Abstract
Background. Coelomycetes are rarely but increasingly reported in association with human infections involving mostly skin and subcutaneous tissues, both in immunocompetent and immunocompromised patients. Coelomycetes constitute a heterogeneous group of filamentous fungi with distinct morphological characteristics in culture, namely an ability to produce asexual spores within fruit bodies.
Methods. We included all cases of proven primary cutaneous and/or subcutaneous infections due to coelomycetes received for identification at the French National Reference Center for Invasive Mycoses and Antifungals between 2005 and 2014. Eumycetoma, chromoblastomycosis, and disseminated infections were excluded.
Results. Eighteen cases were analyzed. The median age was 60.5 years. In all cases, patients originated from tropical or subtropical areas. An underlying immunodepression was present in 89% of cases. Cutaneous and/or subcutaneous lesions, mainly nodules, abscesses, or infiltrated plaques, were observed in distal body areas. Isolates of different genera of coelomycetes were identified: Medicopsis (6), Paraconiothyrium (3), Gloniopsis (3), Diaporthe (3), Peyronellaea (2), Lasiodiplodia (1). Lesion treatment consisted of complete (10) or partial (2) surgical excision and/or the use of systemic antifungal therapy, namely voriconazole (5) and posaconazole (4). Literature review yielded 48 additional cases of cutaneous and/or subcutaneous infections due to coelomycetes.
Conclusions. Infectious diseases physicians should suspect coelomycetes when observing cutaneous and/or subcutaneous infections in immunocompromised hosts from tropical areas; a sequence-based approach is crucial for strains identification but must be supported by consistent phenotypic features; surgical treatment should be favored for solitary, well limited lesions; new triazoles may be used in case of extensive lesions, especially in immunocompromised patients.
Keywords: coelomycetes, cutaneous phaeohyphomycosis, Medicopsis romeroi, Paraconiothyrium sp, subcutaneous abscess
In the past 20 years, the incidence of community-acquired opportunistic infections has steadily risen, and invasive fungal diseases have become a growing source of morbidity and mortality. Rare and even new fungal species, among which melanized fungi and more specifically coelomycetes, are increasingly recognized as significant human pathogens [1].
Coelomycetes are a large and phylogenetically heterogeneous group of filamentous fungi that are grouped together on the basis of their asexual morphs in culture, ie, their ability to produce asexual spores known as conidia, within fruit bodies named conidiomata [2]. In both immunocompetent and immunocompromised patients, coelomycetes have been incriminated in various skin and soft tissue infections, namely cutaneous and subcutaneous phaeohyphomycosis, eumycotic black-grain mycetoma, and even 1 isolated case of chromoblastomycosis [3–6]. The term phaeohyphomycosis was initially coined in 1974 to describe various clinical manifestations caused by melanized fungi. It is defined by the presence of dematiaceous yeast-like cells, pseudo-hyphae-like elements, hyphae, or any combination of these in tissues [4].
In this study, we report 18 cases of cutaneous and/or subcutaneous infections due to coelomycetes that answer that description and can therefore be referred to as cutaneous and/or subcutaneous phaeohyphomycoses. Our aim was to (1) better characterize these rare fungal infections and their causative agents and (2) review treatment options.
MATERIAL AND METHODS
Inclusion and Exclusion Criteria
We performed a retrospective analysis of consecutive cases of cutaneous and/or subcutaneous infections due to coelomycetous fungi that were received for identification at the French National Reference Center for Invasive Mycoses and Antifungals (NRCMA) from 2005 to 2014. Cases were included if they fulfilled the following criteria: (1) proven infection according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria with isolate recovery from abscess drainage, skin biopsy, or subcutaneous tissue samples; (2) absence of dissemination and microbiologically documented deep organ involvement (ie, primary cutaneous and/or subcutaneous infections). Cases of eumycetoma and chromoblastomycosis were excluded. A case of phaeohyphomycosis was defined by clinical findings consistent with that infection and either histopathological evidence of a melanized fungus or a culture positive for a melanized fungus and known agent of phaeohyphomycosis. Procedures were in accordance with the Helsinki Declaration.
Questionnaire and Pathological Analysis
A specific questionnaire was sent to collect epidemiological, clinical, mycological, and therapeutic data as well as follow-up information. Missing information and ambiguous answers were checked by phone with the physician and microbiologist. Tissue biopsies and histological sections (hematoxylin-eosin [HE], Gomori methenamine silver, and periodic acid-Schiff [PAS] stainings) were obtained from pathology laboratories and reanalyzed by a dermatopathologist. When necessary, Fontana-Masson staining was performed to visualize hyphae pigmentation.
Mycological Identification
According to standard practice at the NRCMA, species identification was performed by a polyphasic approach. The purity of all isolates was checked by obtaining single isolated colonies on Sabouraud chloramphenicol agar medium. Colonies were subcultured onto 2% malt agar, potato carrot agar (PC), oatmeal agar (OA) tubes, or autoclaved straw pieces on 2% water agar plates and incubated at 30°C under near-ultraviolet light or at 25°C to promote sporulation. Microscopic preparations were mounted in cotton blue from cultures sporulating on the different media. Molecular identification was performed by sequencing the ITS1-5.8S-ITS2 (ITS) region of the ribosomal deoxyribonucleic acid (rDNA), the D1-D2 domain of the large subunit rDNA (28S), and a small region of the elongation factor (EF)-1α and of the β-tubulin (TUB) genes (described in Supplementary Data).
Antifungal Susceptibility Determination
In vitro susceptibility testing was performed according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) procedure [7] with some modifications [8]. Seven antifungal agents were included: amphotericin B, triazoles (itraconazole, voriconazole, posaconazole), echinocandins (caspofungin, micafungin), and terbinafine. All strains were subcultured on PC or OA for 7 to 30 days at 25°C and 30°C. Conidia were then collected in water, and the suspension was adjusted to 2–5 × 105 colony-forming units/mL.
Review of Reported Cases
We reviewed all cases of cutaneous and/or subcutaneous infections due to coelomycetes published in the literature from 1970 to 2015, excluding eumycetoma and chromoblastomycosis. The keywords used for this search were as follows: phaeohyphomycosis, and cutaneous, subcutaneous, abscess, cyst, skin, coelomycetes, Phoma, Pleurophoma, Rhytidhysteron rufulum, Medicopsis romeroi, Pyrenochaeta, Paraconiothyrium, Phomopsis, Pleurophomopsis, Lasiodiplodia, Colletotrichum, Coniothyrium, Microsphaeropsis, Nattrassia mangiferae, Gloniopsis, and Diaporthe.
RESULTS
Epidemiological Characteristics of the Patients
Among a total of 31 fungal infections due to coelomycetes and received for identification at the NRCMA, 18 cases of proven cutaneous and/or subcutaneous infections were analyzed (Table 1). Patients' median age was 60.5 years (47–78 years). The sex ratio was 3.5:1 (14 of 18, ie, 78% of patients were male).
Table 1.
Clinical and Epidemiological Characteristics of 18 Human Cutaneous and/or Subcutaneous Infections Due to Coelomycetes Seen Between 2005 and 2014 in France
| Treatment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex, Age (Years) | Injury History | Geographical Area | Underlying Risk Factors | Lesion Type | Body Site (Lesion Characteristics) | Histology | Direct Microscopic Examination | Culture | Agent (Dosage, mg/day, Duration) | Surgery | Outcome | Follow-Up |
| 1 | F, 59 | Sri Lanka/ France | Diabetes mellitus/ Polymyalgia rheumatica Prednisone | Subcutaneous | Foot (1 nodule) | Pigmented hyphae, gran inf | Hyphae | Medicopsis romeroi | None | Total excision | Cured | 8 y | |
| 2 | F, 73 | India/France | Giant cell arteritis Prednisone | Subcutaneous | Foot (1 abscess), leg (2 cystic nodules) | Pigmented hyphae, gran inf | Negative | M romeroi | VCZ (400, 3 wk) | Total excision | Cured | 7 y | |
| 3 | M, 65 | West Africa /France | Renal graft IS therapy: Tac, MMF, prednisone |
Subcutaneous | Knee (1 abscess) | Aspecific chronic inf | Negative | M romeroi | POSA (800, 1 mo) | None | Cured | 6 y | |
| 4 | F, 47 | West Africa /France | Diabetes mellitus | Subcutaneous | Foot (1 abscess) | Pigmented hyphae, gran inf | Not done | M romeroi | None | Total excision | Cured | 1 y | |
| 5 | M, 53 | Pakistan /France | Liver graft IS therapy: Tac, MMF, prednisone |
Subcutaneous | Foot (1 abscess) | Not done | Hyphae | M romeroi | POSA (800, 2 wk) followed by LAMB (3 mg/kg, 2 mo) | 2 abscess drainages | Relapse under POSA. Cured by LAMB + total excision | 10 mo | |
| 6 | M, 54 | Farmer / injury | West Indies/ France | Chronic hepatitis C | Subcutaneous | Forearm (1 abscess) | Pigmented hyphae, gran inf | Hyphae | Gloniopsis sp | None | Total excision | Cured | 8 y |
| 7 | M, 63 | West Africa /France | Renal graft IS therapy: cyclosporine |
Subcutaneous | Hand (1 abscess) | Not done | Hyphae | Gloniopsis sp | POSA (800, 2 mo) | Relapse treated by total excision | Relapse 19 mo after POSA. Cured by excision | 5 y | |
| 8 | M, 53 | Sheperd /injury | West Africa /France | Acute B-cell leukemia, neutropenia |
Subcutaneous | Finger (1 infiltrated plaque), foot (2 abscesses) | Not done | Hyphae | Peyronellaea gardenia | LAMB (3 mg/kg, 6 wk) followed by VCZ (400, 6 wk) | None | Cured | 6 y |
| 9 | M, 58 | Farmer | West Africa /France | Renal graft IS therapy: Tac, MMF, prednisone Diabetes mellitus |
Subcutaneous | Foot (1 abscess) | Pigmented hyphae, gran inf | Hyphae | P gardeniae | VCZ (400, 13 mo) | Total excision | Cured | 5 y |
| 10 [9] | M, 71 | Gardener | West Indies (Guadeloupe) /France | Renal graft IS therapy: Tac, MMF, prednisone |
Subcutaneous | Elbow (1 nodule) | Not done | Hyphae | Paraconiothyrium cyclothyrioides | LAMB (3 mg/kg, 4 wk) and VCZ (400, 7 wk) | None | Died of underlying condition | 2 mo |
| 11 | M, 78 | West Indies (Guadeloupe) | Diabetes mellitus | Subcutaneous | Foot (1 nodule) | Not done | Hyphae | Diaporthe sp | ITRA (200, 18 mo) | None | Relapse 6 mo after ITRA | 2 y | |
| 12 | M, 51 | Foot trauma | West Africa /France | Renal graft IS therapy: Tac, MMF, prednisone Diabetes mellitus |
Subcutaneous | Finger (1 nodule), foot (2 nodules) | Pigmented hyphae, gran inf | Hyphae | Diaporthe raonikayaporum | None | Total excision | Cured | 2 y |
| 13 | M, 62 | West Africa /France | Renal graft IS therapy: Tac, prednisone |
Subcutaneous | Knee (multiple nodules with diffuse skin infiltration) | Pigmented hyphae, gran inf | Hyphae | Ascomycete order Pleosporales | POSA (800, 4 y) | 3 rounds of partial excision | 2 early relapses in the first 6 mo | 4 y | |
| 14 | M, 70 | West Africa /France | None | Subcutaneous | Elbow (1 aponeurotic cyst) | Pigmented hyphae, gran inf | Not done | Gloniopsis sp | None | Total excision | Cured | 3 mo | |
| 15 | M, 55 | La Réunion/ France | Renal graft IS therapy: Tac, MMF, prednisone Diabetes mellitus Chronic hepatitis B and C/cirrhosis |
Cutaneous | Foot sole (1 pigmented budding infiltrated plaque with central nodule) | Pigmented hyphae, gran inf | Hyphae | P cyclothyrioides | LAMB (3 mg/kg, 4 wk) and Caspofungin (50, 1 wk) |
None | Died of underlying condition | 1 mo | |
| 16 [10] | M, 68 | West Indies (Martinique) | B cell lymphoma, chemotherapy, methylprednisolone, neutropenia | Cutaneous | Foot sole (1 pigmented infiltrated plaque) |
Not done | Hyphae | P cyclothyrioides | VCZ (200, 10 wk) and corticosteroids discontinuation | None | Lesion regression. Died of underlying condition | 3 mo | |
| Cutaneous | Heel (1 pigmented infiltrated plaque) | Not done | Hyphae | Diaporthe sojae | |||||||||
| 17 [11] | M, 66 | Central Africa /France | Renal graft IS therapy: Tac, MMF, prednisolone |
Cutaneous | Foot sole (1 plantar wart) | Pigmented hyphae, gran inf | Hyphae | M romeroi | Reduction of IS therapy | total excision | Cured | 4 y | |
| 18 | F, 47 | West Indies (Martinique) | 2nd/3rd degree burn lesions over 60% of total BSA | Cutaneous | Forearm (3d degree necrotic burn lesion) | Not done | Negative | Lasiodiplodia theobromae species complex | None | Excision of necrotic tissues | Died of underlying condition | 2 wk | |
Abbreviations: BSA, body surface area; gran, granulomatous; inf, inflammation; IS, immunosuppressive; ITRA, itraconazole; LAMB, liposomal amphotericin B; MMF, mycophenolate mofetil; POSA, posaconazole; Tac, tacrolimus; VCZ, voriconazole.
Geographical Distribution
Patients all originated from tropical and subtropical regions, and most had traveled there in the past 18 months: Africa (10 cases), Asia (3 cases), West Indies (5 cases). Five patients (28%) were from rural areas, engaged in farming or with a history of soil or plant trauma 1 to 3 months before lesion appearance. However, 3 immunocompromised patients without trauma history had not left France for 5 to 10 years before lesion appearance.
Underlying Diseases and Risk Factors
Underlying diseases were reported in 16 cases (89%). Nine patients (50%) received solid organ transplants (SOT) (8 kidneys, 1 liver); 6 patients suffered from diabetes mellitus, and 2 patients suffered from hematological malignancies. Topical/oral steroids and immunosuppressive agents (mycophenolate mofetil, tacrolimus, cyclosporine) were used in 11 and 9 cases, respectively. Two patients (11%) had no apparent underlying disease.
Clinical Signs and Symptoms
Fourteen patients (patients 1 to 14) displayed subcutaneous lesions, usually 1 solitary nodule (Figure 1), sometimes associated with local inflammation and mimicking an abscess (8 cases). One patient had multiple cutaneous and subcutaneous lesions on the knee (patient 13; Figure 2), and 1 patient had an aponeurotic cyst (patient 14). Three patients (patients 15 to 17) presented with a cutaneous form of the disease, exhibiting infiltrated, pigmented, budding, or necrotic plaques, scaly and wart-like when localized on the foot sole. Solitary lesions were the most frequent (12 patients, 67% of cases). Two patients displayed concomitant cutaneous/subcutaneous infections involving 2 (Paraconiothyrium cyclothyrioides and Phaeoacremonium parasiticum [9], patient 10) or 3 fungi (Phomopsis longicolla reidentified as Diaporthe sojae, P cyclothyrioides and Cunninghamella bertholletiae [10], patient 16). Lesions involved exclusively distal areas of the upper limbs in 7 cases (39%) and the lower limbs in 13 cases (72%). The foot was the site most frequently involved (14 lesions).
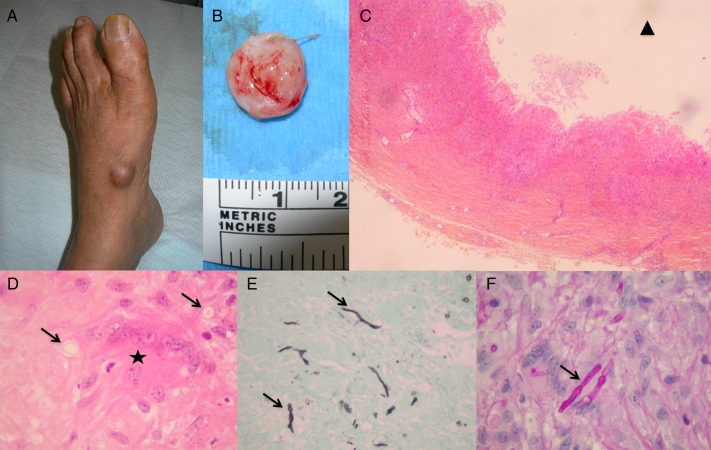
Figure 1.
Patient 1. (A and B) Painless subcutaneous cyst of the left foot containing a puriform liquid in a 59-year-old woman with polymyalgia rheumatica treated by corticosteroid therapy. (C) Hematoxylin-eosin staining showed a deep dermal abscess mixed with granulomatous inflammation (×40). The black triangle labels the cyst lumen. (D) Hematoxylin-eosin staining (×1000) with high magnification of a multinucleated cell (black star) and pigmented fungal hyphae (black arrows) (×1000). (E) Gomori methenamine silver stain and (F) periodic acid-Schiff staining ([E], ×400; [F], ×1000) revealed globose or elongated septate hyphal elements (black arrows).
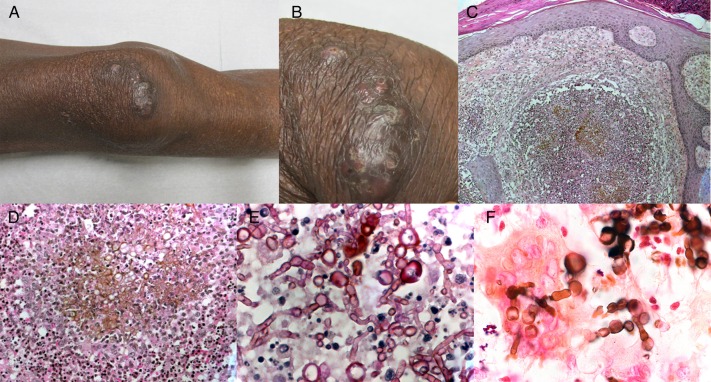
Figure 2.
Patient 13. Pigmented infiltrated plaque of the right knee associated with diffuse subcutaneous infiltration in a 62-year-old kidney transplant recipient. (A) Full and (B) close-up views (courtesy of Camille Frances). (C and D) Hematoxylin-eosin staining revealed a dense dermal infiltrate with granulomatous inflammation, associating neutrophils, lymphocytes, epithelioid, and multinucleated cells, as well as pigmented fungal hyphae ([C], ×100; [D], ×400). (E) Periodic acid-Schiff staining showed septate fungal hyphae (×1000). (F) Fontana-Masson staining confirmed the pigmented character of fungal structures (×1000).
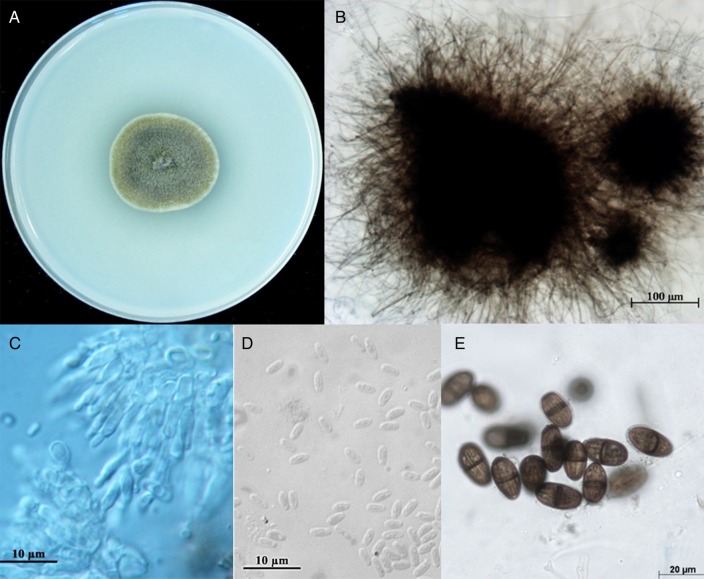
Figure 3.
Macroscopic aspect of Medicopsis romeroi on oatmeal agar (OA), 28°C, 14 days (A); immersed pycnidium of strain CNRMA11.1115 on OA medium (B); conidiophores and conidiogenous cells from a pycnidium of M romeroi (C) small, hyaline conidia of Gloniopsis sp strain (D); mature, septate, striated conidia of Lasiodiplodia theobromae species.
In all 11 cases in which a pathological examination of skin tissue was performed, an aspect highly suggestive of infection was observed with a granulomatous dermal infiltrate containing epithelioid and multinucleated giant cells. In 10 of 11 cases, stainings revealed elongated septate and branched hyphae or globose fungal structures (HE, PAS, and Gomori methenamine silver stainings; Figures 1 and 2). Pigmentation of the fungal structures was confirmed using HE and Fontana-Masson stainings.
Mycological Identification
Twenty-two isolates were available for the 18 patients, but the same fungus was identified in the 4 cases in which we had 2 isolates. Microscopy of the initial cultures revealed septate-melanized hyphae in the majority of isolates (Figure 3). Pycnidial conidiomata were seen for 13 of 22 isolates after at least 3 weeks of subculture on special media (Supplementary Table S1). In parallel, a multilocus sequence-based analysis was performed for all clinical isolates, resulting in the identification of 11 isolates to the species level: M romeroi (6 cases), P cyclothyrioides (3 cases), Diaporthe raonikayaporum (1 case), and D sojae (1 case). Identification to the genus level was achieved for 4 isolates: Diaporthe sp (1 case) and Gloniopsis sp (3 cases). One isolate belonged to the Lasiodiplodia theobromae species complex, and a possible identity of Peyronellaea gardeniae was found for 2 isolates. Finally, the identity of isolate CNRMA11.1115 remained uncertain due to the absence of sequence entries on the curated databases for known taxa (Supplementary Table S1).
In Vitro Antifungal Susceptibility Testing
Minimum inhibitory concentrations (MICs) were determined for the 11 strains that produced enough conidia (Supplementary Table S2). Low MICs of amphotericin B (0.06 to 1 µg/mL), voriconazole (0.03 to 0.5 µg/mL), and terbinafine (0.06 to 1 µg/mL) were observed for all the strains tested with the exception of species CNRMA13.515, which exhibited a terbinafine MIC of 4 µg/mL. The P cyclothyrioides and the 2 P gardeniae isolates exhibited low MICs of all antifungals tested except for echinocandins and posaconazole, respectively. The 3 M romeroi strains and the 1 belonging to the L theobromae species complex had high itraconazole MICs (4 to ≥8 µg/mL). The lowest triazole and echinocandin MICs were observed for P cyclothyrioides and D sojae, respectively.
Treatment and Follow-Up
Systemic antifungals were prescribed in 11 of the 18 cases (61%) for a median duration of 2.5 months: voriconazole (5 cases), posaconazole or liposomal amphotericin B (4 cases each), and itraconazole (1 case). In 1 patient, liposomal amphotericin B therapy was followed by voriconazole administration. Another patient was switched to liposomal amphotericin B after 2 weeks of inefficient posaconazole treatment. Liposomal amphotericin B was combined with caspofungin or voriconazole (1 case each). Six patients were treated by antifungals alone. Surgery was performed in 12 patients: total excision (10 cases) or partial excision (2 cases). It was the sole treatment in 7 cases (39%).
The mean follow-up was 36 months (2 weeks–96 months). Four patients (22%) died of the underlying disease during the first 3 months. Regression or complete cure was obtained in 13 of the remaining 14 cases where follow-up was available. Seven patients were cured by excision alone, which was the first-line treatment in 6 cases. Of the 6 patients treated by antifungal drugs only, 2 relapsed. Of note, patient 7 relapsed 19 months after a 2-month treatment with posaconazole and was cured by total excision of the relapsing solitary lesion. Of the 4 patients treated by combined surgery and antifungals, 2 patients relapsed but were eventually cured by a second or third line of combined treatment. The diffuse subcutaneous infiltration of the lower limb of patient 13 was controlled by the combination of 3 partial surgical procedures and a prolonged high-dose regimen of posaconazole.
DISCUSSION
Coelomycetes correspond to an artificial group of ascomycete and few basidiomycete fungi able to produce spores (conidia) within fruit bodies (conidiomata) [6, 12]. To date, approximately 1000 genera and 7000 species are included in this group [13]. These fungi have been reported as plant pathogens [14] and implicated in animal and human infections [6, 15]. However, in the past, reports have been limited by the lack of correct identification of these pathogens [1]. The ongoing taxonomical reorganization of some groups of coelomycetes is an additional issue.
Melanized fungi are involved in proven infections in both immunocompromised and immunocompetent individuals. In a review of 72 disseminated phaeohyphomycoses, infection was associated with some degree of immune dysfunction in 76% of patients [16]. Similarly, 89% of our 18 patients were immunocompromised, with 50% of SOT recipients. Our literature search yielded 48 published cases of cutaneous and/or subcutaneous infections (excluding eumycetoma and chromoblastomycosis) attributed to coelomycetes, among which 29 (60%) occurred in immunocompromised patients, including 12 (25%) organ transplant recipients (Table 2). It is interesting to note that 2 patients (patients 10 and 16) in our series displayed multiple concomitant fungal infections, a finding that underlines the favoring role of immune suppression in the emergence of these mycoses.
Table 2.
Clinical and Epidemiological Characteristics of Reported Human Cutaneous and/or Subcutaneous Infections Due to Coelomycetes
| Author (and Reference) | Year of Report | Sex, Age in Years | Injury History | Geographical Area | Underlying Risk Factor | Type of Lesion (SC or C) |
Body Site | Culture | Treatment | Outcome | Follow-Upa |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bakerspigel [S2] | 1970 | F, 22 | Farmer | Ontario, Canada | Topical steroids | C: Erythematous nodule with pustular lesions | Leg | Phoma hibernica | Oral griseofulvin | Regression | 5 y |
| Young [S3] | 1973 | F, 42 | Jamaica/ United States | Renal transplant | SC: Subcutaneous cystic lesion | Heel | Phoma sp | Excision | Cured | 6 mo | |
| Gordon [S4] | 1975 | M, 4 | None | C: Superficial crusted lesion | Ear | Phoma sp | Oral griseofulvin | Cured | ND | ||
| Bakerspigel [S5] | 1981 | M, 1.5 | Ontario, Canada | None | C: Perioral crusted lesion | Face | Phoma eupyrena | Topical clotrimazole | Cured | 2 y | |
| Shukla [S6] | 1984 | F, 18 | India | Typhoid fever | C: Superficial papulovesicular lesions | Face | Phoma minutispora | Topical clotrimazole | Cured | 1 mo | |
| Shukla [S6] | 1984 | M, 20 | Farmer | India | Corticosteroids (chronic sinusitis) | C: Superficial maculopapules | Neck | P minutispora | Topical clotrimazole | Cured | 20 d |
| Baker [S7] | 1987 | M, 75 | Farmer | Dominican Republic | Diabetes mellitus/ Corticosteroids (myasthenia gravis) | SC: Subcutaneous lesion | Foot | Phoma minutella | Amputation | Cured | 1 y |
| Stone [S8] | 1988 | M, 25 | Texas | None | SC: Subcutaneous cystic lesion | Forearm | Phoma sp | Excision/Oral ketoconazole | Cured | 15 mo | |
| Dooley [17, 18] | 1989 | F, 56 | Gardener | Texas | Diabetes mellitus/ Cardiac transplant | SC: Subcutaneous nodules | Thigh, knee, wrist |
Pleurophoma pleurospora Reclassified as Paraconiothyrium maculicutis |
Excision/ Topical miconazole | Cured | ND |
| Rai [S9] | 1989 | M, 24 | India | None | C: Superficial papulovesicular lesions | Face, neck, hands | Phoma sorghina | Topical miconazole | Cured | 1 mo | |
| Rai [S9] | 1989 | M, 19 | India | None | C: Superficial macular lesions | Face | P sorghina | Topical miconazole | Cured | 1 mo | |
| Chabasse [S10] | 1995 | M, 74 | Farmer | France | Corticosteroids (asthma) | SC: Subcutaneous abscess | Leg | Pleurophomopsis lignicolla Petr | Excision. Relapse treated by a second excision. | Relapse after 12 mo. | 12 mon |
| Rosen [S11] | 1996 | F, 24 | Vacations on a farm | Texas | Topical steroids | C: Infiltrated plaque | Face | Phoma sp | Oral ketoconazole | Cured | 2 y |
| Hirsh [S12] | 1996 | M, 45 | Farmer | Hawaii | None | C: Infiltrated plaque, nodules | Hand | Phoma sp | Oral itraconazole | Regression | ND |
| Maslen [S13] | 1996 | F, 40 | Intramuscular injections in buttock | Cambodia/ Australia | None | SC: Indurated plaque with central subcutaneous abscess | Buttock | Lasiodiplodia theobromae | Debridement | Cured | 5 mo |
| Zaitz [S14] | 1997 | M, 63 | Brazil | Corticosteroids (sarcoidosis) | C: Infiltrated plaque, nodules | Sternal region, hand | Pleurophoma cava | Amphotericin B/Oral itraconazole | Cured | ND | |
| Arrese [S15] | 1997 | M, 53 | Owner of a bakery | Morocco/ Belgium | Topical steroids/Short corticosteroid therapies (urticaria, hay fever) | C: Scaly plantar lesion | Foot | Phoma sp | Topical bifonazole/ topical ketoconazole | Persistence | Lost to follow-up |
| Sigler [19] | 1997 | M, 73 | Arizona | Diabetes mellitus/ Corticosteroids therapy | SC: Subcutaneous abscesses and infiltrated plaques | Arm, forearm | Nattrassia mangiferae | Topical miconazole/ Amphotericin B | Relapse | ND | |
| Guarro [S16] | 1998 | M, 56 | Farmer, traumatic injury | Brazil | Diabetes mellitus/ corticosteroids | SC: Several nodular solitary or confluent lesions, macular lesions | Forearm, elbow | Colletotrichum gloeosporioides | None | Persistence | Died of other cause |
| Oh [S17] | 1999 | M, 77 | Farmer | Korea | Topical steroids | C: Indurated plaque | Forearm | Phoma sp | Oral itraconazole | Regression | ND |
| Guarro [S18] | 1999 | F, 59 | Spain | None | C: Scaly, infiltrated plaque with inflammatory border | Shoulder | Microsphaeropsis olivacea | Topical clotrimazole/ oral terbinafine | Cured | 7 mo | |
| O'Quinn [20] | 2001 | M, 34 | Cactus inoculation | Tennessee | Acute lymphocytic leukemia/Chemotherapy | SC: Subcutaneous tender nodule | Forearm | C gloeosporioides | Amphotericin B followed by oral itraconazole | Cured | ND |
| O'Quinn [20] | 2001 | M, 47 | Mississippi | Non-Hodgkin lymphoma/ Chemotherapy/ autologous stem cell transplantation | SC: Subcutaneous tender nodule with central pustule | Arm | Colletotrichum coccodes | Amphotericin B followed by oral itraconazole | Cured | ND | |
| Castro [S19] | 2001 | M, 34 | Gardener | Brazil | Renal transplant | SC: Subcutaneous nodule | Leg | Colletotrichum crassipes | Total excision | Cured | ND |
| Miele [S20] | 2002 | M, 60 | Gardener | Washington DC | Diabetes mellitus/Renal transplant | SC: Subcutaneous abscess | Knee | Coniothyrium-Microsphaeropsis complex | Broad debridement/ Oral itraconazole | Cured | ND |
| Girard [S21] | 2004 | M, 45 | West Africa/ France | Leprosy | SC: Multiple painful non-inflammatory subcutaneous nodules | Legs (2), foot (2) | Pyrenochaeta romeroi | Surgical excision/ Abscess drainage/oral itraconazole | Cured | 1 y | |
| Summerbell [S22] | 2004 | F, 50 | Outdoor injury | Jamaica | None | SC: Ulcer | Leg | Lasiodiplodia theobromae | Broad debridement | Cured | 6 mo |
| Siu [S23] | 2004 | M, 49 | Traumatic abrasion | Hawaii | Diabetes mellitus/Heart transplant | SC: Annular and nodular plaques | Legs, Knees | Coniothyrium-Microsphaeropsis complex | Several relapses following excision and oral treatment (fluconazole, itraconazole). Treated by excision and Amphotericine B |
Cured | 4 mo |
| Godoy [S24] | 2004 | M, 65 | Lived in rural area | Brazil | None | C: Desquamative interdigital lesions | Feet | N mangiferae | ND | ND | ND |
| Padhye [S25] | 2004 | M, 41 | West Africa | Diabetes mellitus/AIDS/ chronic hepatitis/active tuberculosis | SC: Tender, mobile, subcutaneous abcess | Arm | Pleurophomopsis lignicolla | Abscess drainage | Healed | ND | |
| Pendle [S26] | 2004 | M, 80 | Australia | Diabetes mellitus/ Inflammatory demyelinating polyneuropathy/ Immunosuppressive therapy |
C: Painless granulomatous plaque | Hand | Microsphaeropsis arundinis | Terbinafine | Cured | 2.5 y | |
| Pendle [S26] | 2004 | M, 56 | Australia | Diabetes mellitus/ Ankylosing spondylarthropathy/ Immunosuppressive therapy |
SC: Necrotic ulcers | Feet | M arundinis | Amputation and itraconazole | Cured | 10 mo | |
| Suh [S27] | 2005 | M, 19 | Korea | Unknown status | C: Verrucous plaque | Face | Phoma sp | AmB | Regression | ND | |
| Balajee [21] | 2007 | M, 3 | ND | Liver transplant | C: Crusted nodules | Leg | Paraconiothyrium cyclothyrioides | ND | ND | ND | |
| Badali [22] | 2010 | F, 45 | India | None | SC: Verrucous plaque, subcutaneous cyst | Forearm | Pyrenochaeta romeroi | Excision | Cured | 1 y | |
| Khan [S28] | 2011 | F, 47 | India/ Kuwait | Acute lymphoblastic leukemia/Chemotherapy | SC: Subcutaneous nodule with central necrosis | Finger | P romeroi | Cyst drainage | Partial regression | ND | |
| Gordon [23] | 2012 | M, 49 | Texas | Renal transplant/ Diabetes mellitus | C: Crusted ulcerated plaques | Legs | P cyclothyrioides | No response to Voriconazole. Posaconazole | Cured | ND | |
| Severo [S29] | 2012 | M, 53 | Brazil | Lung transplant | SC: Necrotic ulcerated subcutaneous cyst | Knee | C gloeosporioides | Total excision | Cured | Died | |
| Mattei [24] | 2013 | M, 43 | Farmer | Brazil | Renal transplant/ Diabetes mellitus | C: Indurated plaques | Arm, leg | Diaporthe phaseolorum | Oral itraconazole and surgical excision | Cured | 5 mo |
| Hsiao [25] | 2013 | M, 78 | Farmer | Taiwan | None | C: Verrucous plaque | Forearm, dorsal hand | P romeroi | No response to oral itraconazole and surgical excision. Amphotericin B | Cured | 6 mo |
| Hall [S30] | 2013 | M, 70 | Florida | Renal transplant | C: Crusted, ulcerated plaque and papules | Finger, forearm | M arundinis | Posaconazole | Cured | 6 mo | |
| Mahajan [26] | 2014 | M, 72 | India | Diabetes mellitus | SC: Subcutaneous swelling | Foot | Rhytidhysteron rufulum | No response to a combination of surgical excision, itraconazole and terbinafine. Intralesional liposomal amphotericin B | Cured | 1 y | |
| Chan [S31] | 2014 | M, 55 | China | Renal transplant | SC: Painless nodular subcutaneous cyst | Thigh | P romeroi | Several relapses after repeated attempts at total excision. Oral Itraconazole continued until death. |
Relapse after tapering of itraconazole. Remission on resuming full dose. | 5 y | |
| Ogawa [S32] | 2014 | M, 68 | Brazil | Renal transplant | SC: Papulo-nodular lesion | Finger | C gloeosporioides | Total excision | Cured | 1 y | |
| Asahina [27] | 2015 | F, 57 | Japan | Systemic lupus erythematosus Autoimmune hepatitis Immunosuppressive therapy |
C: Erythematous scaling plaques and nodules | Finger, forearm, knee, leg, abdomen | M arundinis | Itraconazole, fluconazole, liposomal amphotericin B ineffective. Local thermotherapy. |
Resolution after local therapy | ND | |
| Asahina [27] | 2015 | F, 74 | Japan | Temporal arteritis Hypogammaglobulinemia Immunosuppressive therapy Diabetes mellitus |
C: Erythematous indurated plaques and papules | Hand | M arundinis | Itraconazole and local thermotherapy. | Regression | ND | |
| Papacostas [S33] | 2015 | M, 59 | Inoculation | Kenya/ Australia | None | SC: Subcutaneous swelling | Foot | Lasiodiplodia theobromae | Excision and Voriconazole. | Cured | 3 mo |
| Yadav [S34] | 2015 | F, 50 | India | Diabetes mellitus | SC: Painless subcutaneous cyst | Foot | P romeroi | Drainage and Itraconazole | Cured | 3 mo |
Abbreviations: AIDS, acquired immune deficiency syndrome; AmB, amphotericin B; C, cutaneous; ND, not described; SC, subcutaneous.
All 18 of our cases occurred in patients originating from tropical and subtropical areas, conversely to literature review with only 29 of 48 (60%) patients living in tropical and subtropical regions (Table 2). Indeed, 14 (29%) cases were described in North American countries, United States (mostly southern states), Canada, or European countries (Italy, Spain, France). Four (8%) cases were reported in Northern Asia (Korea, Japan).
Coelomycete infections are frequently reported after inoculation or in patients from rural areas engaged in farming (28% of our patients; 39.5% [19 of 48] of literature cases). In our series, lesions involved mostly distal parts of the limbs, with foot involvement in 11 (61%) patients. Likewise, most of the 48 published cases occurred on exposed areas (face, neck, legs, and arms). These findings are suggestive of the role of minor trauma and inoculation, which is compatible with an environmental source of these fungi. Delay between inoculation and disease may depend on the inoculum size, the extent of the injury sustained, and the underlying disease. Coelomycetes are incriminated as well in keratitis, with case reports suggesting the role of corneal trauma [28]. Rare synovium or lung infections have also been reported [29, 30].
Until recently, the study of coelomycetes phylogeny was based on classic taxonomy that relied on morphology. However, some important distinctive morphological characteristics (conidiation or pigmentation) are inconstant when fungi are cultured on artificial media, rendering phenotypical identification of genera and species difficult. The introduction of molecular techniques such as the sequencing and analysis of fungal ribosomal operons (ITS, 28S) and several protein-coding genes (actin, TUB, EF-1, calmodulin, etc) has considerably helped in resolving species complexes and generic boundaries of some coelomycetes [31–37], resulting in a complete taxonomical reorganization. Many genera still have to be analyzed using only molecular techniques [12]. By now, the mycological community should be aware that important nomenclatural changes are taking place since the publication of the “Amsterdam Declaration of Fungal Nomenclature” [38], which in part abolished the separate naming of anamorphs and teleomorphs of the same fungus. The following online databases may be helpful for clarification of the presently accepted names of fungal species (http://www.mycobank.org/ http://www.indexfungorum.org).
In this study, we performed a polyphasic approach that takes into account morphological features, cultural characteristics, and several molecular targets. However, 7 of the strains could not be identified to the species level due to lack of sporulation, despite the test of varied culture conditions and lack of sequence homology in the public databases. These strains are now included in an ongoing taxonomical study describing novel taxa in coelomycetes.
Nevertheless, in this study, we report new emerging pathogens. Gloniopsis sp was isolated in 3 patients and to date had never been reported as agent of cutaneous or subcutaneous infections. Paraconiothyrium cyclothyrioides, here incriminated in 3 cases of cutaneous plaques or abscesses, was previously described in only 2 cases of skin lesions [21, 23]. Of note, a reported case involving Paraconiothyrium maculicutis had initially been identified as Pleurophoma pleurospora [17, 18]. The most frequently isolated coelomycete in our series (6 cases) was M romeroi, previously named Pyrenochaeta romeroi. This fungus is usually associated with eumycetomas [6]. Only 6 additional cases of phaeohyphomycoses have been attributed to this fungus (Table 2).
Limited data on the in vitro antifungal susceptibilities of coelomycetes are available in the literature, mainly inferred from clinical cases, with potential variations due to the use of different methodologies [2, 3, 22]. Here all strains were tested using a microdilution method slightly adapted from EUCAST [7, 8]. Knowing the extreme phylogenetic diversity of these fungi, we were not surprised to see marked differences in the in vitro susceptibility results according to genus and species, keeping in mind that there are no defined EUCAST breakpoints for coelomycetes and no established correlation between MIC and clinical outcome. Nevertheless, low MIC values were uniformly obtained for voriconazole. These results are in line with Ahmed et al [39] study.
Treatment of coelomycete infections is obviously not standardized. In the literature, therapeutic management varies according to clinical presentation (subcutaneous vs cutaneous). The vast majority (20 of 25) of subcutaneous forms (described as abscesses, cystic lesions or ulcers) were treated by excision or drainage (Table 2). Surgery was the only treatment in 11 of the 20 cases, whereas it was combined with antifungal therapy in 9 cases (itraconazole [7 cases], amphotericin B [2 cases], or ketoconazole, terbinafine, fluconazole, voriconazole [1 case each]). Excision combined with itraconazole followed by terbinafine failed for 1 infection due to R rufulum, which was eventually cured by intralesional amphotericin B injections [26]. The remaining subcutaneous cases were successfully treated by antifungal therapy alone: systemic amphotericin B therapy (3 cases) followed or not by itraconazole [19, 20]. In our series, surgery was performed in 10 of 14 (71%) cases, either alone (7 cases) or combined with antifungal therapy. However, conversely to literature reports in which itraconazole is the first-line therapy, voriconazole (5 cases), posaconazole, or liposomal amphotericin B (4 cases each) were mostly used in our patients.
Concerning the 23 cutaneous forms reported in the literature (described as plaques, papules, or crusted lesions; Table 2), surgical excision was performed in only 2 cases [24, 25] combined with itraconazole in a Diaporthe infection [24], and with amphotericin B in a M romeroi infection [25]. Two cases due to Microsphaeropsis arundinis were treated by local thermotherapy alone or associated to itraconazole [27]. The other 19 cases were treated by antifungal therapy only: itraconazole or topical clotrimazole (3 cases each), terbinafine, griseofulvin, topical miconazole or posaconazole (2 cases each), ketoconazole or amphotericin B (1 case each). Cure was obtained in 16 of 17 cases in which evolution was reported. Lesions persisted in 1 case treated topically. In our series, surgery was performed in 2 cases; voriconazole or liposomal amphotericin B were used in 2 cases.
CONCLUSIONS
In summary, whatever the species identified, coelomycetes are responsible for cutaneous and/or subcutaneous infections, which should be suspected in immunocompromised hosts harboring cutaneous plaques or nodules and originating from the tropics. After skin biopsy, a polyphasic approach combining morphological and molecular analysis is mandatory for definitive mycological identification, which should be provided by an expert laboratory. Antifungal susceptibility results vary between coelomycete genera and strains without any confirmed therapeutic relevance. Therefore, surgery should be the first-line treatment of solitary subcutaneous lesions. In case of multiple or relapsing solitary lesions, antifungal therapy (posaconazole or voriconazole) is warranted. Liposomal amphotericin B is an alternative for management of refractory cases. In SOT recipients, reduction of immunosuppression should also be considered, at least in case of multiple lesions.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Jean-Charles Gantier (French National Reference Center for Invasive Mycoses and Antifungals [NRCMA]) for morphological identification and Damien Hoinard (NRCMA) for technical assistance.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Members of the French Mycosis Study Group who enrolled patients in the present study are as follows: Boulogne-Billancourt (N. Ait-Ammar, J. Dunand, B. Levy, L. Moulonguet), Clichy (V. Zarrouk), Corbeil-Essonnes (S. Kubab, C. Thépot), Guadeloupe (G. Gendrey, M. Beaubrun), Lyon (A. L. Bienvenu, S. Euvrard), Fort-de-France (N. Desbois, J. C. Meniane), Reims (S. Diallo, D. Toubas), Toulouse (S. Cassaing, J. Guitard). Paris: Bichat (C. Chochillon, C. Rioux), Cochin (N. Dupin, A. Paugam), Croix-Saint-Simon (V. Zeller), Necker (M. E. Bougnoux, C. Charlier), Pitié (A. Fekkar, J. Tourret), Saint-Louis (A. Alanio, S. Bretagne, S. Gallien, E. Raffoux), Tenon (C. Frances).
Contributor Information
Collaborators: the French Mycosis Study Group, N. Ait-Ammar, J. Dunand, B. Levy, L. Moulonguet, V. Zarrouk, S. Kubab, C. Thépot, G. Gendrey, M. Beaubrun, A. L. Bienvenu, S. Euvrard, N. Desbois, J. C. Meniane, S. Diallo, D. Toubas, S. Cassaing, J. Guitard, C. Chochillon, C. Rioux, N. Dupin, A. Paugam, V. Zeller, M. E. Bougnoux, C. Charlier, A. Fekkar, J. Tourret, A. Alanio, S. Bretagne, S. Gallien, E. Raffoux, and C. Frances
References
- 1.Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev 2010; 23:884–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stchigel A, Sutton D. Coelomycete fungi in the clinical lab. Current Fungal Infection Reports 2013; 7:171–91. [Google Scholar]
- 3.Garcia-Reyne A, López-Medrano F, Morales JM et al. Cutaneous infection by Phomopsis longicolla in a renal transplant recipient from Guinea: first report of human infection by this fungus. Transpl Infect Dis 2011; 13:204–7. [DOI] [PubMed] [Google Scholar]
- 4.Ajello L, Georg LK, Steigbigel RT, Wang CJ. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia 1974; 66:490–8. [PubMed] [Google Scholar]
- 5.McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol 1983; 8:1–16. [DOI] [PubMed] [Google Scholar]
- 6.Sutton DA. Coelomycetous fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Rev Iberoam Micol 1999; 16:171–9. [PubMed] [Google Scholar]
- 7.Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 2008; 14:982–4. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Hermoso D, Hoinard D, Gantier JC et al. Molecular and phenotypic evaluation of Lichtheimia corymbifera (formerly Absidia corymbifera) complex isolates associated with human mucormycosis: rehabilitation of L. ramosa. J Clin Microbiol 2009; 47:3862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombier MA, Alanio A, Denis B et al. Dual invasive infection with Phaeoacremonium parasiticum and Paraconiothyrium cyclothyrioides in a renal transplant recipient: case report and comprehensive review of the literature of Phaeoacremonium phaeohyphomycosis. J Clin Microbiol 2015; 53:2084–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quillet-Dye C, Meniane JC, Quist D, Desbois N. [Triple cutaneous mycosis (Cunninghamella bertholletiae, Phomopsis spp. and Paraconiothyrium spp.) in an immonucompromised patient: a Martinican case report]. J Mycol Med 2012; 22:357–61. [DOI] [PubMed] [Google Scholar]
- 11.Ocampo MA, Kanitakis J, Bienvenu AL et al. Phaeohyphomycosis caused by Pyrenochaeta romeroi mimicking a plantar wart in a kidney transplant recipient. Transpl Infect Dis 2012; 14:E173–4. [DOI] [PubMed] [Google Scholar]
- 12.Wijayawardene NN, McKenzie E, Chukeatirote E et al. The future of Coelomycete studies. Cryptogamie Mycologie 2012; 33:381–91. [Google Scholar]
- 13.Kirk PM, Cannon PF, Minter DW, Stalpers JA, eds. Dictionary of Fungi, 10th ed. Wallingford: CAB International; 2008: pp 771. [Google Scholar]
- 14.Wijayawardene NN, McKenzie E, Chukeatirote E et al. Coelomycetes. Cryptogamie Mycologie 2012; 33:215–44. [Google Scholar]
- 15.Cano J, Guarro J, Gene J. Molecular and morphological identification of Colletotrichum species of clinical interest. J Clin Microbiol 2004; 42:2450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revankar SG, Patterson JE, Sutton DA et al. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis 2002; 34:467–76. [DOI] [PubMed] [Google Scholar]
- 17.Dooley DP, Beckius ML, Jeffery BS et al. Phaeohyphomycotic cutaneous disease caused by Pleurophoma in a cardiac transplant patient. J Infect Dis 1989; 159:503–7. [DOI] [PubMed] [Google Scholar]
- 18.de Gruyter J, Woudenberg JH, Aveskamp MM et al. Redisposition of Phoma-like anamorphs in Pleosporales. Stud Mycol 2013; 75:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sigler L, Summerbell RC, Poole L et al. Invasive Nattrassia mangiferae infections: case report, literature review, and therapeutic and taxonomic appraisal. J Clin Microbiol 1997; 35:433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Quinn RP, Hoffmann JL, Boyd AS. Colletotrichum species as emerging opportunistic fungal pathogens: a report of 3 cases of phaeohyphomycosis and review. J Am Acad Dermatol 2001; 45:56–61. [DOI] [PubMed] [Google Scholar]
- 21.Balajee SA, Sigler L, Brandt ME. DNA and the classical way: identification of medically important molds in the 21st century. Med Mycol 2007; 45:475–90. [DOI] [PubMed] [Google Scholar]
- 22.Badali H, Chander J, Gulati N et al. Subcutaneous phaeohyphomycotic cyst caused by Pyrenochaeta romeroi. Med Mycol 2010; 48:763–8. [DOI] [PubMed] [Google Scholar]
- 23.Gordon RA, Sutton DA, Thompson EH et al. Cutaneous phaeohyphomycosis caused by Paraconiothyrium cyclothyrioides. J Clin Microbiol 2012; 50:3795–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattei AS, Severo CB, Guazzelli LS et al. Cutaneous infection by Diaporthe phaseolorum in Brazil. Med Mycol Case Rep 2013; 2:85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsiao YW, Chia JH, Lu CF, Chung WH. Molecular diagnosis and therapeutic experience of subcutaneous Pyrenochaeta romeroi infection: a case report and review of the literature. Int J Dermatol 2013; 52:1237–40. [DOI] [PubMed] [Google Scholar]
- 26.Mahajan VK, Sharma V, Prabha N et al. A rare case of subcutaneous phaeohyphomycosis caused by a Rhytidhysteron species: a clinico-therapeutic experience. Int J Dermatol 2014; 53:1485–9. [DOI] [PubMed] [Google Scholar]
- 27.Asahina A, Kobayashi M, Nakano K et al. Deep cutaneous infection with Microsphaeropsis arundinis: report of two Japanese cases. Acta Derm Venereol 2015; 95:855–7. [DOI] [PubMed] [Google Scholar]
- 28.Rishi K, Font RL. Keratitis caused by an unusual fungus, Phoma species. Cornea 2003; 22:166–8. [DOI] [PubMed] [Google Scholar]
- 29.Everett JE, Busick NP, Sielaff T et al. A deeply invasive Phoma species infection in a renal transplant recipient. Transplant Proc 2003; 35:1387–9. [DOI] [PubMed] [Google Scholar]
- 30.Balis E, Velegraki A, Fragou A et al. Lung mass caused by Phoma exigua. Scand J Infect Dis 2006; 38:552–5. [DOI] [PubMed] [Google Scholar]
- 31.de Gruyter J, Aveskamp MM, Woudenberg JH et al. Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res 2009; 113:508–19. [DOI] [PubMed] [Google Scholar]
- 32.Aveskamp MM, Verkley GJ, de Gruyter J et al. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 2009; 101:363–82. [DOI] [PubMed] [Google Scholar]
- 33.Cai L, Hyde KD, Taylor PW et al. A polyphasic approach for studying Colletotrichum. Fungal Divers 2009; 39:183–204. [Google Scholar]
- 34.Aveskamp MM, de Gruyter J, Woudenberg JH et al. Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 2010; 65:1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdollahzadeh J, Javadi A, Mohammadi Goltapeh E et al. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 2010; 25:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udayanga D, Liu X, McKenzie E et al. The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Divers 2011; 50:189–225. [Google Scholar]
- 37.Verkley GJ, Dukik K, Renfurm R et al. Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 2014; 32:25–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawksworth DL, Crous PW, Redhead SA et al. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2011; 2:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed SA, de Hoog GS, Stevens DA et al. In vitro antifungal susceptibility of coelomycete agents of black grain eumycetoma to eight antifungals. Med Mycol 2015; 53:295–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.