Abstract
RIDL (release of insects with dominant lethality) and Wolbachia are 2 potentially powerful tools in the fight against Zika, but their technological advancement is being hampered by policy barriers. In this study, we discuss what could be done to overcome these regulatory deadlocks.
Keywords: health policy, RIDL, vector control, Wolbachia, Zika
After a historical series of localized epidemics within Africa and Oceania [1], the vector-borne Zika virus has emerged as a rising threat in Brazil [2]. The World Health Organization (WHO) recently announced that it is expecting 3–4 million cases this year in the Americas due to its rapid spread and transmission potential. Concomitantly, increasing cases of documented infant microcephaly have been linked to maternal Zika infection [3].
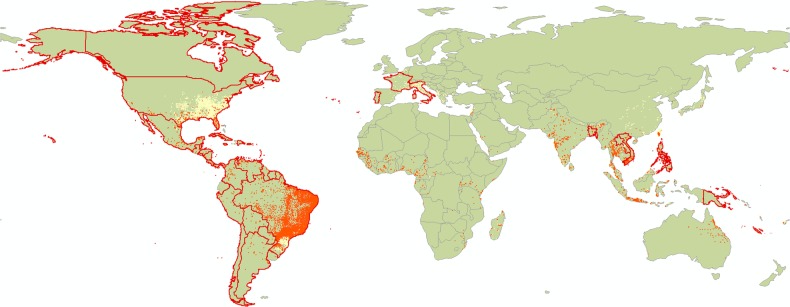
Zika is now a global public health emergency [4] with 48 countries reporting confirmed cases of local transmission in the past 9 months (as of April 29, 2016, data from the European Centre for Disease Prevention and Control). It also has the potential to have seasonal incursions to areas such as the United States, southern Europe, and Australia, where its main reported mosquito vectors, Aedes aegypti and Aedes albopictus, are present [5] (see Figure 1 for vector distribution and current countries affected). Its vector profile is thought to be shared with dengue but could potentially expand to include other more prevalent mosquitoes. Ongoing research at the Oswaldo Cruz Foundation suggests that Culex quinquefasciatus could be a carrier [6], possibly contributing towards the noticeable increase in the rate of Zika spread. Of particular concern is the upcoming Rio Olympic Games in August, which is expected to facilitate disease spread by increasing the number of infectious travelers.
Figure 1.
The distribution of Aedes aegypti and Aedes Albopictus, and countries with confirmed local Zika transmission. The distribution of the 2 main Zika vectors in the tropics and subtropics (Aedes Aegypti in orange, Aedes Albopictus in yellow) overlaid with locations of past and ongoing outbreaks (confirmed local transmission in countries in the last 9 months as of April 29, 2016 shown with red border (data from the European Centre for Disease Prevention and Control).
Postepidemic Versus Preventative
Although the race for a vaccine is underway, no candidate is available in the foreseeable future, making vector management essential for containing Zika to prevent further epidemics. With an incomplete map of transmission pathways and multiple global genetic strains, which could include many vectors and sexual contact, the true severity of Zika's spread and distribution of past to current outbreaks is difficult to establish. Compounding this is the poor understanding of the public health and economic burden of disease vectors [7], which raises the importance of long-term and cost-effective solutions that are safe and preventative.
Traditional control methods have involved (1) habitat management with the application of larvicides and removal of breeding habitats or (2) preventative measures such as indoor residual spraying and wide-area fogging [8]. Although these principal methods show short-term efficacy and should be continued, by themselves they will have a limited and transient effect on the global spread of Zika, as evidenced by current dengue endemicity. Aedes aegypti prefers to breed in small, frequently obscure, habitats, and being both diurnal and anthropophilic, they requires considerable human intervention. With issues such as competing application priorities and widespread insecticide resistance, the consequent high accumulated project costs often results in delayed postepidemic action. Therefore, new complementary approaches that are both longer lasting and large scale are required. Two promising breakthroughs include the engineering of RIDL (release of insects with dominant lethality) by Oxitec (Abingdon, UK) [9, 10] and Wolbachia-infected mosquitoes in the Eliminate Dengue Project [10, 11].
Large-Scale Integrated Vector Management
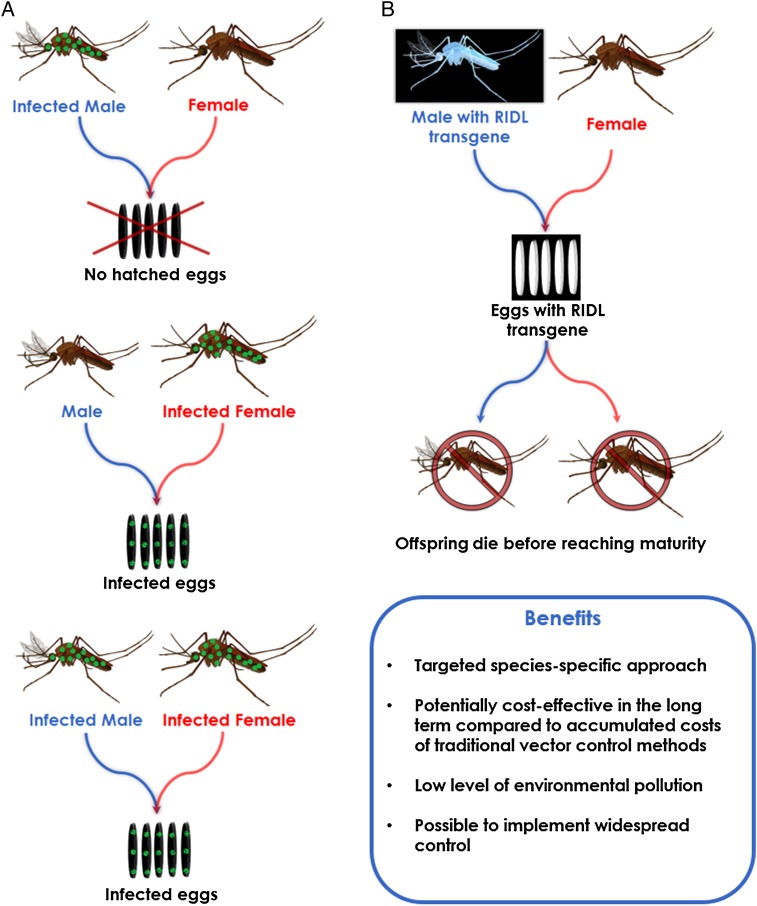
The bacterium Wolbachia naturally occurs in many different insect species, and when transferred into Aedes aegypti, it causes cytoplasmic incompatibility (see Figure 2A for Wolbachia mechanisms). Over a small number of generations, it is able to spread, replace the wild population, and reduce reproductive capability. Currently, product development and deployment strategy is being optimized [12]. RIDL also offers highly species-specific control, using a lethal gene that causes the premature death of progeny (see Figure 2B for RIDL mechanisms). Unlike Wolbachia, RIDL is a suppression strategy, with dramatic reductions in native mosquito populations observed in a few weeks [13].
Figure 2.
Mechanisms and benefits of RIDL (release of insects with dominant lethality) and Wolbachia. (A) Outcomes of different mating combinations with Wolbachia-infected mosquitoes (12): after the release of Wolbachia-infected males, their mating events with wild-type females lead to no eggs hatching. Any wild-type males that mate with Wolbachia-infected females will result in all progeny carrying Wolbachia. Infected males that mate with infected females will also observe this consequence. The pairings highlighted above demonstrate where sterility occurs and how Wolbachia is able to spread down generations. (B) Effect of the RIDL transgene in mosquito populations (inset, Aedes aegypti mosquito) (13): mass reared Aedes aegypti males carry a lethal mutant gene, which when reared in a laboratory setting can be controlled, allowing them to survive normally. Once released, however, the progeny they create when mating with wild females are unable to survive past the pupal stage. Their delayed death maintains density dependence, which keeps competition for resources high and maintains natural death rates. By preventing wild females from laying viable young, population reduction occurs at sites of RIDL mosquito release.
These techniques only release males, which do not increase transmission risk because only females bite. Their proponents envision for a targeted yet large-scale campaign, expanding our arsenal of current vector control tools. However, they face many obstacles.
Issues include the persistence of their releases, mosquito-rearing schemes, and community acceptance. Yet other issues are particularly damaging. Stigma created by spurious media claims of a direct link between RIDL and Zika can have a long-lasting effect, denormalizing the technology to the public, and evoking opposition. This severely undermines the scientific process by disregarding the undergoing thorough safety testing behind these projects. The lack of structured and objective information in the public forum could stifle the development of policy measures, even when widespread intrinsic opposition does not exist.
Risk Assessments Are in Place
RIDL and Wolbachia have been utilized outside of the laboratory. RIDL has been tested in Grand Cayman and Malaysia and has trials undergoing in Brazil [14], whereas Wolbachia mosquitoes have been released in small-scale trials in Vietnam, Indonesia, Colombia, and Singapore and expanded trials in Australia and Brazil [15]. The vector control community is unanimous that health and safety are the primary concern, and stringent guidelines are in place to ensure public well being.
The main public concerns revolve around (1) the unintended contamination by these mechanisms in both humans and the environment and (2) the unknown consequences of such events. Scientists have taken these concerns into account, which has led to engagement with external risk assessment experts, and they continue to carry out field safety tests, acknowledging that public concern can be constructive, driving innovation and good working practices. RIDL utilizes a fail-safe biocontainment feature with confined mosquito spread risk, and site-specific studies including biological assays found no significant ecological impacts of the technology [14, 16]. For Wolbachia, a panel of national independent experts outside the Eliminate Dengue Project gave a conservative estimate of “negligible risk” in using the bacterium [17].
Although no negative impacts have been recorded, RIDL has faced a mixed public response with accusations of incomplete risk assessment procedures, lack of transparency regarding results, and political agendas. Despite this, RIDL has continued to increase its client base with many visible supporters from the scientific community actively engaging with the public. The WHO has recently begun backing trials of genetically modified (GM) mosquitoes, showing its support for “the use of both old and new approaches to mosquito control as the most immediate line of defence”. On the contrary, being non-GM, Wolbachia technologies have fewer public relation barriers. Their success is also attributed to the thorough engagement and communication campaigns across their target sites and a slow and cautious scaling-up program.
Recommendations
To combat Zika more effectively and quickly, these technologies require more government funding and the highly efficient allocation of resources. This calls for cross-border collaboration among scientists, politicians, and the public, integrating bottom-up, community-led projects with the top-down technological approaches. Initial investment is key for emerging technologies with long-term aspirations at the forefront. Progress can be stifled if bureaucratic barriers are not overcome, having the detrimental effect of creating regulatory deadlocks, draining funds, and wasting time. The risks in not using GM technology should be discussed on par with their use on a timescale that allows for containment of Zika before it reaches remote areas and becomes extremely difficult to manage; this should include conflicts zones, rural segregated populations, and stigmatized communities.
Genetically modified crops, which are grown in 28 countries [18], can serve as a template for Zika regulatory policies, taking on lessons through years of discussion and debate. The technologies are not the final solution, and they face similar problems of resistance to current methods, but they can contribute towards a durable and sustainable control outcome if used in a functional regulatory framework that meshes science with policy. This requires continued spatial modeling, examining the effects of different release patterns across time and space, the costs of different schemes, and effective deployment and monitoring strategies. Theoretical exploration continues with field testing where policymakers can aid scientists to use the technologies on a larger scale.
Moreover, control efforts should complement the distributional severity of the outbreak. In this case, Brazil should be targeted because it is the current Zika epicenter. The current lack of a global strategy is cost-ineffective because both hosts and vectors are spatially mobile, causing endemic infection and seasonally driven outbreaks. Thus, problems of host population reinfection and vector resurgence can continually occur even if local eradication is achieved. Therefore, it is important to bring large-scale technologies such as RIDL and Wolbachia forward in the policy realm. If implemented widely, they not only offer long-term cost-saving benefits, but they also contribute towards community-level protection by reducing the number of transmissible infection pathways.
CONCLUSIONS
Implementation of these policies can provide the technology to vulnerable nations, regardless of their wealth, and accelerates innovation as they develop specific strains for their sites. As demand for these products increases, competitive markets will emerge, leading to higher production standards, larger mosquito-rearing facilities, and regional transport dissemination methods. New technologies for vector control will mainly be utilized in middle- to low-income countries that lack the research infrastructure available elsewhere, and therefore their reliance on international regulatory pathways makes international cooperation critical. If this is not feasible, then it becomes necessary to implement research to commercialization pipelines within borders so that individual countries will have the power to control localized Zika transmission as effectively as possible. However, the global route is more appealing because it shares scientific and labor resources, but this requires the enabling of extensive testing at more field sites and the streamlining of regulation and approval processes.
Acknowledgments
Financial support. B. L. D. and L. R. C. received funding from the Ministry of Health, Singapore (Communicable Disease Public Health Research Grant CDPHRG14NOV007). A. R. C. and J. Y. received funding from the Ministry of Health, Singapore (Communicable Disease Public Health Research Grant CDPHRG12NOV021). A. R. C. also received funding from the Ministry of Defence, Singapore.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Duffy MR, Chen TH, Hancock T et al. . Zika virus outbreak on Yap Island, federated states of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 2.Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 2015; 21:1885–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira Melo AS, Malinger G, Ximenes R et al. . Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016; 47:6–7. [DOI] [PubMed] [Google Scholar]
- 4.Gulland A. Zika virus is a global public health emergency, declares WHO. BMJ 2016; 352:i657. [DOI] [PubMed] [Google Scholar]
- 5.Kraemer MU, Sinka ME, Duda KA et al. . The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015; 4:e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Guardian. Zika virus can be carried by more common mosquito, scientists say. Available at: http://www.theguardian.com/world/2016/mar/03/zika-virus-carried-more-common-mosquito-scientists-say Accessed 7 March 2016.
- 7.Bhatt S, Gething PW, Brady OJ et al. . The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ooi E, Goh K, Gubler DJ. Dengue preventation and 35 years of vector control. Emerg Infect Dis 2006; 12:887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson MP, Su Z, Alphey N et al. . Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system. Proc Natl Acad Sci U S A 2007; 104:9540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakob L, Walker T. Zika virus outbreak in the Americas: the need for novel mosquito control methods. Lancet Glob Health 2016; 4:e148–9. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann AA, Montgomery BL, Popovici J et al. . Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011; 476, 454–61. [DOI] [PubMed] [Google Scholar]
- 12.Lambrechts L, Ferguson NM, Harris E et al. . Assessing the epidemiological effect of Wolbachia for dengue control. Lancet Infect Dis 2015; 15:862–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris A, Nimmo D, McKemey AR et al. . Field performance of engineered male mosquitoes. Nat Biotechnol 2011; 29:1034–7. [DOI] [PubMed] [Google Scholar]
- 14.Oxitec. Aedes aegypti OX513A. Available at: www.oxitec.com/health/our-products/aedes-agypti-ox513a Accessed 13 February 2016.
- 15.Eliminate Dengue Program. Our Research. Available at: www.eliminatedengue.com/our-research Accessed 13 February 2016.
- 16.Nordin O, Donald W, Ming WH et al. . Oral ingestion of transgenic RIDL Ae. Aegypti larvae has no negative effect on two predator Toxorhynchites species. PLoS One 2013; 8:e58805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoc TQ, UyenNinh T, Tuat NV et al. . Risk assessment of the pilot release of Aedes aegypti mosquitoes containing Wolbachia. Available at: www.eliminatedengue.com/library/publication/document/july_2011_ra_report_eng.pdf Accessed 13 February 2016.
- 18.International Service for the Acquisition of Agri-Bioteach Applications. Global Status of Commercialized Biotech/GM Crops: 2014. Available at: https://www.isaaa.org/resources/publications/briefs/49/executivesummary/default.asp. Accessed 16 February 2016.