The median age of HIV-infected adults on ART is progressively increasing, and most of the issues for HIV specialists involve the follow-up of stable patients and screening, prevention and treatment of age-related conditions. Clinical paradigms of long-term treated HIV infected patients are evolving and warrant incorporation of geriatric principles and expertise in prevention and management of cardiovascular disease, cancer screening and other comorbidities.
Keywords: aging, cardiovascular disease, frailty, HIV, polypharmacy
Abstract
In the modern antiretroviral therapy (ART) era, motivated people living with human immunodeficiency virus (HIV) who have access to therapy are expected to maintain viral suppression indefinitely and to receive treatment for decades. Hence, the current clinical scenario has dramatically shifted since the early 1980s, from treatment and prevention of opportunistic infections and palliative care to a new scenario in which most HIV specialists focus on HIV primary care, ie, the follow up of stable patients, surveillance of long-term toxicities, and screening and prevention of age-related conditions. The median age of HIV-infected adults on ART is progressively increasing. By 2030, 3 of every 4 patients are expected to be aged 50 years or older in many countries, more than 80% will have at least 1 age-related disease, and approximately one third will have at least 3 age-related diseases. Contemporary care of HIV-infected patients is evolving, and questions about how we might monitor and perhaps even treat HIV-infected adults have emerged. Through key published works, this review briefly describes the most prevalent comorbidities and age-associated conditions and highlights the differential features in the HIV-infected population. We also discuss the most critical aspects to be considered in the care of patients with HIV for the management and prevention of age-associated disease.
Cardiovascular morbidity and mortality is increasing among people aging with human immunodeficiency virus (HIV) [1]. Several cohort studies have shown that this population exhibits, among other cardiovascular diseases, excess risk of ischemic heart disease and heart failure [2]. Most studies suggest that HIV-infected subjects are at 1.5- to 2-fold-increased risk of acute myocardial infarction (MI) [3, 4], and the impact of cardiovascular disease is likely to increase because the median age of people living with HIV is progressively increasing in most countries with unrestricted access to antiretroviral therapy (ART) [5].
DYSLIPIDEMIA
Lipid abnormalities are frequently found in patients infected with HIV. Patients on ART may show modest improvements of lipids when switching to a regimen with more favorable lipid profile, especially when switching from old boosted protease inhibitors (PIs) to newer ones (ie, darunavir and atazanavir), and especially to integrase inhibitors and rilpivirine, which have more neutral effects on lipids [6]. The mainstay of treatment of dyslipidemia is the use of statins, which have been shown to decrease mortality in cohort studies [7]. In addition, given its anti-inflammatory effects, statins might even prove beneficial for several age-associated conditions such as chronic kidney disease (CKD) [8] and atherosclerosis [9], which seem to be more prevalent in the aging HIV-infected population. Drug interactions with ART is the most common clinical problem. For example, the use of sinvastatin is contraindicated with ritonavir or cobicistat. Atorvastatin, rosuvastatin, and pitavastatin, at starting doses of 10, 10, and 4 mg, respectively, are 3 reasonable options. Pitavastatin might be the drug of choice in patients receiving boosted PIs, given the absence of significant interactions with these agents. The experience with atorvastatin in patients not receiving boosted PIs is wide, and although emerging data suggest that rosuvastatin has positive effects in other surrogate markers of disease progression in HIV-infected patients (ie, carotid intima-media regression [9] and coronary calcium [10]), the potential for mild insulin resistance increase and new-onset diabetes mellitus stills exists [11].
ARTERIAL HYPERTENSION
It is unclear whether HIV infection or its treatment is associated with increased incidence of hypertension [12]. In general, blood pressure measurements slightly rise after ART initiation [13], which might be attributable to the weight gain and improvement of overall status. Special attention is needed when initiating calcium channel blockers, indapamide, and doxazosine given the risk of potential drug-to-drug interactions. Maraviroc is associated with orthostatic hypotension, which needs to be considered when initiating antihypertensive drugs.
ISCHEMIC HEART DISEASE
Primary prevention of cardiovascular events is crucial in the general management of patients infected with HIV. Hence, cardiovascular risk stratification is warranted to define clinical objectives. The previous versions of the American College of Cardiology/American Heart Association (ACC/AHA) guidelines relied on low-density lipoprotein (LDL) cholesterol level thresholds as clinical objectives to be met according to an individual's CVD risk categorization [14]. A number of equations have been calibrated to predict the risk of adverse cardiovascular outcomes in specific populations, such as SCORE, for European individuals. Even more, a risk equation developed from HIV-infected populations is available [15]. Overall, the Framingham risk equation [16] is the most widely implemented, and it classifies patients at high risk when the 10-year predicted risk of MI exceeds 20%. In this situation, the European AIDS Clinical Society recommends an aggressive management of cardiovascular risk factors and proactive ART swithcing to regimens with the most favorable metabolic profile [17].
The 2013 ACC/AHA guidelines have abandoned LDL and non-high-density lipoprotein cholesterol thresholds and goals and instead identify 4 groups likely to benefit from statin therapy. Benefit groups include the following individuals: age 21 or older with clinical atherosclerotic cardiovascular disease (ASCVD); age 21 or older with LDL 190 mg/dL or higher; age 40–75 with diabetes and LDL 70–189 mg/dL; and age 40–75 with a 10-year ASCVD risk score—that is, percentage risk of nonfatal MI, coronary death, nonfatal/fatal stroke within the next 10 years—7.5% or higher by the Pooled Cohort Equations calculator [18].
Given that HIV infection implies an additional, significant CVD risk factor not included in these equations, several studies have addressed the performance of CVD risk equations and collectively suggest that in HIV-infected patients, the equations underestimate the actual risk [15, 19–23]. A recent study evaluated the presence of high-risk morphology coronary plaque without known CVD, and the study found that although the 2013 ACC/AHA cholesterol guidelines recommend statin therapy for a higher percentage of subjects with and without high-risk plaque relative to 2004 guidelines, statin therapy still would not be recommended for the majority (74%) of HIV-infected subjects with high-risk plaques [21]. Although the performance of cardiovascular risk equations might not be optimal and outcome studies are needed to determine the utility of new statin recommendations among HIV-infected subjects, these equations provide at least a starting point for assessing the risk.
Primary prevention of ischemic heart disease is based on the screening and early detection of subclinical disease and aggressive management of cardiovascular risk factors, including exercise and diet modifications, smoking cessation, diagnosis and management of dyslipidemia, diabetes and hypertension, and aspirin use, when indicated. In secondary prevention, besides the strict control of cardiovascular risk factors, antiplatelet and/or anticoagulant therapy, beta blockers, and arterial vasodilators (calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers) are generally recommended. Hence, a number of drugs are commonly needed, and a thorough assessment of potential interactions with ART must be routinely performed [17, 24, 25].
ATRIAL FIBRILLATION
There is no evidence of excess risk of atrial fibrillation or arterial embolization in HIV-infected patients. However, it must be noted that boosted PIs and elvitegravir/cobicistat show clinically relevant interactions with the majority of drugs used for the management of this condition. More importantly, their concomitant use with rivaroxaban is contraindicated given the increased risk of major bleeding. First-generation nonnucleoside reverse-transcriptase inhibitors (efavirenz, etravirine, and nevirapine) also show potential interactions with acenocoumarol, warfarin, rivaroxaban, and clopidogrel, warranting close clinical monitoring. Raltegravir, dolutegravir, and maraviroc do not significantly interact with antiplatelet or anticoagulant agents; therefore, integrase inhibitors are a better choice in these situations.
HEART FAILURE
Human immunodeficiency virus-associated myocardiopathy is a common complication in low-income countries. Although the prevalence of systolic dysfunction has decreased, the prevalence of diastolic dysfunction is increasing after the introduction of highly active ART (HAART) in 1996. There are no specific recommendations for the management of heart failure in the HIV-infected patient beyond the assessment of drug interactions with ART [26]. Diuretics do not show significant interactions with antiretroviral drugs. Boosted PIs significantly increase digoxin levels via P-glycoprotein inhibition [27]. Tolvaptan, a vasopressin V2-receptor antagonist, and eplerenone, an aldosterone receptor antagonist, can potentially interact with antiretroviral drugs with effects on liver CYP3A4 activity.
STROKE
Stroke events have been classically more difficult to analyze in HIV populations because the pathogenesis of cerebral infarction is more diverse than that of MI, and its diagnosis is harder to validate. For coronary heart disease, traditional risk factors have classically been more prevalent among patients infected with HIV, and additional conditions, including infections and substance abuse, might also play a role.
There is evidence suggesting that HIV infection can increase an individual's risk of stroke [28, 29]. Several cohort studies have analyzed specific risk factors among HIV-infected populations. Beyond classic risk factors, the risk appears to be higher among subjects with detectable HIV ribonucleic acid (RNA), low CD4 counts, drug use, non-Hispanic blacks, and, according to recent data, in women [30–32]. In a recent study in Spain, marked differences in stroke incidence trends were reported when HIV-monoinfected and hepatitis C virus (HCV)-coinfected individuals were compared [33]. Stroke incidence was initially much higher in monoinfection and declined throughout the study period, whereas the opposite trend was seen in coinfection. By the end of the study period, incidence of both hemorrhagic and ischemic stroke had become higher in coinfected individuals. Overall, these studies highlight stroke as another very relevant comorbidity in HIV, for which uncontrolled viral load, low CD4, drug use, and HCV infection are important factors. For many other non-acquired immune deficiency syndrome (AIDS)-defining conditions, this risk might approach that of the general population in the subgroup of patients with long-term suppressed viremia and high CD4+ T-cell counts [29, 34]; therefore, beyond the control of traditional risk factors, early ART initiation and prolonged control of HIV replication are critical interventions to decrease the risk of stroke.
NONACQUIRED IMMUNE DEFICIENCY SYNDROME-DEFINING CANCERS
Non-AIDS-defining cancers have emerged as a leading cause of mortality among people aging with HIV and are likely to gain importance in the forthcoming years as the median age of HIV-infected patients continues to increase [5, 35, 36]. It has been estimated that 10% of HIV-infected patients develop cancer [37]. Although during the pre-HAART era AIDS-defining cancers (Kaposi sarcoma, cervical cancer, and non-Hodgkin lymphoma [HL]) were the most prevalent malignancies, several non-AIDS-defining cancers now account for the overall excess risk of cancer [38]. However, not all non-AIDS-defining cancers show increased prevalence in the HIV-infected population. Although some are dramatically increased, ie, anal cancer (from 10-fold to 30-fold higher risk) or lung cancer, melanoma and hepatocellular carcinoma ([HCC] from 2-fold to 5-fold higher risk), for reasons that remain poorly understood HIV-infected patients might be protected against some cancers, ie, prostate, breast, and colorectal cancer [38, 39]. A higher burden of risk factors, such as tobacco use or alcohol consumption, and a higher prevalence of coinfections associated with cancer, such as human papillomavirus, HCV, or Epstein-Barr virus (EBV), are likely determinants of the increased risk of cancer. In contrast, as it has been observed in other inflammatory conditions (ie, rheumatoid arthritis [40]), the sustained proinflammatory state associated with chronic infection might protect against the development of some neoplasias. The incidence and standardized incidence ratios of cancers most commonly diagnosed in HIV-infected individuals in the post-HAART era is summarized in Table 1. Note that the risk of many of these cancers is highly dependent on the risk factors of the population studied (ie, HCV coinfection in the case of liver cancer or tobacco use in the case of lung cancer). In the sections below, we will focus on the non-AIDS-defining cancers whose risk is more prominently increased in treated HIV-infected patients.
Table 1.
Cancer Event-Rates per 100 000 Persons-Year and Standardized Incidence Ratios in HIV-Infected Individuals After 1996
| Event | Events-Rate per 100 000 Persons-Year | Standardized Incidence Ratio |
|---|---|---|
| Any Non-AIDS-defining cancer [37, 39, 41, 42] | 670–724 | 1.7–2.7 |
| Anus [37, 43–46] | 60–130 | 20–79 |
| Hodgkin lymphoma [37, 43, 47, 48] | 47–60 | 11.5–31.7 |
| Lung [42, 44, 45, 48–50] | 64 | 1.1–3.0 |
| Prostate [37, 42, 48] | 60–97 | +0.5 |
| Colorectal [42, 43, 45] | 48 | 0.3–1.4 |
| Liver [39, 42, 43] | 8–26 | 3.0–7.7 |
| Melanoma [41–45, 48, 51] | 10–60 | 0.5–1.5 |
| Oropharyngeal [42, 43, 45, 47, 48] | 37 | 0.9–1.6 |
| Breast cancer [42, 43, 47, 48] | 18 | 0.7–3.2 |
| Cervix [42, 44, 47] | 11–24 | 24 |
| Vagina/vulva [43, 47] | 10–16 | 5.9–6.8 |
Abbreviations: AIDS, acquired immune deficiency syndrome; HIV, human immunodeficiency virus.
Anal Cancer
Albeit uncommon in the general population, anal cancer is an emerging cancer in patients infected with HIV. This excess risk is especially marked in (1) men who have sex with men (MSM), (2) women with a history of receptive anal intercourse, (3) women with a history of abnormal cervical Pap smear, and (4) men and women with genital warts, although there is increased risk of anal cancer regardless sexual orientation [52, 53]. Given the success of cervical cytology in preventing cervical cancer, screening for anal cancer is supported by HIV-specific clinical guidelines with digital rectal examination and anal cytology in at-risk populations, including HIV-infected (1) MSM, (2) women with a history of receptive anal intercourse, (3) women with a history of abnormal cervical Pap smear, and (4) men and women with genital warts. As recommended by experts, high-resolution anoscopy with biopsy should be performed if available in the presence of cytologic abnormalities [17]. High-grade squamous intraepithelial lesions (HSIL) can progress to anal cancer, although the rate of progression is low (estimated in 1 of 377 per year) [54]. Hence, treatment should be offered to patients with anal HSIL, and a variety of modalities are available, including topical therapy, immune modulation with imiquimod, infrared coagulation, and anoscopy-directed lesion ablation. However, given the limitations of current screening methods and the lack of clinical trials assessing the efficacy and safety of current diagnostic and therapeutic approaches, the Centers for Disease Control and Prevention does not routinely recommend anal cancer screening with anal cytology in persons living with HIV [55]. The Anchor Study (NCT02135419) is an ongoing trial of anal cancer screening and prevention, which will address many of the key issues.
Hodgkin Lymphoma
Hodgkin lymphoma is among the most frequent non-AIDS-defining cancers, with an incidence 15- to 30-fold higher than in the general population [56]. Although the risk of other immunodeficiency-related conditions has clearly decreased with the use of ART, it seems, however, that the incidence of HL has not declined among HIV-infected subjects. There is even conflicting evidence suggesting a potential increase of HL in the ART era [57, 58]. Risk factors include advanced infection, with CD4 counts typically below 100 cells/mL or a history of low nadir CD4, and high viral loads. Because most patients infected with HIV show seropositivity for EBV, some HL might be a consequence of persistent defects of the adaptive immunity to control this pathogen, which is able to exert lymphoproliferative effects. Most HIV-associated HL show unfavorable histology, with a predominance (approximately 60% of cases) of mixed cellularity HL [59] and more advanced disease at diagnosis. Treatment of advanced HL includes initiation of ART and the use of a standard chemotherapy regimen such as ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) [60].
Lung Cancer
Patients infected with HIV show approximately 2- to 4-fold increased risk of lung cancer [43, 56, 57, 61]. Tobacco use is the main risk factor for lung cancer in the general population and among patients infected with HIV [62], and the prevalence of smoking is increased in the HIV-infected population [63]. Patients infected with HIV lose more life years through smoking than through HIV [62], but HIV infection is associated with excess risk of cancer even after adjustment for tobacco use [49]. Human immunodeficiency virus infection might also influence the clinical course of lung cancer, because non-small cell lung cancer seems to behave more aggressively among patients infected with HIV [64]. For these reasons, patients infected with HIV can particularly benefit from smoking cessation, which should be aggressively pursued among this cohort as the most effective measure to prevent lung cancer. Many expert groups now advocate for lung cancer screening with annual low-dose computerized tomography in patients with high-risk criteria, ie, age 55 to 74 years, a history of smoking at least 30 pack-years and, if a former smoker, having quit within the previous 15 years [65–68], although there is a significant risk of overdiagnosis and unnecessary invasive studies. Lung cancer screening in HIV-infected patients fulfilling these high-risk criteria might be especially justified given the additional risk independently associated with HIV infection, but there are no specific data for lung cancer screening in patients infected with HIV.
Hepatocellular Carcinoma
The incidence of HCC is 8-fold higher in patients infected with HIV compared with the general population [44], and it is an emerging cancer in different cohorts of patients infected with HIV [69–71]. For example, in Spain the incidence of this cancer increased from 0.1 to 1.1/1000 persons-years between 1999 and 2009 [70]. Compared with individuals with HCV infection alone, HIV/HCV coinfection is associated with accelerated progression of liver fibrosis to cirrhosis and HCC and to excess mortality [72].
All HIV-infected patients should be screened for HCV infection using enzyme immunoassays to detect anti-HCV antibodies. In addition, any HIV-infected patient with cirrhosis, regardless the etiology, should be systematically screened at 6-monthly intervals with hepatic ultrasound or computerized tomography, in the case of nodules [17, 24, 25]. Of note, the first preventive measure for HCC is HCV eradication. Recent data from trials assessing the efficacy of new anti-HCV therapies in HIV/HCV-infected patients suggest that HCV cure would be achieved in the majority of subjects with access to new treatments in a near future [73, 74].
Risk of Drug Interactions Between Chemotherapy and Antiretroviral Therapy During Treatment of Cancer
Initiation of chemotherapy in the HIV-infected patient has classically been challenging given a number of additive toxicities and drug interactions. Because most ART-associated toxicities aggravated with chemotherapy were associated with older drugs (such as neurotoxicity with didanosine and stavudine, bone marrow suppression with zidovudine, or hepatotoxicity with first nucleoside reverse-transcriptase inhibitors zidovudine, didanosine, and stavudine), most problems seen today with modern and safer ART are mainly driven by a wide spectrum of drug interactions with chemotherapy agents given the narrow therapeutic window, or they are determined by the diverse effects of antiretroviral drugs on the cytochrome P450 system as substrates, inhibitors, or inducers. Consultation of reference databases of drug interactions helps to guide clinical decisions [24]. There is a low level of evidence to guide clinical decisions regarding ART and chemotherapy given that HIV-infected patients with cancer have been classically excluded from clinical trials. Nonetheless, it is generally recommended to maintain ART given the significant risks associated with stopping ART [75]. A close collaboration between the HIV specialists and the oncologist is mandatory for the optimal management of these patients. It is common clinical practice to switch ART regimens to minimize drug interactions. Raltegravir, dolutegravir, and maraviroc have low risk of interactions and are reasonable options [76].
KIDNEY DISEASE
There is a higher than expected risk of renal disease among HIV-infected individuals relative to age- and sex-matched populations. Although the risk of acute kidney injury is decreasing, the prevalence and incidence of chronic and end-stage kidney disease is projected to rise, as the prevalence of HIV infection and the median age of the HIV-infected population continues to rise [77, 78]. This excess risk is driven by both HIV-related conditions, including HIV-associated nephropathy, immune complex-mediated glomerulonephritis, drug toxicities, and glomerulonephritis due to HCV coinfection, and by HIV-unrelated conditions, including traditional cardiovascular risk factors (such as diabetes and hypertension) and incomplete recovery from an episode of acute kidney failure [77–79]. Human immunodeficiency virus-related factors for progressive CKD include HCV coinfection and high HIV RNA loads [80]. Although ART seems to slow the rate of renal function decline, tenofovir disoproxil fumarate (TDF) and boosted PIs have been shown to impair kidney function [81–85].
Patients infected with HIV should be screened for early identification of CKD [17, 25, 86]. Individuals infected with HIV should have their glomerular filtration rate (GFR) evaluated at least twice yearly using a creatinine-based estimate. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) GFR equation seems to be the most accurate for use in HIV-positive adults on stable ART [86–88]. Several common drugs in HIV management, including cobicistat, dolutegravir, rilpivirine, and cotrimoxazole, can decrease creatinine tubular secretion, so creatinine changes of <0.1 mg/dL after initiation of these drugs with respect to baseline values would probably lack clinical significance. Patients should also have a urinalysis or quantitative measurement of urine protein excretion at least once yearly. The definitions of the most prevalentmodalities of kidney impairmentin HIV-infected patients are summarized in Table 2. The estimated incidence of acute kidney impairment is approximately 6–10 cases per 100 patients-year in outpatients [89], and it is generally driven by concomitant conditions (fever, sepsis, dehydration).
Table 2.
Definitions to Characterize the Presence of Kidney Function Impairment
| Condition | Definition |
|---|---|
| Acute kidney impairment | Significant (>25%) and rapid (<2–7 d) eGFR decline. It is generally driven by concomitant conditions (fever, sepsis, dehydration) |
| Chronic kidney disease | Estimated eGFR that persists below 60 mL/min/1.73 m2 (CKD stages 1–2) for more than 3 months or the presence of proteinuria (protein/creatinine ratio >300 mg/g) even in the presence of eGFR >60 mL per min/m2 |
| Proximal tubular dysfunction, with or without eGFR impairment | At least 2 of the kidney alterations observed in the Fanconi syndrome: euglycemic glycosuria, phosphaturia, uricosuria, decreased fractional phosphate excretion, and/or uric acid or hypophosphatemia |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
The prevalence of CKD (defined by GFR <60 mL per minute/1.73 m2) among patients infected with HIV in North America and Europe ranges from 4.7% to 9.7%, and higher rates have been reported when CKD was defined by either reduced GFR or proteinuria [82, 90, 91]. Glomerular harm is usually reflected by albuminuria, whereas tubular abnormalities cause low-grade albuminuria, as reflected by an albumin/protein urine ratio <0.4. Albumin-to-creatinine ratio in random urine samples (ideally the first morning sample) is the preferred marker for staging CKD [92].
Proximal tubular dysfunction, with or without estimated GFR (eGFR) impairment, is usually due to direct drug toxicity on tubular epithelial cells, as observed with tenofovir. Tenofovir disoproxil fumarate has been consistently associated with proximal tubular dysfunction and progressive eGFR decline. In the presence of concomitant risk factors for kidney impairment, it is usually challenging to elucidate the extent of renal impairment driven by TDF. Several candidate biomarkers are under evaluation and may help to anticipate TDF-induced proximal tubular dysfunction.
Clinical efforts should focus on the prevention of kidney disease by early detection and aggressive treatment of risk factors, including diabetes, hypertension, and smoking. The clear association between high plasma HIV RNA levels and low CD4+ T-cell counts with excess risk of kidney disease also provides a rationale for early ART initiation. Clinical recommendations for the management of particular situations of kidney impairment are summarized in Table 3.
Table 3.
Clinical Recommendations in the Setting of Kidney Impairment
| Condition | Recommendation |
|---|---|
| Proteinuria with preserved eGFR (>60 mL/min) |
|
| Progressive tubular dysfunction |
|
| Progressive eGFR decline |
|
| Chronic kidney disease with eGFR <60 mL/min |
|
| End-stage kidney disease (eGFR <10 mL/min or dialysis) |
|
| Kidney transplant |
|
Abbreviations: AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitors; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate.
OSTEOPENIA, OSTEOPOROSIS, AND CALCIUM DISORDERS
Osteopenia and Osteoporosis
There is increasing awareness of a higher prevalence of low bone mineral density (BMD) and excess risk of bone fractures among patients infected with HIV, either ART-treated or untreated. In the HIV Outpatient Study (HOPS), a prospective cohort at 10 HIV clinics throughout the United States, age-adjusted fractures were 2.0–3.7 times higher than in the general population [95]. The prevalence of osteopenia and osteoporosis in a meta-analysis including 884 HIV-infected patients demonstrated that 67% had osteopenia and 15% had osteoporosis [96]. The pathogenesis of osteoporosis is usually multifactorial, and traditional risk factors likely act in concert with HIV-related risk factors. Human immunodeficiency virus particles activate osteoclasts, alter vitamin D metabolism, and induce increased systemic inflammation, which negatively impact on BMD [97, 98]. In addition, a biphasic BMD loss is usually appreciated after ART initiation, with a faster decline during the first 6 months and a slower but sustained decay thereafter. Tenofovir elicits greater BMD loss than other antiretrovirals of the same class (such as abacavir). Protease inhibitors might also cause BMD loss, although the evidence supporting this association is weaker [99].
Dual-energy x-ray absorptiometry (DXA) is recommended as the test of choice for screening of osteoporosis in all HIV-infected post-menopausic women and men older than 50 years [100]. The optimal time for rescreening is controversial, and it seems reasonable to base the time on the results of baseline DXA. A diagnostic workup should exclude secondary causes of osteoporosis. Therefore, the following laboratory parameters should be measured: complete blood count, albumin, calcium, phosphorus, creatinine, alanine transaminase, alkaline phosphatase, 25-OH-vitamin D, intact parathyroid hormone (PTH), thyroid-stimulating hormone, morning total testosterone (in men), and 24-hour urine calcium and creatinine. Measuring 25-hydroxyvitamin D levels, which indicate the body's vitamin D stores, may be especially useful given the high prevalence of vitamin D insufficiency reported in the HIV-infected population.
Treatment of osteoporosis and osteopenia does not differ from recommendations in the general population, although it is less supported by the few clinical trials in patients infected with HIV [100]. According to the National Osteoporosis Foundation guidelines, pharmacologic treatment of osteoporosis is recommended for postmenopausal women and men >50 years with a T-score of the femoral neck or lumbar spine less than or equal to −2.5, patients with a history of fragility fracture of the spine or hip, or patients with low bone mass (T-score between −1 and −2.5 at the femoral neck or lumbar spine) and high probability of a major fracture, based on the World Health Organization's Fracture Risk Assessment Tool (FRAX) (a significant risk includes a 10-year probability of fracture that is >3% for the hip or >20% for any major osteoporotic fracture) [101]. Encouraging life-style changes is important to minimize the impact of secondary causes, including avoiding smoking cessation, performing weight-bearing exercise, and consuming a diet rich in calcium and vitamin D. Bisphosphonates are generally considered the first-line pharmacological therapy for osteoporosis [102–104]. There are studies in HIV-infected patients supporting the efficacy and safety of weekly alendronic acid by mouth, and, more recently, annual intravenous zoledronic acid showed efficacy in 2 small studies [105, 106]. However, pharmacologic therapy for osteoporosis in patients infected with HIV warrants careful considerations before initiation, given the lack of prospective data on fracture in this population and the lack of data regarding long-term safety. There is no evidence that switching ART regimen from tenofovir or PI-containing regimens will decrease the risk of bone fractures in those with established osteoporosis, although it seems reasonable to discontinue tenofovir when other options are available.
Hypophosphatemia, Hyperparathyroidism, and Calcium Metabolism Disorders
Impaired parathyroid (PTH) secretion and action has been described in patients infected with HIV [107], independently of vitamin D levels [108]. However, the clinical significance of this observation is uncertain. In contrast, hyperparathyroidism usually reflects vitamin D deficiency (<20 ng/mL) or insufficiency (<30 ng/mL), which can be found in more than two thirds of individuals. In addition to classic risk factors for low vitamin D levels [109], patients initiating ART that includes efavirenz have been found to have a 5 ng/mL reduction in vitamin D levels compared with patients starting ART without efavirenz [110, 111]. Potential mechanisms are efavirenz-induced inhibition of CYP2RI, an enzyme implicated in the 25-hydroxilation of vitamin D, and up-regulation of the catabolism of vitamin D into its inactive metabolites. Hypocalcemia has been described in 6.5% of individuals infected with HIV after adjusting for serum albumin, in relation with vitamin D deficiency [112]. Hyperparathyroidism can also be due to hypophosphatemia, which is relatively common during both untreated (approximately 10%) and treated (20%–30%) HIV disease. Tenofovir disoproxil fumarate has been associated with hypophosphatemia by altering phosphate tubular reabsorption in the setting of proximal tubular dysfunction and Fanconi syndrome. A urine phosphate excretion >100 mg/24 hours and a fractional excretion of phosphate ≥5% indicate phosphate urine loss, which is a cause of secondary hyperparathyroidism (PHT levels >65 pg/mL).
Although the prevalence of vitamin D insufficiency is higher among patients infected with HIV, it is challenging to make recommendations for its monitoring given the lack of evidence showing a clinical benefit of vitamin D supplementation in the absence of low BMD. The Osteo Renal Exchange Program recommends that vitamin D status should be determined by serum 25-hydroxy vitamin D levels in (1) HIV-infected patients with a history of low BMD and/or fracture and in (2) patients with any of the major risk factors for low vitamin D levels, including dark skin, dietary deficiency, avoidance of sun exposure, malabsorption, obesity, CKD, or treatment with regimens containing efavirenz, although the health benefit of identification and correction of vitamin deficiency in these groups is unclear [17]. Supplementary vitamin D should be given to HIV-infected patients with vitamin D insufficiency or deficiency, particularly if the deficiency is associated with compensatory hyperparathyroidism. Vitamin D intake should be titrated to achieve a serum 25-hydroxy vitamin level of approximately 30 ng/mL, and a suitable maintenance dose should be administered thereafter to sustain this level. Vitamin D deficiency can blunt bone response to bisphosphonate treatment; therefore, the target serum 25-hydroxy vitamin D level of 30 ng/mL should be achieved before initiating therapy with an antiresorptive drug [100]. Clinical trials assessing the optimal dose in patients infected with HIV are not available. In accordance with the Institute of Medicine of the National Academies, at least 800 international units of vitamin D should be administered daily, as recommended for older HIV-seronegative adults [113].
HUMAN IMMUNODEFICIENCY VIRUS-ASSOCIATED NEUROCOGNITIVE DISORDERS
Neurocognitive impairment has been commonly observed since the beginning of the AIDS epidemic among HIV-infected individuals. Although more characteristic of advanced stages of the disease, these conditions, ranging from mild neuropsychological deficits to profoundly disabling dementia, can also occur in patients with asymptomatic HIV infection. The pathophysiology of HIV-associated neurocognitive disorders (HAND) is an area of ongoing debate. It has been proposed that brain macrophages, microglial cells, and astrocytes, which can support HIV infection, are involved in the pathogenesis of HAND [114]. Some studies have suggested that even despite years of ART-mediated viral suppression, chronic activation of the innate immunity in the periphery [115, 116] and latent or persistent HIV infection in the brain may sustain chronic macrophage and microglia activation [117, 118]. Whether HIV infection is associated to increased prevalence of HAND independently of ART use or disease stage remains unclear [119–121]. The widespread of ART has been associated with a decrease in the prevalence of more severe neurocognitive deficits. However, overall neurocognitive deficits remain common in various cohort studies (20%–69%) even in the setting of HIV suppression [122, 123].
In clinical settings, HAND is mainly characterized by cognitive deficits, including attention, executive functioning, and speed of informational processing [124]. In 2007, the HIV Neurobehavioral Research Center published a classification scheme commonly referred as the “Frascati criteria”, which has been validated and widely adopted [125], and include the following conditions, for which an alternative cause explaining the neurocognitive defects must be ruled out (Table 4).
Table 4.
Classification Scheme of HAND Based on the “Frascati Criteria”
| Condition | Definition |
|---|---|
| Asymptomatic neurocognitive impairment | A score of at least 1 standard deviation below the mean on at least 2 cognitive areas of standardized neuropsychological testing without this deficit causing an observable functional impairment |
| Mild neurocognitive disorder | A score of one standard deviation below the mean on at least 2 cognitive areas of standardized neuropsychological testing with at least mild impairment of daily functioning |
| HIV-associated dementia | A score of at least 2 standard deviations below the mean on at least 2 cognitive areas of standardized neuropsychological testing with marked associated impairment in activities of daily living |
Abbreviations: HIV, human immunodeficiency virus.
The European AIDS Clinical Society recommends assessing for the presence of symptoms of neurocognitive impairment in all HIV-infected patients with no confounding conditions, and HAND screening can be performed quickly during a clinical visit [17], as described in Table 5.
Table 5.
HAND Screening During a Clinical Visit Recommended by the European AIDS Clinical Societya
| Action | Description |
|---|---|
| Rule out confounding conditions |
|
| Screen for HAND | Three questions:
|
Abbreviations: AIDS, acquired immune deficiency syndrome; HAND, HIV-associated neurocognitive disorders; HIV, human immunodeficiency virus.
a For each question, answers could be as follows: (a) never, (b) hardly ever, or (c) yes, definitely. Persons who are HIV positive are considered to have an “abnormal” result when answering “yes, definitely” on at least 1 question, which would require further evaluation [17].
Effective ART is the principal treatment of HAND. Other issues to be considered during the therapeutic approach include management of associated psychiatric conditions, central nervous system drug penetration, and the possibility of central nervous system viral escape. In the case of ART-treated patients diagnosed with HAND, a diagnostic work-up including magnetic resonance imaging and cerebrospinal fluid (CSF) examination is recommended for assessment for additional causes of HAND. If technically available, some experts recommend measuring HIV RNA and HIV genotype in CSF to evaluate HIV escape (either CSF HIV RNA >50 and plasma HIV RNA <50 c/mL or both CSF and plasma HIV RNA >50 c/mL, with CSF HIV RNA >1 log10 higher than plasma HIV RNA). Given the differential ability of antiretroviral drugs to cross the blood-brain barrier to penetrate the central nervous system, if CSF escape is confirmed, a switch to regimens with greater CSF penetration should be considered. Drugs with demonstrated adequate CSF penetration include zidovudine, abacavir, efavirenz, nevirapine, lopinavir/ritonavir, darunavir/ritonavir, dolutegravir, and maraviroc [17].
MULTIMORBIDITY, POLYPHARMACY, AND FRAILTY
Multimorbidity
As described, patients infected with HIV experience increased risk of a variety of non-AIDS comorbidities, which seem to be accentuated and/or accelerated by HIV [126–129]. Cohort studies have shown that, for a given age stratum, HIV infection represents an independent risk factor for the risk of comorbidities, and the number of comorbidities is higher in treated HIV-infected individuals compared with age- and sex-matched HIV-uninfected population and approach the prevalence of comorbidities observed among persons 10 years older [35, 130]. The increasing awareness of multimorbidity in HIV has raised 2 new concerns: the harm associated with polypharmacy and the development of frailty.
Polypharmacy
Among emerging clinical problems in the aging HIV-infected population, polypharmacy is likely an overlooked source of harm. It has been repeatedly shown how polypharmacy negatively impacts on medication adherence and increases the risk of adverse outcomes, including drug toxicities, drug interactions, hospitalization, geriatric syndromes, and mortality [131, 132]. Although polypharmacy has multiple definitions, it is most commonly considered as (1) the use of 6 or more medications or (2) the use of a potentially inappropriate drug for which the medication does not match the diagnosis. Risk factors for clinically significant drug interactions with ART include regimens with 3 or more antiretroviral drugs or a PI [133]. The consequences of polypharmacy are notable in older subjects living with HIV, and although the actual prevalence of adverse drug events and drug-drug interactions in this subset of patients has not been well studied, the modeling study by Smit et al [5] suggest that in 2030, 40% of patients could have complications with the currently recommended first-line regimens due to drug interactions or to contraindications.
The list of relevant interactions with concomitant ART is overwhelming and beyond the scope of this review. It is common in clinical practice to consult updated databases to check for interactions with ART [24]. The list of medications must be thoroughly reviewed, and a drug simplification effort should be pursued at every visit. In general, many physicians often hesitate to stop medications, especially if other health provider initiated treatment. Simplicity might be a solution, and ideally the number of physicians addressing patient's care should be minimized [131].
Frailty
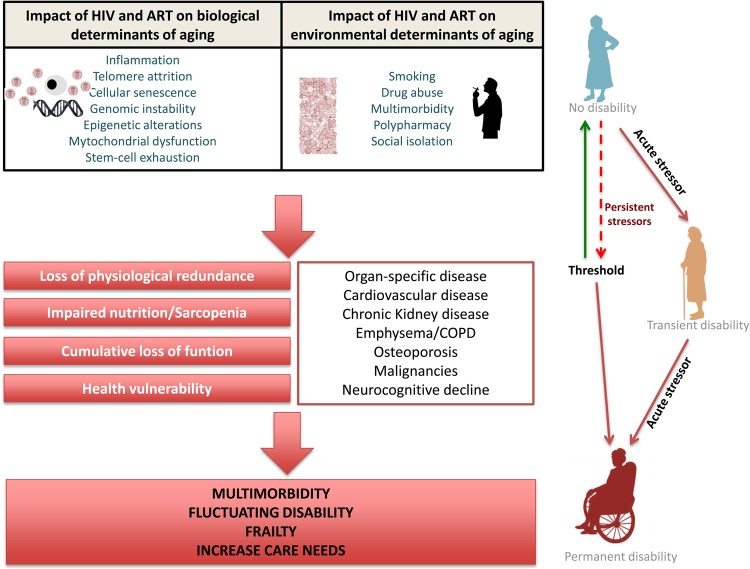
As the HIV-infected population ages, recognition of frailty has become increasingly important. Most definitions of frailty describe a syndrome characterized by loss of function, strength, physiologic reserve, and increased vulnerability to stressors due to age-associated declines across neuromuscular, metabolic, and immune systems [134, 135]. In general, frail subjects often suffer fluctuating disability, falls, delirium, and alterations in mobility, strength, endurance, nutrition, and physical activity [135]. Human immunodeficiency virus disease can contribute to this frail phenotype through direct and indirect effects. The most studied contribution to the aging process implicated the immune systems. Many, but not all, of the immunologic alterations that persist during treated HIV infection are similar to those observed in the elderly [136, 137], and it is now widely accepted that HIV-related immunosenescence contributes to disease progression and adverse outcomes [138, 139]. Mitochondrial dysfunction, a common adverse effect of first antiretrovirals [140], can contribute to sarcopenia [141], which is a common finding among HIV-infected individuals [142]. Inflammation persists in patients on long-term ART, and inflammation is associated with anorexia and catabolism of skeletal muscle and adipose tissue, which might contribute to the nutritional and functional impairment that characterize frailty [143, 144]. The CD4/CD8 ratio, a surrogate marker of immunosenescence, correlates with a frailty phenotype in HIV-infected women [145] and with the risk of non-AIDS-associated mortality [137, 146–148]. Other risk factors for frailty include polypharmacy, multimorbidity, and social isolation. Human immunodeficiency virus disease is now a chronic disease with many of these factors. For example, HIV disease is associated with accumulation of comorbidities in multiple systems, which ultimately lead to geriatric syndromes, including frailty, polypharmacy, and even falls an average of 15–20 years earlier than the general population [130, 149–151]. Hence, treated HIV disease is now a chronic condition with many risk factors for frailty. Several studies have shown that frailty is more frequent in treated HIV disease than in the general population [152, 153]. A hypothesized model illustrating how direct and indirect HIV-effects might impact on the development of frailty is illustrated in Figure 1.
Figure 1.
A model of the pathophysiology of frailty in human immunodeficiency virus (HIV)-disease. ART, antiretroviral therapy; COPD, chronic obstructive pulmonary disease.
Screening of frailty is rarely performed in most HIV clinics. However, the growing burden of HIV disease in geriatric populations [134] might demand new models for healthcare delivery. Screening of frailty would identify patients at high risk of adverse clinical outcomes who might benefit from closer monitoring, with the goal of preventing loss of independence. Over the past several years, a plethora of frailty screening tools have been developed and used to identify elderly at risk of adverse events, such as the 5-item FRAIL (fatigue, resistance, ambulation, illnesses, and loss of weight) questionnaire, to allow physicians to objectively recognize frail individuals [154].
However, there is limited evidence on interventions designed to improve prognosis in frail patients. Exercise is believed to be the best strategy to improve quality of life and functionality in the elderly [155]. Many researchers in the field believe that this simple but difficult to implement intervention might improve clinical outcomes in treated HIV-infected subjects [156]. Recent data have shown that higher physical activity improves psychomotor and executive functioning [157, 158] and inflammatory (interleukin-6, high-sensitivity C-reactive protein), metabolic (leptin), and cardiovascular (hyperemic velocity) markers [159]. Hormonal and supplemental interventions, including testosterone replacement [160], growth hormone [161], and vitamin D [162] supplementation have been investigated in the general population. Overall, data are very limited to recommend its clinical use to attenuate frailty, and none of these interventions have been investigated in patients infected with HIV.
FINAL CONSIDERATIONS
Mounting evidence suggest that persons infected with HIV might have close to normal lifespans [163–165], and the impact of HIV on the ageing process might be smaller than expected [36], especially among patients who initiate ART early [166]. As recently illustrated by the Strategic Timing of Antiretroviral Treatment (START) study, in which early ART initiation was associated with a 57% reduction of both serious AIDS and non-AIDS illnesses, initiating ART soon after HIV infection remains a priority to improve long-term outcomes [167]. The benefits of early ART initiation might be even greater in the subgroup of older patients diagnosed with HIV, who were not well represented in START study, who typically display greater defects in the innate and adaptive immunity repeatedly associated with age-associated conditions [136].
CONCLUSIONS
Still, severe age-associated diseases are highly and increasingly prevalent among treated HIV-infected adults. In the framework of increasing health problems and multimorbidity in the aging HIV population, there is now a vivid debate about how the care should be delivered. Arguably, management of AIDS-defining conditions is no longer the dominant problem in many parts of the world, and HIV care warrants new skills. In the following years, HIV specialists will still require expertise on ART use, yet they will also need specific knowledge in the screening, prevention, and management of age-associated conditions. We think that the HIV specialist should ideally provide integrative care and bring together the screening, prevention, and treatment of most frequent comorbidities. Although this approach is increasingly observed in clinical guidelines [17], it can become unfeasible in routine care due to time and resource constrictions. We propose a model in which at-risk patients, ie, those older than 50 years, with 2 or more comorbidities or polypharmacy periodically undergo a directed and specific assessment (Table 6). In a near future, studies aimed at treating persistent inflammation, such as the REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV; ACTG5332) trial evaluating the effect of statins in HIV-infected subjects with no indication or statin use on the incidence of cardiovascular events or the ACTG5336 to reduce inflammation in treated subjects, will probably shed new light on how to prevent non-AIDS comorbidities linked with chronic HIV.
Table 6.
Proposed Assessment of Comorbidities in Stable HIV-Infected Patients >50 Years
| Assessment | Tool | Follow-up Frequency | Comment |
|---|---|---|---|
| Lifestyle and prevention | |||
| Diet | Dietary survey | Annual | |
| Body composition | Body mass index | Annual | |
| Exercise | Advise to engage in aerobic physical activity to reduce LDL-c, non-HDL-c, and blood pressure. Frequency: 3–4 sessions a week. Intensity: moderate to vigorous. Duration: 40 min on average | ||
| Tobacco use | Guided assessment | At each visit | Pursue smoking cessation in smokers. |
| Alcohol and illicit drugs | Guided assessment | At each visit | |
| Pharmacology | |||
| Polipharmacy | Guided assessment | At each visit | |
| Optimize comedications | Clinical judgment | At each visit | |
| Drug-drug interactions | Interaction checker | At each visit | For example, at www.hiv-druginteractions.org |
| Comorbidities | |||
| Cardiovascular disease | |||
| Risk assessment | Framingham/ASCVD/SCORE | ||
| Hypertension | Blood pressure | Annual | |
| Diabetes | Serum glucose | Annual | Consider oral glucose tolerance test/HbA1c if fasting glucose levels of 5.7–6.9 mmol/L (100–125 mg/dL) |
| Lipids | TC, HDL-c, LDL-c, TG | Annual | |
| Liver disease | |||
| Liver function | ALT/AST, ALP, Bilirubin | ||
| Staging of liver fibrosis | FibroScan, serum fibrosis markers | In HCV and/or HBV-coinfected persons | |
| Portal hypertension assessment | Hepatic ultrasound | 6 mo | In HCV-coinfected persons with liver cirrhosis Child Pugh class A or B and Child Pugh class C awaiting liver transplantation; and in HBV-coinfected persons irrespective of fibrosis stage |
| Pulmonary disease | |||
| Lung Imaging | Chest x-ray | As indicated | |
| Lung function | Spirometry | As indicated | |
| Kidney | |||
| glomerular filtration rate | CKD-EPI | 6 mo | |
| Kidney damage | Urine dipstick analysis, Urine albumin/creatinine ratio, protein/creatinine ratio | Annual | In individuals treated with tenofovir disoproxil fumarate, more specific markers of tubular function (ie, fractional excretions of phosphate and uric acid, urine concentrations of low molecular weight proteins) and more frequent monitoring may be needed, particularly in patients at high risk of renal toxicity. |
| Bone disease | |||
| Bone profile | ALT, calcium, phosphate, vitamin D | 6–12 mo | Due to cost constraints, it is controversial to universally screen vitamin D levels. In patients with low BMD or with tubular dysfunction, it seems reasonable to measure 25-OH-vitamin D levels and, eventually, parathyroid hormone levels before vitamin D supplementation. |
| Osteopenia/osteoporosis | Dual-energy x-ray absorptiometry | Every 10 y if BMD T score <−1.5 SD. Every 5 y if T score >−1.5 to −1.99). Every 1–2 y if T score <−2.5. | Recommended in all HIV-infected patients above 50 y. |
| Neurocognitive impairment | |||
| Screening questionnaire | HIV Dementia Score | 2 y | |
| Depression | |||
| screening questionnaire | PHQ-2 questionnaire | As indicated | |
| Cancer screening | |||
| Anal | Rectal exam, anal citology and eventually high-resolution anoscopy | Annual | In MSM or women with history of high-grade cervical, vulvar, vaginal dysplasia, or cancer. Evidence of benefit unknown. |
| Lung | Low-dose radiation chest scan | Annual | Controversial. Promoting smoking cessation is likely to have a greater impact on cancer prevention. High-risk criteria for participation in the NLST were age 55 to 74 y, a history of smoking at least 30 pack-years and, if a former smoker, had quit within the previous 15 y. Lung cancer is commonly diagnosed earlier in HIV-infected patients, who might benefit from initiating screening at earlier age. |
| Cervical | Cervical Papanicolay | 1–3 y | |
| Breast | Mammography | 1–3 y | |
| Prostate | PSA | 2 y | Controversial. Discuss the small risk reduction against the potential harms. |
| Liver | Ultrasound and α-foetoprotein | 6 mo | Controversial. Patients with cirrhosis and persons with HBV irrespective of fibrosis stage |
| Colorectal | Fecal occult blood testing, sigmoidoscopy, or colonoscopy at age 50 y. The risks and benefits of these screening methods vary. | As indicated | If average risk: screen in >50 y and continue up to 75 y. The risks and benefits depends upon the method used. |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; ASCVD, arteriosclerotic cardiovascular disease; AST, aspartate aminotransferase; BMD, bone mineral density; CKD-EPI, chronic kidney disease epidemiology collaboration; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL-c, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-c, low-density lipoprotein cholesterol; MSM, men who have sex with men; NLST, National Lung Screening Trial; PHQ-2, Patient Health Questionaire-2; PSA, prostate-specific antigen; SD; standard deviation; TC, total cholesterol; TG, triglycerides.
Acknowledgments
Potential conflicts of interest. S. S.-V. has received honoraria for the following: speaking at symposia organized on behalf of ViiV Healthcare and Janssen; developing educational materials for MSD and Gilead; and board membership from Gilead. A. R. and S. M. have received honoraria for the following: speaking at symposia organized on behalf of Janssen, Abbvie, Gilead, MSD, ViiV, and BMS; developing educational materials for Janssen, Abbvie, Gilead, MSD, ViiV, and BMS; research from Janssen, Abbvie, Gilead, MSD, ViiV, and BMS; and board membership from Janssen, Abbvie, Gilead, MSD, ViiV, and BMS. J. B. has received the following: research grants from Abbvie, BMS, GILEAD, MSD and ViiV; and honoraria for advisory boards and talks from Abbvie, BMS, GILEAD, Janssen, MSD, and ViiV. F. G. has received funds for the following: speaking at symposia organized on behalf of ViiV, Janssen, Gilead, and BMS; developing educational materials for ViiV; research from MSD; and board membership from ViiV, Janssen, Gilead, and BMS. E. M. has received research funding from lecture sponsorships and has served on advisory boards for MSD and Janssen. C. M. has received funds for the following: speaking at symposia organized on behalf of Gilead, MSD, Abbvie, ViiV, and Janssen; developing educational materials for Merck; and board membership from Janssen, Gilead, and Merck. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
PANEL OF EXPERTS CONSULTED FOR THE ELABORATION OF THIS REVIEW
Ignacio Bernardino, Hospital La Paz, Madrid, Spain
José Ramón Blanco, Hospital San Pedro, La Rioja, Spain
José Luis Casado, Hospital Ramón y Cajal, Madrid, Spain.
Vicente Estrada, Hospital Clínico San Carlos.
Josefa Galindo, Hospital La Fe, Valencia, Spain.
Carmen Hidalgo, Hospital Virgen de las Nieves, Granada, Spain
Fernando Lozano, Hospital de Valme, Sevilla, Spain.
Julián Olalla, Hospital Costa del Sol, Málaga, Spain.
Enric Pedrol, Hospital de Figureres, Gerona, Spain.
Eugenia Negredo, Hospital Germans Trias I Pujol, Barcelona, Spain.
José Sanz Moreno, Hospital Príncipe de Asturias, Madrid, Spain.
Rosario Palacios, Hospital de Málaga, Málaga, Spain.
Carmen Ricart, Hospital Dr. Peset, Valencia, Spain.
Rafael Rubio, Hospital Doce de Octubre, Madrid, Spain.
Eulalia Valencia, Hospital La Paz, Madrid, Spain.
References
- 1.Smith C, Sabin CA, Lundgren JD et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010; 24:1537–48. [DOI] [PubMed] [Google Scholar]
- 2.Butt AA, Chang CC, Kuller L et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011; 171:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CC, Kuller LH et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smit M, Brinkman K, Geerlings S et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 3099:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currier JS. Management of cardiovascular risk (including dyslipidemia) in the HIV-infected patient. UpToDate, Post TW. (ed), UptoDate, Waltham, MA: 2015. [Google Scholar]
- 7.Lang S, Lacombe JM, Mary-Krause M et al. Is impact of statin therapy on all-cause mortality different in HIV-infected individuals compared to general population? Results from the FHDH-ANRS CO4 cohort. PLoS One 2015; 10:e0133358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longenecker CT, Hileman CO, Funderburg NT et al. Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: the SATURN-HIV trial. Clin Infect Dis 2014; 59:1148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longenecker CT, Jian Y, Debanne SM, McComsey GA. Rosuvastatin arrests progression of carotid intima-media thickness in treated HIV. In: Conference on Retroviruses and Opportunistic Infections Seattle, Washington 23–26 February 2015 (Abstract 137). [Google Scholar]
- 10.Lo J, Lu MT, Ihenachor EJ et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015; 2:e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Danielson E, Fonseca FA et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–207. [DOI] [PubMed] [Google Scholar]
- 12.Dillon DG, Gurdasani D, Riha J et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol 2013; 42:1754–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seaberg EC, Muñoz A, Lu M et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 2005; 19:953–60. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Bairey Merz CN et al. Implications of recent clinical trials for the National Cholesterol Education Program adult treatment panel III guidelines. Circulation 2004; 110:227–39. [DOI] [PubMed] [Google Scholar]
- 15.Friis-Møller N, Thiébaut R, Reiss P et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil 2010; 17:491–501. [DOI] [PubMed] [Google Scholar]
- 16.D'Agostino RB, Vasan RS, Pencina MJ et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117:743–53. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines of the European AIDS Clinical Society. EACS Guidelines 2015; 48:206. [Google Scholar]
- 18.Stone NJ, Robinson JG, Lichtenstein AH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129 (25 Suppl 2):1–45. [DOI] [PubMed] [Google Scholar]
- 19.Law MG, Friis-Møller N, El-Sadr WM et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med 2006; 7:218–30. [DOI] [PubMed] [Google Scholar]
- 20.Serrano-Villar S, Estrada V, Gomez-Garre D et al. Diagnosis of subclinical atherosclerosis in HIV-infected patients: higher accuracy of the D:A:D risk equation over Framingham and SCORE algorithms. Eur J Prev Cardiol 2014; 21:739–48. [DOI] [PubMed] [Google Scholar]
- 21.Zanni MV, Fitch KV, Feldpausch M et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS 2014; 28:2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chew KW, Bhattacharya D, McGinnis KA et al. Short Communication: coronary heart disease risk by Framingham risk score in hepatitis C and HIV/hepatitis C-coinfected persons. AIDS Res Hum Retroviruses 2015; 31:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinstein MJ, Drozd DR, Ning H et al. Observed versus predicted myocardial infarction among individuals with human immunodeficiency virus by American College of Cardiology/American Heart Association pooled cohort equation risk strata: center for AIDS research network of integrated clinical systems. Available at: http://circ.ahajournals.org/content/132/Suppl_3/A17992.short Accessed 22 December 2015. [Google Scholar]
- 24.Liverpool HIV Pharmacology Group (LHPG). Drug Interact Charts 2015. Available at: http://www.hiv-druginteractions.org/. Accessed 8 November 2015. [Google Scholar]
- 25.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. DHSS: Department of Health and Human Services agencies, 2015. Available at: http://aidsinfo.nih.gov/guidelines. Accessed 8 November 2015. [Google Scholar]
- 26.Remick J, Georgiopoulou V, Marti C et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation 2014; 129:1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt C, Kaeser B, Riek M et al. Effect of saquinavir/ritonavir on P-glycoprotein activity in healthy volunteers using digoxin as a probe. Int J Clin Pharmacol Ther 2010; 48:192–9. [DOI] [PubMed] [Google Scholar]
- 28.d'Arminio A, Sabin CA, Phillips AN et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS 2004; 18:1811–7. [DOI] [PubMed] [Google Scholar]
- 29.Marcus JL, Leyden WA, Chao CR et al. HIV infection and incidence of ischemic stroke. AIDS 2014; 28:1911–9. [DOI] [PubMed] [Google Scholar]
- 30.Chow F, Wilson MR, Wu K et al. Stroke incidence highest in women and black HIV-infected participants in ALLRT cohort. In: Conference on Retroviruses and Opportunistic Infections 22–25 February 2016 Boston, Massachusetts (Abstract 43). [Google Scholar]
- 31.Chow F, Regan S, Looby SE et al. Persistently increased ischemic stroke risk in HIV-infected women. In: Conference on Retroviruses and Opportunistic Infections 22–25 February 2016 Boston, Massachusetts (Abstract 638). [Google Scholar]
- 32.Crane HM, Chow F, Becker KJ et al. Design, implementation, and findings of next generation stroke adjudication in HIV. In: Conference on Retroviruses and Opportunistic Infections 22–25 February 2016 Boston, Massachusetts (Abstract 636). [Google Scholar]
- 33.Berenguer J, Álvaro-Meca A, Díaz A et al. Stroke in HIV-infected patients in the combination antiretroviral therapy era. In: Conference on Retroviruses and Opportunistic Infections 22–25 February 2016 Boston, Massachusetts (Abstract 639). [Google Scholar]
- 34.Chow FC, Bacchetti P, Kim AS et al. Effect of CD4+ cell count and viral suppression on risk of ischemic stroke in HIV infection. AIDS 2014; 28:2573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schouten J, Wit FW, Stolte IG et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen LD, May MT, Kronborg G et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV 2015; 2:e288–98. [DOI] [PubMed] [Google Scholar]
- 37.Crum-Cianflone N, Hullsiek KH, Marconi V et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS 2009; 23:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigel K, Dubrow R, Silverberg M et al. Cancer screening in patients infected with HIV. Curr HIV/AIDS Rep 2011; 8:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiels M, Cole S, Poole C et al. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 2009; 52:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther 2008; 10:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engels EA, Pfeiffer RM, Goedert JJ et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS 2006; 20:1645–54. [DOI] [PubMed] [Google Scholar]
- 42.Silverberg MJ, Chao C, Leyden WA et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009; 23:2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370:59–67. [DOI] [PubMed] [Google Scholar]
- 44.Patel P, Hanson DL, Sullivan PS et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008; 148:728–36. [DOI] [PubMed] [Google Scholar]
- 45.Van Leeuwen MT, Vajdic CM, Middleton MG et al. Continuing declines in some but not all HIV-associated cancers in Australia after widespread use of antiretroviral therapy. AIDS 2009; 23:2183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverberg MJ, Lau B, Achenbach CJ et al. Cumulative incidence of cancer among persons with HIV in North America. Ann Intern Med 2015; 163:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salters K, Cescon A, Zhang W et al. Cancer incidence among HIV-positive women in British Columbia, Canada: heightened risk of virus-related malignancies. HIV Med 2016; 17:188–95. [DOI] [PubMed] [Google Scholar]
- 48.Herida M, Mary-Krause M, Kaphan R et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol 2003; 21:3447–53. [DOI] [PubMed] [Google Scholar]
- 49.Kirk GD, Merlo C, O'Driscoll P et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis 2007; 45:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiels MS, Cole SR, Wegner S et al. Effect of HAART on incident cancer and noncancer AIDS events among male HIV seroconverters. J Acquir Immune Defic Syndr 2008; 48:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsen CM, Knight LL, Green AC. Risk of melanoma in people with HIV/AIDS in the pre- and post-HAART eras: a systematic review and meta-analysis of cohort studies. PLoS One 2014; 9:e95096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverberg MJ, Lau B, Justice AC et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis 2012; 54:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piketty C, Cochand-Priollet B, Lanoy E et al. Lack of regression of anal squamous intraepithelial lesions despite immune restoration under cART. AIDS 2013; 27:401–6. [DOI] [PubMed] [Google Scholar]
- 54.Machalek DA, Poynten M, Jin F et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13:487–500. [DOI] [PubMed] [Google Scholar]
- 55.Park IU, Introcaso C, Dunne EF. Human papillomavirus and genital warts: a review of the evidence for the 2015 centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 2015; 61(Suppl 8):S849–55. [DOI] [PubMed] [Google Scholar]
- 56.Deeken JF, Tjen-A-Looi A, Rudek MA et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis 2012; 55:1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powles T, Robinson D, Stebbing J et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 2009; 27:884–90. [DOI] [PubMed] [Google Scholar]
- 58.Clifford GM, Rickenbach M, Lise M et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood 2009; 113:5737–42. [DOI] [PubMed] [Google Scholar]
- 59.Hentrich M, Berger M, Wyen C et al. Stage-adapted treatment of HIV-associated Hodgkin lymphoma: results of a prospective multicenter study. J Clin Oncol 2012; 30:4117–23. [DOI] [PubMed] [Google Scholar]
- 60.Bower M, Collins S, Cottrill C et al. British HIV Association guidelines for HIV-associated malignancies 2008. HIV Med 2008; 9:336–88. [DOI] [PubMed] [Google Scholar]
- 61.Franceschi S, Lise M, Clifford GM et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer 2010; 103:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Helleberg M, May MT, Ingle SM et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America: The ART Cohort Collaboration. AIDS 2015; 29:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clifford GM, Lise M, Franceschi S et al. Lung cancer in the Swiss HIV Cohort Study: role of smoking, immunodeficiency and pulmonary infection. Br J Cancer 2012; 106:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigel K, Crothers K, Dubrow R et al. Prognosis in HIV-infected patients with non-small cell lung cancer. Br J Cancer 2013; 109:1974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(5 Suppl):e78S–92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wender R, Fontham ET, Barrera E et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013; 63:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Church TR, Black WC, Aberle DR et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013; 368:1980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.US Preventive Services Task Force. Lung Cancer: Screening. Summary of Recommendation and Evidence. Available at: http://www.uspreventiveservicestaskforce.org/page/document/updatesummaryfinal/lung-cancer-screening. Accessed 10 November 2015.
- 69.Bräu N, Fox RK, Xiao P et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol 2007; 47:527–37. [DOI] [PubMed] [Google Scholar]
- 70.Merchante N, Merino E, López-Aldeguer J et al. Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin Infect Dis 2013; 56:143–50. [DOI] [PubMed] [Google Scholar]
- 71.Salmon-Ceron D, Rosenthal E, Lewden C et al. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol 2009; 50:736–45. [DOI] [PubMed] [Google Scholar]
- 72.Weber R, Sabin CA, Friis-Møller N et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 73.Sulkowski MS, Eron JJ, Wyles D et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1. JAMA 2015; 313:1223–31. [DOI] [PubMed] [Google Scholar]
- 74.Osinusi A, Townsend K, Kohli A et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA 2015; 313:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodger AJ, Lodwick R, Schechter M et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 2013; 27:973–9. [DOI] [PubMed] [Google Scholar]
- 76.Rudeck M, Ambinder R, Flexner C, Deeken J. Systemic therapy for malignancy in patients on anti-retroviral medications. UpToDate, Post TW. (ed), UptoDate Waltham, MA: 2015. [Google Scholar]
- 77.Lucas GM, Ross MJ, Stock PG et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e96–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rasch MG, Helleberg M, Feldt-Rasmussen B et al. Increased risk of dialysis and end-stage renal disease among HIV patients in Denmark compared with the background population. Nephrol Dial Transplant 2014; 29:1232–8. [DOI] [PubMed] [Google Scholar]
- 79.Szczech LA, Gange SJ, Van Der Horst C et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int 2002; 61:195–202. [DOI] [PubMed] [Google Scholar]
- 80.Peters L, Grint D, Lundgren JD et al. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS 2012; 26:1917–26. [DOI] [PubMed] [Google Scholar]
- 81.Kalayjian RC, Lau B, Mechekano RN et al. Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS 2012; 26:1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mocroft A, Kirk O, Gatell J et al. Chronic renal failure among HIV-1-infected patients. AIDS 2007; 21:1119–27. [DOI] [PubMed] [Google Scholar]
- 83.Mocroft A, Kirk O, Reiss P et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS 2010; 24:1667–78. [DOI] [PubMed] [Google Scholar]
- 84.Scherzer R, Estrella M, Li Y et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 2012; 26:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ryom L, Mocroft A, Kirk O et al. Predictors of advanced chronic kidney disease and end-stage renal disease in HIV-positive persons. AIDS 2014; 28:187–99. [DOI] [PubMed] [Google Scholar]
- 86.George E, Lucas GM, Nadkarni GN et al. Kidney function and the risk of cardiovascular events in HIV-1-infected patients. AIDS 2010; 24:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vrouenraets SM, Fux CA, Wit FW et al. A comparison of measured and estimated glomerular filtration rate in successfully treated HIV-patients with preserved renal function. Clin Nephrol 2012; 77:311–20. [DOI] [PubMed] [Google Scholar]
- 88.Inker LA, Wyatt C, Creamer R et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. J Acquir Immune Defic Syndr 2012; 61:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wikman P, Safont P, Del Palacio M et al. The significance of antiretroviral-associated acute kidney injury in a cohort of ambulatory human immunodeficiency virus-infected patients. Nephrol Dial Transplant 2013; 28:2073–81. [DOI] [PubMed] [Google Scholar]
- 90.Fernando SK, Finkelstein FO, Moore BA, Weissman S. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci 2008; 335:89–94. [DOI] [PubMed] [Google Scholar]
- 91.Choi AI, Rodriguez RA, Bacchetti P et al. Racial differences in end-stage renal disease rates in HIV infection versus diabetes. J Am Soc Nephrol 2007; 18:2968–74. [DOI] [PubMed] [Google Scholar]
- 92.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3:4–4. [DOI] [PubMed] [Google Scholar]
- 93.Roland ME, Barin B, Carlson L et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant 2008; 8:355–65. [DOI] [PubMed] [Google Scholar]
- 94.Stock PG, Barin B, Murphy B et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med 2010; 363:2004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Young B, Dao CN, Buchacz K et al. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis 2011; 52:1061–8. [DOI] [PubMed] [Google Scholar]
- 96.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006; 20:2165–74. [DOI] [PubMed] [Google Scholar]
- 97.Bedimo R, Cutrell J, Zhang S et al. Mechanisms of bone disease in HIV and hepatitis C virus: impact of bone turnover, tenofovir exposure, sex steroids and severity of liver disease. AIDS 2016; 30:601–8. [DOI] [PubMed] [Google Scholar]
- 98.Warriner AH, Mugavero M, Overton ET. Bone alterations associated with HIV. Curr HIV/AIDS Rep 2014; 11:233–40. [DOI] [PubMed] [Google Scholar]
- 99.Brown TT, Moser C, Currier JS et al. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 2015; 212:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown TT, Hoy J, Borderi M et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis 2015; 60:1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cosman F, de Beur SJ, LeBoff MS et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25:2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mondy K, Powderly WG, Claxton SA et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr 2005; 38:426–31. [DOI] [PubMed] [Google Scholar]
- 103.Guaraldi G, Orlando G, Madeddu G et al. Alendronate reduces bone resorption in HIV-associated osteopenia/osteoporosis. HIV Clin Trials 2004; 5:269–77. [DOI] [PubMed] [Google Scholar]
- 104.McComsey GA, Kendall MA, Tebas P et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007; 21:2473–82. [DOI] [PubMed] [Google Scholar]
- 105.Negredo E, Bonjoch A, Pérez-Álvarez N et al. Comparison of two different strategies of treatment with zoledronate in HIV-infected patients with low bone mineral density: single dose versus two doses in 2 years. HIV Med 2015; 16:441–8. [DOI] [PubMed] [Google Scholar]
- 106.Bolland MJ, Grey A, Horne AM et al. Effects of intravenous zoledronate on bone turnover and bone density persist for at least five years in HIV-infected men. J Clin Endocrinol Metab 2012; 97:1922–8. [DOI] [PubMed] [Google Scholar]
- 107.Hellman P, Albert J, Gidlund M et al. Impaired parathyroid hormone release in human immunodeficiency virus infection. AIDS Res Hum Retroviruses 1994; 10:391–4. [DOI] [PubMed] [Google Scholar]
- 108.Havens PL, Stephensen CB, Hazra R et al. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis 2012; 54:1013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lerma E, Molas ME, Montero MM et al. Prevalence and factors associated with vitamin D deficiency and hyperparathyroidism in HIV-infected patients treated in Barcelona. ISRN AIDS 2012; 2012:485307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther 2010; 15:425–9. [DOI] [PubMed] [Google Scholar]
- 111.Allavena C, Delpierre C, Cuzin L et al. High frequency of vitamin D deficiency in HIV-infected patients: effects of HIV-related factors and antiretroviral drugs. J Antimicrob Chemother 2012; 67:2222–30. [DOI] [PubMed] [Google Scholar]
- 112.Kuehn EW, Anders HJ, Bogner JR et al. Hypocalcaemia in HIV infection and AIDS. J Intern Med 1999; 245:69–73. [DOI] [PubMed] [Google Scholar]
- 113.Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine Consensus Report. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed 8 November 2015. [Google Scholar]
- 114.González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol 2005; 5:69–81. [DOI] [PubMed] [Google Scholar]
- 115.Lyons JL, Uno H, Ancuta P et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 2011; 57:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burdo TH, Lo J, Abbara S et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Edén A, Price RW, Spudich S et al. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis 2007; 196:1779–83. [DOI] [PubMed] [Google Scholar]
- 118.Kamat A, Lyons JL, Misra V et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 2012; 60:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McDonnell J, Haddow L, Daskalopoulou M et al. Minimal cognitive impairment in UK HIV-positive men who have sex with men: effect of case definitions and comparison with the general population and HIV-negative men. J Acquir Immune Defic Syndr 2014; 67:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Crum-Cianflone NF, Moore DJ, Letendre S et al. Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology 2013; 80:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mateen FJ, Shinohara RT, Carone M et al. Neurologic disorders incidence in HIV+ vs HIV− men: Multicenter AIDS Cohort Study, 1996–2011. Neurology 2012; 79:1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simioni S, Cavassini M, Annoni JM et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010; 24:1243–50. [DOI] [PubMed] [Google Scholar]
- 123.Robertson KR, Smurzynski M, Parsons TD et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21:1915–21. [DOI] [PubMed] [Google Scholar]
- 124.Schouten J, Cinque P, Gisslen M et al. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS 2011; 25:561–75. [DOI] [PubMed] [Google Scholar]
- 125.Antinori A, Arendt G, Becker JT et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Effros RB, Fletcher CV, Gebo K et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 2008; 47:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 2013; 69:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Althoff KN, McGinnis KA, Wyatt CM et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2014; 60:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brothers TD, Rockwood K. Biologic aging, frailty, and age-related disease in chronic HIV infection. Curr Opin HIV AIDS 2014; 9:412–8. [DOI] [PubMed] [Google Scholar]
- 130.Guaraldi G, Orlando G, Zona S et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 131.Gleason LJ, Luque AE, Shah K. Polypharmacy in the HIV-infected older adult population. Clin Interv Aging 2013; 8:749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Edelman EJ, Gordon KS, Glover J et al. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging 2013; 30:613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miller CD, El-Kholi R, Faragon JJ, Lodise TP. Prevalence and risk factors for clinically significant drug interactions with antiretroviral therapy. Pharmacotherapy 2007; 27:1379–86. [DOI] [PubMed] [Google Scholar]
- 134.Walston J, Hadley EC, Ferrucci L et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001. [DOI] [PubMed] [Google Scholar]
- 135.Clegg A, Young J, Iliffe S et al. Frailty in elderly people. Lancet 2013; 381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serrano-Villar S, Sainz T, Lee SA et al. HIV-Infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Papagno L, Spina CA, Marchant A et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol 2004; 2:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012; 24:501–6. [DOI] [PubMed] [Google Scholar]
- 140.Benbrik E, Chariot P, Bonavaud S et al. Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured human muscle cells. J Neurol Sci 1997; 149:19–25. [DOI] [PubMed] [Google Scholar]
- 141.Hiona A, Sanz A, Kujoth GC et al. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One 2010; 5:e11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Buehring B, Kirchner E, Sun Z, Calabrese L. The frequency of low muscle mass and its overlap with low bone mineral density and lipodystrophy in individuals with HIV--a pilot study using DXA total body composition analysis. J Clin Densitom 2012; 15:224–32. [DOI] [PubMed] [Google Scholar]
- 143.Erlandson KM, Allshouse AA, Jankowski CM et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 2013; 208:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 2006; 119:526.e9–17. [DOI] [PubMed] [Google Scholar]
- 145.Terzian AS, Holman S, Nathwani N et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009; 18:1965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Serrano-Villar S, Pérez-Elías MJ, Dronda F et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 2014; 9:e85798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mussini C, Lorenzini P, Cozzi-lepri A et al. CD4/CD8 ratio normalisation and non-AIDS related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–106. [DOI] [PubMed] [Google Scholar]
- 148.May M. Association of CD4:CD8 with cause-specific mortality in patients on long term ART. In: Conference of Retrovirus and Opportunistic Infections Seattle, Washington, 23-26 February 2015. (Abstract 579). [Google Scholar]
- 149.Pathai S, Lawn SD, Gilbert CE et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. AIDS 2013; 27:2375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hasse B, Ledergerber B, Furrer H et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53:1130–9. [DOI] [PubMed] [Google Scholar]
- 151.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Desquilbet L, Jacobson LP, Fried LP et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol Ser A Biol Sci Med Sci 2007; 62:1279–86. [DOI] [PubMed] [Google Scholar]
- 153.Onen NF, Agbebi A, Shacham E et al. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect 2009; 59:346–52. [DOI] [PubMed] [Google Scholar]
- 154.Morley JE, Vellas B, van Kan GA et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Keysor JJ. Does late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidence. Am J Prev Med 2003; 25:129–36. [DOI] [PubMed] [Google Scholar]
- 156.d'Ettorre G, Ceccarelli G, Giustini N et al. Taming HIV-related inflammation with physical activity: a matter of timing. AIDS Res Hum Retroviruses 2014; 30:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Monroe A, Zhang L, Jacobson LP et al. The impact of physical activity on cognition in men with and without HIV. In: Conference on Retroviruses and Opportunistic Infections Seattle, Washington 23–26 February 2015 (Abstract 489). [Google Scholar]
- 158.Basco B, Ortega M, Ances B et al. Aerobic exercise attenuates cognitive decline and brain volume loss associated with HIV. In: Conference on Retroviruses and Opportunistic Infections Seattle, Washington 23–26 February 2015 (Abstract 488). [Google Scholar]
- 159.Dirajlal-Fargo S, Webel AR, Kinley B et al. The effect of physical activity on cardiometabolic health and inflammation in HIV. In: Conference on Retroviruses and Opportunistic Infections Seattle, Washington 23–26 February 2015. (Abstract 745). [Google Scholar]
- 160.Tenover JS. Androgen replacement therapy to reverse and/or prevent age-associated sarcopenia in men. Baillieres Clin Endocrinol Metab 1998; 12:419–25. [DOI] [PubMed] [Google Scholar]
- 161.Lamberts SW. The somatopause: to treat or not to treat? Horm Res 2000; 53(Suppl 3):42–3. [DOI] [PubMed] [Google Scholar]
- 162.Morley JE, Kim MJ, Haren MT. Frailty and hormones. Rev Endocr Metab Disord 2005; 6:101–8. [DOI] [PubMed] [Google Scholar]
- 163.Young J, Psichogiou M, Meyer L et al. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med 2012; 9:e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lewden C, Gabillard D, Minga A et al. CD4-specific mortality rates among HIV-infected adults with high CD4 counts and no antiretroviral treatment in West Africa. J Acquir Immune Defic Syndr 2012; 59:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Samji H, Cescon A, Hogg RS et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wada NI, Jacobson LP, Margolick JB et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lundgren JD, Babiker AG, Gordin F et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]