a substantial amount of crosstalk between these cell types. LSECs provide instructive signals both during the early, inductive phase of regeneration, which is characterized by strong hepatocyte proliferation, and the subsequent angiogenic phase, which generates blood vessels required for liver reconstitution. Hepatocyte growth factor (HGF) released by ECs triggers the proliferation of hepatocytes in the vicinity by activating NF-κB [49, 50]. Hepatocytes, in return, release VEGF that activates the cognate receptors in LSECs and thereby promotes angiogenesis [49, 51]. Expression of angiopoietin-2 (Ang2), a secreted ligand for the Tie2 receptor with important roles in vascular integrity and growth, is dynamically regulated after partial hepatectomy in mice (Figure 3). In the early inductive phase, down-regulated expression of Ang2 is accompanied by reduced LSEC expression of transforming growth factor β1 (TGFβ1), a known inhibitor of hepatocyte proliferation [52]. Later in the regenerative process, the recovery of Ang2 expression initiates the proliferative angiogenic phase by upregulating endothelial VEGFR2 receptor [58]. Moreover, VEGFR2 and, downstream of this receptor, the transcription factor Id1 in LSECs are also required for the initial burst of hepatocyte proliferation after partial hepatectomy (Figure 3) [59]. Reconstitution of the hepatovascular mass was reduced after inducible inactivation of the gene encoding VEGFR2 in LSECs or in Id1-/- mice. This was linked to diminished expression of LSEC-derived angiocrine factors such HGF and Wnt2, which promote hepatocyte proliferation (Figure 3). In addition, VEGFR2-Id1-dependent proliferative angiogenesis contributes to the biphasic liver reconstitution [53].
Figure 3. Liver endothelium in regeneration and fibrosis.
Liver sinusoidal ECs play critical roles after liver injury in mediating regeneration and fibrosis. LSECs release hepatocyte growth factor and, in turn, hepatocytes release VEGF (top). During the inductive phase, hepatocyte proliferation is enabled by decreased endothelial expression of Ang2 leading to reduced TGFβ1 secretion. Vegfr2-Id1 mediated release of Wnt2 and HGF by LSECs also promotes growth of hepatocytes. Recovery in Ang2 levels upregulates VEGFR2 and thereby promotes vessel growth in the angiogenic phase. LSEC release SDF1 (Cxcl12) to attract hepatic stellate cells, which also release SDF1 to attract immune cells. Hepatic Stellate Cells also express high levels of HGF after partial hepatectomy to overcome the inhibitoy effect of TGFβ1. While CXCR7 in LSECs promotes angiocrine signaling and regeneration, CXCR4 generates a pro-fibrotic niche. Bottom: Liver regeneration involves inductive and angiogenic phases that are tightly controlled by LSECs. Angiocrine factors make the decision between fibrosis and regeneration after injury.
Despite the exceptional regenerative potential of liver, chronic insults result in fibrosis leading to cirrhosis and, ultimately, liver failure. The chemokine SDF1/Cxcl12 and its two G-protein coupled receptors, CXCR4 and CXCR7, play a key role in the decision between fibrosis and regeneration [60]. After acute injury, CXCR7 upregulation in LSECs promoted endothelial Id1 expression and regeneration by enabling release of pro-regenerative angiocrine factors. Accordingly, EC-specific deletion of CXCR7 reduced Id1-mediated regenerative signaling. The CXCR7-dependent pro-regenerative response was counterbalanced by constitutive FGFR1 signalling and upregulated CXCR4 expression in LSECs, which led to liver fibrosis. EC-specific, genetic inactivation of FGFR1 or CXCR4 restored the regenerative potential of liver [54]. Thus, the balance between regeneration and fibrosis is set by the ratio of pro-regenerative, angiocrine and pro-fibrotic pathways in the vascular niche (Figure 3).
Despite these exciting findings, processes in the regenerating liver remain incompletely understood and involve crosstalk between many cell types. LSECs are located in direct proximity to hepatic stellate cells, which are located in the perisinusoidal space between hepatocytes and the endothelium. Upon injury, hepatic stellate cells get activated and secrete factors and ECM to modulate the functions of LSECs and hepatocytes, which is considered to be a major cause of fibrosis [55]. LSECs release SDF1/Cxcl12 to attract hepatic stellate cells to the endothelium [56]. Hepatic stellate cells also release Cxcl12 to attract immune cells upon injury and express high levels of HGF after partial hepatectomy to overcome the antiproliferative effect of TGFβ1 (Figure 3) [57]. In the terminal stages of liver regeneration, hepatic stellate cells participate in the termination of hepatocyte proliferation, which is thought to involve higher expression of TGFβ1 [57].
Coupling of angiogenesis and osteogenesis in the skeletal system
Structural support for the vertebrate body is provided by the skeletal system, which remains surprisingly dynamic after the completion of development and is renewed throughout adult life. This maintenance involves the continuous production of mature bone cells (osteocytes) and their osteoblast progenitors. The osteogenic formation of new bone occurs in balance with osteoclastic degradation, and both processes are crucial for homeostasis and repair of the skeleton [58]. In humans and most other vertebrate species, bone also harbors the hematopoietic system, which provides a lifelong supply of blood cells that are derived from self-renewing HSCs [59]. Bone is a highly vascularized tissue and ECs play essential roles in osteogenesis and hematopoiesis (Figure 4).
Figure 4. Functional roles of the bone vasculature.
Angiogenesis in bone is promoted by VEGF secretion from chondrocytes and osteoblasts. Two novel vessel subtypes, termed type H and type L, have been identified in the skeletal system. Type H vessels require Notch and HIF signaling for their maintenance and secrete osteogenic factors and Noggin acting on osteoprogenitor cells and chondrocytes, which are important sources of VEGF leading to coupling of angiogenesis and osteogenesis. Angiocrine factors (such as SDF1/Cxcl12 and SCF) released by endothelium and pericytes have been also shown to support HSCs, which express the cognate receptors CXCR4 and c-Kit, respectively. Although ECs, perivascular, neural, and mesenchymal cells are involved in HSC maintenance, the exact composition and localization of stem cell niches as well as the roles of vessel subtypes require further investigation.
During development of the skeletal system, the formation of rudimental bone in the embryo requires the vascularization of mesenchymal condensations (intramembranous ossification) or cartilaginous elements (endochondral ossification). Vascular invasion coincides with the appearance of bone forming osteoblasts, bone resorbing osteoclasts and hematopoietic cells [60]. Chondrocytes and osteoblast lineage cells are important sources of ECM molecules, which are likely to affect EC behavior, but chondrocytes also show high expression of VEGF and thereby provide a crucial pro-angiogenic signal [61–63]. The hypoxia-inducible factor (HIF) pathway, an important positive transcriptional regulator of VEGF expression, has been also shown to regulate angiogenesis, osteogenesis and chondrogenesis [64, 65].
Blood vessels in the skeletal system are very heterogeneous and functionally specialized. In addition to feeding arteries and draining veins, bone marrow (BM) capillaries have been described as a highly branched, irregular and discontinuous sinusoidal network that is surrounded by hematopoietic cells [66, 67]. Recently, bone capillaries have been further divided into two specialized vessel subtypes, termed type H and type L (Figure 4) [68]. Type H endothelium, which shows high expression of the markers CD31/Pecam1 and endomucin, is found at the vascular growth front in postnatal long bone and secretes factors that promote osteogenesis by acting both on chondrocytes and the osteoblast lineage [68, 69]. Type H ECs also show high proliferative activity and, as genetic lineage tracing experiments have shown, are hierarchically upstream of type L ECs. In contrast, type L ECs form the sinusoidal vessels of the BM cavity and are not associated with osteoblastic cells. Interestingly, while the total number of ECs is not significantly different in juvenile, adult and aged mice, the fraction of type H declines after adolescence [68], which might contribute to the well-known loss of osteogenic capacity and bone mass during ageing. Notch and HIF signaling have identified as pathways that promote the expansion of type H vessels (Figure 4) together with associated osteoprogenitor cells [68, 69].
It has long been proposed that osteogenesis and angiogenesis are coupled processes. Osteoblasts express VEGF that promotes angiogenesis and, conversely, ECs express pro-osteogenic factors. Defective angiogenesis and osteogenesis was reported for mutant mice lacking matrix-binding isoforms of VEGF [70]. Bone morphogenic proteins, important osteogenic factors, also promote angiogenesis through the expression of VEGF in osteoblasts [71]. Levels of BMP and their receptors differentially modulate bone formation [72] highlighting the importance of appropriate BMP activity. Genetic inactivation of Noggin, a secreted BMP antagonist, led to compromised skeletal patterning, cartilage hyperplasia, fused joints and deregulated ossification [73]. Indicating the importance of Noggin levels, both osteoblast-specific inactivation and overexpression of Noggin in the postnatal skeleton led to osteopenia, the loss of bone mass [74, 75]. Endothelial Noggin expression is strongly modulated by Notch signaling (Figure 4) [69]. EC-specific Notch loss-of-function mutants displayed reduced Noggin expression, which, presumably in an angiocrine fashion, compromised osteogenesis and chondrocyte maturation [69]. The latter impaired chondrocyte VEGF expression leading to strong angiogenesis defects, which were rescued by Noggin administration [69]. Thus, signaling interactions between endothelial and non-endothelial cell types couple vascular growth and osteogenesis in the skeletal system.
Vascular niche for hematopoiesis
Niche microenvironments involving mesenchymal, osteoblastic, vascular and neural cells have been proposed to control HSC self-renewal and function [76]. ECs and vessel-associated leptin receptor-expressing stromal cells have been shown to maintain HSCs through the release of stem cell factor (SCF/Kitl) and Cxcl12 (Figure 4). In contrast, inactivation of the gene for SCF in osteoprogenitors and other mesenchymal cells did not affect HSC maintenance suggesting that HSCs reside in a perivascular niche [77]. Earlier work had already shown that so-called Cxcl12 abundant reticular (CAR) cells, which are located near sinusoidal ECs and in the endosteum, maintain the HSC pool through Cxcl12-CXCR4 signaling [78]. The exact relationship between different perivascular cell populations associated with the BM vasculature and their precise functions require further investigation.
Other blood vessel-derived signals also contribute to the vascular HSC niche. The growth factor pleiotrophin (PTN) is another secreted component required for the retention and self-renewal of HSCs in the vascular niche. Accordingly, HSCs were significantly decreased and hematopoietic regeneration impaired in Ptn-/- mice [79]. The cell adhesion molecule E-selectin is expressed exclusively in ECs and controls HSC maintenance. Genetic inactivation or pharmacological inhibition of glycosphingolipid synthesis, which prevents the production of E-selectin–binding ligands, enhanced HSC quiescence and self-renewal potential [80]. Thus, E-selectin function in the vascular niche promotes HSC proliferation. In contrast, EC-specific deletion of the Notch ligand Jagged1 led to decreased hematopoiesis and exhaustion of the HSC pool [81]. Recently, it has been reported that arteriolar niches and, in particular, perivascular cells expressing the chondroitin sulfate proteoglycan NG2 control HSC quiescence. Depletion of NG2+ cells increased cycling and reduced the long-term repopulating capacity of HSCs [82]. Thus, instructive, vessel-derived signals regulate the maintenance of BM HSCs.
The vascular niche for neurogenesis in the adult nervous system
Neurogenesis in the mammalian brain is mainly confined to development, but neuron production persists in the adult subventricular zone (SVZ) and in the hippocampus, which contain neural stem cells (NSCs). NSC self-renewal and function require a local niche microenvironment involving ECs. Dividing neuroprogenitors in the subgranule zone of the hippocampus were found in close association with vessels suggesting an angiogenic niche for neurogenesis [83]. Blood vessels of the SVZ neural stem cell niche were reported to be straight (non-tortuous) and form a specialized planar plexus. Low rates of blood flow in this plexus have been associated with hypoxic conditions in the ependymal layer [84]. While ECs in the central nervous system are generally tightly associated with pericytes and astrocyte endfeet, which together form the blood-brain-barrier that prevents the entry of immune cells and potentially harmful, blood-borne substances [85, 86], the vascular plexus in the adult SVZ lacks astrocyte and pericyte coverage. Instead, dividing NSCs and transit-amplifying progeny make direct contact with the SVZ endothelium [87, 88]. Arguing further for a key role of the endothelium in the neurogenic niche, ECs but not vascular smooth muscle cells were found to release soluble factors that stimulate NSC self-renewal and enhance neuron production in co-culture experiments [89].
Numerous studies have focused on the nature of the molecular crosstalk between ECs and NSCs in the neurogenic niche. Arguing for reciprocal interactions, it was shown that neural stem/progenitor cells constitutively express HIF-1α and VEGF, which was enhanced in vitro by conditions mimicking ischemia [90]. Intracerebrally transplanted neural stem/progenitor cells provided vasculotrophic support and promoted higher microvessel densities in a mouse model of mild focal ischemia [90]. In addition to VEGF, brain-derived neurotrophic factor (BDNF) is involved in the crosstalk between NSCs and ECs in vitro. Both growth factors were shown to trigger eNOS activation and generation of nitric oxide, a known stimulator of angiogenesis [91]. VEGF treatment of cultured ECs enhanced expression of multiple cytokines and induced human NSC migration [92]. The biological function of VEGF is not confined to angiogenesis and controls the development of newly born olfactory bulb interneurons and neuronal plasticity in the adult hippocampus [93, 94]. Another VEGF family member, VEGF-C, is a regulator of neurogenesis but not angiogenesis in the adult brain. Overexpression of VEGF-C stimulated NSCs, which express the receptor VEGFR3, and thereby promoted SVZ neurogenesis without affecting the local vasculature. Conversely, inactivation of the Vegfr3 gene in neural cells or administration of VEGFR3 blocking antibodies reduced neurogenesis [95].
Pigment epithelium-derived factor (PEDF) has been identified as an EC-derived signal promoting self-renewal of adult NSCs in vitro (Figure 5) [96]. Intraventricular infusion of PEDF led to the activation of slowly dividing stem cells, whereas blockade of endogenous PEDF had the opposite effect [96]. More recently, PEDF function was linked to enhancement of Notch-dependent transcription in astroglia-like NSCs, which changed the output of asymmetric divisions to the production of two self-renewing cells [97]. While most studies have focused on the role of secreted signals, endothelial expression of the transmembrane proteins ephrin-B2 and Jagged-1 in the adult SVZ was found to suppress NSC proliferation and induce the expression of stemness genes (Figure 5). Accordingly, EC-specific inactivation of the Efnb2 and Jag1 genes led to aberrant activation and depletion of quiescent stem cells [98].
Figure 5. Vascular niche for neurogenesis.
Neural stem/progenitor cells in the adult reside in a specialized vascular niche and constitutively express VEGF to promote vasoprotection and angiogenesis. Endothelial expression of growth factors such as BDNF, VEGF and PEDF has neuroprotective function and promotes NSC self-renewal. ECs also regulate stem cell quiescence and promote stemness through membrane-anchored (ephrin-B2, Jagged1) or secreted (SDF1) signals.
The interactions between ECs and NSCs might be of particular importance after local brain damage. Focal demyelination in the adult corpus callosum led to increased blood vessel number and ramification. SVZ-derived neuroprogenitors were physically associated with vessels, and progenitor cell recruitment to lesions was significantly reduced by VEGF inhibition [99]. SVZ expression of netrin 1, a protein involved in axon guidance, was upregulated after demyelination injury. Blocking antibody experiments indicate that netrin-1 controls local angiogenesis and progenitor cell migration [99]. Transplanted proliferative SVZ progenitor cells were found to home to vessels, which is mediated by SDF1-CXCR4 signaling. SDF1 has distinct effects on different progenitor cell populations. While exposure to high SDF1 levels promoted stem cell quiescence, the chemokine also facilitated the exit from the adult SVZ vascular niche and increased neuroblast migration toward the olfactory bulb [87]. Indicating that EC-derived factors can have damaging effects, endothelial production of TGFβ enhances neural stem/progenitor cell apoptosis in ageing and irradiated mice. Accordingly, neurogenesis was improved by TGFβ inhibitors [100].
Concluding remarks
The examples provided in this review highlight the important roles of ECs in tissue development, patterning, homeostasis, and regeneration. The endothelium often takes a central position in these processes and there are many reasons why ECs are ideally positioned as the source of important instructive, angiocrine signals. The vascular transport network extends into every organ system and needs to be embedded in those tissues in a certain spacing or pattern, which places ECs in central and therefore strategic positions for the regulation of morphogenesis and organ homeostasis. As ECs and other cell types frequently form functional units, such as kidney glomeruli, liver lobules or lung alveoli, the assembly, differentiation and function of the different cellular components needs to be tightly coordinated. Because circulating blood cells extensively rely on the vascular conduit system and frequently interact with the endothelium, it is perhaps not surprising that ECs contribute to niche microenvironments. During tissue repair, proliferative cell expansion processes are sometimes temporally separated from cell differentiation and tissue patterning events. The latter has to involve the restoration of a fully functional vascular network so that ECs appear ideally suited as the source of molecular signals that can trigger or suppress processes in the surrounding tissue. Many of the interactions of ECs with other cell populations are not unidirectional but part of a complex, interdependent network of signaling processes. During tissue growth and repair, positive or negative feedback loops in the communication between different cell populations provide robustness and presumably help to prevent dysfunctional overgrowth or misguided differentiation. This also raises the important question whether treatments aiming at ECs and their angiocrine activity might be utilized for therapeutic purposes in the future. Many results in animal models suggest that this might be the case, but, of course, further validation and studies with human cells or tissue samples are required. While it is obvious that ECs are functionally specialized in different organ systems [89], the molecular basis of organ-specific differentiation and the extent of EC heterogeneity remain largely unknown. It is, for example, feasible that ECs are programmed by transcription factors or other signals in a hardwired, predetermined fashion that is distinct for different organs. Alternatively, specific endothelial phenotypes and angiocrine signatures might result from plasticity in response to tissue-derived signals that are specific for certain local microenvironments. These and other important questions need to be resolved to utilize the full potential of ECs in tissue repair and regeneration, which would undoubtedly facilitate new, exciting opportunities in tissue engineering and medicine.
Highlights.
Endothelial cells lining blood vessels induce organ formation and other morphogenetic processes in the embryo
Blood vessels are also an important source of paracrine (angiocrine) signals acting on other cell types in organ regeneration
Vascular niches and endothelial cell-derived signals generate microenvironments for stem and progenitor cells
Acknowledgments
The authors thank the Max Planck Society, the University of Münster, the DFG cluster of excellence ‘Cells in Motion’, and the European Research Council (AdG 339409 AngioBone) for funding and support.
References
- 1.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 3.Ehling M, et al. Notch controls retinal blood vessel maturation and quiescence. Development. 2013;140:3051–3061. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- 4.Korn C, et al. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development. 2014;141:1757–1766. doi: 10.1242/dev.104422. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medvinsky A, et al. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 7.Kattman SJ, et al. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Lin CJ, et al. Partitioning the heart: mechanisms of cardiac septation and valve development. Development. 2012;139:3277–3299. doi: 10.1242/dev.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misfeldt AM, et al. Endocardial cells are a distinct endothelial lineage derived from Flk1+ multipotent cardiovascular progenitors. Dev Biol. 2009;333:78–89. doi: 10.1016/j.ydbio.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 10.de la Pompa JL, Epstein JA. Coordinating tissue interactions: Notch signaling in cardiac development and disease. Dev Cell. 2012;22:244–254. doi: 10.1016/j.devcel.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemmens K, et al. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–960. doi: 10.1161/CIRCULATIONAHA.107.690487. [DOI] [PubMed] [Google Scholar]
- 12.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 13.Crivellato E, et al. Contribution of endothelial cells to organogenesis: a modern reappraisal of an old Aristotelian concept. J Anat. 2007;211:415–427. doi: 10.1111/j.1469-7580.2007.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu B, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151:1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majumdar A, Drummond IA. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev Genet. 1999;24:220–229. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<220::AID-DVG5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Gerber HP, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 17.Eremina V, et al. Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol. 2006;17:724–735. doi: 10.1681/ASN.2005080810. [DOI] [PubMed] [Google Scholar]
- 18.Serluca FC, et al. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr Biol. 2002;12:492–497. doi: 10.1016/s0960-9822(02)00694-2. [DOI] [PubMed] [Google Scholar]
- 19.Bjarnegard M, et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 20.Reidy K, Tufro A. Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol. 2011;26:1407–1412. doi: 10.1007/s00467-011-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miner JH. The glomerular basement membrane. Exp Cell Res. 2012;318:973–978. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto K, et al. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 23.Field HA, et al. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol. 2003;253:279–290. doi: 10.1016/s0012-1606(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 24.Zong Y, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han S, et al. An endothelial cell niche induces hepatic specification through dual repression of Wnt and Notch signaling. Stem Cells. 2011;29:217–228. doi: 10.1002/stem.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battle MA, et al. Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc Natl Acad Sci U S A. 2006;103:8419–8424. doi: 10.1073/pnas.0600246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammert E, et al. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 28.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 29.Reinert RB, et al. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62:4154–4164. doi: 10.2337/db13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsumoto K, Kume S. Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development. 2011;138:1947–1955. doi: 10.1242/dev.058719. [DOI] [PubMed] [Google Scholar]
- 31.Kinkel MD, Prince VE. On the diabetic menu: zebrafish as a model for pancreas development and function. Bioessays. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar M, et al. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- 33.Edsbagge J, et al. Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme. Development. 2005;132:1085–1092. doi: 10.1242/dev.01643. [DOI] [PubMed] [Google Scholar]
- 34.Sand FW, et al. Growth-limiting role of endothelial cells in endoderm development. Dev Biol. 2011;352:267–277. doi: 10.1016/j.ydbio.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Saito D, et al. The dorsal aorta initiates a molecular cascade that instructs sympatho-adrenal specification. Science. 2012;336:1578–1581. doi: 10.1126/science.1222369. [DOI] [PubMed] [Google Scholar]
- 36.DeLisser HM, et al. Loss of PECAM-1 function impairs alveolarization. J Biol Chem. 2006;281:8724–8731. doi: 10.1074/jbc.M511798200. [DOI] [PubMed] [Google Scholar]
- 37.Jakkula M, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 38.Metzger RJ, et al. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarus A, et al. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lassus P, et al. Vascular endothelial growth factor in human preterm lung. Am J Respir Crit Care Med. 1999;159:1429–1433. doi: 10.1164/ajrccm.159.5.9806073. [DOI] [PubMed] [Google Scholar]
- 41.Lassus P, et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164:1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 42.Berse B, et al. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maniscalco WM, et al. Vascular endothelial growth factor mRNA increases in alveolar epithelial cells during recovery from oxygen injury. Am J Respir Cell Mol Biol. 1995;13:377–386. doi: 10.1165/ajrcmb.13.4.7546767. [DOI] [PubMed] [Google Scholar]
- 44.Thebaud B, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 45.Compernolle V, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 46.Kumar PA, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeCouter J, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 50.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tam BY, et al. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 52.Hu J, et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 53.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding BS, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kordes C, Haussinger D. Hepatic stem cell niches. J Clin Invest. 2013;123:1874–1880. doi: 10.1172/JCI66027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin C, et al. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 59.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117:5281–5288. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- 60.Maes C, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eshkar-Oren I, et al. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development. 2009;136:1263–1272. doi: 10.1242/dev.034199. [DOI] [PubMed] [Google Scholar]
- 62.Gerber HP, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 63.Maes C, et al. Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J. 2010;29:424–441. doi: 10.1038/emboj.2009.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bentovim L, et al. HIF1alpha is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development. 2012;139:4473–4483. doi: 10.1242/dev.083881. [DOI] [PubMed] [Google Scholar]
- 65.Maes C, et al. VEGF-independent cell-autonomous functions of HIF-1alpha regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J Bone Miner Res. 2012;27:596–609. doi: 10.1002/jbmr.1487. [DOI] [PubMed] [Google Scholar]
- 66.Pannarale L, et al. SEM corrosion-casts study of the microcirculation of the flat bones in the rat. Anat Rec. 1997;247:462–471. doi: 10.1002/(SICI)1097-0185(199704)247:4<462::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 67.Zamboni L, Pease DC. The vascular bed of red bone marrow. J Ultrastruct Res. 1961;5:65–85. doi: 10.1016/s0022-5320(61)80006-3. [DOI] [PubMed] [Google Scholar]
- 68.Kusumbe AP, et al. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramasamy SK, et al. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maes C, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 71.Deckers MM, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 72.Chen G, et al. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tylzanowski P, et al. The Noggin null mouse phenotype is strain dependent and haploinsufficiency leads to skeletal defects. Dev Dyn. 2006;235:1599–1607. doi: 10.1002/dvdy.20782. [DOI] [PubMed] [Google Scholar]
- 74.Canalis E, et al. Conditional inactivation of noggin in the postnatal skeleton causes osteopenia. Endocrinology. 2012;153:1616–1626. doi: 10.1210/en.2011-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devlin RD, et al. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144:1972–1978. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- 76.Nagasawa T, et al. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol. 2011;32:315–320. doi: 10.1016/j.it.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Ding L, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugiyama T, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 79.Himburg HA, et al. Pleiotrophin regulates the retention and selfrenewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2:964–975. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winkler IG, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 81.Poulos MG, et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 2013;4:1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palmer TD, et al. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 84.Culver JC, et al. A specialized microvascular domain in the mouse neural stem cell niche. PLoS One. 2013;8:e53546. doi: 10.1371/journal.pone.0053546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 86.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 90.Roitbak T, et al. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J Cereb Blood Flow Metab. 2008;28:1530–1542. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q, et al. Modeling the neurovascular niche: VEGF- and BDNF- mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt NO, et al. Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res. 2009;1268:24–37. doi: 10.1016/j.brainres.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 93.Licht T, et al. VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development. 2010;137:261–271. doi: 10.1242/dev.039636. [DOI] [PubMed] [Google Scholar]
- 94.Licht T, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calvo CF, et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011;25:831–844. doi: 10.1101/gad.615311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramirez-Castillejo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 97.Andreu-Agullo C, et al. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 98.Ottone C, et al. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. 2014 doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cayre M, et al. Netrin 1 contributes to vascular remodeling in the subventricular zone and promotes progenitor emigration after demyelination. Development. 2013;140:3107–3117. doi: 10.1242/dev.092999. [DOI] [PubMed] [Google Scholar]
- 100.Pineda JR, et al. Vascular-derived TGF-beta increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol Med. 2013;5:548–562. doi: 10.1002/emmm.201202197. [DOI] [PMC free article] [PubMed] [Google Scholar]